The Gut Microbiome Role in Multiple Myeloma: Emerging Insights and Therapeutic Opportunities
Abstract
1. Introduction
1.1. Multiple Myeloma
1.2. The Gut Microbiome
2. Methods
3. Microbiome and Hematologic Cancers
3.1. Role of the Microbiome in Hematological Cancers
3.2. Systemic Immunity and Inflammation
3.3. The Effect of Gut Microbiome on the Immune System
3.4. Link Between the Gut Microbiota and Other Cancers
4. Interplay Between the Gut Microbiome and Multiple Myeloma
4.1. Microbiota Alteration in Multiple Myeloma
4.2. Microbiota-Derived Metabolites in Multiple Myeloma
4.2.1. Short-Chain Fatty Acids
4.2.2. L-Glutamine
4.3. Microbiota and Immune Regulation in Multiple Myeloma
5. Role of the Microbiome in Multiple Myeloma Treatment
5.1. Microbiome-Based Therapies in Multiple Myeloma
5.1.1. Fecal Microbiota Transplantation (FMT)
| Therapy/Exposure Type | Type (Clinical/Preclinical) | Design | Sample Size (n) | Population/Model | Intervention/Exposure | Outcomes Measured | Key Findings | Adverse Events/Safety | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Autologous FMT | Clinical | Prospective feasibility study | 7 | Adults undergoing HSCT | Re-infusion of the patient’s own pre-treatment stool (autologous FMT) | Feasibility, gut microbiota restoration, incidence of infections, GVHD, engraftment | Auto-FMT was feasible, safe, and restored gut microbiota diversity with early clinical benefits. | No FMT-related serious adverse events; overall well-tolerated | [97] |
| Fecal microbiota diversity changes after auto-HCT | Clinical | Prospective, multicenter observational cohort study | 1325 | Adults undergoing autologous HCT for hematologic malignancies (incl. multiple myeloma, lymphoma) | Fecal microbiome profiling (16S rRNA sequencing) before and after auto-HCT | Microbiota diversity (Shannon index), associations with overall survival, relapse, infectious complications | Gut microbiota diversity loss after auto-HCT predicted poorer survival and higher non-relapse mortality. | Not applicable (observational, sequencing only) | [98] |
| Gut microbiome perturbation and ASCT outcomes | Clinical | Prospective observational pilot study | 30 | Patients with multiple myeloma undergoing ASCT | Longitudinal fecal microbiome profiling (16S rRNA sequencing) during ASCT | Microbial diversity, engraftment, infectious complications, treatment response | Post-ASCT microbial diversity loss delayed engraftment, increased infections, and altered treatment response. | Not applicable (observational, sequencing only) | [41] |
| Gut microbiome alterations in MM | Clinical | Cross-sectional case–control study | 37 | Newly diagnosed MM patients vs. age-matched controls | Stool microbiome sequencing (16S rRNA & metagenomics) | Microbiome composition, metabolic pathways, and nitrogen metabolism | MM patients showed nitrogen-recycling bacteria enrichment, potentially accelerating disease progression. | Not applicable (observational, sequencing only) | [64] |
| Gut microbiome diversity and HSCT outcomes | Clinical | Prospective observational cohort study | 80 | Adults undergoing allogeneic HSCT (incl. MM) | Longitudinal fecal microbiome profiling (16S rRNA sequencing) | GVHD incidence, overall survival, and relapse rates | Higher gut microbial diversity post-HSCT predicted lower mortality and reduced GVHD incidence. | Not applicable (observational, sequencing only) | [99] |
| Microbiota diversity and allo-HSCT survival | Clinical | Multicenter observational cohort study (US, EU) | 1362 | Hematologic malignancies (incl. MM) undergoing allo-HSCT | Fecal microbiome profiling (16S rRNA sequencing) | Overall survival, GVHD, infections | Microbial diversity loss strongly predicted increased mortality post-HSCT. | Not applicable (observational, sequencing only) | [100] |
5.1.2. Probiotics and Prebiotics
5.1.3. Personalized Medicine
5.2. Safety, Regulation, and Interactions of Microbiota with Standard MM Therapies
6. Limitations of Current Studies
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, J.; Lu, Y.; Wei, W.; Ma, G.; Li, C.; Li, L.; Wang, Y.; Wang, Y.; Xu, R.; Cui, S. Ferroptosis: A novel pharmacological mechanism against multiple myeloma. Front. Pharmacol. 2025, 16, 1606804. [Google Scholar] [CrossRef]
- Liu, J.; Xie, F.; Yi, Z.G.; Ma, T.; Tie, W.T.; Li, Y.H.; Bai, J.; Zhang, L.S. Gut microbiota deficiency ameliorates multiple myeloma and myeloma-related bone disease by Th17 cells in mice models. J. Cancer 2023, 14, 3191–3202. [Google Scholar] [CrossRef]
- Parrondo, R.D.; Ailawadhi, S.; Cerchione, C. Bispecific antibodies for the treatment of relapsed/refractory multiple myeloma: Updates and future perspectives. Front. Oncol. 2024, 14, 1394048. [Google Scholar] [CrossRef]
- van de Donk, N.; Pawlyn, C.; Yong, K.L. Multiple myeloma. Lancet 2021, 397, 410–427. [Google Scholar] [CrossRef]
- Cerchione, C.; Usmani, S.Z.; Stewart, A.K.; Kaiser, M.; Rasche, L.; Kortum, M.; Mateos, M.V.; Spencer, A.; Sonneveld, P.; Anderson, K.C. Gene Expression Profiling in Multiple Myeloma: Redefining the Paradigm of Risk-Adapted Treatment. Front. Oncol. 2022, 12, 820768. [Google Scholar] [CrossRef]
- Malard, F.; Neri, P.; Bahlis, N.J.; Terpos, E.; Moukalled, N.; Hungria, V.T.M.; Manier, S.; Mohty, M. Multiple myeloma. Nat. Rev. Dis. Primers 2024, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef] [PubMed]
- Abduh, M.S. An overview of multiple myeloma: A monoclonal plasma cell malignancy’s diagnosis, management, and treatment modalities. Saudi J. Biol. Sci. 2024, 31, 103920. [Google Scholar] [CrossRef]
- Kyle, R.A.; Larson, D.R.; Therneau, T.M.; Dispenzieri, A.; Kumar, S.; Cerhan, J.R.; Rajkumar, S.V. Long-Term Follow-up of Monoclonal Gammopathy of Undetermined Significance. N. Engl. J. Med. 2018, 378, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Qu, Y.; Wang, M.; Chu, B.; Chen, W.; Zheng, Y.; Niu, T.; Qian, Z. Pathogenesis and treatment of multiple myeloma. MedComm 2022, 3, e146. [Google Scholar] [CrossRef]
- Al-Mansoori, L.; Al-Jaber, H.; Prince, M.S.; Elrayess, M.A. Role of Inflammatory Cytokines, Growth Factors and Adipokines in Adipogenesis and Insulin Resistance. Inflammation 2022, 45, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Habib, C.N.; Ali, A.E.; Anber, N.H.; George, M.Y. Lactoferrin ameliorates carfilzomib-induced renal and pulmonary deficits: Insights to the inflammasome NLRP3/NF-kappaB and PI3K/Akt/GSK-3beta/MAPK axes. Life Sci. 2023, 335, 122245. [Google Scholar] [CrossRef]
- Zakaria, N.; Menze, E.T.; Elsherbiny, D.A.; Tadros, M.G.; George, M.Y. Lycopene mitigates paclitaxel-induced cognitive impairment in mice; Insights into Nrf2/HO-1, NF-kappaB/NLRP3, and GRP-78/ATF-6 axes. Prog. Neuropsychopharmacol. Biol. Psychiatry 2025, 137, 111262. [Google Scholar] [CrossRef]
- Morgan, G.J.; He, J.; Tytarenko, R.; Patel, P.; Stephens, O.W.; Zhong, S.; Deshpande, S.; Bauer, M.; Weinhold, N.; Schinke, C.; et al. Kinase domain activation through gene rearrangement in multiple myeloma. Leukemia 2018, 32, 2435–2444. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Ali, T.A.; Faiyaz, A.; Khan, O.S.; Raza, S.S.; Kulinski, M.; Omri, H.E.; Bhat, A.A.; Uddin, S. Cytokine-Mediated Dysregulation of Signaling Pathways in the Pathogenesis of Multiple Myeloma. Int. J. Mol. Sci. 2020, 21, 5002. [Google Scholar] [CrossRef]
- Garcia-Ortiz, A.; Rodriguez-Garcia, Y.; Encinas, J.; Maroto-Martin, E.; Castellano, E.; Teixido, J.; Martinez-Lopez, J. The Role of Tumor Microenvironment in Multiple Myeloma Development and Progression. Cancers 2021, 13, 217. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Liao, M.; Bai, J.; Li, Y.; Chen, Y.; Zhang, L.; Guo, X.; Li, L.; Zhang, L. Exploring the causal relationship between gut microbiota and multiple myeloma risk based on Mendelian randomization and biological annotation. Front. Microbiol. 2024, 15, 1310444. [Google Scholar] [CrossRef]
- Padala, S.A.; Barsouk, A.; Barsouk, A.; Rawla, P.; Vakiti, A.; Kolhe, R.; Kota, V.; Ajebo, G.H. Epidemiology, Staging, and Management of Multiple Myeloma. Med. Sci. 2021, 9, 3. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Zhang, L.; Xiang, Y.; Li, Y.; Zhang, J. Gut microbiome in multiple myeloma: Mechanisms of progression and clinical applications. Front. Immunol. 2022, 13, 1058272. [Google Scholar] [CrossRef]
- Rosinol, L.; Oriol, A.; Rios, R.; Sureda, A.; Blanchard, M.J.; Hernandez, M.T.; Martinez-Martinez, R.; Moraleda, J.M.; Jarque, I.; Bargay, J.; et al. Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood 2019, 134, 1337–1345. [Google Scholar] [CrossRef]
- Botta, C.; Martino, E.A.; Conticello, C.; Mendicino, F.; Vigna, E.; Romano, A.; Palumbo, G.A.; Cerchione, C.; Martinelli, G.; Morabito, F.; et al. Treatment of Lenalidomide Exposed or Refractory Multiple Myeloma: Network Meta-Analysis of Lenalidomide-Sparing Regimens. Front. Oncol. 2021, 11, 643490. [Google Scholar] [CrossRef] [PubMed]
- George, M.Y.; Dabour, M.S.; Rashad, E.; Zordoky, B.N. Empagliflozin Alleviates Carfilzomib-Induced Cardiotoxicity in Mice by Modulating Oxidative Stress, Inflammatory Response, Endoplasmic Reticulum Stress, and Autophagy. Antioxidants 2024, 13, 671. [Google Scholar] [CrossRef]
- Dabour, M.S.; George, M.Y.; Grant, M.K.O.; Zordoky, B.N. Canagliflozin differentially modulates carfilzomib-induced endoplasmic reticulum stress in multiple myeloma and endothelial cells. Arch. Toxicol. 2025, 99, 729–744. [Google Scholar] [CrossRef]
- Durer, C.; Durer, S.; Lee, S.; Chakraborty, R.; Malik, M.N.; Rafae, A.; Zar, M.A.; Kamal, A.; Rosko, N.; Samaras, C.; et al. Treatment of relapsed multiple myeloma: Evidence-based recommendations. Blood Rev. 2020, 39, 100616. [Google Scholar] [CrossRef] [PubMed]
- Group, N.H.W.; Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; et al. The NIH Human Microbiome Project. Genome Res. 2009, 19, 2317–2323. [Google Scholar] [CrossRef]
- Muguerza-Rodriguez, L.; Mier, A.; Ponce-Gonzalez, J.G.; Casals, C.; Corral-Perez, J. Systematic Review on the Importance of Gut Microbiota in the Regulation of Type 2 Diabetes Through Physical Activity and Exercise. Curr. Issues Mol. Biol. 2025, 47, 505. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Gamal, N.K.; El-Naga, R.N.; Ayoub, I.M.; George, M.Y. Neuromodulatory effect of troxerutin against doxorubicin and cyclophosphamide-induced cognitive impairment in rats: Potential crosstalk between gut-brain and NLRP3 inflammasome axes. Int. Immunopharmacol. 2025, 149, 114216. [Google Scholar] [CrossRef]
- Xu, K.; Motiwala, Z.; Corona-Avila, I.; Makhanasa, D.; Alkahalifeh, L.; Khan, M.W. The Gut Microbiome and Its Multifaceted Role in Cancer Metabolism, Initiation, and Progression: Insights and Therapeutic Implications. Technol. Cancer Res. Treat. 2025, 24, 1–15. [Google Scholar] [CrossRef]
- Fattizzo, B.; Cavallaro, F.; Folino, F.; Barcellini, W. Recent insights into the role of the microbiome in malignant and benign hematologic diseases. Crit. Rev. Oncol. Hematol. 2021, 160, 103289. [Google Scholar] [CrossRef]
- Reddy, B.S.; Narisawa, T.; Wright, P.; Vukusich, D.; Weisburger, J.H.; Wynder, E.L. Colon carcinogenesis with azoxymethane and dimethylhydrazine in germ-free rats. Cancer Res. 1975, 35, 287–290. [Google Scholar] [PubMed]
- Dapito, D.H.; Mencin, A.; Gwak, G.Y.; Pradere, J.P.; Jang, M.K.; Mederacke, I.; Caviglia, J.M.; Khiabanian, H.; Adeyemi, A.; Bataller, R.; et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 2012, 21, 504–516. [Google Scholar] [CrossRef]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.B.; Zhou, Y.L.; Fang, J.Y. Gut Microbiota in Cancer Immune Response and Immunotherapy. Trends Cancer 2021, 7, 647–660. [Google Scholar] [CrossRef]
- Ahmed, N.; Ghannoum, M.; Gallogly, M.; de Lima, M.; Malek, E. Influence of gut microbiome on multiple myeloma: Friend or foe? J. Immunother. Cancer 2020, 8, e000576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gu, J.; Liu, J.; Huang, B.; Li, J. Fecal Microbiota Taxonomic Shifts in Chinese Multiple Myeloma Patients Analyzed by Quantitative Polimerase Chain Reaction (QPCR) and 16S rRNA High-Throughput Sequencing. Med. Sci. Monit. 2019, 25, 8269–8280. [Google Scholar] [CrossRef]
- Calcinotto, A.; Brevi, A.; Chesi, M.; Ferrarese, R.; Garcia Perez, L.; Grioni, M.; Kumar, S.; Garbitt, V.M.; Sharik, M.E.; Henderson, K.J.; et al. Microbiota-driven interleukin-17-producing cells and eosinophils synergize to accelerate multiple myeloma progression. Nat. Commun. 2018, 9, 4832. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, A.; Arroyo, A.; Garcia-Vicente, R.; Morales, M.L.; Gomez-Gordo, R.; Justo, P.; Cuellar, C.; Sanchez-Pina, J.; Lopez, N.; Alonso, R.; et al. Short-Chain Fatty Acid Production by Gut Microbiota Predicts Treatment Response in Multiple Myeloma. Clin. Cancer Res. 2024, 30, 904–917. [Google Scholar] [CrossRef]
- D’Angelo, C.R.; Sudakaran, S.; Callander, N.S. Clinical effects and applications of the gut microbiome in hematologic malignancies. Cancer 2021, 127, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Jung, J.; Lee, J.A.; Lee, E.; Lee, H.; Eom, H.S.; Park, H.J. Understanding gut Microbiome changes in Korean children, adolescents, and young adults with hematologic malignancies. Ann. Hematol. 2025, 104, 2947–2961. [Google Scholar] [CrossRef]
- Helmink, B.A.; Khan, M.A.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The microbiome, cancer, and cancer therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef]
- Kim, D.; Zeng, M.Y.; Núñez, G. The interplay between host immune cells and gut microbiota in chronic inflammatory diseases. Exp. Mol. Med. 2017, 49, e339. [Google Scholar] [CrossRef]
- Deshmukh, H.S.; Liu, Y.; Menkiti, O.R.; Mei, J.; Dai, N.; O’Leary, C.E.; Oliver, P.M.; Kolls, J.K.; Weiser, J.N.; Worthen, G.S. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat. Med. 2014, 20, 524–530. [Google Scholar] [CrossRef]
- Purcell, R.V.; Pearson, J.; Aitchison, A.; Dixon, L.; Frizelle, F.A.; Keenan, J.I. Colonization with enterotoxigenic Bacteroides fragilis is associated with early-stage colorectal neoplasia. PLoS ONE 2017, 12, e0171602. [Google Scholar] [CrossRef]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 Protein of Fusobacterium nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors from Immune Cell Attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum Potentiates Intestinal Tumorigenesis and Modulates the Tumor-Immune Microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef]
- Wu, H.-J.; Ivanov, I.I.; Darce, J.; Hattori, K.; Shima, T.; Umesaki, Y.; Littman, D.R.; Benoist, C.; Mathis, D. Gut-Residing Segmented Filamentous Bacteria Drive Autoimmune Arthritis via T Helper 17 Cells. Immunity 2010, 32, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Ohnmacht, C.; Park, J.-H.; Cording, S.; Wing, J.B.; Atarashi, K.; Obata, Y.; Gaboriau-Routhiau, V.; Marques, R.; Dulauroy, S.; Fedoseeva, M.; et al. The microbiota regulates type 2 immunity through RORγt+T cells. Sciences (Am. Assoc. Adv. Sci.) 2015, 349, 989–993. [Google Scholar] [CrossRef]
- He, Z.; Gharaibeh, R.Z.; Newsome, R.C.; Pope, J.L.; Dougherty, M.W.; Tomkovich, S.; Pons, B.; Mirey, G.; Vignard, J.; Hendrixson, D.R.; et al. Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin. Gut 2019, 68, 289–300. [Google Scholar] [CrossRef]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum Promotes Colorectal Carcinogenesis by Modulating E-Cadherin/β-Catenin Signaling via its FadA Adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.N.; Araújo-Pérez, F.; Azcárate-Peril, A.; Yeh, J.J.; Sandler, R.S.; Keku, T.O. Fusobacterium Is Associated with Colorectal Adenomas. PLoS ONE 2013, 8, e53653. [Google Scholar] [CrossRef] [PubMed]
- Mima, K.; Nakagawa, S.; Sawayama, H.; Ishimoto, T.; Imai, K.; Iwatsuki, M.; Hashimoto, D.; Baba, Y.; Yamashita, Y.-i.; Yoshida, N.; et al. The microbiome and hepatobiliary-pancreatic cancers. Cancer Lett. 2017, 402, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.M.; Fox, J.G.; Anver, M.R.; Haines, D.C.; George, C.V.; Collins, M.J.; Gorelick, P.L.; Nagashima, K.; Gonda, M.A.; Gilden, R.V.; et al. Chronic Active Hepatitis and Associated Liver Tumors in Mice Caused by a Presistent Bacterial Infection With a Novel Helicobacter Species. JNCI J. Natl. Cancer Inst. 1994, 86, 1222–1227. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, X.G.; Wang, Z.M.; Zhou, J.H.; Tian, X.F.; Li, N. Identification of helicobacter species in human liver samples from patients with primary hepatocellular carcinoma. J. Clin. Pathol. 2004, 57, 1273–1277. [Google Scholar] [CrossRef]
- Grąt, M.; Wronka, K.M.; Krasnodębski, M.; Masior, Ł.; Lewandowski, Z.; Kosińska, I.; Grąt, K.; Stypułkowski, J.; Rejowski, S.; Wasilewicz, M.; et al. Profile of Gut Microbiota Associated with the Presence of Hepatocellular Cancer in Patients with Liver Cirrhosis. Transplant. Proc. 2016, 48, 1687–1691. [Google Scholar] [CrossRef]
- Scanu, T.; Spaapen, R.M.; Bakker, J.M.; Pratap, C.B.; Wu, L.-E.; Hofland, I.; Broeks, A.; Shukla, V.K.; Kumar, M.; Janssen, H.; et al. Salmonella Manipulation of Host Signaling Pathways Provokes Cellular Transformation Associated with Gallbladder Carcinoma. Cell Host Microbe 2015, 17, 763–774. [Google Scholar] [CrossRef]
- Farrell, J.J.; Zhang, L.; Zhou, H.; Chia, D.; Elashoff, D.; Akin, D.; Paster, B.J.; Joshipura, K.; Wong, D.T.W. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 2012, 61, 582–588. [Google Scholar] [CrossRef]
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human oral microbiome and prospective risk for pancreatic cancer: A population-based nested case-control study. Gut 2018, 67, 120–127. [Google Scholar] [CrossRef]
- Pianko, M.J.; Golob, J.L. Host-microbe interactions and outcomes in multiple myeloma and hematopoietic stem cell transplantation. Cancer Metastasis Rev. 2022, 41, 367–382. [Google Scholar] [CrossRef]
- Jian, X.; Zhu, Y.; Ouyang, J.; Wang, Y.; Lei, Q.; Xia, J.; Guan, Y.; Zhang, J.; Guo, J.; He, Y.; et al. Alterations of gut microbiome accelerate multiple myeloma progression by increasing the relative abundances of nitrogen-recycling bacteria. Microbiome 2020, 8, 74. [Google Scholar] [CrossRef]
- Konishi, H.; Saito, T.; Takahashi, S.; Tanaka, H.; Okuda, K.; Akutsu, H.; Dokoshi, T.; Sakatani, A.; Takahashi, K.; Ando, K.; et al. The butyrate derived from probiotic Clostridium butyricum exhibits an inhibitory effect on multiple myeloma through cell death induction. Sci. Rep. 2025, 15, 11919. [Google Scholar] [CrossRef] [PubMed]
- Pianko, M.J.; Devlin, S.M.; Littmann, E.R.; Chansakul, A.; Mastey, D.; Salcedo, M.; Fontana, E.; Ling, L.; Tavitian, E.; Slingerland, J.B.; et al. Minimal residual disease negativity in multiple myeloma is associated with intestinal microbiota composition. Blood Adv. 2019, 3, 2040–2044. [Google Scholar] [CrossRef]
- Khanna, S.; Tosh, P.K. A Clinician’s Primer on the Role of the Microbiome in Human Health and Disease. Mayo Clin. Proc. 2014, 89, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Chen, D.; Zhang, K.; Zhang, W.; Liu, T.; Wang, S.; Dai, X.; Wang, B.; Zhong, W.; Cao, H. Gut microbiota-derived short-chain fatty acids and colorectal cancer: Ready for clinical translation? Cancer Lett. 2022, 526, 225–235. [Google Scholar] [CrossRef]
- Mirzaei, R.; Afaghi, A.; Babakhani, S.; Sohrabi, M.R.; Hosseini-Fard, S.R.; Babolhavaeji, K.; Khani Ali Akbari, S.; Yousefimashouf, R.; Karampoor, S. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed. Pharmacother. 2021, 139, 111619. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Park, J.; Kim, M. Gut Microbiota-Derived Short-Chain Fatty Acids, T Cells, and Inflammation. Immune Netw. 2014, 14, 277. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef]
- Kodama, M.; Nakayama, K.I. A second Warburg-like effect in cancer metabolism: The metabolic shift of glutamine-derived nitrogen. BioEssays 2020, 42, 2000169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bai, C.; Ruan, Y.; Liu, M.; Chu, Q.; Qiu, L.; Yang, C.; Li, B. Coordinative metabolism of glutamine carbon and nitrogen in proliferating cancer cells under hypoxia. Nat. Commun. 2019, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Bolzoni, M.; Chiu, M.; Accardi, F.; Vescovini, R.; Airoldi, I.; Storti, P.; Todoerti, K.; Agnelli, L.; Missale, G.; Andreoli, R.; et al. Dependence on glutamine uptake and glutamine addiction characterize myeloma cells: A new attractive target. Blood 2016, 128, 667–679. [Google Scholar] [CrossRef]
- Chiu, M.; Toscani, D.; Marchica, V.; Taurino, G.; Costa, F.; Bianchi, M.G.; Andreoli, R.; Franceschi, V.; Storti, P.; Burroughs-Garcia, J.; et al. Myeloma Cells Deplete Bone Marrow Glutamine and Inhibit Osteoblast Differentiation Limiting Asparagine Availability. Cancers 2020, 12, 3267. [Google Scholar] [CrossRef]
- Niess, J.H.; Reinecker, H.-C. Dendritic cells in the recognition of intestinal microbiota. Cell. Microbiol. 2006, 8, 558–564. [Google Scholar] [CrossRef]
- Rescigno, M. Intestinal Dendritic Cells. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 2010; pp. 109–138. [Google Scholar] [CrossRef]
- Knochelmann, H.M.; Dwyer, C.J.; Bailey, S.R.; Amaya, S.M.; Elston, D.M.; Mazza-McCrann, J.M.; Paulos, C.M. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell. Mol. Immunol. 2018, 15, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Kageyama, T.; Fang, V.; Kedmi, R.; Martinez, C.S.; Talbot, J.; Chen, A.; Cabrera, I.; Gorshko, O.; Kurakake, R.; et al. Redundant cytokine requirement for intestinal microbiota-induced Th17 cell differentiation in draining lymph nodes. Cell Rep. 2021, 36, 109608. [Google Scholar] [CrossRef]
- Chang, S.H. T helper 17 (Th17) cells and interleukin-17 (IL-17) in cancer. Arch. Pharmacal Res. 2019, 42, 549–559. [Google Scholar] [CrossRef]
- Rossi, M.; Altomare, E.; Botta, C.; Gallo Cantafio, M.E.; Sarvide, S.; Caracciolo, D.; Riillo, C.; Gaspari, M.; Taverna, D.; Conforti, F.; et al. miR-21 antagonism abrogates Th17 tumor promoting functions in multiple myeloma. Leukemia 2021, 35, 823–834. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Holstein, S.A.; McCarthy, P.L. Immunomodulatory Drugs in Multiple Myeloma: Mechanisms of Action and Clinical Experience. Drugs 2017, 77, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Colley, A.; Brauns, T.; Sluder, A.E.; Poznansky, M.C.; Gemechu, Y. Immunomodulatory drugs: A promising clinical ally for cancer immunotherapy. Trends Mol. Med. 2024, 30, 765–780. [Google Scholar] [CrossRef]
- Nobels, A.; van Marcke, C.; Jordan, B.F.; Van Hul, M.; Cani, P.D. The gut microbiome and cancer: From tumorigenesis to therapy. Nat. Metab. 2025, 7, 895–917. [Google Scholar] [CrossRef]
- Rastogi, S.; Singh, A. Gut microbiome and human health: Exploring how the probiotic genus Lactobacillus modulate immune responses. Front. Pharmacol. 2022, 13, 1042189. [Google Scholar] [CrossRef]
- Martino, M.; Canale, F.A.; Alati, C.; Vincelli, I.D.; Moscato, T.; Porto, G.; Loteta, B.; Naso, V.; Mazza, M.; Nicolini, F.; et al. CART-Cell Therapy: Recent Advances and New Evidence in Multiple Myeloma. Cancers 2021, 13, 2639. [Google Scholar] [CrossRef]
- Nicolini, F.; Bravaccini, S.; Mazza, M.; Gruszka, A.M.; Tazzari, M.; MartIn-Antonio, B.; Juan, M.; Ibrahim, T.; Maltoni, R.; Martinelli, G.; et al. CAR T cells targeting options in the fight against multiple myeloma. Panminerva Med. 2021, 63, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Swan, D.; Madduri, D.; Hocking, J. CAR-T cell therapy in Multiple Myeloma: Current status and future challenges. Blood Cancer J. 2024, 14, 206. [Google Scholar] [CrossRef]
- Schoultz, I.; Claesson, M.J.; Dominguez-Bello, M.G.; Fak Hallenius, F.; Konturek, P.; Korpela, K.; Laursen, M.F.; Penders, J.; Roager, H.; Vatanen, T.; et al. Gut microbiota development across the lifespan: Disease links and health-promoting interventions. J. Intern. Med. 2025, 297, 560–583. [Google Scholar] [CrossRef]
- Shen, Y.; Fan, N.; Ma, S.X.; Cheng, X.; Yang, X.; Wang, G. Gut Microbiota Dysbiosis: Pathogenesis, Diseases, Prevention, and Therapy. MedComm (2020) 2025, 6, e70168. [Google Scholar] [CrossRef] [PubMed]
- Vinterberg, J.E.; Oddsdottir, J.; Nye, M.; Pinton, P. Management of Recurrent Clostridioides difficile Infection (rCDI): A Systematic Literature Review to Assess the Feasibility of Indirect Treatment Comparison (ITC). Infect. Dis. Ther. 2025, 14, 327–355. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.S.; Kang, Z.R.; Chen, Y.X.; Fang, J.Y. The gut microbiome modulate response to immunotherapy in cancer. Sci. China Life Sci. 2025, 68, 381–396. [Google Scholar] [CrossRef]
- Lei, W.; Zhou, K.; Lei, Y.; Li, Q.; Zhu, H. Gut microbiota shapes cancer immunotherapy responses. npj Biofilms Microbiomes 2025, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Daillere, R.; Vetizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.M.; Flament, C.; Lepage, P.; Roberti, M.P.; et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity 2016, 45, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Chirlaque, C.; Aranda, C.J.; Ocon, B.; Capitan-Canadas, F.; Ortega-Gonzalez, M.; Carrero, J.J.; Suarez, M.D.; Zarzuelo, A.; Sanchez de Medina, F.; Martinez-Augustin, O. Germ-free and Antibiotic-treated Mice are Highly Susceptible to Epithelial Injury in DSS Colitis. J. Crohns Colitis 2016, 10, 1324–1335. [Google Scholar] [CrossRef]
- Li, A.; Bowen, J.M.; Ball, I.A.; Wilson, S.; Yong, A.; Yeung, D.T.; Lee, C.H.; Bryant, R.V.; Costello, S.P.; Ryan, F.J.; et al. Autologous Faecal Microbiota Transplantation to Improve Outcomes of Haematopoietic Stem Cell Transplantation: Results of a Single-Centre Feasibility Study. Biomedicines 2023, 11, 3274. [Google Scholar] [CrossRef]
- Khan, N.; Lindner, S.; Gomes, A.L.C.; Devlin, S.M.; Shah, G.L.; Sung, A.D.; Sauter, C.S.; Landau, H.J.; Dahi, P.B.; Perales, M.A.; et al. Fecal microbiota diversity disruption and clinical outcomes after auto-HCT: A multicenter observational study. Blood 2021, 137, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Taur, Y.; Coyte, K.; Schluter, J.; Robilotti, E.; Figueroa, C.; Gjonbalaj, M.; Littmann, E.R.; Ling, L.; Miller, L.; Gyaltshen, Y.; et al. Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci. Transl. Med. 2018, 10, 460. [Google Scholar] [CrossRef]
- Peled, J.U.; Gomes, A.L.C.; Devlin, S.M.; Littmann, E.R.; Taur, Y.; Sung, A.D.; Weber, D.; Hashimoto, D.; Slingerland, A.E.; Slingerland, J.B.; et al. Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2020, 382, 822–834. [Google Scholar] [CrossRef]
- Routy, B.; Lenehan, J.G.; Miller, W.H., Jr.; Jamal, R.; Messaoudene, M.; Daisley, B.A.; Hes, C.; Al, K.F.; Martinez-Gili, L.; Puncochar, M.; et al. Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: A phase I trial. Nat. Med. 2023, 29, 2121–2132. [Google Scholar] [CrossRef]
- Ding, Y.; Hou, Y.; Lao, X. The Role of Akkermansia muciniphila in Disease Regulation. Probiotics Antimicrob. Proteins 2025, 17, 2027–2038. [Google Scholar] [CrossRef]
- Al-Adham, I.S.I.; Agha, A.; Al-Akayleh, F.; Al-Remawi, M.; Jaber, N.; Al Manasur, M.; Collier, P.J. Prebiotics Beyond the Gut: Omics Insights, Artificial Intelligence, and Clinical Trials in Organ-Specific Applications. Probiotics Antimicrob. Proteins 2025, 17, 2500–2521. [Google Scholar] [CrossRef]
- Hughes, R.L.; Alvarado, D.A.; Swanson, K.S.; Holscher, H.D. The Prebiotic Potential of Inulin-Type Fructans: A Systematic Review. Adv. Nutr. 2022, 13, 492–529. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Eze, U.J.; Lal, A.; Elkoush, M.I.; Halytska, M.; Atif, S. Recurrent Lactobacillus Rhamnoses Bacteremia and Complications in an Immunocompromised Patient with History of Probiotic Use: A Case Report. Cureus 2024, 16, e54879. [Google Scholar] [CrossRef]
- Devi, L.S.; Broor, S.; Rautela, R.S.; Grover, S.S.; Chakravarti, A.; Chattopadhya, D. Increasing Prevalence of Escherichia coli and Klebsiella pneumoniae Producing CTX-M-Type Extended-Spectrum Beta-Lactamase, Carbapenemase, and NDM-1 in Patients from a Rural Community with Community Acquired Infections: A 3-Year Study. Int. J. Appl. Basic Med. Res. 2020, 10, 156–163. [Google Scholar] [CrossRef]
- Aghamajidi, A.; Maleki Vareki, S. The Effect of the Gut Microbiota on Systemic and Anti-Tumor Immunity and Response to Systemic Therapy against Cancer. Cancers 2022, 14, 3563. [Google Scholar] [CrossRef]
- Zaplana, T.; Miele, S.; Tolonen, A.C. Lachnospiraceae are emerging industrial biocatalysts and biotherapeutics. Front. Bioeng. Biotechnol. 2023, 11, 1324396. [Google Scholar] [CrossRef] [PubMed]
- Kriss, M.; Hazleton, K.Z.; Nusbacher, N.M.; Martin, C.G.; Lozupone, C.A. Low diversity gut microbiota dysbiosis: Drivers, functional implications and recovery. Curr. Opin. Microbiol. 2018, 44, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Alkharabsheh, O.; Sidiqi, M.H.; Aljama, M.A.; Gertz, M.A.; Frankel, A.E. The Human Microbiota in Multiple Myeloma and Proteasome Inhibitors. Acta Haematol. 2020, 143, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wei, Y.; Zhu, Y.; Guo, J.; Zhang, J.; He, Y.; Li, X.; Liu, J.; Zhou, W. The Interaction between Gut Microbiota and Host Amino Acids Metabolism in Multiple Myeloma. Cancers 2023, 15, 1942. [Google Scholar] [CrossRef] [PubMed]
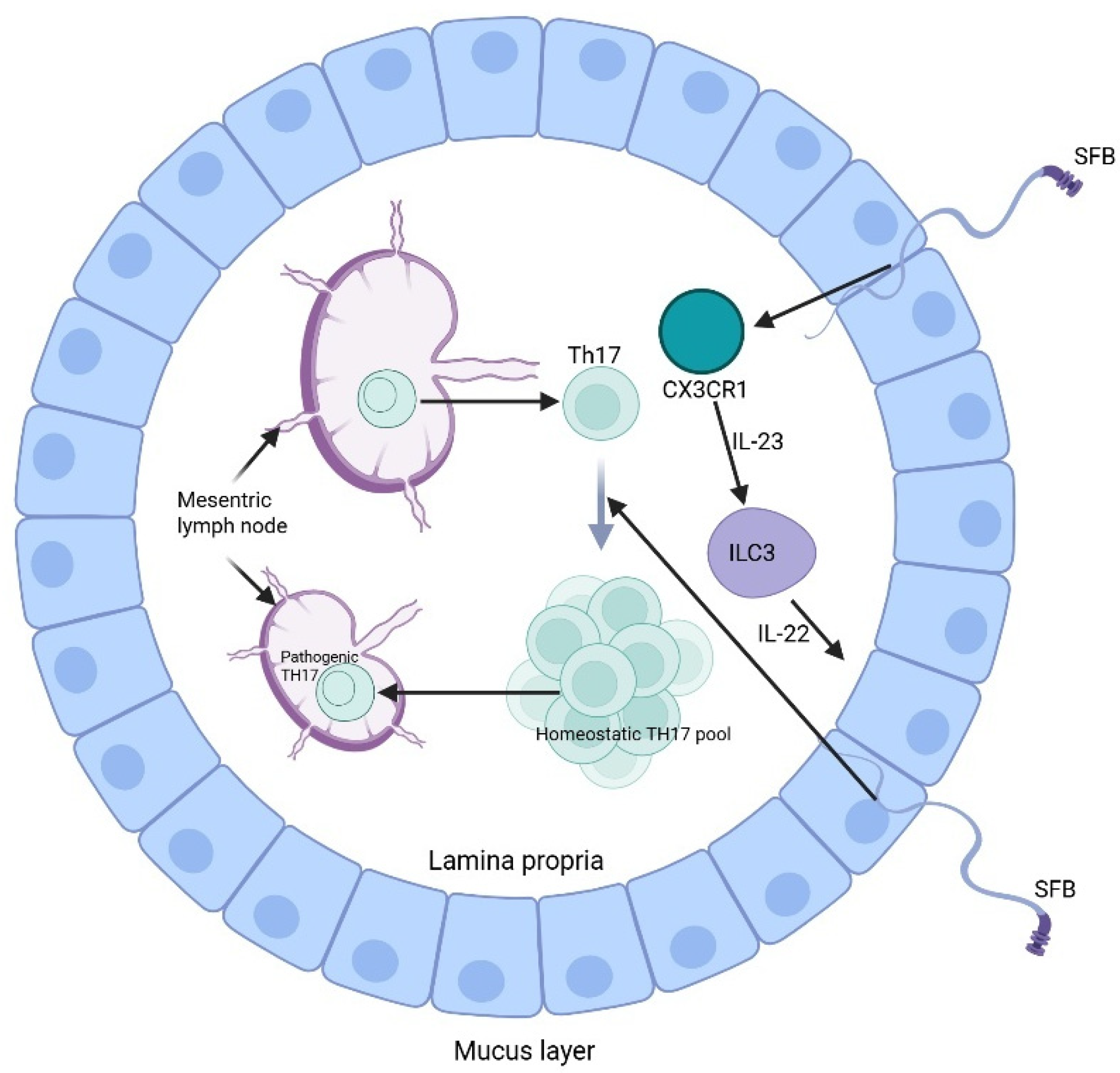
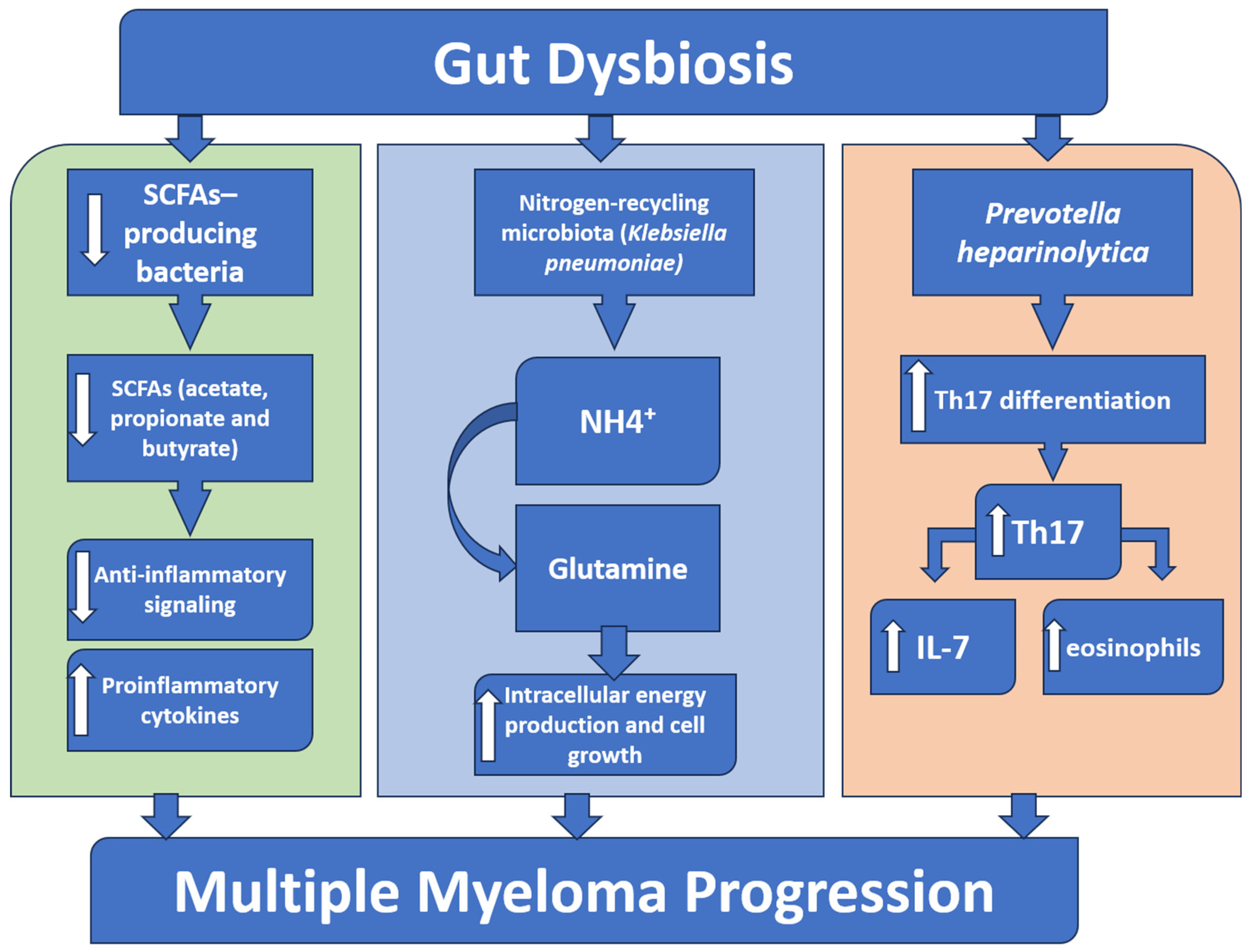

| Microbial Groups | Key Roles | Experimental Outcomes | References |
|---|---|---|---|
| Nitrogen-recycling bacteria (Klebsiella pneumoniae) | Produce glutamine, supplying the proliferating cells with their metabolic demand | Promoted cell growth and boosted the disease progression | [64] |
| Prevotella heparinolytica | Promotes intestinal Th17 cell differentiation and its migration to the bone marrow |
| [39] |
| SCFA-producing bacteria such as (Eubacterium halii, Faecalibacterium prausnitzii, and Clostridium butyricum) | Produce SCFA as butyrate, propionate, and acetate that maintain the intestinal barrier integrity and hinder NF-κB activation and inflammation | Their depletion in MM resulted in:
| [65,66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
George, M.Y.; Gamal, N.K.; Mansour, D.E.; Famurewa, A.C.; Bose, D.; Messiha, P.A.; Cerchione, C. The Gut Microbiome Role in Multiple Myeloma: Emerging Insights and Therapeutic Opportunities. Hematol. Rep. 2025, 17, 56. https://doi.org/10.3390/hematolrep17060056
George MY, Gamal NK, Mansour DE, Famurewa AC, Bose D, Messiha PA, Cerchione C. The Gut Microbiome Role in Multiple Myeloma: Emerging Insights and Therapeutic Opportunities. Hematology Reports. 2025; 17(6):56. https://doi.org/10.3390/hematolrep17060056
Chicago/Turabian StyleGeorge, Mina Y., Nada K. Gamal, Daniel E. Mansour, Ademola C. Famurewa, Debalina Bose, Peter A. Messiha, and Claudio Cerchione. 2025. "The Gut Microbiome Role in Multiple Myeloma: Emerging Insights and Therapeutic Opportunities" Hematology Reports 17, no. 6: 56. https://doi.org/10.3390/hematolrep17060056
APA StyleGeorge, M. Y., Gamal, N. K., Mansour, D. E., Famurewa, A. C., Bose, D., Messiha, P. A., & Cerchione, C. (2025). The Gut Microbiome Role in Multiple Myeloma: Emerging Insights and Therapeutic Opportunities. Hematology Reports, 17(6), 56. https://doi.org/10.3390/hematolrep17060056








