Abstract
Background: Clinical trials comparing novel oral anticoagulants (NOACs) with warfarin reported a lower mortality rate and a reduced incidence of bleeding with NOACs. However, these studies do not allow for final conclusions about safety. Moreover, direct comparisons among NOACs are not available. Objectives: The aim of this study was to analyze data from EudraVigilance in order to compare OAC safety profiles. Methods: We searched for all suspected adverse drug reactions (ADRs) from OACs collected in the EudraVigilance up to March 2019. We calculated the reporting odds ratios (RORs) in order to assess the risk of reporting specific ADRs among drugs. Moreover, OAC safety profiles were investigated through correspondence analysis and visualized in contribution biplots. Results: A total of 244,149 individual case safety reports (ICSRs; 431,354 ADRs) related to OACs were retrieved from EudraVigilance. About 80% of ICSRs refer to NOACs, especially rivaroxaban. Gastrointestinal (Gastr) and central nervous system (Nerv) disorders were the most represented categories. More than 90% of ADRs were serious and almost 9% fatal, with the highest ROR reported for dabigatran. Both fatal and non-fatal ADRs reported for Vitamin K Antagonists (VKAs) differed from those reported for NOACs. Among the latter, dabigatran and rivaroxaban showed similar profiles, while apixaban differed from all other OACs, even in the case of fatal ADRs. Conclusions: As expected, data collected from EudraVigilance showed differences among drugs, probably related to their specific characteristics and/or the peculiar utilization in clinical practice. Further investigations are needed to better compare the safety profile of OACs.
1. Introduction
Anticoagulants represent the drugs of choice for the prevention and treatment of venous thromboembolism and to reduce the risk of stroke in patients with atrial fibrillation [1,2,3,4,5,6]. Novel oral anticoagulants (NOACs) have changed the landscape of treatment in these clinical conditions, demonstrating non-inferiority in efficacy and safety compared with warfarin in randomized controlled trials (RCTs) with fewer treatment-related issues [2,3,7,8,9]. The primary safety concern with all oral anticoagulants (OACs) is obviously the risk of bleeding, which is strictly related to the pharmacological effect [10,11,12,13,14]. Intracranial bleeding is the most feared adverse drug reaction (ADR), due to irreversible sequelae and a high rate of mortality, whereas gastrointestinal bleeding is more common but less often fatal. Differences in bleeding sites have been observed with NOACs in comparison to Vitamin K Antagonists (VKAs) [15].
More generally, beyond the differences in terms of the mechanisms of action, food effect, drug–drug interactions, and renal clearance, indirect comparisons and network meta-analyses demonstrated that NOACs have generally similar efficacy but different safety profiles [16,17,18,19].
However, until now, there have been no head-to-head RCTs comparing NOACs [20]. Even if available, clinical trials do not allow for final conclusions about safety, and are usually designed to evaluate efficacy above all, and include a small and selected well-monitored population, which do not reflect real-world conditions [21,22,23]. Therefore, the most commonly used approach for post-marketing surveillance still relies on spontaneous reporting systems: despite multiple limitations, such as underreporting and reporting biases, lack of information about drug exposure, and difficulty in distinguishing ADRs from other events, this approach remains the most efficient way to collect safety data [23,24].
This study aims to analyze data from a spontaneous reporting system in order to compare OAC safety profiles in real-world and long-term use.
2. Materials and Methods
We analyzed all suspected ADRs related to the use of the main OACs (warfarin, acenocumarol, dabigatran, rivaroxaban, apixaban, edoxaban) reported on EudraVigilance up to March 2019.
EudraVigilance is the European database maintained by the European Medicines Agency (EMA) since 2001 [25,26], which collects, manages and analyzes suspected ADRs to medicines authorized in the European Economic Area (EEA), submitted by European national regulatory authorities (or by marketing-authorization holders for ADRs occurring outside Europe). Each individual case safety report (ICSR) is composed of at least one report, which might be integrated with the follow-up. Non-serious ADRs occurring outside of the EEA are not included.
Aggregated data are freely accessible from the “European Database of Suspected Adverse Drug Reaction Reports” (http://www.adrreports.eu/, accessed on 14 October 2025). They are coded by the Medical Dictionary for Regulatory Activities (MedDRA), and classified by age classes, sex, system organ classes (SOCs), seriousness (serious, non-serious, not specified) and outcome (fatal, not recovered, not specified, recovered, recovered with sequelae, recovering, unknown) [27].
According to criteria from international guidelines, serious ADRs are those resulting in death, life-threatening events, requiring or prolonging hospitalization, resulting in persistent or significant disability/incapacity or congenital anomaly/birth defect, or any other medical event deemed to be important due to major clinical consequences [28].
The Reporting Odds Ratio (ROR) was calculated as a measure of disproportionality to compare the risk of reporting specific ADRs among drugs [29].
Moreover, we calculated the indexed residuals for each specific drug–ADR pair, defined as the difference between the observed and expected values. Positive and negative values indicated that the observed values were higher and lower than expected, respectively. Expected values were obtained from the relative frequencies of an individual ADR and all the ADRs for a given drug (i.e., multiplying all column totals with all row totals in Tables S1 and S2).
Similarities in the safety profiles of OACs were investigated through correspondence analysis (CA) [29] and visualized with a contribution biplot [30]. CA is a multivariate dimensionality reduction method that provides a means to summarize data and visualize them in a two-dimensional space. In this way, new coordinates are obtained for each observation (i.e., the OACs), and the closer the points are, the more similar their safety profiles are. Moreover, variables (i.e., the ADRs) that contribute the most to the overall variability in the original dataset can be shown in a two-dimensional plot (called a contribution biplot) as vectors, together with OAC coordinates, allowing an easier interpretation of which ADR is mainly accountable for the separation of safety profiles. Indeed, the longer the vector in the plot, the higher the contribution of the specific ADR to the definition of the safety profile representation [30].
We performed CA and generated the contribute biplots using R statistical software (R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing Vienna, Austria. URL https://www.R-project.org/, accessed on 14 October 2025) and the libraries FactoMineR [31] and ggplot2 [32]. The minimum number of dimensions to analyze were selected according to Bendixen, Mike in 2003 [33], while the most important variables were identified using the average expected contribution calculated as a threshold, on the assumption of a uniform contribution for all the variables as the reciprocal of the number of variables (in other words: 1/number of considered ADRs).
In order to minimize the effect of possible confounders, we excluded the ADRs reported as ‘social circumstances’ (including conditions such as ‘elderly’, ‘family stress’, ‘economic problem’, ‘low income’) and fatal ADRs that are considered implausible or explainable by other conditions (such as ‘ear’ and ‘eye disorders’, ‘investigation’, ‘surgical procedures’, and ‘product issues’).
3. Results
A total of 244,149 individual cases related to OACs were retrieved from the EudraVigilance database, corresponding to 431,354 ADRs (Table 1 and Table 2). About 80% of ICSRs refer to NOACs: in particular, rivaroxaban (41.6%). Comparing the number of ICRSs reported by European countries, we found that France and Italy represent the ones with the highest number of reports (more than 20,000; Figure 1), followed by Germany and the UK. In contrast with the other European nations, the drug with the highest rate of ADRs in Italy is warfarin (57.2%; Table 3), followed by dabigatran (15.8%).

Table 1.
Population characteristics as indicated in the individual case safety reports (ICSRs), according to drug. F: female; M: male; NS: not specified.

Table 2.
Seriousness and outcome of suspected adverse drug reactions (ADRs) associated with oral anticoagulants. ADR: adverse drug reactions; NS: not specified.

Figure 1.
European countries with more than 10,000 ICSRs.

Table 3.
Percentage of individual case safety reports (ICSRs), according to European country and drug.
Males and females are equally represented, with most of them belonging to the 65–85 age group. Moreover, a significant proportion of ICSRs have been reported for patients who were 85 and over (14.6%), in particular for edoxaban and acenocumarol (19.5% and 19.4%, respectively).
More than 90% of ADRs are serious, with rivaroxaban having the highest (95.5%; OR 1.95 IC 95 1.89–2.00) and edoxaban the lowest rate (70.9%; OR 0.15 IC 95 0.14–0.16). ADRs with a fatal outcome are overall 38,250 (8.9%), with the highest percentage reported for dabigatran (12.4%; OR 1.67 IC 95 1.62–1.71).
‘Gastrointestinal’ (‘Gastr’) and ‘nervous system’ (‘Nerv’) disorders are the most represented categories, accounting for 30.3% of ADRs and 39.0% of fatal ADRs (Supplementary Tables S1 and S2).
Table 4 and Table 5 show the percentages of indexed residuals for ADRs categorized according to SOCs. The larger positive differences between observed and expected ADRs have been found for warfarin and acenocumarol with ‘pregnancy, puerperium and perinatal conditions’ (‘Preg’; 255.4% and 149.8%, respectively) and ‘investigations’ (‘Inv’; 104.0% and 128.0%, respectively), for apixaban with ‘surgical and medical procedures’ (‘Surg’; 156.1%) and for edoxaban with ‘skin and subcutaneous tissue disorders’ (‘Skin’; 122.6%).

Table 4.
Percentages of indexed residuals for ADRs, categorized according to system organ classes (SOCs).

Table 5.
Percentages of indexed residuals for fatal ADRs categorized according to system organ classes (SOCs).
Dabigatran and rivaroxaban are the only two OACs where ‘Gastr’ (one of the most reported ADRs) has been found to be more likely (27.1% and 15.2%) than expected.
Large differences have been found associated between ‘Preg’ and warfarin (386.1%) and acenocumarol (72.1%), while also considering only fatal ADRs. In this case, the ‘Preg’ ADR is less likely observed with NOACs, with the only exception being edoxaban (208.8% more likely than expected). Fatal ‘general disorders and administration site conditions’ (‘Genrl’) have been found to be more likely for apixaban (95.3%) and less likely for all the other OACs. The same has been observed for ‘Gastr’ fatal ADR and dabigatran (53.9%). On the contrary, large positive differences between expected and observed values in rivaroxaban ADRs were not identified.
Using CA, we were able to separate ADRs in a space defined by only two dimensions (dm1 and dm2), accounting for about 80% of the whole dataset variance (79.2% and 85.1% for overall and fatal ADRs, respectively; see Figure 2A,B and Methods for further details). In particular, for overall ADRs, dm1 accounted for more than half (52.7%) and dm2 for about one third (26.5%) of the observed variance.
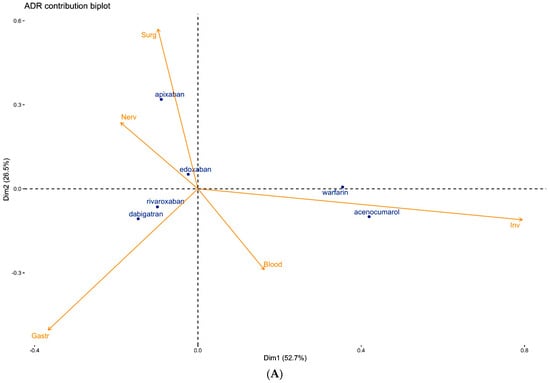

Figure 2.
(A) Contribution biplot showing the determinant role of ADRs in separating the safety profiles of OACs. (B) Contribution biplot showing the determinant role of fatal ADRs in separating the safety profiles of OACs.
In Figure 2A, warfarin and acenocumarol are well separated along the positive pole of dm1, rivaroxaban, and dabigatran in the negative quadrant of dm1 and dm2, apixaban far from all the other OACs in the further positive region of dm2, and edoxaban close to the origin.
The top 5 ADRs are represented by vectors, the length of which determines their contribution in the separation of OACs. ‘Inv’ is the main ADR responsible for the separation of VKAs from NOACs along dm1, while ‘Surg’ and ‘Gastr’ (dm2) show a determinant role in separating the apixaban safety profile from dabigatran and rivaroxaban. The exclusion of ‘Inv’ maintains the initial clustering (Supplementary Figure S1), although the two dimensions explain only about 60% of the overall variance. In this case, even edoxaban is separated from the origin dragged by ‘Nerv’.
The contribution biplot for fatal ADRs (Figure 2B) demonstrates again the separation along dm1 between VKAs and NOACs, with a greater distance between acenocumarol and warfarin. In this case, rivaroxaban and edoxaban, being close to the origin, have been playing a minor role in the analysis. Dabigatran and apixaban are further away in the negative poles of dm1 and dm2, respectively. The top fatal ADRs are ‘Genrl’, which is strongly associated with the negative pole of dm2, and therefore separating apixaban from the others OACs; ‘Gastr’, which equally contributes to both dimensions in the direction along which dabigatran drifted; ‘Nerv’; and ‘Psychiatric disorders’ (‘Psych’), which is involved in the separation of warfarin. The last relevant fatal ADRs identified by this analysis are ‘injury, poisoning and procedural complications’ (‘Inj&P’), which are partially associated with acenocumarol and warfarin.
Figure 3A,B show the ROR of the top 5 ADRs, which characterize the OAC safety profile according to our analysis (Supplementary Tables S3 and S4).

Figure 3.
(A) ROR and their 95% confidence intervals for specific ADRs. ADRs reported into EudraVigilance for each drug have been compared with the related categories reported for the others. (B) ROR and their 95% confidence intervals for fatal ADRs. ADRs with a fatal outcome were 38,250 (8.9%) overall, and differences in the likelihood of reporting them were found among OACs.
4. Discussion
OACs are included in the World Health Organization Model List of Essential Medicines [34], which represents a list of the most efficacious, safe and cost–effective medicines, selected on the basis of current and future public health relevance. Although it is still difficult to be treat some categories of patients with OAC [35], their use has increased over time and is expected to grow further, due to aging populations and the prevalence of chronic cardiovascular disease and AF risk factors [36]. Indeed, target patients are predominantly elderly, with high rates of cardiovascular risk factors and morbidity [37]. For more than 50 years since it was first approved for human use, warfarin was the only leading oral anticoagulant available [38]. NOACs have been approved by the European Union since 2008 [39], and the first authorization for the prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (NVAF) was granted for dabigatran in 2011. These drugs have overcome many of the practical limitations of VKAs, appearing to have at least similar efficacy, with fewer major bleeding events [20,40], while VKAs still mantain the main indication for splancnic thrombosis, deep vein thrombosis, antiphospholypid syndrome or prosthetic heart valve [41,42,43,44,45,46,47,48].
Several systematic reviews have concluded that the risk of major bleeding in NOACs is generally equivalent to or less than that with warfarin [49,50,51,52]. Therefore, they surpassed warfarin as the drug of choice, particularly for the management of AF [51], and a steady decrease in initiations of warfarin and an increase in NOACs were observed over time. This can, at least in part, explain the greater number of ADRs reported for NOACs on EudraVigilance compared to VKAs, despite their shorter post-marketing period and their better safety profile. However, we found differences in the rate of ADRs among the top European countries, with Italy being the only one with warfarin as the main drug according to the number of ADRs. As reported by the Italian Medicines Agency (AIFA) in the National Report on Medicines use in Italy [53], despite the progressive reduction in their use since the introduction of NOACs in 2013, in 2018 VKAs still represented the most prescribed OACs in Italy (DDD/1000 inhabitants/die = 4.6), followed by rivaroxaban (DDD/1000 inhabitants/die = 3.2), apixaban (DDD/1000 inhabitants/die = 3.0), dabigatran (DDD/1000 inhabitants/die = 2.2) and edoxaban (DDD/1000 inhabitants/die = 1.0) [54]. Comprehensive data about patients’ exposure in different countries are not available. The European and national guidelines did not favor any particular NOAC and did not seem to have influenced the choice to prescribe one of them [55]. A European cross-national drug utilization study reported [39] an increase in NOAC users (incidence from 8.7 per 10,000 in Spain to 27.6 per 10,000 in Denmark), with the highest increase observed for apixaban, followed by rivaroxaban. Overall, rivaroxaban presented the highest incidence, followed by apixaban and dabigatran. The authors correlate the differences detected among countries with different national or regional recommendations, prescription patterns and characteristics of the selected databases.
Interestingly, several studies reported that different factors affected the choice of NOACs in the observed population. The oldest patients were preferably treated with apixaban or edoxaban [56,57,58]. Moreover, in patients with comorbidities and higher stroke and bleeding risk, the drug of choice is apixaban, whereas those on rivaroxaban showed an intermediate clinical profile between apixaban and dabigatran [59]. These studies did not include edoxaban, the newest agent, with fewer available real-life data and a shorter follow-up duration. Comparative evaluations of NOACs have shown that, while dabigatran, rivaroxaban and apixaban provide similar efficacy in preventing stroke and systemic embolism in patients with atrial fibrillation, differences in their safety profiles are clinically relevant. In adjusted indirect comparisons of pivotal randomized trials, apixaban was associated with a significantly lower risk of major hemorrhage when compared with dabigatran and rivaroxaban [60]. Real-world studies further corroborate these findings, showing that bleeding rates, particularly gastrointestinal and intracranial events, vary across agents, even after careful propensity-score adjustment [61].
Data about Italian patients with NVAF who were treated with NOACs were collected into the AIFA database of monitoring registries and were published, including factors associated with treatment choice, treatment discontinuation and switches among drugs [59,62,63]. The observed population included very elderly patients at high risk of events, treated mostly with rivaroxaban (33.8% of treatments), followed by apixaban (31.1%), dabigatran (28.6%) and edoxaban (6.5%); the last was introduced on the market in 2016. Even this study showed that different factors affected the choice of a specific NOAC in the observed population, e.g., oldest patients were preferably treated with apixaban or edoxaban, in line with previous observations [56,57,58]; a CHA2DS2-VASc score equal to three or higher was more strongly associated with apixaban or dabigatran and a HAS-BLED score higher than four, with a higher frequency of prescription for apixaban and rivaroxaban. It is known that the occurrence of ADRs can be influenced by several factors: for example, being a frail elderly patient with multi-morbidity and exposure to polypharmacy [64,65]. Therefore, the different baseline characteristics that were demonstrated to influence the choice of NOACs could determine a different risk of reporting adverse events, as observed in this study. We have no data on concomitant medications or diseases, but we found a high prevalence of ADRs in elderly and very elderly subjects with all medications: in particular, with edoxaban and acenocumarol. This may be explained by the fact that OACs are commonly prescribed in older adults, due to their approved indications [66]. ADRs due to underlying diseases or patient characteristics, as well as those related to medication errors, drug–drug interactions, poor adherence and poor monitoring of patients, may be preventable [67]. An observational study recently conducted in five European countries [68] raised concerns about the level of adherence in clinical practice to restrictions, special warnings and precautions based on the medicines’ summary of product characteristics (SPCs), with a consequent possible increase in ADRs risk. Moreover, available ‘real world’ data suggest variable adherence to NOACs (from 38% to 99%) [69]. Strict adherence is crucial, as non-adherence to NOACs has been associated with increased risks of stroke, bleeding and death [70,71]. The poor adherence to NOACs can be explained by the absence of the need for routine coagulation monitoring [55,72]. This is one of the main advantages compared to VKAs, but can represent a risk for older and frail patients who require vigilance in order to balance the bleeding risk and anticoagulant effect and prevent potentially severe complications [69,73]. In this regard, the guidelines [69,74] recommend to regularly review patients treated with NOAC: in particular, after one month initially, and at least every three to four months thereafter. As clinical experience with NOACs grows, follow-up intervals may become longer, based on individual (patient-specific) or local (center-specific) factors.
Our analysis confirms the similarity of the safety profile of VKAs, significantly associated with the ADRs ‘Inv’ and ‘Inj&P’. This is an expected result, as a frequently reported ADR with VKA is related to indentification of international normalized ratios (INR) outside the therapeutic range [75]. Warfarin safety, indeed, is highly dependent on maintaining an optimal time in the therapeutic range (TTR), which can vary considerably across populations. This variability is influenced by numerous factors, including patient adherence, comorbidities, pharmacogenetic differences, concomitant medications, and, importantly, the healthcare system’s ability to provide adequate monitoring, education and support. Pharmacologic interactions also play a critical role. Therefore, differences in TTR may reflect not only individual patient characteristics but also the overall quality and structure of anticoagulation management programs across countries.
Moreover, our results suggest the similarity of the overall safety profile of dabigatran and rivaroxaban, which differs from that of the other NOACs, for the risk of reporting, above all, the ‘Gastr’ and ‘Nerv’ ADRs. Previously conflicting results have been reported [52,76,77,78,79,80,81]. Relative differences in bleeding sites among NOACs were found in previous studies [15,82,83,84,85,86]. Gastrointestinal bleeding remains a major concern of NOAC use, being potentially severe and even fatal [87,88]. At the same time, the possible non-adherence to NOACs due to gastrointestinal bleeding may increase the risk of stroke and VTE [89]. However, existing findings from meta-analyses of RCTs are heterogeneous [90].
Our findings are supported by the results published by EMA in 2019 about an observational study conducted on six databases covering regionally/nationally representative populations in five European countries, assessing the risk of major bleeding with the NOACs apixaban, dabigatran and rivaroxaban in patients with NVAF, in comparison with other OACs [68]. This study showed differences in the risk of major bleeding among these medicines. In particular, apixaban was not associated with an increased risk of gastrointestinal bleeding and seemed to be associated with the lowest risk of major events compared to dabigatran and rivaroxaban. Edoxaban was not included in the analysis. The separation of apixaban is guided by ‘Surg’ events. A lot of ADRs included in this cathegory are measures to manage ADRs (such as transfusions) or probably concomitant events (cardioversion or other cardiac procedures, dental care, surgery procedures not specified) without a clear causal/effect explaination. According to international guidelines, patient characteristics and surgical factors need to be taken into account to determine when to discontinue and restart a NOAC [69,74], in order to balance the risk of procedural bleeding with the risk for thromboembolism [90]. The PAUSE trial demonstrated that in patients treated with NOAC, a simple standardized perioperative management approach (without heparin bridging or coagulation function testing) is associated with low rates of bleeding and thromboembolism [91]. While invasive interventions require temporary discontinuation, less invasive procedures with a low bleeding risk do not necessarily require the suspension of treatment. In general, minor surgical procedures and those procedures where bleeding is easily controllable can be performed 12–24 h after the last NOAC intake, whereas in case of invasive procedures with a high risk of major bleeding, it is recommended to take the last NOAC dose 48 h or longer before surgery. After a procedure with immediate and complete haemostasis, NOACs can generally be restarted 6–8 h after the end of the intervention, with some exceptions. If an emergency intervention is required, the NOAC should be discontinued immediately. There are no specific indications to treat patients undergoing surgery with one NOAC or another. Therefore, the higher ADR-reporting in apixaban should be further investigated. Interestingly, we found differences in terms of fatal ADRs among drugs, with dabigatran associated with the highest risk of reporting an ADR with a fatal outcome. As expected, the most frequently reported fatal ‘Gastr’ and ‘Nerv’ ADRs were bleeding. Even in this case, the most important fatal ADR reported for apixaban (‘Genrl’) is not clearly explainable, being related to a generic ‘death’. This raises some concern about the quality of data and the need for further investigations in order to clarify if this event should have been classified as the outcome of another specific ADR, or should represent an ADR itself. We can observe a clear signal for Fatal ‘Psych’ ADRs and warfarin, almost all represented by ‘suicide’. Warfarin overdoses (intentional or unintentional) are relatively uncommon [92], but represent a major concern due to the potential significant morbidity and mortality. Emergency treatment is complicated by the risk of thromboembolic events, in the case of complete anticoagulation reversal [93]. Previous studies showed a high prevalence of depressive symptoms in patients treated with anticoagulants, with detrimental effects on anticoagulation therapy, due to all the above risks of poor adherence and consequent adverse medical events [94]. Screening for depression and appropriate treatment should be included in the management of these patients. Previous studies found non-bleeding adverse events associated with NOACs to be linked to multiple organ systems, including the reproductive system, endocrine disorders, and psychiatric disorders, which requires high vigilance in clinical practice [95].
Large variations have been found for fatal ‘Preg’ ADRs (in particular, ‘fetal death’ and ‘stillbirth’) and warfarin and acenocumarol use. Pregnant women frequently require anticoagulation during pregnancy or in postpartum, due to an increased risk of venous thromboembolism [96,97]. The use of anticoagulants during pregnancy is challenging; due to the potential teratogenic effects of drugs and the pharmacokinetic changes, there is a consequent need for complex dose adjustments [98]. VKAs are not considered to be acceptable in pregnancy [99], as they are able to cross the placenta and cause a typical gestation embryopathy, as well as other adverse fetal outcomes and bleeding [100]. Moreover, current guidelines advise against NOAC use too, even if their efficacy and safety profile during pregnancy is unknown, due to the exclusion of pregnant women from clinical trials [74,101,102], and low-molecular-weight heparin is still the treatment of choice during pregnancy [103].
Finally, the fewer rates of ADRs (and, in particular, of ‘Preg’ and ‘Hepatobiliary disorders’) might be responsible for the large deviation detected for edoxaban, and it is difficult to draw any conclusions. The differences observed in adverse event profiles suggest that OAC may have practical relevance for treatment individualization. In particular, the lower reporting rates of major gastrointestinal and central nervous system events with apixaban suggest a potentially safer option in elderly or frail patients, or in those with prior bleeding events. Conversely, the higher rates of fatal gastrointestinal ADRs associated with dabigatran and rivaroxaban highlight the importance of careful patient selection, avoidance of concomitant gastrotoxic agents, and regular reassessment of renal function. The distinct pattern of procedural and surgical-related events with apixaban further underlines the need for optimized perioperative management and clinician awareness of drug-specific risks. The present observations and the differences in ADRs’ sites found in this study help to understand the safety of anticoagulants in real life. Studies conducted on the spontaneous reporting system are affected by important limitations [104]. Interpretation of data found in the pharmacovigilance databases may be difficult due to a lot of biases, such as stimulated reporting, selective reporting and under-reporting [105]. Generally, only a small fraction of ADRs are reported [106], and the number of ADRs may be influenced by many different factors: first of all, the use of the product, the country-specific limitation of prescription, the frequency and the seriousness of reactions. Moreover, it is not always possible to assess the use of other suspected or concomitant medications that interact with the drug or contribute to the development of the ADR or underlying diseases, such as gastrointestinal tumors [107,108,109,110,111,112]. Neither clinical details of patients nor information on possible confounding factors and causality assessment are available. Furthermore, we found some other criticism for data interpretation: in particular, in the case of ADRs reported with apixaban. Despite these limitations, post-marketing surveillance still represents an important instrument to evaluate the real-world effectiveness and safety of drugs, especially in long-term use by unselected patients [106,113,114], as already demonstrated by our group in other settings [25,26,27,115,116,117,118]. To our knowledge, these are the first spontaneous reports related to all OACs identified in the EudraVigilance database. Future pharmacovigilance efforts should aim to integrate spontaneous reporting data with clinical and demographic information derived from electronic health records or dedicated registries. Such linkage would enable more robust causal inference, stratification by comorbidities, and assessment of confounding factors such as concomitant drug use, adherence and renal function. In addition, prospective real-world studies and active surveillance programs could help confirm the observed safety differences among individual oral anticoagulants and refine evidence-based prescribing recommendations.
Our study is informative and provides important data about overall and specific drug reporting in Europe. Indeed, EudraVigilance is one of the biggest spontaneous reporting systems in the world, with more than 14.5 million ICSRs collected till 2018 [119]. Our study is coherent with results from pre- and post-marketing studies, even though we identified some new potential safety signals and/or biases, for which further in-depth analysis should be performed. In the future, it would be useful to analyze nationwide data in order to investigate the difference in terms of ADR-reporting among European countries according to the possible variability in OACs use in Europe.
5. Conclusions
Our analysis of EudraVigilance data confirms that oral anticoagulants are associated with a high proportion of serious and fatal adverse drug reactions, with relevant differences among individual agents. VKAs showed a distinctive safety profile compared with NOACs, while within NOACs, dabigatran and rivaroxaban appeared more similar, and apixaban emerged as distinct, particularly regarding surgical and gastrointestinal events. These findings underline the importance of post-marketing surveillance to capture real-world safety signals that are not evident in randomized clinical trials. Further pharmacoepidemiological studies linking spontaneous reports with clinical data are warranted to refine the risk–benefit assessment and support safer therapeutic strategies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/hematolrep17050054/s1, Table S1: Frequencies of individual ADRs, categorized according to system organ classes (SOCs), with percentages in brackets. Table S2: Frequencies of individual fatal ADRs, categorized according to system organ classes (SOCs), with percentages in brackets. Table S3: Rrisk of reporting (ROR) and theirs 95% confidence intervals (LCL, lower control limit, and UCL, upper control limit) for ADRs. ADRs reported into EudraVigilance for each drug were categorized according to system organ classes (SOCs), and have been compared with the related categories reported for the others. Table S4: Rrisk of reporting (ROR) and theirs 95% confidence intervals (LCL, lower control limit, and UCL, upper control limit) for fatal ADRs. ADRs reported into EudraVigilance for each drug were categorized according to system organ classes (SOCs), and have been compared with the related categories reported for the others. Figure S1: Contribution biplot without ‘Inv’.
Author Contributions
P.P.O. contributed to conceptualization, formal analysis, methodology, supervision, data curation, and writing—original draft. F.E.P., G.G., C.G., A.D., C.V., D.G.C., G.A.P., S.S. and F.D. contributed to supervision and writing—review and editing. E.M., E.L.S., G.L.R. and G.E.L. contributed to conceptualization, methodology, supervision, and writing—original draft. L.G. contributed to conceptualization, formal analysis, data curation, methodology, supervision, and writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in the context of the regional pharmacovigilance project AIFA 2010/2011 project ADR-648 “Monitoring of safety and efficacy of drugs prescribed under Law 648/96”.
Institutional Review Board Statement
According to the guidelines for observational studies provided by the Italian Medicines Agency, approval from the Ethics Committee is not required for studies based on databases. In particular, the EudraVigilance database collects data from pharmacovigilance reports, made available for the public as cumulative data (https://www.adrreports.eu/en/search.html, accessed on 14 October 2025).
Informed Consent Statement
Informed consent for publication was not required, as the data were obtained from the EudraVigilance database, which contains pharmacovigilance reports made publicly available in cumulative form (https://www.adrreports.eu/en/search.html, accessed on 14 October 2025).
Data Availability Statement
The data that support the findings of this study are available at http://www.adrreports.eu/en/search.html (accessed on 14 October 2025). These data were derived from EudraVigilance, the European database of suspected adverse drug reaction reports, published by European Medicines Agency (EMA).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ADR | Adverse Drug Reaction |
| AF | Atrial Fibrillation |
| CA | Correspondence Analysis |
| CI | Confidence Interval |
| EEA | European Economic Area |
| EMA | European Medicines Agency |
| ICSR | Individual Case Safety Report |
| MedDRA | Medical Dictionary for Regulatory Activities |
| NOAC | Novel Oral Anticoagulant |
| OAC | Oral Anticoagulant |
| OR | Odds Ratio |
| ROR | Reporting Odds Ratio |
| SOC | System Organ Class |
| VKA | Vitamin K Antagonist |
References
- Vinogradova, Y.; Coupland, C.; Hill, T.; Hippisley-Cox, J. Risks and Benefits of Direct Oral Anticoagulants versus Warfarin in a Real World Setting: Cohort Study in Primary Care. BMJ 2018, 362, k2505. [Google Scholar] [CrossRef]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Kearon, C.; Ageno, W.; Cannegieter, S.C.; Cosmi, B.; Geersing, G.-J.; Kyrle, P.A. Categorization of Patients as Having Provoked or Unprovoked Venous Thromboembolism: Guidance from the SSC of ISTH. J. Thromb. Haemost. 2016, 14, 1480–1483. [Google Scholar] [CrossRef]
- Barnes, G.D.; Ageno, W.; Ansell, J.; Kaatz, S. Recommendation on the Nomenclature for Oral Anticoagulants: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2015, 13, 1154–1156. [Google Scholar] [CrossRef]
- Husted, S.; de Caterina, R.; Andreotti, F.; Arnesen, H.; Bachmann, F.; Huber, K.; Lip, G.Y.H.; Morais, J.; Rasmussen, L.H.; Siegbahn, A.; et al. Non-Vitamin K Antagonist Oral Anticoagulants (NOACs): No Longer New or Novel. Thromb. Haemost. 2017, 117, 1002–1004. [Google Scholar] [CrossRef]
- Olesen, J.B.; Sørensen, R.; Hansen, M.L.; Lamberts, M.; Weeke, P.; Mikkelsen, A.P.; Køber, L.; Gislason, G.H.; Torp-Pedersen, C.; Fosbøl, E.L. Non-Vitamin K Antagonist Oral Anticoagulation Agents in Anticoagulant Naïve Atrial Fibrillation Patients: Danish Nationwide Descriptive Data 2011–2013. EP Eur. 2015, 17, 187–193. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Villines, T.C.; Peacock, W.F. Safety of Direct Oral Anticoagulants: Insights from Postmarketing Studies. Am. J. Emerg. Med. 2016, 34, 9–13. [Google Scholar] [CrossRef]
- Salazar, C.A.; del Aguila, D.; Cordova, E.G. Direct Thrombin Inhibitors versus Vitamin K Antagonists for Preventing Cerebral or Systemic Embolism in People with Non-Valvular Atrial Fibrillation. Cochrane Database Syst. Rev. 2014, 2014, 9–13. [Google Scholar] [CrossRef]
- Bruins Slot, K.M.H.; Berge, E. Factor Xa Inhibitors versus Vitamin K Antagonists for Preventing Cerebral or Systemic Embolism in Patients with Atrial Fibrillation. Cochrane Database Syst. Rev. 2018, 3, CD008980. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the Efficacy and Safety of New Oral Anticoagulants with Warfarin in Patients with Atrial Fibrillation: A Meta-Analysis of Randomised Trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Chatterjee, S.; Sardar, P.; Biondi-Zoccai, G.; Kumbhani, D.J. New Oral Anticoagulants and the Risk of Intracranial Hemorrhage: Traditional and Bayesian Meta-Analysis and Mixed Treatment Comparison of Randomized Trials of New Oral Anticoagulants in Atrial Fibrillation. JAMA Neurol. 2013, 70, 1486–1490. [Google Scholar] [CrossRef]
- Cabarrot, A.; Montastruc, J.L.; Chebane, L.; Rousseau, V.; Bondon-Guitton, E.; Moulis, F.; Durrieu, G.; Bagheri, H.; Montastruc, F. Neurological and Digestive Bleeding with Direct Oral Anticoagulants versus Vitamin K Antagonists: The Differences Do Not Stop There! A Pharmacovigilance Study. Pharmacol. Res. 2017, 118, 119–120. [Google Scholar] [CrossRef]
- López-López, J.A.; Sterne, J.A.C.; Thom, H.H.Z.; Higgins, J.P.T.; Hingorani, A.D.; Okoli, G.N.; Davies, P.A.; Bodalia, P.N.; Bryden, P.A.; Welton, N.J.; et al. Oral Anticoagulants for Prevention of Stroke in Atrial Fibrillation: Systematic Review, Network Meta-Analysis, and Cost Effectiveness Analysis. BMJ 2017, 359, j5058. [Google Scholar] [CrossRef]
- Almutairi, A.R.; Zhou, L.; Gellad, W.F.; Lee, J.K.; Slack, M.K.; Martin, J.R.; Lo-Ciganic, W.-H. Effectiveness and Safety of Non-Vitamin K Antagonist Oral Anticoagulants for Atrial Fibrillation and Venous Thromboembolism: A Systematic Review and Meta-Analyses. Clin. Ther. 2017, 39, 1456–1478.e36. [Google Scholar] [CrossRef]
- Cameron, C.; Coyle, D.; Richter, T.; Kelly, S.; Gauthier, K.; Steiner, S.; Carrier, M.; Coyle, K.; Bai, A.; Moulton, K.; et al. Systematic Review and Network Meta-Analysis Comparing Antithrombotic Agents for the Prevention of Stroke and Major Bleeding in Patients with Atrial Fibrillation. BMJ Open 2014, 4, e004301. [Google Scholar] [CrossRef]
- Bellesini, M.; Bianchin, M.; Corradi, C.; Donadini, M.P.; Raschi, E.; Squizzato, A. Drug–Drug Interactions between Direct Oral Anticoagulants and Hepatitis C Direct-Acting Antiviral Agents: Looking for Evidence Through a Systematic Review. Clin. Drug Investig. 2020, 40, 1001–1008. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Keshishian, A.; Li, X.; Hamilton, M.; Masseria, C.; Gupta, K.; Luo, X.; Mardekian, J.; Friend, K.; Nadkarni, A.; et al. Effectiveness and Safety of Oral Anticoagulants Among Nonvalvular Atrial Fibrillation Patients. Stroke 2018, 49, 2933–2944. [Google Scholar] [CrossRef]
- Umscheid, C.A.; Margolis, D.J.; Grossman, C.E. Key Concepts of Clinical Trials: A Narrative Review. Postgrad. Med. 2011, 123, 194–204. [Google Scholar] [CrossRef]
- Philip, W.; James, T.; Caterson, I.D.; Coutinho, W.; Finer, N.; Van Gaal, L.F.; Maggioni, A.P.; Torp-Pedersen, C.; Sharma, A.M.; Shepherd, G.M.; et al. Effect of Sibutramine on Cardiovascular Outcomes in Overweight and Obese Subjects. N. Engl. J. Med. 2010, 363, 905–917. [Google Scholar]
- Menzella, F.; Ballarin, A.; Sartor, M.; Floriani, A.F.; Corsi, L.; Dartora, C.; Tonin, S.; Romagnoli, M. Comparison between Clinical Trials and Real-World Evidence Studies on Biologics for Severe Asthma. J. Int. Med. Res. 2022, 50, 3000605221133689. [Google Scholar] [CrossRef]
- Montastruc, J.-L.; Sommet, A.; Bagheri, H.; Lapeyre-Mestre, M. Benefits and Strengths of the Disproportionality Analysis for Identification of Adverse Drug Reactions in a Pharmacovigilance Database. Br. J. Clin. Pharmacol. 2011, 72, 905–908. [Google Scholar] [CrossRef]
- Gozzo, L.; Vetro, C.; Brancati, S.; Longo, L.; Vitale, D.C.; Romano, G.L.; Mauro, E.; Fiumara, P.F.; Maugeri, C.; Parisi, M.S.; et al. Off-Label Use of Venetoclax in Patients with Acute Myeloid Leukemia: Single Center Experience and Data From Pharmacovigilance Database. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Vetro, C.; Duminuco, A.; Gozzo, L.; Maugeri, C.; Parisi, M.; Brancati, S.; Longo, L.; Vitale, D.C.; Romano, G.L.; Ciuni, R.; et al. Pegylated Asparaginase-Induced Liver Injury: A Case-Based Review and Data From Pharmacovigilance. J. Clin. Pharmacol. 2022, 62, 1142–1150. [Google Scholar] [CrossRef]
- Gozzo, L.; Nardo, A.; Brancati, S.; Judica, A.; Duminuco, A.; Maugeri, C.; Parisi, M.; Longo, L.; Vitale, D.C.; Ruscica, R.; et al. Severe Gastrointestinal Toxicity Following the Use of Gilteritinib: A Case Series and Analysis of Postmarketing Surveillance Data. Healthcare 2023, 11, 1479. [Google Scholar] [CrossRef]
- European Medicines Agency Science Medicines Health. Guideline on Good Pharmacovigilance Practices (GVP)-Module VI: Collection, Management and Submission of Reports of Suspected Adverse Reactions to Medicinal Products (Rev 2); European Medicines Agency Science Medicines Health: London, UK, 2017. [Google Scholar]
- Greenacre, M. Correspondence Analysis in Medical Research. Stat. Methods Med. Res. 1992, 1, 97–117. [Google Scholar] [CrossRef]
- Greenacre, M. Contribution Biplots. J. Comput. Graph. Stat. 2013, 22, 107–122. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Valero-Mora, P.M. Ggplot2: Elegant Graphics for Data Analysis. J. Stat. Softw. Book Rev. 2010, 35, 1–3. [Google Scholar] [CrossRef]
- Bendixen, M.T. A Practical Guide to the Use of Correspondence Analysis in Marketing Research. Mark. Bull. 1996, 14, 16–38. [Google Scholar]
- World Health Organization. World Health Organization World Health Organization Model List of Essential Medicines, 21st List; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Napolitano, M.; Valore, L.; Malato, A.; Saccullo, G.; Vetro, C.; Mitra, M.E.; Fabbiano, F.; Mannina, D.; Casuccio, A.; Lucchesi, A.; et al. Management of Venous Thromboembolism in Patients with Acute Leukemia at High Bleeding Risk: A Multi-Center Study. Leuk. Lymphoma 2016, 57, 116–119. [Google Scholar] [CrossRef]
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.-H.; McAnulty, J.H.; Zheng, Z.-J.; et al. Worldwide Epidemiology of Atrial Fibrillation. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef]
- Steinberg, B.A.; Gao, H.; Shrader, P.; Pieper, K.; Thomas, L.; Camm, A.J.; Ezekowitz, M.D.; Fonarow, G.C.; Gersh, B.J.; Goldhaber, S.; et al. International Trends in Clinical Characteristics and Oral Anticoagulation Treatment for Patients with Atrial Fibrillation: Results from the GARFIELD-AF, ORBIT-AF I, and ORBIT-AF II Registries. Am. Heart J. 2017, 194, 132–140. [Google Scholar] [CrossRef]
- Eriksson, B.I.; Quinlan, D.J.; Eikelboom, J.W. Novel Oral Factor Xa and Thrombin Inhibitors in the Management of Thromboembolism. Annu. Rev. Med. 2011, 62, 41–57. [Google Scholar] [CrossRef]
- Ibáñez, L.; Sabaté, M.; Vidal, X.; Ballarin, E.; Rottenkolber, M.; Schmiedl, S.; Heeke, A.; Huerta, C.; Martin Merino, E.; Montero, D.; et al. Incidence of Direct Oral Anticoagulant Use in Patients with Nonvalvular Atrial Fibrillation and Characteristics of Users in 6 European Countries (2008–2015): A Cross-National Drug Utilization Study. Br. J. Clin. Pharmacol. 2019, 85, 2524–2539. [Google Scholar] [CrossRef]
- Graham, D.J.; Baro, E.; Zhang, R.; Liao, J.; Wernecke, M.; Reichman, M.E.; Hu, M.; Illoh, O.; Wei, Y.; Goulding, M.R.; et al. Comparative Stroke, Bleeding, and Mortality Risks in Older Medicare Patients Treated with Oral Anticoagulants for Nonvalvular Atrial Fibrillation. Am. J. Med. 2019, 132, 596–604.e11. [Google Scholar] [CrossRef]
- Duminuco, A.; Au Yeung, J.; Vaghela, R.; Virdee, S.; Woodley, C.; Asirvatham, S.; Curto-Garcia, N.; Sriskandarajah, P.; O’Sullivan, J.; de Lavallade, H.; et al. Development of a Natural Language Processing Pipeline for Assessment of Cardiovascular Risk in Myeloproliferative Neoplasms. Hemasphere 2024, 8, e143. [Google Scholar] [CrossRef]
- Duminuco, A.; Vaghela, R.; Virdee, S.; Woodley, C.; Asirvatham, S.; Curto-Garcia, N.; Sriskandarajah, P.; O’Sullivan, J.; de Lavallade, H.; Radia, D.; et al. QRISK3 Score Is Predictive of Thrombotic Risk in Patients with Myeloproliferative Neoplasms. Leukemia 2025, 39, 2384–2390. [Google Scholar] [CrossRef]
- Duminuco, A.; Harrington, P.; Del Fabro, V.; Scalisi, E.; Santuccio, G.; Santisi, A.; Sbriglione, A.; Garibaldi, B.; Markovic, U.; Di Raimondo, F.; et al. Old Therapy, New Questions: Rethinking Phlebotomy in a Pharmacologic Landscape. Pharmaceuticals 2025, 18, 1212. [Google Scholar] [CrossRef]
- Duminuco, A.; Nardo, A.; Giuffrida, G.; Leotta, S.; Markovic, U.; Giallongo, C.; Tibullo, D.; Romano, A.; Di Raimondo, F.; Palumbo, G.A. Myelofibrosis and Survival Prognostic Models: A Journey between Past and Future. J. Clin. Med. 2023, 12, 2188. [Google Scholar] [CrossRef]
- Duminuco, A.; Del Fabro, V.; De Luca, P.; Leotta, D.; Limoli, M.C.; Longo, E.; Nardo, A.; Santuccio, G.; Petronaci, A.; Stanzione, G.; et al. Emergencies in Hematology: Why, When and How I Treat? J. Clin. Med. 2024, 13, 7572. [Google Scholar] [CrossRef]
- Keeling, D.; Mackie, I.; Moore, G.W.; Greer, I.A.; Greaves, M.; British Committee for Standards in Haematology. Guidelines on the Investigation and Management of Antiphospholipid Syndrome. Br. J. Haematol. 2012, 157, 47–58. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease: Developed by the Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Duminuco, A.; Torre, E.; Palumbo, G.A.; Harrison, C. A Journey Through JAK Inhibitors for the Treatment of Myeloproliferative Diseases. Curr. Hematol. Malig. Rep. 2023, 18, 176–189. [Google Scholar] [CrossRef]
- Miller, C.S.; Pappas, S.C. Risk of Gastrointestinal Bleeding in Patients Taking Non-Vitamin K Antagonist Oral Anticoagulants: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2017, 15, 1674–1683.e3. [Google Scholar] [CrossRef]
- Deitelzweig, S.; Tang, C.-H.; Cheng, R.W.-Y.; Wang, M.Y.-H.; Hung, K.-Y. Risk of Major Bleeding in Patients with Non-Valvular Atrial Fibrillation Treated with Oral Anticoagulants: A Systematic Review of Real-World Observational Studies. Curr. Med. Res. Opin. 2017, 33, 1583–1594. [Google Scholar] [CrossRef]
- Generalova, D.; Gallini, A.; Montastruc, F.; Coley, N.; Montastruc, J.; Vellas, B.; Andrieu, S.; Gardette, V. A Systematic Review of Clinicians’ Views and Experiences of Direct-Acting Oral Anticoagulants in the Management of Nonvalvular Atrial Fibrillation. Br. J. Clin. Pharmacol. 2018, 84, 2692–2703. [Google Scholar] [CrossRef]
- Burr, N.; Lummis, K.; Sood, R.; Kane, J.S.; Corp, A.; Subramanian, V. Risk of Gastrointestinal Bleeding with Direct Oral Anticoagulants: A Systematic Review and Network Meta-Analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 85–93. [Google Scholar] [CrossRef]
- Italian Medicines Agency. National Report on Medicines Use in Italy; Italian Medicines Agency: Roma, Italy, 2020.
- Osservatorio Nazionale sull’impiego dei Medicinali. L’uso Dei Farmaci in Italia. Rapporto Nazionale. Anno 2019; Osservatorio Nazionale sull’impiego dei Medicinali: Roma, Italy, 2020.
- Komen, J.; Forslund, T.; Hjemdahl, P.; Andersen, M.; Wettermark, B. Effects of Policy Interventions on the Introduction of Novel Oral Anticoagulants in Stockholm: An Interrupted Time Series Analysis. Br. J. Clin. Pharmacol. 2017, 83, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Staerk, L.; Fosbøl, E.L.; Gadsbøll, K.; Sindet-Pedersen, C.; Pallisgaard, J.L.; Lamberts, M.; Lip, G.Y.H.; Torp-Pedersen, C.; Gislason, G.H.; Olesen, J.B. Non-Vitamin K Antagonist Oral Anticoagulation Usage According to Age among Patients with Atrial Fibrillation: Temporal Trends 2011–2015 in Denmark. Sci. Rep. 2016, 6, 31477. [Google Scholar] [CrossRef]
- Lamberts, M.; Staerk, L.; Olesen, J.B.; Fosbøl, E.L.; Hansen, M.L.; Harboe, L.; Lefevre, C.; Evans, D.; Gislason, G.H. Major Bleeding Complications and Persistence with Oral Anticoagulation in Non-Valvular Atrial Fibrillation: Contemporary Findings in Real-Life Danish Patients. J. Am. Heart Assoc. 2025, 6, e004517. [Google Scholar] [CrossRef]
- Tepper, P.G.; Mardekian, J.; Masseria, C.; Phatak, H.; Kamble, S.; Abdulsattar, Y.; Petkun, W.; Lip, G.Y.H. Real-World Comparison of Bleeding Risks among Non-Valvular Atrial Fibrillation Patients Prescribed Apixaban, Dabigatran, or Rivaroxaban. PLoS ONE 2018, 13, e0205989. [Google Scholar] [CrossRef]
- Olimpieri, P.P.; Di Lenarda, A.; Mammarella, F.; Gozzo, L.; Cirilli, A.; Cuomo, M.; Gulizia, M.M.; Colivicchi, F.; Murri, G.; Gabrielli, D.; et al. Non-Vitamin K Antagonist Oral Anticoagulation Agents in Patients with Atrial Fibrillation: Insights from Italian Monitoring Registries. IJC Heart Vasc. 2020, 26, 100465. [Google Scholar] [CrossRef]
- Schneeweiss, S.; Gagne, J.J.; Patrick, A.R.; Choudhry, N.K.; Avorn, J. Comparative Efficacy and Safety of New Oral Anticoagulants in Patients with Atrial Fibrillation. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Andersson, N.W.; Svanström, H.; Lund, M.; Pasternak, B.; Melbye, M. Comparative Effectiveness and Safety of Apixaban, Dabigatran, and Rivaroxaban in Patients with Non-Valvular Atrial Fibrillation. Int. J. Cardiol. 2018, 268, 113–119. [Google Scholar] [CrossRef]
- Gozzo, L.; Di Lenarda, A.; Mammarella, F.; Olimpieri, P.P.; Cirilli, A.; Cuomo, M.; Gulizia, M.M.; Colivicchi, F.; Murri, G.; Kunutsor, S.K.; et al. Starting Dose and Dose Adjustment of Non-Vitamin K Antagonist Oral Anticoagulation Agents in a Nationwide Cohort of Patients with Atrial Fibrillation. Sci. Rep. 2021, 11, 20689. [Google Scholar] [CrossRef] [PubMed]
- Breccia, M.; Celant, S.; Olimpieri, P.P.; Olimpieri, O.M.; Pane, F.; Iurlo, A.; Cirilli, A.; Colatrella, A.; Gozzo, L.; Pugliese, S.; et al. Mortality Rate in Patients with Chronic Myeloid Leukemia in Chronic Phase Treated with Frontline Second Generation Tyrosine Kinase Inhibitors: A Retrospective Analysis by the Monitoring Registries of the Italian Medicines Agency (AIFA). Ann. Hematol. 2021, 100, 481–485. [Google Scholar] [CrossRef]
- Davies, E.A.; O’Mahony, M.S. Adverse Drug Reactions in Special Populations–the Elderly. Br. J. Clin. Pharmacol. 2015, 80, 796–807. [Google Scholar] [CrossRef]
- Marconi, G.; Petracci, E.; Lanzarone, G.; Vetro, C.; Martelli, M.P.; Papayannidis, C.; Audisio, E.; Minetto, P.; Riva, C.; Guolo, F.; et al. Impact of Pre-Treatment Comorbidity Burden on Survival in Patients Receiving Venetoclax Plus Hypomethylating Agents. Am. J. Hematol. 2025, 100, 708–711. [Google Scholar] [CrossRef]
- Mitchell, A.; Snowball, J.; Welsh, T.J.; Watson, M.C.; McGrogan, A. Prescribing of Direct Oral Anticoagulants and Warfarin to Older People with Atrial Fibrillation in UK General Practice: A Cohort Study. BMC Med. 2021, 19, 189. [Google Scholar] [CrossRef]
- Formica, D.; Sultana, J.; Cutroneo, P.M.; Lucchesi, S.; Angelica, R.; Crisafulli, S.; Ingrasciotta, Y.; Salvo, F.; Spina, E.; Trifirò, G. The Economic Burden of Preventable Adverse Drug Reactions: A Systematic Review of Observational Studies. Expert. Opin. Drug Saf. 2018, 17, 681–695. [Google Scholar] [CrossRef]
- DTB Team. EMA reviews bleeding risk with direct oral anticoagulants. Drug Ther. Bull. 2019, 57, 88. [Google Scholar] [CrossRef]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef]
- Polymeris, A.A.; Albert, V.; Hersberger, K.E.; Engelter, S.T.; Schaedelin, S.; Arnet, I.; Lyrer, P.A. Protocol for MAAESTRO: Electronic Monitoring and Improvement of Adherence to Direct Oral Anticoagulant Treatment—A Randomized Crossover Study of an Educational and Reminder-Based Intervention in Ischemic STROke Patients Under Polypharmacy. Front. Neurol. 2018, 9, 1134. [Google Scholar] [CrossRef]
- Yao, X.; Abraham, N.S.; Alexander, G.C.; Crown, W.; Montori, V.M.; Sangaralingham, L.R.; Gersh, B.J.; Shah, N.D.; Noseworthy, P.A. Effect of Adherence to Oral Anticoagulants on Risk of Stroke and Major Bleeding Among Patients with Atrial Fibrillation. J. Am. Heart Assoc. 2025, 5, e003074. [Google Scholar] [CrossRef]
- Grymonprez, M.; Capiau, A.; Steurbaut, S.; Mehuys, E.; Boussery, K.; De Backer, T.L.; Lahousse, L. Adherence and Persistence to Oral Anticoagulants in Patients with Atrial Fibrillation: A Belgian Nationwide Cohort Study. Front. Cardiovasc. Med. 2022, 9, 994085. [Google Scholar] [CrossRef]
- Damanti, S.; Braham, S.; Pasina, L. Anticoagulation in Frail Older People. J. Geriatr. Cardiol. 2019, 16, 844–846. [Google Scholar]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. EP Eur. 2021, 23, 1612–1676. [Google Scholar] [CrossRef]
- Caldeira, D.; Rodrigues, R.; Abreu, D.; Anes, A.M.; Rosa, M.M.; Ferreira, J.J. Suspected Adverse Drug Reaction Reports with Oral Anticoagulants in Portugal: A Pharmacovigilance Study. Expert. Opin. Drug Saf. 2018, 17, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Bitar, Y.d.S.L.; Neto, M.G.; Filho, J.A.L.; Pereira, L.V.; Travassos, K.S.O.; Akrami, K.M.; Roever, L.; Duraes, A.R. Comparison of the New Oral Anticoagulants and Warfarin in Patients with Atrial Fibrillation and Valvular Heart Disease: Systematic Review and Meta-Analysis. Drugs R&D 2019, 19, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.S.; Singh, S.; Alexander, G.C.; Heien, H.; Haas, L.R.; Crown, W.; Shah, N.D. Comparative Risk of Gastrointestinal Bleeding with Dabigatran, Rivaroxaban, and Warfarin: Population Based Cohort Study. BMJ Br. Med. J. 2015, 350, h1857. [Google Scholar] [CrossRef]
- Sharma, M.; Cornelius, V.R.; Patel, J.P.; Davies, J.G.; Molokhia, M. Efficacy and Harms of Direct Oral Anticoagulants in the Elderly for Stroke Prevention in Atrial Fibrillation and Secondary Prevention of Venous Thromboembolism. Circulation 2015, 132, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.B.; Skjøth, F.; Nielsen, P.B.; Kjældgaard, J.N.; Lip, G.Y.H. Comparative Effectiveness and Safety of Non-Vitamin K Antagonist Oral Anticoagulants and Warfarin in Patients with Atrial Fibrillation: Propensity Weighted Nationwide Cohort Study. BMJ 2016, 353, i3189. [Google Scholar] [CrossRef]
- Halvorsen, S.; Ghanima, W.; Fride Tvete, I.; Hoxmark, C.; Falck, P.; Solli, O.; Jonasson, C. A Nationwide Registry Study to Compare Bleeding Rates in Patients with Atrial Fibrillation Being Prescribed Oral Anticoagulants. Eur. Heart J. Cardiovasc. Pharmacother. 2017, 3, 28–36. [Google Scholar] [CrossRef]
- Shimada, K.; Hasegawa, S.; Nakao, S.; Mukai, R.; Sasaoka, S.; Ueda, N.; Kato, Y.; Abe, J.; Mori, T.; Yoshimura, T.; et al. Adverse Reaction Profiles of Hemorrhagic Adverse Reactions Caused by Direct Oral Anticoagulants Analyzed Using the Food and Drug Administration Adverse Event Reporting System (FAERS) Database and the Japanese Adverse Drug Event Report (JADER) Database. Int. J. Med. Sci. 2019, 16, 1295–1303. [Google Scholar] [CrossRef]
- Pardo-Cabello, A.J.; Manzano-Gamero, V.; Luna, J.d.D. Comparative Study of Adverse Drug Reactions among Direct-Acting Oral Anticoagulants and Vitamin K Antagonists Using the EudraVigilance Database. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Moudallel, S.; van den Eynde, C.; Malý, J.; Rydant, S.; Steurbaut, S. Retrospective Analysis of Gastrointestinal Bleedings with Direct Oral Anticoagulants Reported to EudraVigilance. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, R.J.; Nolting, L.; Dolginsky, M.; Kym, E.; Orrico, K.B. Dabigatran Versus Warfarin for Atrial Fibrillation in Real-World Clinical Practice. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 126–134. [Google Scholar] [CrossRef]
- Dunn, A. In Older Patients with AF and Frailty, Switching from VKA to NOAC Therapy Increased a Composite of Major or CRNM Bleeding at 12 Mo. Ann. Intern. Med. 2024, 177, JC57. [Google Scholar] [CrossRef]
- Monaco, L.; Biagi, C.; Conti, V.; Melis, M.; Donati, M.; Venegoni, M.; Vaccheri, A.; Motola, D. Safety Profile of the Direct Oral Anticoagulants: An Analysis of the WHO Database of Adverse Drug Reactions. Br. J. Clin. Pharmacol. 2017, 83, 1532–1543. [Google Scholar] [CrossRef]
- Amaraneni, A.; Chippa, V.; Goldin, J.; Rettew, A.C. Anticoagulation Safety; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Saviano, A.; Brigida, M.; Petruzziello, C.; Candelli, M.; Gabrielli, M.; Ojetti, V. Gastrointestinal Bleeding Due to NOACs Use: Exploring the Molecular Mechanisms. Int. J. Mol. Sci. 2022, 23, 13955. [Google Scholar] [CrossRef]
- Holthuis, E.; Smits, E.; Spentzouris, G.; Beier, D.; Enders, D.; Gini, R.; Bartolini, C.; Mazzaglia, G.; Penning-van Beest, F.; Herings, R. Increased Risk of Stroke Due to Non-Adherence and Non-Persistence with Direct Oral Anticoagulants (DOACs): Real-World Analyses Using a Nested Case–Control Study from The Netherlands, Italy and Germany. Drugs Real. World Outcomes 2022, 9, 597–607. [Google Scholar] [CrossRef]
- He, Y.; Wong, I.C.K.; Li, X.; Anand, S.; Leung, W.K.; Siu, C.W.; Chan, E.W. The Association between Non-Vitamin K Antagonist Oral Anticoagulants and Gastrointestinal Bleeding: A Meta-Analysis of Observational Studies. Br. J. Clin. Pharmacol. 2016, 82, 285–300. [Google Scholar] [CrossRef]
- Douketis, J.D.; Spyropoulos, A.C.; Duncan, J.; Carrier, M.; Le Gal, G.; Tafur, A.J.; Vanassche, T.; Verhamme, P.; Shivakumar, S.; Gross, P.L.; et al. Perioperative Management of Patients with Atrial Fibrillation Receiving a Direct Oral Anticoagulant. JAMA Intern. Med. 2019, 179, 1469–1478. [Google Scholar] [CrossRef]
- Levine, M.; Pizon, A.F.; Padilla-Jones, A.; Ruha, A.-M. Warfarin Overdose: A 25-Year Experience. J. Med. Toxicol. 2014, 10, 156–164. [Google Scholar] [CrossRef]
- Isbister, G.K.; Hackett, L.P.; Whyte, I.M. Intentional Warfarin Overdose. Ther. Drug Monit. 2003, 25, 715–722. [Google Scholar] [CrossRef]
- Michal, M.; Prochaska, J.H.; Ullmann, A.; Keller, K.; Gobel, S.; Coldewey, M.; Münzel, T.; Wiltink, J.; Beutel, M.E.; Wild, P.S. Relevance of Depression for Anticoagulation Management in a Routine Medical Care Setting: Results from the ThrombEVAL Study Program. J. Thromb. Haemost. 2014, 12, 2024–2033. [Google Scholar] [CrossRef]
- Guo, G.; Song, Y.; Chang, S.; Zhang, J. Signal Mining for Non-Bleeding Adverse Event in Novel Oral Anticoagulants: A Pharmacovigilance Study Based on FAERS Database. Naunyn Schmiedebergs Arch. Pharmacol. 2025. [Google Scholar] [CrossRef]
- Middeldorp, S. How I Treat Pregnancy-Related Venous Thromboembolism. Blood 2011, 118, 5394–5400. [Google Scholar] [CrossRef]
- James, A.H. Prevention and Management of Venous Thromboembolism in Pregnancy. Am. J. Med. 2007, 120, S26–S34. [Google Scholar] [CrossRef]
- Alshawabkeh, L.; Economy, K.E.; Valente, A.M. Anticoagulation During Pregnancy: Evolving Strategies with a Focus on Mechanical Valves. JACC 2016, 68, 16. [Google Scholar] [CrossRef]
- Bates, S.M.; Rajasekhar, A.; Middeldorp, S.; McLintock, C.; Rodger, M.A.; James, A.H.; Vazquez, S.R.; Greer, I.A.; Riva, J.J.; Bhatt, M.; et al. American Society of Hematology 2018 Guidelines for Management of Venous Thromboembolism: Venous Thromboembolism in the Context of Pregnancy. Blood Adv. 2018, 2, 3317–3359. [Google Scholar] [CrossRef]
- Bates, S.M.; Middeldorp, S.; Rodger, M.; James, A.H.; Greer, I. Guidance for the Treatment and Prevention of Obstetric-Associated Venous Thromboembolism. J. Thromb. Thrombolysis 2016, 41, 92–128. [Google Scholar] [CrossRef]
- Lameijer, H.; Aalberts, J.J.J.; van Veldhuisen, D.J.; Meijer, K.; Pieper, P.G. Efficacy and Safety of Direct Oral Anticoagulants during Pregnancy; a Systematic Literature Review. Thromb. Res. 2018, 169, 123–127. [Google Scholar] [CrossRef]
- Beyer-Westendorf, J.; Michalski, F.; Tittl, L.; Middeldorp, S.; Cohen, H.; Abdul Kadir, R.; Jayakody Arachchillage, D.; Arya, R.; Ay, C.; Marten, S. Pregnancy Outcome in Patients Exposed to Direct Oral Anticoagulants-and the Challenge of Event Reporting. Thromb. Haemost. 2017, 117, 1956–1963. [Google Scholar] [CrossRef]
- Ginsberg, J.S.; Bates, S.M. Management of Venous Thromboembolism during Pregnancy. J. Thromb. Haemost. 2003, 1, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Caterina, P.; Christian, L.; Serafina, C.; Giuseppina, M.; Sabrina, S.; Lucrezia, B.; Rosaria, M.L.; Orietta, S.; Antonio, S.; Francesca, S.; et al. Limitations and Obstacles of the Spontaneous Adverse Drugs Reactions Reporting: Two “Challenging” Case Reports. J. Pharmacol. Pharmacother. 2013, 4, S66–S72. [Google Scholar] [CrossRef]
- Gozzo, L. Pharmacovigilance and Appropriate Drug Use. Healthcare 2024, 12, 669. [Google Scholar] [CrossRef]
- Carmela, M.; Stefania, E.; Adele E, D.F.; Annalisa, C.; Emilio, R.; Sarro, D. Giovambattista Pharmacovigilance in Italy: An Overview. J. Pharmacol. Pharmacother. 2013, 4, S20–S28. [Google Scholar] [CrossRef]
- Vetro, C.; Bonanno, G.; Giulietti, G.; Romano, A.; Conticello, C.; Chiarenza, A.; Spina, P.; Coppolino, F.; Cunsolo, R.; Di Raimondo, F. Rare Gastrointestinal Lymphomas: The Endoscopic Investigation. World J. Gastrointest. Endosc. 2015, 7, 928–949. [Google Scholar] [CrossRef]
- Vetro, C.; Romano, A.; Chiarenza, A.; Conticello, C.; Donnarumma, D.; Gorgone, A.; Coppolino, F.; Palumbo, G.A.; Bonanno, G.; Di Raimondo, F. Endoscopic Ultrasonography in Gastric Lymphomas: Appraisal on Reliability in Long-Term Follow-Up. Hematol. Oncol. 2012, 30, 180–185. [Google Scholar] [CrossRef]
- Vetro, C.; Chiarenza, A.; Romano, A.; Amico, I.; Calafiore, V.; Di Raimondo, C.; Coppolino, F.; Di Raimondo, F. Prognostic Assessment and Treatment of Primary Gastric Lymphomas: How Endoscopic Ultrasonography Can Help in Tailoring Patient Management. Clin. Lymphoma Myeloma Leuk. 2014, 14, 179–185. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Yamashita, Y.; Morimoto, T.; Chatani, R.; Kaneda, K.; Ikeda, N.; Kobayashi, Y.; Ikeda, S.; Kim, K.; Inoko, M.; et al. Direct Oral Anticoagulant-Associated Bleeding Complications in Patients with Gastrointestinal Cancer and Venous Thromboembolism. Eur. J. Intern. Med. 2024, 127, 74–83. [Google Scholar] [CrossRef]
- Muñoz Martín, A.J.; Soto Alsar, J.; Ortega Morán, L. Up and down in Gastrointestinal Cancer and Bleeding with Direct Oral Anticoagulants. Eur. J. Intern. Med. 2024, 127, 41–42. [Google Scholar] [CrossRef]
- Cheung, K.S.; Leung, W.K. Gastrointestinal Bleeding in Patients on Novel Oral Anticoagulants: Risk, Prevention and Management. World J. Gastroenterol. 2017, 23, 1954–1963. [Google Scholar] [CrossRef]
- Sportiello, L.; Rafaniello, C.; Scavone, C.; Vitale, C.; Rossi, F.; Capuano, A. The Importance of Pharmacovigilance for the Drug Safety: Focus on Cardiovascular Profile of Incretin-Based Therapy. Int. J. Cardiol. 2016, 202, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Rafaniello, C.; Ferrajolo, C.; Sullo, M.G.; Sessa, M.; Sportiello, L.; Balzano, A.; Manguso, F.; Aiezza, M.L.; Rossi, F.; Scarpignato, C.; et al. Risk of Gastrointestinal Complications Associated to NSAIDs, Low-Dose Aspirin and Their Combinations: Results of a Pharmacovigilance Reporting System. Pharmacol. Res. 2016, 104, 108–114. [Google Scholar] [CrossRef]
- Palumbo, F.E.; Duminuco, A.; Longo, L.; Vitale, D.C.; Maugeri, C.; Brancati, S.; Parisi, M.S.; Palumbo, G.A.; Romano, G.L.; Drago, F.; et al. Length of Washout Period After Remission Does Not Influence Relapse Risk in Patients with Acute Myeloid Leukemia Treated with Hypomethylating Agents Combined with Venetoclax. J. Clin. Med. 2025, 14, 5007. [Google Scholar] [CrossRef] [PubMed]
- Longo, E.; Palumbo, F.E.; Duminuco, A.; Longo, L.; Vitale, D.C.; Brancati, S.; Maugeri, C.; Parisi, M.S.; Palumbo, G.A.; Romano, G.L.; et al. Does the Timing of Response Impact the Outcome of Relapsed/Refractory Acute Myeloid Leukemia Treated with Venetoclax in Combination with Hypomethylating Agents? A Proof of Concept from a Monocentric Observational Study. J. Clin. Med. 2025, 14, 5586. [Google Scholar] [CrossRef]
- Palumbo, F.E.; Vetro, C.; Calafiore, V.; Duminuco, A.; Maugeri, C.; Parisi, M.; Palumbo, G.A.; Gozzo, L.; Longo, L.; Vitale, D.C.; et al. Extramedullary Acute Myeloid Leukemia: Evaluating Azacitidine—Venetoclax Combination Outcomes and the Role of Molecular Genetics and Bone Marrow Involvement. Eur. J. Haematol. 2025, 115, 505–509. [Google Scholar] [CrossRef]
- Gozzo, L.; Leotta, S.; Romano, G.L.; Vetro, C.; Duminuco, A.; Milone, G.; Cupri, A.; Palumbo, F.E.; Brancati, S.; Ruscica, R.; et al. Early Access for Medicines in ITALY: The Case of Ruxolitinib for Patients with Graft-Versus-Host Disease. J. Clin. Med. 2024, 13, 4273. [Google Scholar] [CrossRef]
- European Medicines Agency. Annual Report on EudraVigilance; 2019 EMA/906394/2019; European Medicines Agency: London, UK, 2019. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).