Diagnostic Challenges in Acute Leukemia: From Dental Pain to Catastrophic Intracerebral Hemorrhage
Abstract
1. Introduction
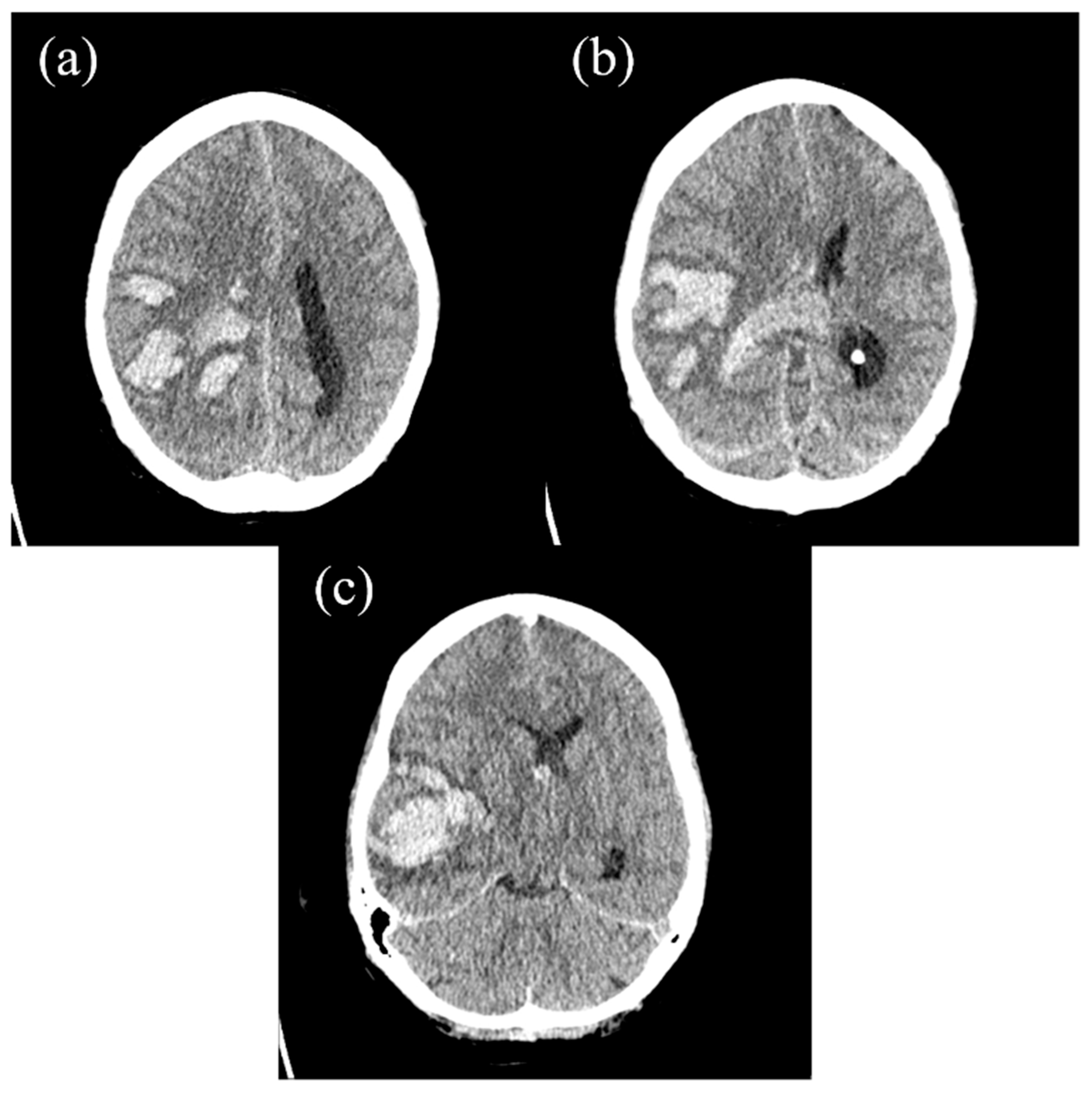
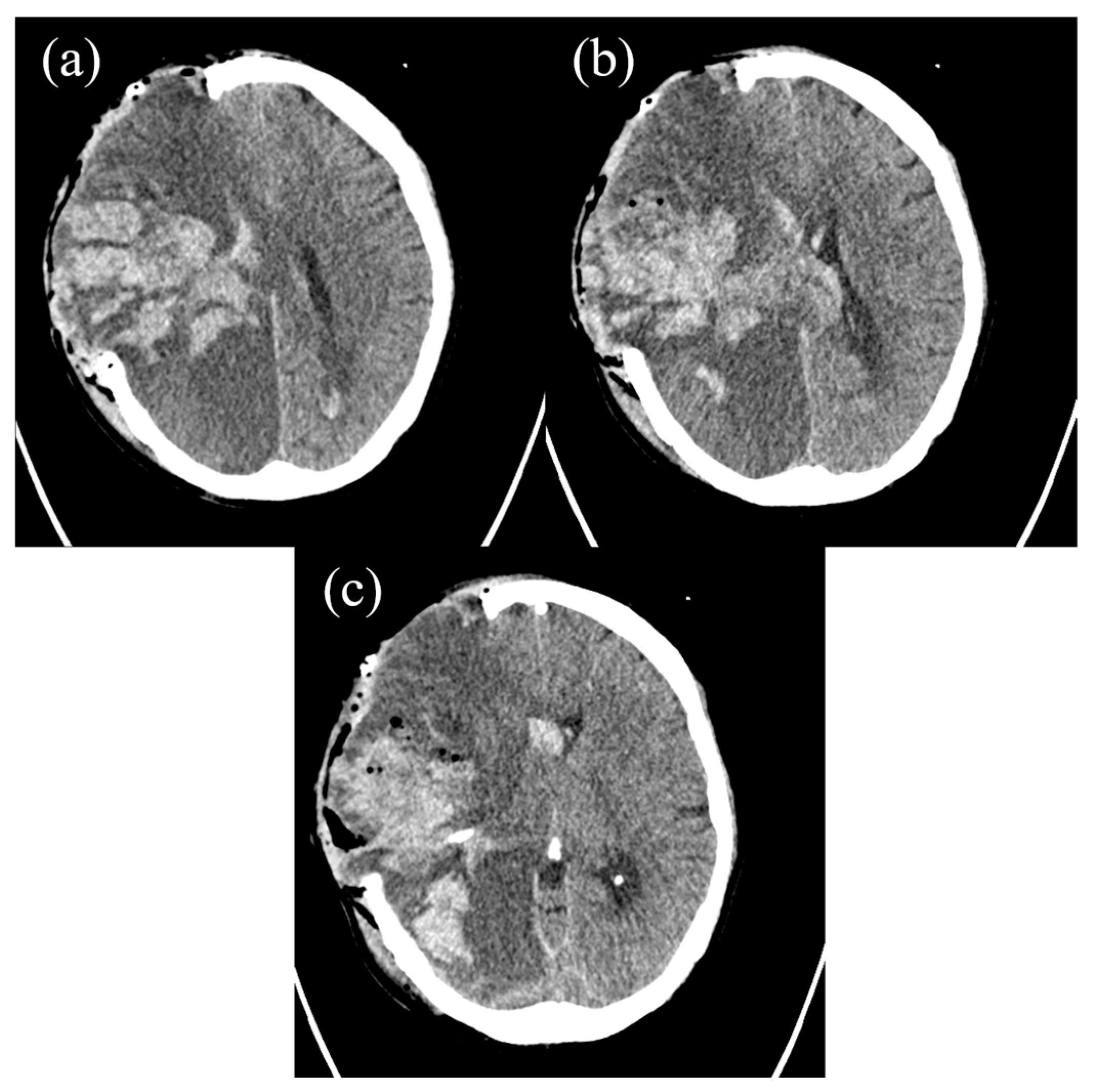
2. The Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AML | Acute Myeloid Leukemia |
| ALL | Acute Lymphoblastic Leukemia |
| DIC | Disseminated Intravascular Coagulopathy |
| APL | Acute Promyelocytic Leukemia |
| ICH | Intracerebral Hemorrhage |
| CT | Computed Tomography |
| CRP | C-Reactive Protein |
| ASAT | Aspartate Aminotransferase |
| GGT | Gamma-Glutamyl Transferase |
| INR | International Normalized Ratio |
| CBC | Complete Blood Count |
| CD | Cluster of Differentiation |
| PCC | Prothrombin Complex Concentrate |
| L | Liter |
References
- Fatahzadeh, M.; Krakow, A.M. Manifestation of acute monocytic leukemia in the oral cavity: A case report. Spec. Care Dent. 2008, 28, 190–194. [Google Scholar] [CrossRef]
- Reenesh, M.; Munishwar, S.; Rath, S.K. Generalised leukaemic gingival enlargement: A case report. J. Oral Maxillofac. Res. 2012, 3, e5. [Google Scholar] [CrossRef]
- Quispe, R.A.; Aguiar, E.M.; de Oliveira, C.T.; Neves, A.C.X.; Santos, P. Oral manifestations of leukemia as part of early diagnosis. Hematol. Transfus. Cell Ther. 2022, 44, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fantasia, J.E.; Kaplan, R. Oral manifestations of acute myelomonocytic leukemia: A case report and review of the classification of leukemias. J. Periodontol. 2002, 73, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.L.; Tsai, C.C. Primary gingival enlargement as a diagnostic indicator in acute myelomonocytic leukemia. A case report. J. Periodontol. 1988, 59, 852–855. [Google Scholar] [CrossRef] [PubMed]
- Weckx, L.L.; Hidal, L.B.; Marcucci, G. Oral manifestations of leukemia. Ear Nose Throat J. 1990, 69, 341–342, 345–346. [Google Scholar]
- Bastos Silveira, B.; Di Carvalho Melo, L.; Amorim Dos Santos, J.; Ferreira, E.B.; Reis, P.E.D.; De Luca Canto, G.; Acevedo, A.C.; Massignan, C.; Guerra, E.N.S. Oral manifestations in pediatric patients with leukemia: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2024, 155, 858–870.e830. [Google Scholar] [CrossRef]
- Suárez-Cuenca, J.A.; Arellano-Sánchez, J.L.; Scherling-Ocampo, A.A.; Sánchez-Hernández, G.; Pérez-Guevara, D.; Chalapud-Revelo, J.R. Rapidly progressing, fatal and acute promyelocytic leukaemia that initially manifested as a painful third molar: A case report. J. Med. Case Rep. 2009, 3, 102. [Google Scholar] [CrossRef]
- Chen, C.Y.; Tai, C.H.; Tsay, W.; Chen, P.Y.; Tien, H.F. Prediction of fatal intracranial hemorrhage in patients with acute myeloid leukemia. Ann. Oncol. 2009, 20, 1100–1104. [Google Scholar] [CrossRef]
- Koschade, S.E.; Stratmann, J.A.; Miesbach, W.; Steffen, B.; Serve, H.; Finkelmeier, F.; Brandts, C.H.; Ballo, O. Intracranial hemorrhage in newly diagnosed non-promyelocytic acute myeloid leukemia patients admitted for intensive induction chemotherapy. Eur. J. Haematol. 2022, 108, 125–132. [Google Scholar] [CrossRef]
- Rose-Inman, H.; Kuehl, D. Acute leukemia. Emerg. Med. Clin. N. Am. 2014, 32, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Tai, C.H.; Cheng, A.; Wu, H.C.; Tsay, W.; Liu, J.H.; Chen, P.Y.; Huang, S.Y.; Yao, M.; Tang, J.L.; et al. Intracranial hemorrhage in adult patients with hematological malignancies. BMC Med. 2012, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.; Lo, Y.Y.; Crawford, J.R. Intracerebral haemorrhage as an initial manifestation of T-cell acute lymphoblastic leukaemia in a paediatric patient. BMJ Case Rep. 2022, 15, e252594. [Google Scholar] [CrossRef] [PubMed]
- Michalak, E.; Dudzik, A.; Śręba, J.; Kęsek, B.; Darczuk, D. Oral manifestations of leukaemia: Cooperation between dentist and haematologist. Hematol. Clin. Pract. 2022, 13, 55–61. [Google Scholar] [CrossRef]
- Lim, H.C.; Kim, C.S. Oral signs of acute leukemia for early detection. J. Periodontal Implant. Sci. 2014, 44, 293–299. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Li, Y.; Ma, Y.-S.; Sun, X.-J.; Liu, Y.-Z.; Yin, Y.-K.; Hu, B.; Su, M.-H.; Li, Q.-L.; Mi, Y.-C.; et al. Clinical characteristics and prognostic factors in intracranial hemorrhage patients with hematological diseases. Ann. Hematol. 2022, 101, 2617–2625. [Google Scholar] [CrossRef]
- Estey, E.; Döhner, H. Acute myeloid leukaemia. Lancet 2006, 368, 1894–1907. [Google Scholar] [CrossRef]
- Wu, M.Y.; Lin, C.H.; Hou, Y.T.; Lin, P.C.; Yiang, G.T.; Tien, Y.C.; Yeh, H.C. Syncope as Initial Presentation in an Undifferentiated Type Acute Myeloid Leukemia Patient with Acute Intracranial Hemorrhage. Brain Sci. 2019, 9, 207. [Google Scholar] [CrossRef]
- Patil, S.; Nourbakhsh, A.; Thakur, J.D.; Khan, I.S.; Guthikonda, B. Fatal intracranial hemorrhage as the initial presentation of acute lymphocytic leukemia: A case report. Turk. Neurosurg. 2013, 23, 568–571. [Google Scholar] [CrossRef]
- Van de Louw, A. Effect of leukapheresis on blood coagulation in patients with hyperleukocytic acute myeloid leukemia. Transfus. Apher. Sci. 2017, 56, 214–219. [Google Scholar] [CrossRef]
- Kawanami, T.; Kurita, K.; Yamakawa, M.; Omoto, E.; Kato, T. Cerebrovascular disease in acute leukemia: A clinicopathological study of 14 patients. Intern. Med. 2002, 41, 1130–1134. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.; Wei, Z.; Ye, X.; Zhu, L.; Xie, M.; Xie, W.; Zhu, J.; Li, L.; Zhou, D.; et al. Risk factors and clinical characteristics of non-promyelocytic acute myeloid leukemia of intracerebral hemorrhage: A single center study in China. J. Clin. Neurosci. 2017, 44, 203–206. [Google Scholar] [CrossRef]
- Yu, Z.; Zheng, J.; Guo, R.; Ma, L.; You, C.; Li, H. Prognostic impact of leukocytosis in intracerebral hemorrhage: A PRISMA-compliant systematic review and meta-analysis. Medicine 2019, 98, e16281. [Google Scholar] [CrossRef]
- Baharoglu, M.I.; Cordonnier, C.; Al-Shahi Salman, R.; de Gans, K.; Koopman, M.M.; Brand, A.; Majoie, C.B.; Beenen, L.F.; Marquering, H.A.; Vermeulen, M.; et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): A randomised, open-label, phase 3 trial. Lancet 2016, 387, 2605–2613. [Google Scholar] [CrossRef]
- Röllig, C.; Ehninger, G. How I treat hyperleukocytosis in acute myeloid leukemia. Blood 2015, 125, 3246–3252. [Google Scholar] [CrossRef]
- Koi, S.; Nogami, A.; Fujiwara, H.; Bando, K.; Saito, M.; Osada, Y.; Tanaka, K.; Sakashita, C.; Yoshifuji, K.; Okada, K.; et al. Acute promyelocytic leukemia with asymptomatic cerebral hemorrhage. Rinsho Ketsueki 2023, 64, 1426–1430. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinchuk, A.; Roch, S.P.; Mawrin, C.; Behme, D.; Stein, K.-P.; Neyazi, B.; Mikusko, M.; Sandalcioglu, I.E.; Rashidi, A. Diagnostic Challenges in Acute Leukemia: From Dental Pain to Catastrophic Intracerebral Hemorrhage. Hematol. Rep. 2025, 17, 36. https://doi.org/10.3390/hematolrep17040036
Pinchuk A, Roch SP, Mawrin C, Behme D, Stein K-P, Neyazi B, Mikusko M, Sandalcioglu IE, Rashidi A. Diagnostic Challenges in Acute Leukemia: From Dental Pain to Catastrophic Intracerebral Hemorrhage. Hematology Reports. 2025; 17(4):36. https://doi.org/10.3390/hematolrep17040036
Chicago/Turabian StylePinchuk, Anatoli, Stefan P. Roch, Christian Mawrin, Daniel Behme, Klaus-Peter Stein, Belal Neyazi, Martin Mikusko, Ibrahim Erol Sandalcioglu, and Ali Rashidi. 2025. "Diagnostic Challenges in Acute Leukemia: From Dental Pain to Catastrophic Intracerebral Hemorrhage" Hematology Reports 17, no. 4: 36. https://doi.org/10.3390/hematolrep17040036
APA StylePinchuk, A., Roch, S. P., Mawrin, C., Behme, D., Stein, K.-P., Neyazi, B., Mikusko, M., Sandalcioglu, I. E., & Rashidi, A. (2025). Diagnostic Challenges in Acute Leukemia: From Dental Pain to Catastrophic Intracerebral Hemorrhage. Hematology Reports, 17(4), 36. https://doi.org/10.3390/hematolrep17040036





