Case-Based Insights into Enteropathy-Associated T-Cell Lymphoma—Single-Center Experience
Abstract
1. Introduction
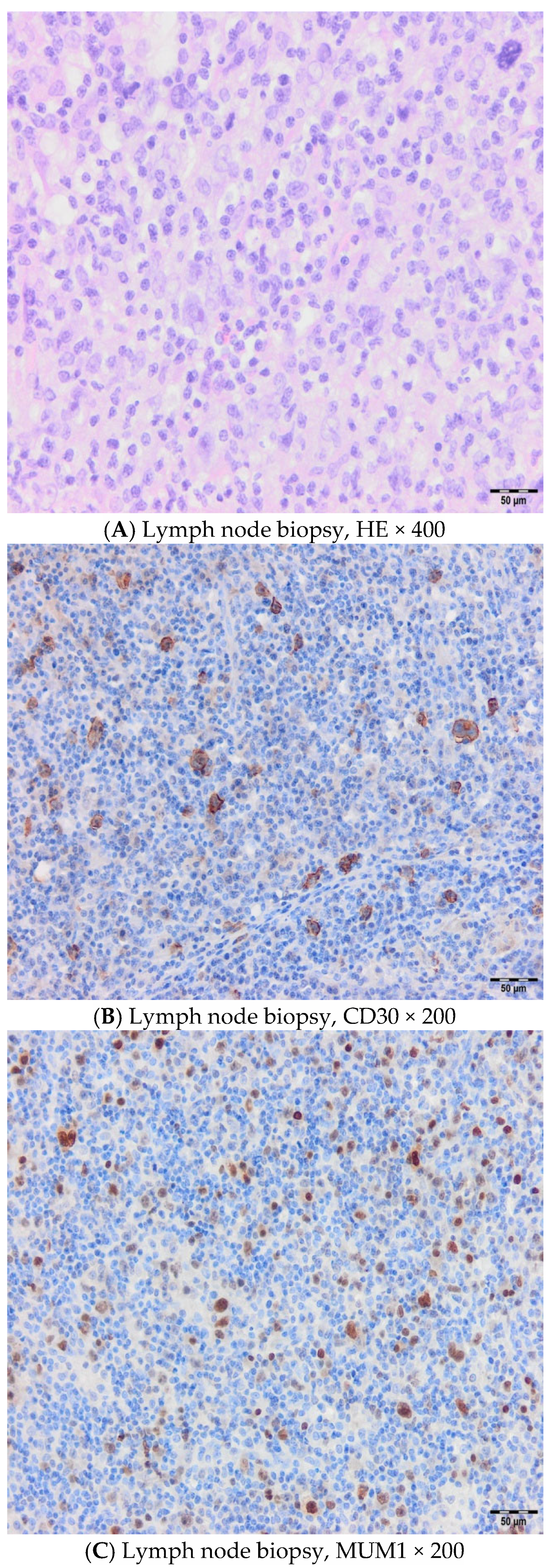
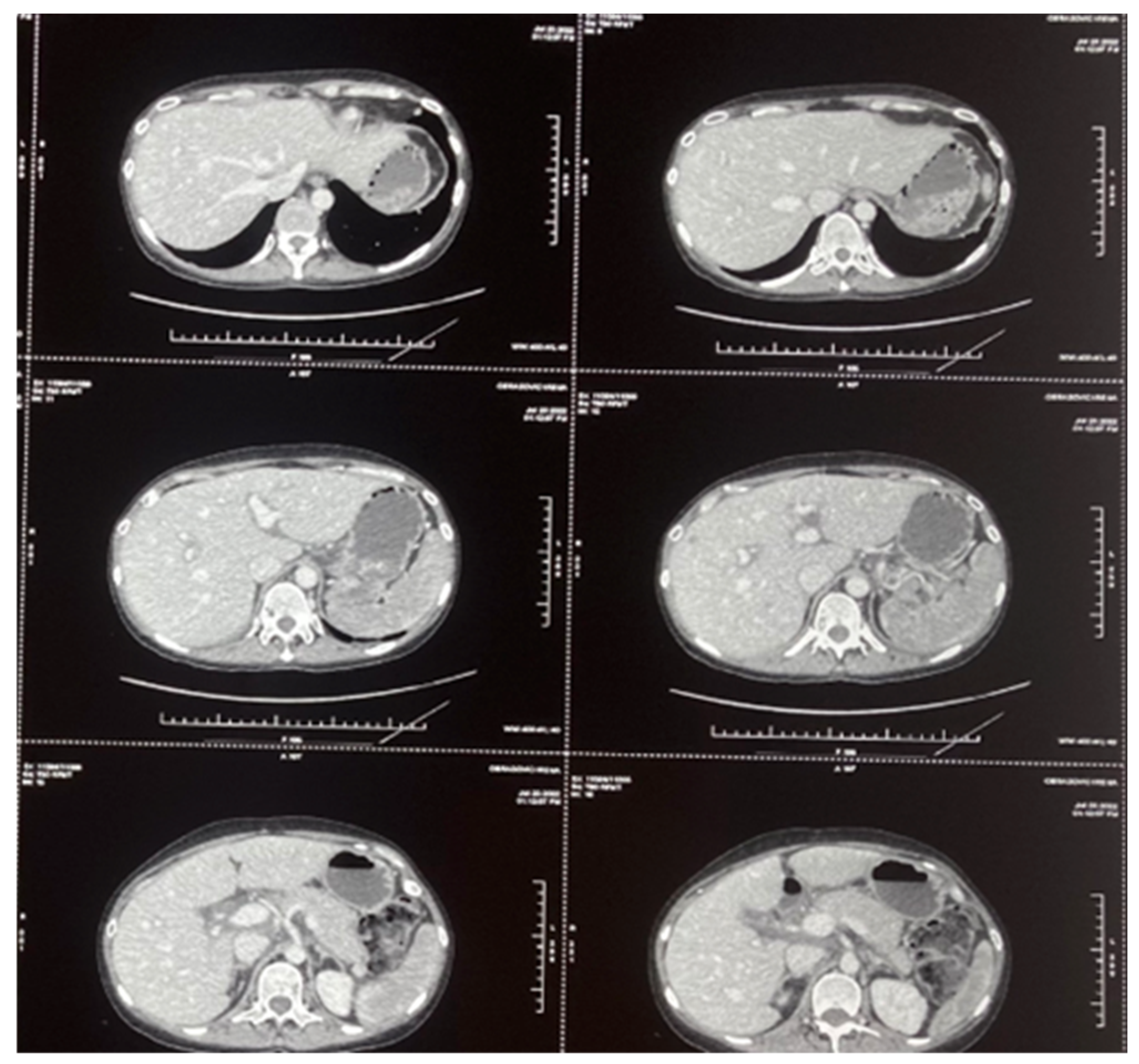
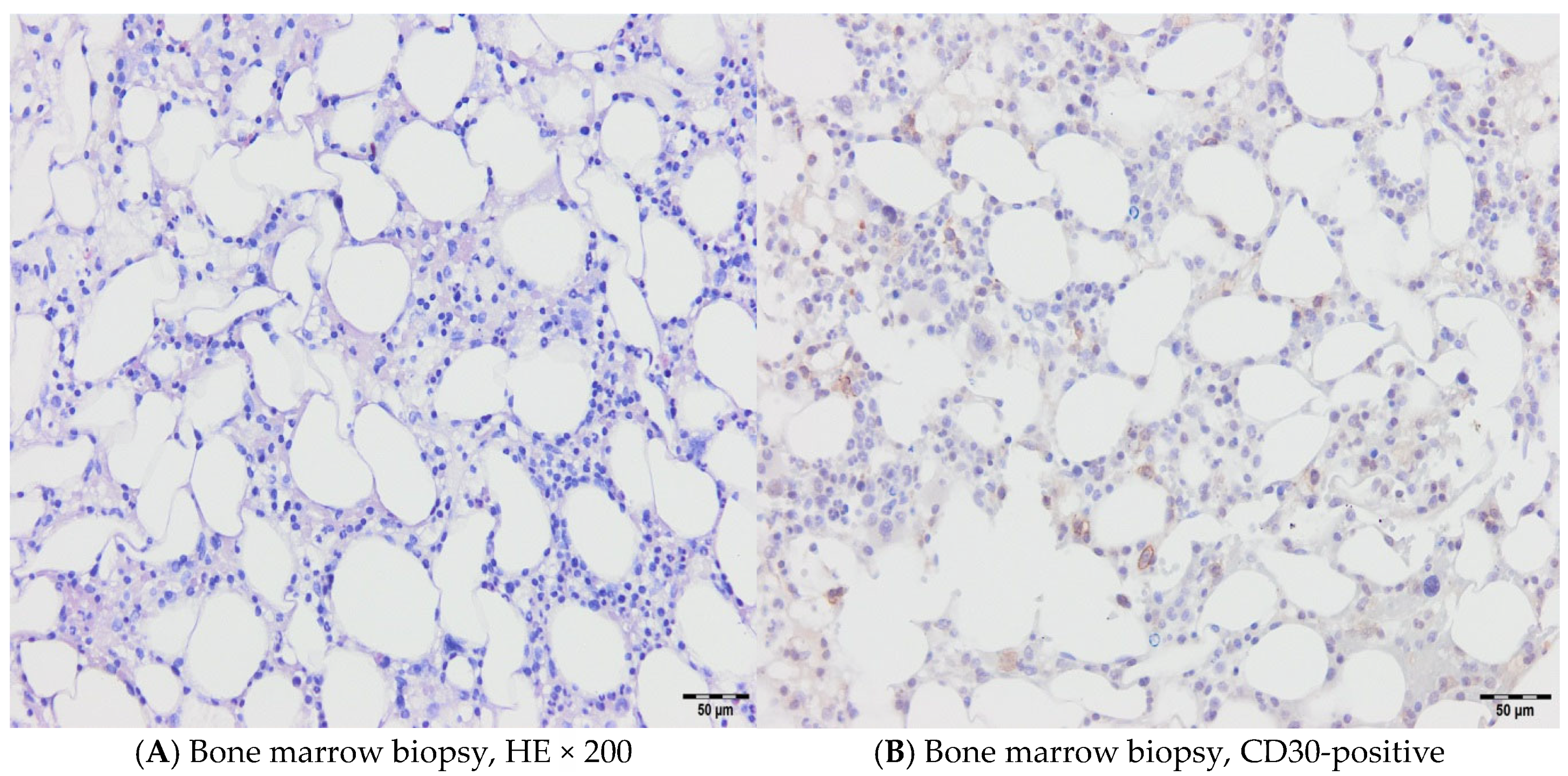
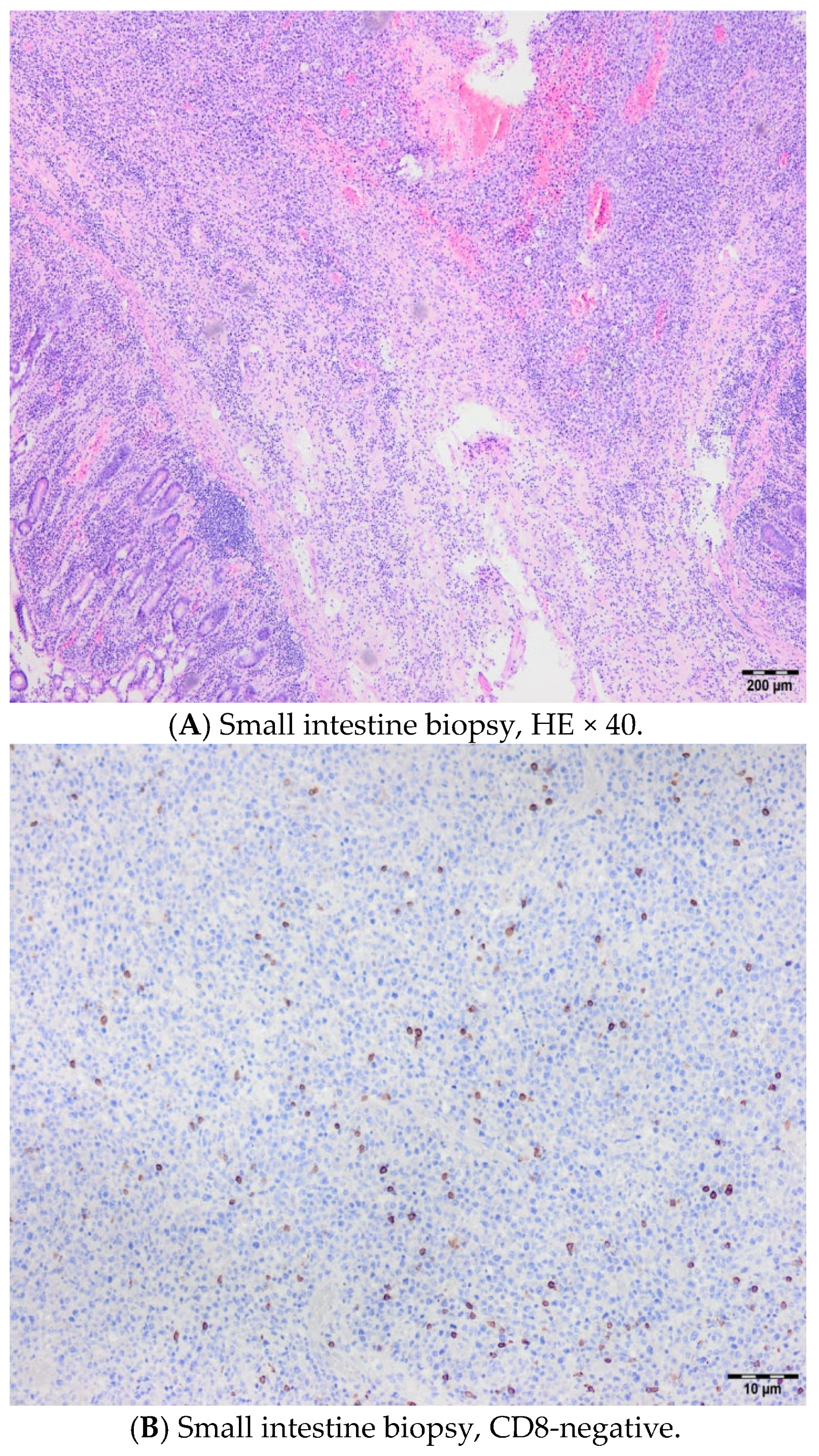
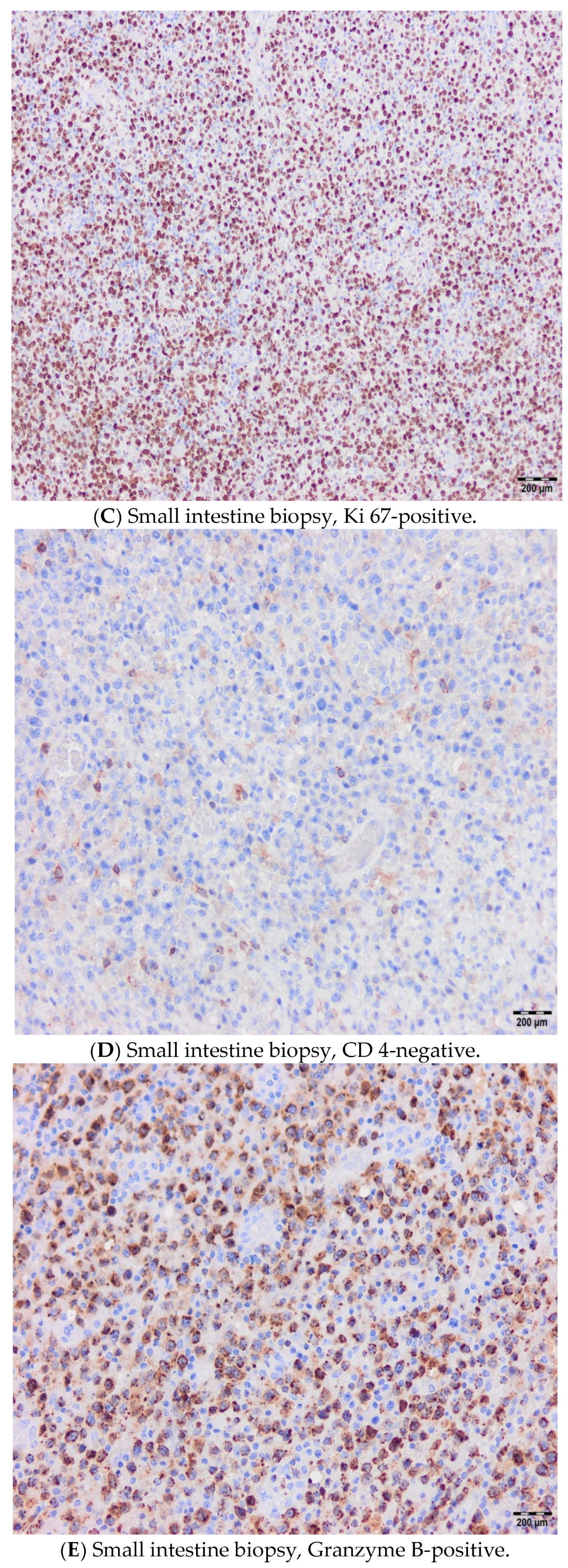
2. Cases
2.1. Case 1
2.2. Case 2
2.3. Case 3
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delabie, J.; Holte, H.; Vose, J.M.; Ullrich, F.; Jaffe, E.S.; Savage, K.J.; Connors, J.M.; Rimsza, L.; Harris, N.L.; Müller-Hermelink, K.; et al. Enteropathy- associated T cell Lymphoma: Clinical and histological findings from the International Peripheral T-Cell Lymphoma Project. Blood 2011, 118, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Bearzi, I.; Holmes, G.K. Association of celiac disease and intestinal lymphomas and other cancers. Gastroenterology 2005, 128 (Suppl. 1), 79–86. [Google Scholar] [CrossRef] [PubMed]
- O′Farrelly, C.; Feighery, C.; O′Briain, D.S.; Stevens, F.; Connolly, C.E.; McCarthy, C.; Weir, D.G. Humoral response to wheat protein in patients with celiac disease and enteropathy associated T cell lymphoma. Br. Med. J. 1986, 293, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Biagi, F.; Gobbi, P.G.; Corazza, G.R. How I treat enteropathy-associated T-cell lymphoma. Blood J. Am. Soc. Hematol. 2012, 119, 2458–2468. [Google Scholar] [CrossRef]
- Verbeek, W.H.M.; Van De Water, J.M.W.; Al-Toma, A.; Oudejans, J.J.; Mulder, C.J.J.; Coupé, V.M.H. Incidence of enteropathy associated T cell lymphoma: A nation-wide study of population based registry in The Nederlands. Scand. J. Gastroenterol. 2008, 43, 1322–1328. [Google Scholar] [CrossRef]
- Tomita, S.; Kikuti, Y.Y.; Carreras, J.; Kojima, M.; Ando, K.; Takasaki, H.; Sakai, R.; Takata, K.; Yoshino, T.; Bea, S.; et al. Genomic and immunohistochemical profiles of enteropathy-associated T-cell lymphoma in Japan. Mod. Pathol. 2015, 28, 1286–1296. [Google Scholar] [CrossRef]
- Fei, F.; Reddy, V.; Patel, C.R.; Dhall, D.; Lee, G.; Meng-Jun, X.; Al Diffalha, S. Monomorphic Epitheliotropic Intestinal T-cell Lymphoma: A Study of Four Cases and Review of Literature. Ann. Clin. Lab. Sci. 2020, 50, 806–812. [Google Scholar]
- Al Somali, Z.; Hamadani, M.; Kharfan-Dabaja, M.; Sureda, A.; El Fakih, R.; Aljurf, M. Enteropathy- Associated T cell Lymphoma. Curr. Hematol. Malig. Rep. 2021, 16, 140–147. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.D.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M. The 5th edition of WHO Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Arps, D.P.; Smith, L.B. Classic Versus Type II Enteropathy- Associated T-Cell Lymphoma: Diagnostic Considerations. Arch. Pathol. Lab. Med. 2013, 137, 1227–1231. [Google Scholar] [CrossRef]
- Bhansali, R.S.; Barta, S.K. SOHO State of the Art Updates and Next Questions|Challenging Cases in Rare T-Cell Lymphomas. Clin. Lymphoma Myeloma Leuk. 2023, 23, 642–650. [Google Scholar] [CrossRef]
- Nijeboer, P.; de Baaij, L.R.; Visser, O.; Witte, B.I.; Cillessen, S.A.; Mulder, C.J.; Bouma, G. Treatment response in enteropathy associated T cell lymphoma: Survival in large multicenter cohort. Am. J. Hematol. 2015, 90, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Kronsten, V.T.; Gosson, C.; Al-Khatib, A.; Bagwan, I.; Worthington, T.; Muthalali, S.; Ethell, M.; Lim, G.; Youd, P. Tonsillar Extraintestinal Enteropathy- Associated T- Cell Lymphoma in a Patient With Celiac Disease. ACG Case Rep. J. 2022, 9, e00824. [Google Scholar] [CrossRef] [PubMed]
- Bishton, M.J.; Haynes, A.P. Combination chemotherapy followed with autologous stem cell transplantation for enteropathy- associated T cell lymphoma. Br. J. Haematol. 2007, 136, 111–113. [Google Scholar] [CrossRef]
- Sieniawski, M.K.; Lennard, A.L. Enteropathy- Associated T Cell Lymphoma: Epidemiology, Clinical Features and Current Treatment Strategies. Curr. Hematol. Malig. Rep. 2011, 6, 231–240. [Google Scholar] [CrossRef]
- Sieniawski, M.; Angamuthu, N.; Boyd, K.; Chasty, R.; Davies, J.; Forsyth, P.; Jack, F.; Lyons, S.; Mounter, P.; Revell, P.; et al. Evaluation of enteropathy-associated T-cell lymphoma comparing standard therapies with a novel regimen including autologous stem cell transplantation. Blood 2010, 115, 3664–3670. [Google Scholar] [CrossRef]
- Horwitz, S.; O′Connor, O.A.; Pro, B.; Illidge, T.; Fanale, M.; Advani, R.; Bartlett, N.; Christensen, J.H.; Morschhauser, F.; Domenech, E.D.; et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): A global, double- blind, randomized, phase 3 trial. Lancet 2019, 393, 229–240. [Google Scholar] [CrossRef]
- Gallamini, A.; Zaja, F.; Patti, C.; Billio, A.; Specchia, M.R.; Tucci, A.; Levis, A.; Manna, A.; Secondo, V.; Rigacci, L.; et al. Alemtuzumab (Campath-1H) and CHOP chemotherapy as firstline treatment of peripheral T-cell lymphoma: Results of a GITIL (Gruppo Italiano Terapie Innovative nei Linfomi) prospective multicenter trial. Blood 2007, 110, 2316–2323. [Google Scholar] [CrossRef]
- Soldini, D.; Mora, O.; Cavalli, F.; Zucca, E.; Mazzucchelli, L. Efficacy of alemtuzumab and gemcitabine in a patient with enteropathy-type T-cell lymphoma. Br. J. Haematol. 2008, 142, 484–486. [Google Scholar] [CrossRef]
- Tack, G.J.; Verbeek, W.H.; Al-Toma, A.; Kuik, D.J.; Schreurs, M.W.; Visser, O.; Mulder, C.J. Evaluation of cladribine treatment in refractory celiac disease type II. World J. Gastroenterol. 2011, 17, 506–513. [Google Scholar] [CrossRef]
- Piekarz, R.L.; Frye, R.; Prince, H.M.; Kirschbaum, M.H.; Zain, J.; Allen, S.L.; Jaffe, E.S.; Ling, A.; Turner, M.; Peer, C.J.; et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood 2011, 117, 5827–5834. [Google Scholar] [CrossRef]
- Manso, R.; Rodriguez, M.; Chamizo, C.; Pérez, N.; Alonso-Alonso, R.; Minguez, P.A.; Borregon, J.; Baez-Duran, E.; Pozo, E.M.C.; Piris, M.A.; et al. Intestinal T-Cell Lymphomas: Molecular Integrative Analysis Recognizes Different Therapeutic Targets for Each Subtype. Hematol. Oncol. 2021, 39, 396–425. [Google Scholar] [CrossRef]
- Gooderham, M.J.; Forman, S.B.; Bissonnette, R.; Beebe, J.S.; Zhang, W.; Banfield, C.; Zhu, L.; Papacharalambous, J.; Vincent, M.S.; Peeva, E. Efficacy and Safety of Oral Janus Kinase 1 Inhibitor Abrocitinib for Patients With Atopic Dermatitis: A Phase 2 Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 104. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Ren, T.; Huang, Y.; Ren, C.; Yang, K.; Zhang, H.; Guo, W. Bortezomib induces apoptosis and suppresses cell growth and metastasis by inactivation of Stat3 signaling in chondrosarcoma. Int. J. Oncol. 2017, 50, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Cellier, C.; Bouma, G.; Van Gils, T.; Khater, S.; Malamut, G.; Crespo, L.; Collin, P.; Green, P.H.; Crowe, S.E.; Tsuji, W. Safety and efficacy of AMG 714 in patients with type 2 refractory celiac disease: A phase 2a, randomized, double-blind, placebo-controlled, parallel-group study. Lancet Gastroenterol. Hepatol. 2019, 4, 960–970. [Google Scholar] [CrossRef] [PubMed]
- van de Water, J.M.; Cillessen, S.A.; Visser, O.J.; Verbeek, W.H.; Meijer, C.J.; Mulder, C.J. Enteropathy associated T-cell Lymphoma and its precursor lesions. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 43–56. [Google Scholar] [CrossRef]
- Rampertab, S.D.; Forde, K.A.; Green, P.H.R. Small bowel neoplasia in celiac disease. Gut 2003, 52, 1211–1214. [Google Scholar] [CrossRef]
- Wang, M.; Yu, M.; Kong, W.-J.; Cui, M.; Gao, F. Association between intestinal neoplasms and celiac disease: A review. World J. Gastrointest. Oncol. 2021, 13, 1017–1028. [Google Scholar] [CrossRef]
- Almasri, M.; Maher, N.; Al Deeban, B.; Diop, N.M.; Moia, R.; Gaidano, G. Liquid Biopsy in B and T cell Lymphoma: From Bench to Bedside. Int. J. Mol. Sci. 2025, 26, 4869. [Google Scholar] [CrossRef]
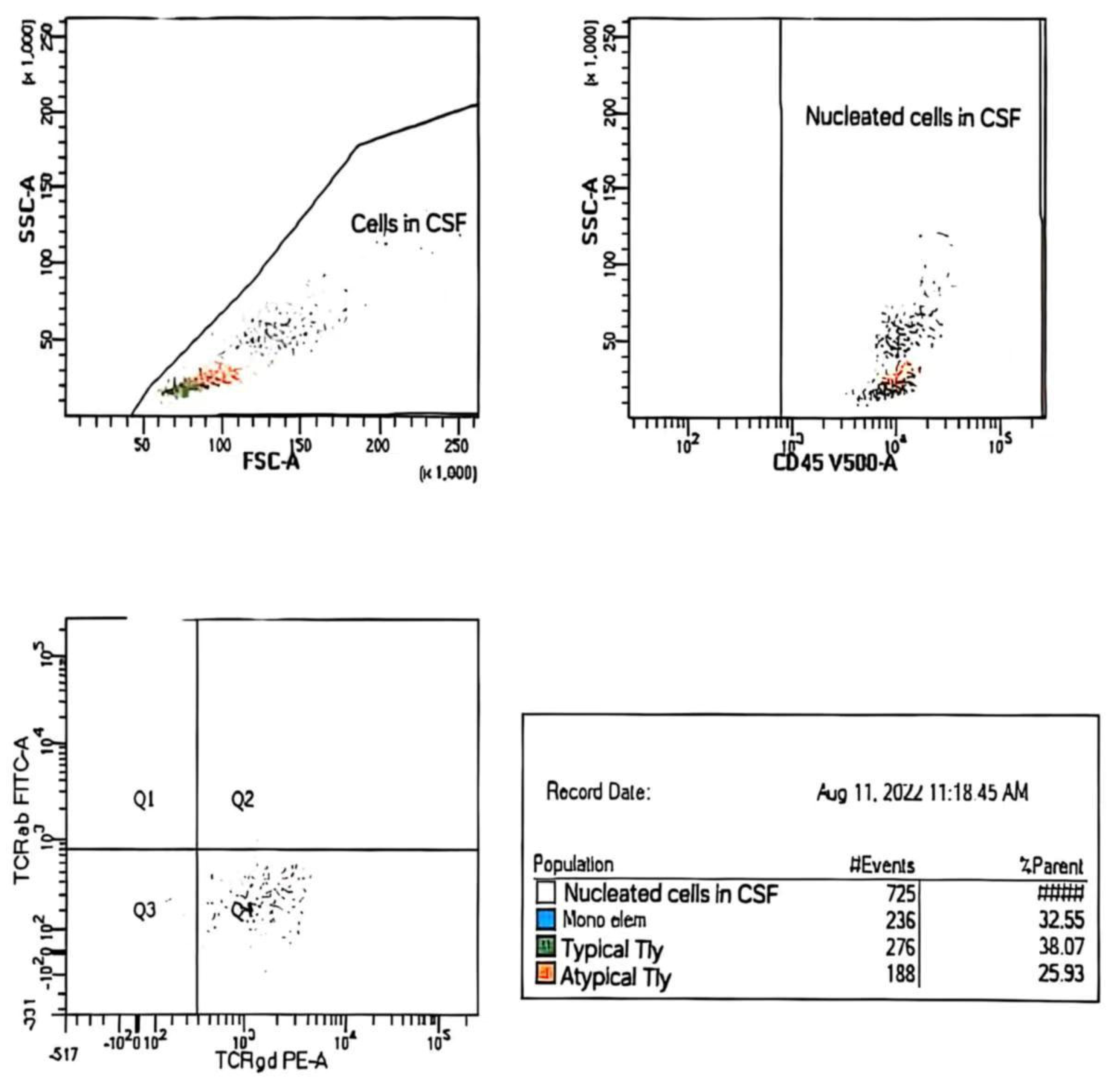
| Sample/Method | CSF,~2 mL/WLLyW/ Multparameter/ flow cytometry/ BD FACSCanto II (4-2-2) | ||
| Description of CSF | Clear; no admixture of red blood cells in sediment | ||
| Analysed combination of Ag | 1. mCD3/CD16/CD56/CD4/CD19/CD8/CD45 2. TCRαβ/TCRγδ/CD4/CD7/CD8/CD14/mCD3/CD45 3. CD2/CD5/CD4/CD7/CD8/mCD3/CD45 4. CD57/CD30/CD4/CD7/CD8/mCD3/CD45 | ||
| No. of analyzed cells ep | 725 nucleated cell in CSF | ||
| Population of cells in CSF | Gating strategies | % SC/number of cells | |
| Nucleated cells | CD45+/SSC | 100% (725 c) | |
| Mono elements | CD14+high CD45+high/SSCmedium | 33% (236/725 c) | |
| Typical T-Ly | mCD+med CD4+ CD8+ CD7+het TCRαβ+ TCRγδ CD45+high/ SSC low | 38% (276/725 c) | |
| Atypical T-Ly | mCD3+low CD2+high CD7+bright TCRγδ+low CD30+low CD4+ CD8+ TCRαβ+ CD57+ CD45+high/SSClow | 26% (188/725 c) | |
| Population of Atypical T-ly (188c–725c) | |||
| Antigen | % positive cells | Antigen | % positive cells |
| CD45+high | 100% | CD4 | 0% |
| mCD3+Low | 100% | CD8 | 0% |
| CD7 +bright | 100% | CD5 | 0% |
| CD2 +high | 100% | CD16 | 0% |
| CD30 +low | 64% | CD56 | 0% |
| TCRγδ+low | 91% | CD57 | 0% |
| TCRαβ | 0% | CD19 | 0% |
| Ag+ = partial exp. – exp. on subpop. cells | |||
| Conclusion: Analysis of CSF sediment revealed the presence of an atypical population of T-Ly with immunophenotypic characteristics corresponding to lymphoma cells (~26% NC → 188c/725c). Parallel analysis of peripheral blood cells revealed the presence of a relatively small population of lymphoma cells (mCD3+low CD2+high CD7+bright TCRγδ+high CD30+het CD4+ CD8+ TCRαβ+ CD45+high / SSClow, ~0.9% Le). | |||
| Trial Name/ID | Intervention(s) | Status | Design/Notes | Key Findings |
|---|---|---|---|---|
| NCT03217643 (EATL-001) | BV + CHP following HDT + ASCT | Phase 2/completed | 14 newly diagnosed CD30 + EATL type I | ORR 79%, CR 64%, 2y PFS 63%, OS 68% |
| SNLG | IVE/MTX + ASCT | Retrospective/completed | 26 transplant eligible EATL | 5y PFS~52%, OS~60%, versus anthracycline- only 7 mo OS |
| NCT00697346 | Alisertib (MLN8237) | Phase 1/completed | Advanced hematologic malignancies, incl. EATL | EATL specific results not detailed |
| NCT00901147 | Bortezomib + panabinostat | Phase 2/completed | R/R PTCL incl. EATL | No separate EATL data reported |
| NIH CD30 CAR-T trial | CD30-CART cells post- fludarabine+ cyclophosphamide | Phase 1/recruiting | CD30+ lymphomas incl. EATL | Ongoing |
| NIH/Mayo Clinic Nivolumab | Nivolumab (anti-PD-1) | Phase 1/recruiting | PTCL incl. EATL | Ongoing |
| AMG714 for RCDII | Anti IL-15 monoclonal antibody | Phase 2a/completed | Type II refractory celiac disease (pre-EATL) | Halted malignant IEL progression, relevant as EATL prevention |
| NCT02588651 | BV monotherapy | Phase 2/recruiting | CD30-low mature T-cell lymphomas, possibly EATL | Ongoing, may include EATL subgroups |
| ASTX660 | ASTX660, Duvelisib combos | Phase I/II/active/terminated | R/R PTCL, some EATL | EATL data pending |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elez, M.; Atanasković, L.; Mirosavljević, S.; Bezmarević, M.; Živojinović, D.; Romanović, R.; Djekić, J.; Krstić, P. Case-Based Insights into Enteropathy-Associated T-Cell Lymphoma—Single-Center Experience. Hematol. Rep. 2025, 17, 43. https://doi.org/10.3390/hematolrep17050043
Elez M, Atanasković L, Mirosavljević S, Bezmarević M, Živojinović D, Romanović R, Djekić J, Krstić P. Case-Based Insights into Enteropathy-Associated T-Cell Lymphoma—Single-Center Experience. Hematology Reports. 2025; 17(5):43. https://doi.org/10.3390/hematolrep17050043
Chicago/Turabian StyleElez, Marija, Lavinika Atanasković, Svetlana Mirosavljević, Mihailo Bezmarević, Dragan Živojinović, Radoslav Romanović, Jelena Djekić, and Predrag Krstić. 2025. "Case-Based Insights into Enteropathy-Associated T-Cell Lymphoma—Single-Center Experience" Hematology Reports 17, no. 5: 43. https://doi.org/10.3390/hematolrep17050043
APA StyleElez, M., Atanasković, L., Mirosavljević, S., Bezmarević, M., Živojinović, D., Romanović, R., Djekić, J., & Krstić, P. (2025). Case-Based Insights into Enteropathy-Associated T-Cell Lymphoma—Single-Center Experience. Hematology Reports, 17(5), 43. https://doi.org/10.3390/hematolrep17050043






