Reversible Platelet Aggregation Induced by Low-Temperature Storage in Heparinized Whole Blood Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects, Anticoagulation of Whole Blood with Heparin or EDTA
2.2. Treatment of the Whole Blood and Blood Cell Count Using an Automated Hematology Analyzer Based on the Electrical Impedance Methods
2.3. Observation of Platelet Morphology in the Peripheral Blood Smears
2.4. Molecular Docking Simulation
2.5. Statistical Analyses
2.6. Ethics Status
3. Results
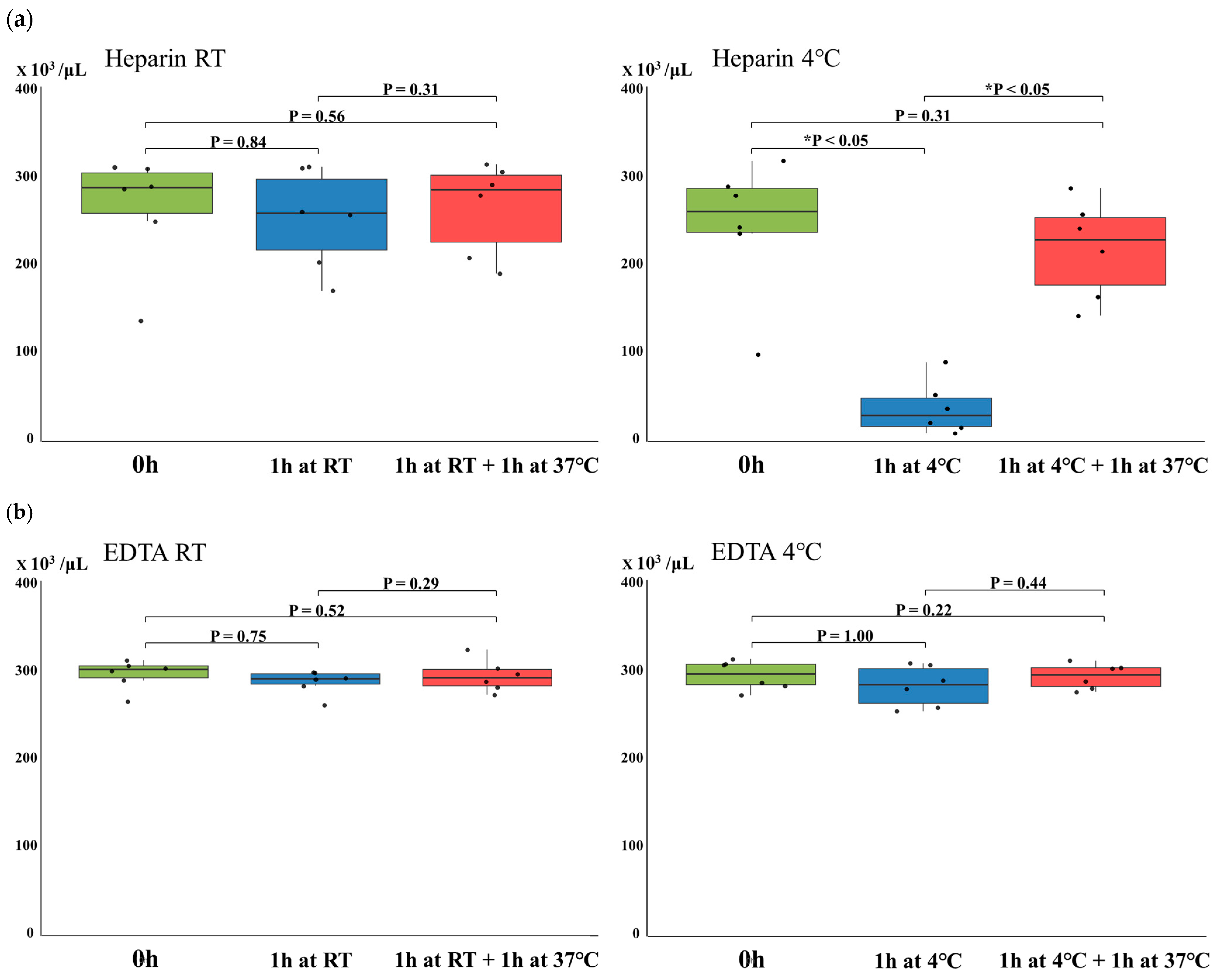
3.1. Changes in Platelet Counts in Heparinized or EDTA-Anticoagulated Whole Blood Due to Temperature Variations
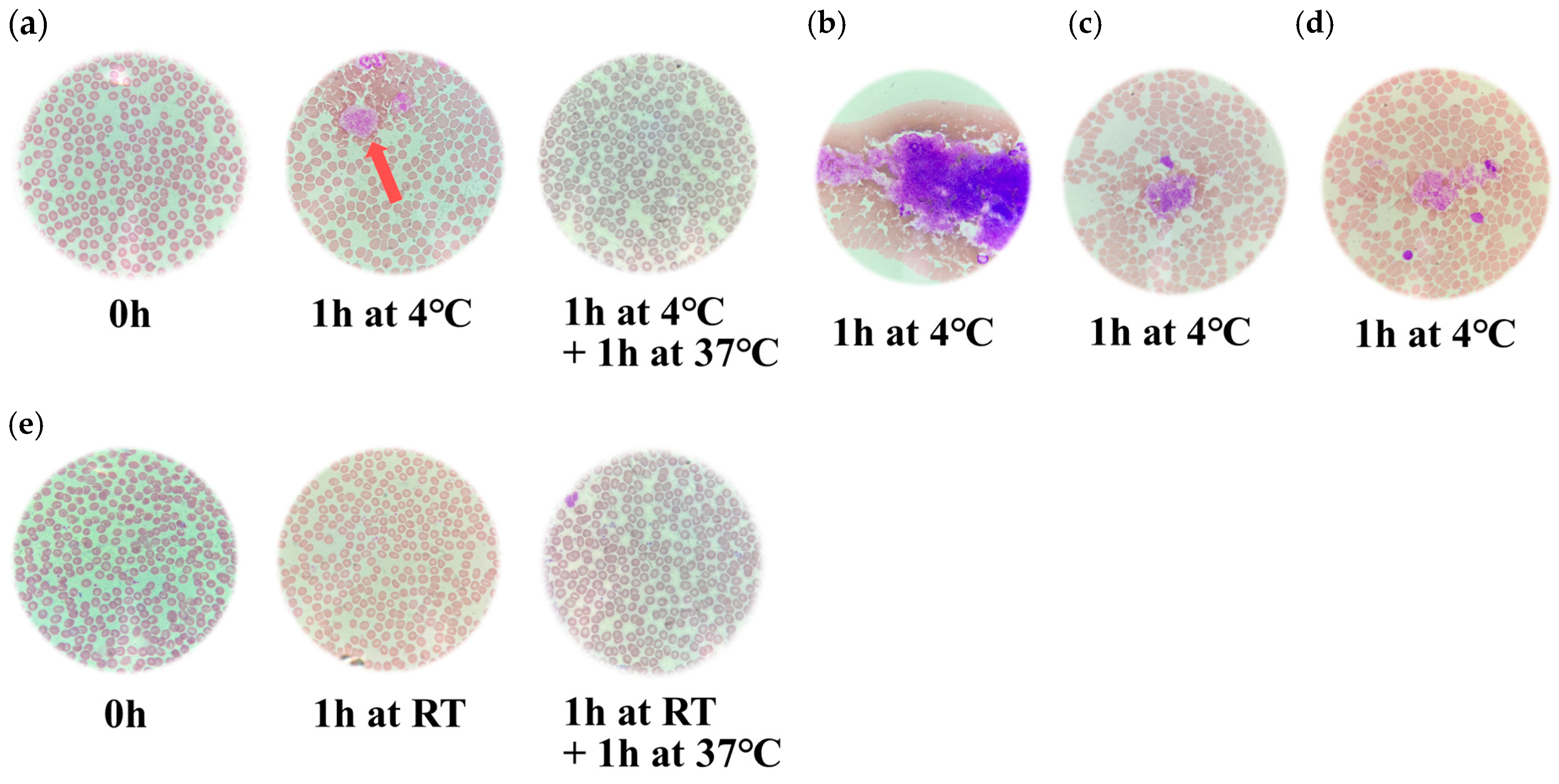
3.2. Microscopic Findings of the Blood Smears Stained with May-Grünwald Giemsa
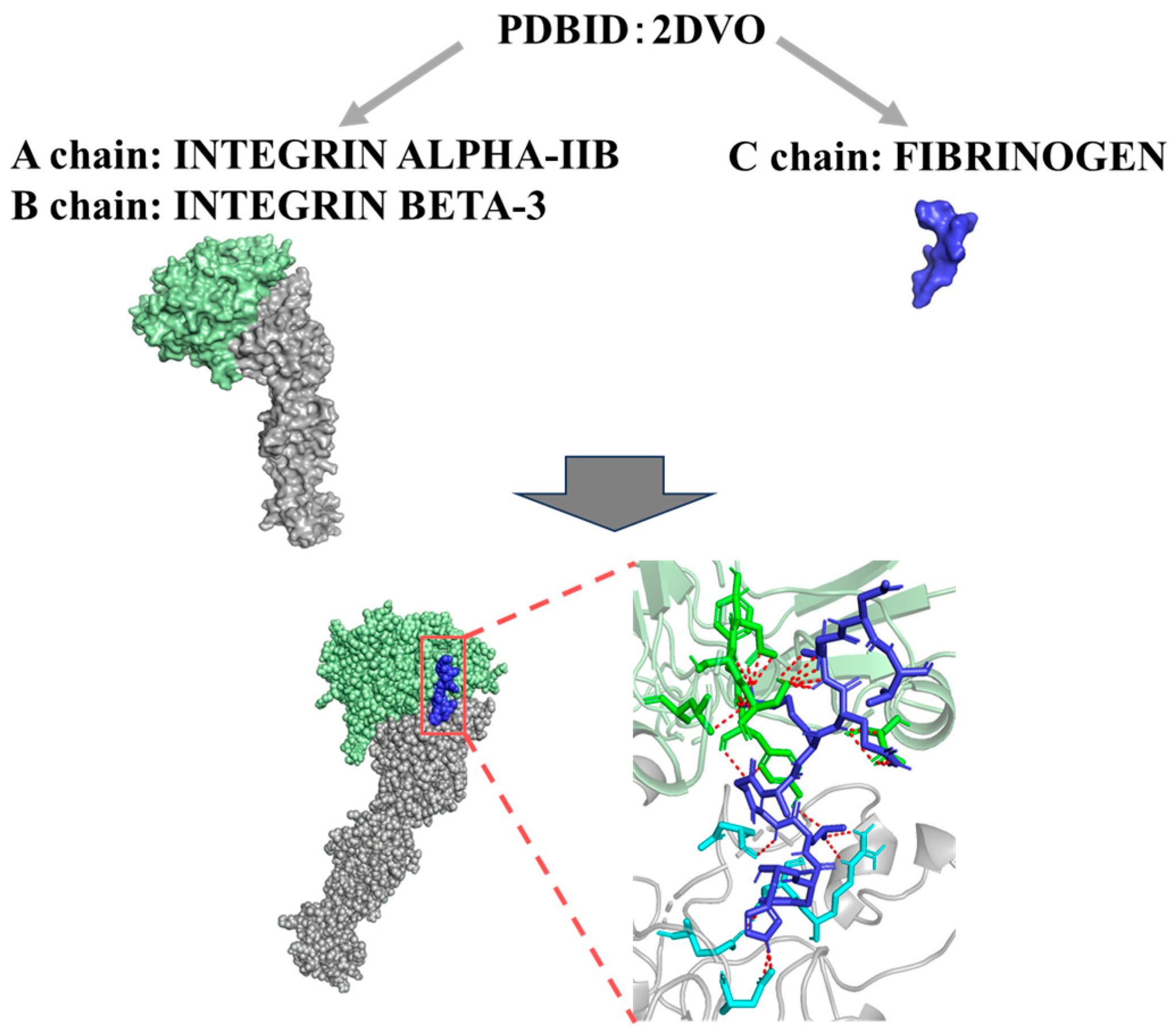
3.3. Evaluation of the Binding Affinities Between Integrin GPIIb/IIIa and Fibrinogen
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Slichter, S.J. Relationship between Platelet Count and Bleeding Risk in Thrombocytopenic Patients. Transfus. Med. Rev. 2004, 18, 153–167. [Google Scholar] [CrossRef]
- Harrison, P.; Goodall, A.H. Studies on Mean Platelet Volume (MPV)-New Editorial Policy. Platelets 2016, 27, 605–606. [Google Scholar] [CrossRef]
- Thomas, S. Platelets: Handle with Care. Transfus. Med. 2016, 26, 330–338. [Google Scholar] [CrossRef]
- Mani, H.; Kirchmayr, K.; Kläffling, C.; Schindewolf, M.; Luxembourg, B.; Linnemann, B.; Lindhoff-Last, E. Influence of Blood Collection Techniques on Platelet Function. Platelets 2004, 15, 315–318. [Google Scholar] [CrossRef]
- Tassi Yunga, S.; Gower, A.J.; Melrose, A.R.; Fitzgerald, M.K.; Rajendran, A.; Lusardi, T.A.; Armstrong, R.J.; Minnier, J.; Jordan, K.R.; McCarty, O.J.T.; et al. Effects of Ex Vivo Blood Anticoagulation and Preanalytical Processing Time on the Proteome Content of Platelets. J. Thromb. Haemost. 2022, 20, 1437–1450. [Google Scholar] [CrossRef]
- Murata, Y.; Kusudo, E.; Kawamoto, S.; Fukuda, K. Effects of Whole Blood Storage in a Polyolefin Blood Bag on Platelets for Acute Normovolemic Hemodilution. Sci. Rep. 2021, 11, 12201. [Google Scholar] [CrossRef]
- Putra, A.A.M.S.; Hernaningsih, Y. Comparison of Complete Blood Count Parameters Using EDTA, Sodium Citrate, and Heparin Anticoagulants. Res. J. Pharm. Technol. 2022, 15, 4687–4691. [Google Scholar] [CrossRef]
- Choudhary, S.; Shankarkatkar, R.; Punneshetty, D.S.; Begum, F.; Shilpa, S.J. EDTA Induced Storage Artefacts–a Study on Peripheral Blood Smears. Int. J. Acad. Med. Pharm. 2022, 5, 183–187. [Google Scholar] [CrossRef]
- Ahn, H.L.; Jo, Y.I.; Choi, Y.S.; Lee, J.Y.; Lee, H.W.; Kim, S.R.; Sim, J.; Lee, W.; Jin, C.J. EDTA-Dependent Pseudothrombocytopenia Confirmed by Supplementation of Kanamycin; a Case Report. Korean J. Intern. Med. 2002, 17, 65–68. [Google Scholar] [CrossRef]
- Berkman, N.; Michaeli, Y.; Or, R.; Eldor, A. EDTA-Dependent Pseudothrombocytopenia: A Clinical Study of 18 Patients and a Review of the Literature. Am. J. Hematol. 1991, 36, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.C.N.; Lim, C.E.D. The Phantom Platelet Problem: Unmasking Ethylenediaminetetraacetic Acid (EDTA)-Induced Pseudo-Thrombocytopenia. Cureus 2025, 17, e81211. [Google Scholar] [CrossRef]
- Bizzaro, N. EDTA-Dependent Pseudothrombocytopenia: A Clinical and Epidemiological Study of 112 Cases, with 10-Year Follow-Up. Am. J. Hematol. 1995, 50, 103–109. [Google Scholar] [CrossRef]
- Lardinois, B.; Favresse, J.; Chatelain, B.; Lippi, G.; Mullier, F. Pseudothrombocytopenia-A Review on Causes, Occurrence and Clinical Implications. J. Clin. Med. 2021, 10, 594. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E. Laboratory Diagnosis of Heparin-Induced Thrombocytopenia. Int. J. Lab. Hematol. 2019, 41 (Suppl. 1), 15–25. [Google Scholar] [CrossRef]
- Hall, M.W.; Goodman, P.D.; Alston, S.M.; Solen, K.A. Hypothermia-Induced Platelet Aggregation in Heparinized Flowing Human Blood: Identification of a High Responder Subpopulation. Am. J. Hematol. 2002, 69, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Boylan, B.; Fang, J.; Wilcox, D.A.; Newman, D.K.; Newman, P.J. Heparin Promotes Platelet Responsiveness by Potentiating αIIbβ3-Mediated Outside-in Signaling. Blood 2011, 117, 4946–4952. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.T.; Richard, B.; Izaguirre, G.; Schedin-Weiss, S.; Gettins, P.G.W. Molecular Mechanisms of Antithrombin-Heparin Regulation of Blood Clotting Proteinases. A Paradigm for Understanding Proteinase Regulation by Serpin Family Protein Proteinase Inhibitors. Biochimie 2010, 92, 1587–1596. [Google Scholar] [CrossRef]
- Shattil, S.J.; Kim, C.; Ginsberg, M.H. The Final Steps of Integrin Activation: The End Game. Nat. Rev. Mol. Cell Biol. 2010, 11, 288–300. [Google Scholar] [CrossRef]
- Xiao, T.; Takagi, J.; Coller, B.S.; Wang, J.-H.; Springer, T.A. Structural Basis for Allostery in Integrins and Binding to Fibrinogen-Mimetic Therapeutics. Nature 2004, 432, 59–67. [Google Scholar] [CrossRef]
- Janus-Bell, E.; Mangin, P.H. The Relative Importance of Platelet Integrins in Hemostasis, Thrombosis and Beyond. Haematologica 2023, 108, 1734–1747. [Google Scholar] [CrossRef]
- Joo, S.-J. Mechanisms of Platelet Activation and Integrin αIIβ3. Korean Circ. J. 2012, 42, 295–301. [Google Scholar] [CrossRef]
- Ma, Y.-Q.; Qin, J.; Plow, E.F. Platelet Integrin αIIbβ3: Activation Mechanisms. J. Thromb. Haemost. 2007, 5, 1345–1352. [Google Scholar] [CrossRef]
- WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy. Available online: https://www.who.int/publications/i/item/9789241599221 (accessed on 25 June 2025).
- Vu, Q.H.; Van, H.T.; Tran, V.T.; Huynh, T.D.P.; Nguyen, V.C.; Le, D.T. Development of a Robust Blood Smear Preparation Procedure for External Quality Assessment. Pract. Lab. Med. 2021, 27, e00253. [Google Scholar] [CrossRef] [PubMed]
- Piaton, E.; Fabre, M.; Goubin-Versini, I.; Bretz-Grenier, M.-F.; Courtade-Saïdi, M.; Vincent, S.; Belleannée, G.; Thivolet, F.; Boutonnat, J.; Debaque, H.; et al. Guidelines for May-Grünwald-Giemsa Staining in Haematology and Non-Gynaecological Cytopathology: Recommendations of the French Society of Clinical Cytology (SFCC) and of the French Association for Quality Assurance in Anatomic and Cytologic Pathology (AFAQAP). Cytopathology 2016, 27, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Akorsu, E.E.; Adjabeng, L.B.; Sulleymana, M.A.; Kwadzokpui, P.K. Variations in the Full Blood Count Parameters among Apparently Healthy Humans in the Ho Municipality Using Ethylenediamine Tetraacetic Acid (EDTA), Sodium Citrate and Lithium Heparin Anticoagulants: A Laboratory-Based Cross-Sectional Analytical Study. Heliyon 2023, 9, e17311. [Google Scholar] [CrossRef] [PubMed]
- Salter, B.S.; Weiner, M.M.; Trinh, M.A.; Heller, J.; Evans, A.S.; Adams, D.H.; Fischer, G.W. Heparin-Induced Thrombocytopenia: A Comprehensive Clinical Review. J. Am. Coll. Cardiol. 2016, 67, 2519–2532. [Google Scholar] [CrossRef]
- Hirsh, J.; Raschke, R. Heparin and Low-Molecular-Weight Heparin: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004, 126, 188S–203S. [Google Scholar] [CrossRef]
- Hsu, E.; Moosavi, L. Biochemistry, Antithrombin III. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Rauova, L.; Zhai, L.; Kowalska, M.A.; Arepally, G.M.; Cines, D.B.; Poncz, M. Role of Platelet Surface PF4 Antigenic Complexes in Heparin-Induced Thrombocytopenia Pathogenesis: Diagnostic and Therapeutic Implications. Blood 2006, 107, 2346–2353. [Google Scholar] [CrossRef]
- Cai, Z.; Yarovoi, S.V.; Zhu, Z.; Rauova, L.; Hayes, V.; Lebedeva, T.; Liu, Q.; Poncz, M.; Arepally, G.; Cines, D.B.; et al. Atomic Description of the Immune Complex Involved in Heparin-Induced Thrombocytopenia. Nat. Commun. 2015, 6, 8277. [Google Scholar] [CrossRef]
- Rauova, L.; Bdeir, K.; Rux, A.H.; Kalathottukaren, M.T.; Oberg, J.; La, C.C.; Lim, D.T.E.; Hayes, V.; Koma, G.T.; Sarkar, A.; et al. Destabilization of PF4-Antigenic Complexes in Heparin-Induced Thrombocytopenia. Blood 2025, 145, 3030–3040. [Google Scholar] [CrossRef]
- Johnston, I.; Sarkar, A.; Hayes, V.; Koma, G.T.; Arepally, G.M.; Chen, J.; Chung, D.W.; López, J.A.; Cines, D.B.; Rauova, L.; et al. Recognition of PF4-VWF Complexes by Heparin-Induced Thrombocytopenia Antibodies Contributes to Thrombus Propagation. Blood 2020, 135, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-H.; Medvedev, N.; Delcea, M.; Greinacher, A. Anti-Platelet Factor 4/Polyanion Antibodies Mediate a New Mechanism of Autoimmunity. Nat. Commun. 2017, 8, 14945. [Google Scholar] [CrossRef]
- Davenport, A. Review Article: Low-Molecular-Weight Heparin as an Alternative Anticoagulant to Unfractionated Heparin for Routine Outpatient Haemodialysis Treatments. Nephrology 2009, 14, 455–461. [Google Scholar] [CrossRef]
- Conners, M.S.; Money, S.R. The New Heparins. Ochsner J. 2002, 4, 41–47. [Google Scholar]
- Merli, G.J.; Groce, J.B. Pharmacological and Clinical Differences Between Low-Molecular-Weight Heparins. Pharm. Ther. 2010, 35, 95–105. [Google Scholar]
- Straub, A.; Krajewski, S.; Hohmann, J.D.; Westein, E.; Jia, F.; Bassler, N.; Selan, C.; Kurz, J.; Wendel, H.P.; Dezfouli, S.; et al. Evidence of Platelet Activation at Medically Used Hypothermia and Mechanistic Data Indicating ADP as a Key Mediator and Therapeutic Target. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1607–1616. [Google Scholar] [CrossRef]
- Sobel, M.; Fish, W.R.; Toma, N.; Luo, S.; Bird, K.; Mori, K.; Kusumoto, S.; Blystone, S.D.; Suda, Y. Heparin Modulates Integrin Function in Human Platelets. J. Vasc. Surg. 2001, 33, 587–594. [Google Scholar] [CrossRef]
- Peerschke, E.; Zucker, M. Fibrinogen Receptor Exposure and Aggregation of Human Blood Platelets Produced by ADP and Chilling. Blood 1981, 57, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Bissantz, C.; Kuhn, B.; Stahl, M. A Medicinal Chemist’s Guide to Molecular Interactions. J. Med. Chem. 2010, 53, 5061–5084. [Google Scholar] [CrossRef] [PubMed]
- Kastritis, P.L.; Bonvin, A.M.J.J. On the Binding Affinity of Macromolecular Interactions: Daring to Ask Why Proteins Interact. J. R. Soc. Interface 2013, 10, 20120835. [Google Scholar] [CrossRef]
- Gayathri, M.; Prajna, K.M.; Chaithanya, U. A Comparative Study of Platelet Count by Manual Method and Automated Analyser: A Retrospective Study. Int. J. Res. Med. Sci. 2024, 12, 3326–3330. [Google Scholar] [CrossRef]
- Friede, K.A.; Infeld, M.M.; Tan, R.S.; Knickerbocker, H.J.; Myers, R.A.; Dubois, L.G.; Thompson, J.W.; Kaddurah-Daouk, R.; Ginsburg, G.S.; Ortel, T.L.; et al. Influence of Sex on Platelet Reactivity in Response to Aspirin. J. Am. Heart Assoc. 2020, 9, e014726. [Google Scholar] [CrossRef] [PubMed]
| Model | ΔG (kcal mol−1) | Kd (M) at °C | |||
|---|---|---|---|---|---|
| 4 °C | Model 1 | −8.9 | 8.9 × 10−8 | ||
| Model 2 | −9.4 | 4.0 × 10−8 | |||
| Model 3 | −9.6 | 2.8 × 10−8 | |||
| Model 4 | −9.2 | 5.5 × 10−8 | |||
| 37 °C | Model 1 | −8.9 | 5.0 × 10−7 | ||
| Model 2 | −9.4 | 2.4 × 10−7 | |||
| Model 3 | −9.6 | 1.8 × 10−7 | |||
| Model 4 | −9.2 | 3.2 × 10−7 | |||
| Minimum | 0.25 | Median | 0.75 | Maximum | |
| 4 °C | 2.8 × 10−8 | 3.7 × 10−8 | 4.8 × 10−8 | 6.4 × 10−8 | 8.9 × 10−8 |
| 37 °C | 1.8 × 10−7 | 2.3 × 10−7 | 2.8 × 10−7 | 3.7 × 10−7 | 5.0 × 10−7 |
| p-value = 0.0286 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, Y.; Miyazaki, M.; Kimura, R.; Arai, R.; Takada, M.; Takahashi, A.; Kimura, H. Reversible Platelet Aggregation Induced by Low-Temperature Storage in Heparinized Whole Blood Samples. Hematol. Rep. 2025, 17, 42. https://doi.org/10.3390/hematolrep17050042
Hayashi Y, Miyazaki M, Kimura R, Arai R, Takada M, Takahashi A, Kimura H. Reversible Platelet Aggregation Induced by Low-Temperature Storage in Heparinized Whole Blood Samples. Hematology Reports. 2025; 17(5):42. https://doi.org/10.3390/hematolrep17050042
Chicago/Turabian StyleHayashi, Yuriko, Manato Miyazaki, Ryusuke Kimura, Ririka Arai, Miu Takada, Ayuko Takahashi, and Hirokazu Kimura. 2025. "Reversible Platelet Aggregation Induced by Low-Temperature Storage in Heparinized Whole Blood Samples" Hematology Reports 17, no. 5: 42. https://doi.org/10.3390/hematolrep17050042
APA StyleHayashi, Y., Miyazaki, M., Kimura, R., Arai, R., Takada, M., Takahashi, A., & Kimura, H. (2025). Reversible Platelet Aggregation Induced by Low-Temperature Storage in Heparinized Whole Blood Samples. Hematology Reports, 17(5), 42. https://doi.org/10.3390/hematolrep17050042







