Genetic Profiling of Acute and Chronic Leukemia via Next-Generation Sequencing: Current Insights and Future Perspectives
Abstract
1. Introduction
2. Conventional Diagnostic Methods in Leukemia
2.1. Bone Marrow Aspiration and Biopsy
2.2. Cytogenetics and Karyotyping
2.3. Fluorescence In Situ Hybridization (FISH)
2.4. Polymerase Chain Reaction (PCR)
2.5. Flow Cytometry
2.6. Other Limitations of Conventional Methods
3. Basics, Techniques, and Procedures of NGS
3.1. Basics of NGS
3.2. Techniques of NGS
3.3. Step-by-Step Procedures of NGS
4. NGS in Acute Lymphoblastic Leukemia (ALL)
5. NGS in Acute Myeloid Leukemia (AML)
6. NGS in Chronic Lymphocytic Leukemia (CLL)
7. NGS in Chronic Myeloid Leukemia (CML)
8. When to Perform NGS in Leukemia Disease Course?
9. Challenges and Future Perspectives
9.1. Standardization and Interpretation Challenges
9.2. Other Barriers to Widespread Clinical Application
9.3. Integration of NGS with Other Omics Technologies
9.4. Potential for Real-Time NGS in Clinical Decision-Making
9.5. Drug Development and Precision Medicine
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chennamadhavuni, A.; Lyengar, V.; Mukkamalla, S.K.R.; Shimanovsky, A. Leukemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Vakiti, A.; Reynolds, S.B.; Mewawalla, P. Acute Myeloid Leukemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Debnath, A.; Nath, S. Prognosis and Treatment in Acute Myeloid Leukemia: A Comprehensive Review. Egypt. J. Med. Hum. Genet. 2024, 25, 91. [Google Scholar] [CrossRef]
- Puckett, Y.; Chan, O. Acute Lymphocytic Leukemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Mukkamalla, S.K.R.; Taneja, A.; Malipeddi, D.; Master, S.R. Chronic Lymphocytic Leukemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Sampaio, M.M.; Santos, M.L.C.; Marques, H.S.; Gonçalves, V.L.d.S.; Araújo, G.R.L.; Lopes, L.W.; Apolonio, J.S.; Silva, C.S.; Santos, L.K.d.S.; Cuzzuol, B.R.; et al. Chronic Myeloid Leukemia-from the Philadelphia Chromosome to Specific Target Drugs: A Literature Review. World J. Clin. Oncol. 2021, 12, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Guilhot, F.; Hehlmann, R. Long-Term Outcomes of Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia. Blood 2025, 145, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Tazi, Y.; Arango-Ossa, J.E.; Zhou, Y.; Bernard, E.; Thomas, I.; Gilkes, A.; Freeman, S.; Pradat, Y.; Johnson, S.J.; Hills, R.; et al. Unified Classification and Risk-Stratification in Acute Myeloid Leukemia. Nat. Commun. 2022, 13, 4622. [Google Scholar] [CrossRef]
- Wemyss, C.; Jones, E.; Stentz, R.; Carding, S.R. Acute Myeloid Leukaemia and Acute Lymphoblastic Leukaemia Classification and Metabolic Characteristics for Informing and Advancing Treatment. Cancers 2024, 16, 4136. [Google Scholar] [CrossRef]
- Aung, M.M.K.; Mills, M.L.; Bittencourt-Silvestre, J.; Keeshan, K. Insights into the Molecular Profiles of Adult and Paediatric Acute Myeloid Leukaemia. Mol. Oncol. 2021, 15, 2253–2272. [Google Scholar] [CrossRef]
- Malone, E.R.; Oliva, M.; Sabatini, P.J.B.; Stockley, T.L.; Siu, L.L. Molecular Profiling for Precision Cancer Therapies. Genome Med. 2020, 12, 8. [Google Scholar] [CrossRef]
- Kiyoi, H.; Kawashima, N.; Ishikawa, Y. FLT3 Mutations in Acute Myeloid Leukemia: Therapeutic Paradigm beyond Inhibitor Development. Cancer Sci. 2020, 111, 312–322. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Cortes, J.E. Mutations in AML: Prognostic and Therapeutic Implications. Hematol. Am. Soc. Hematol. Educ. Program. 2016, 2016, 348–355. [Google Scholar]
- Downing, J.R. Targeted Therapy in Leukemia. Mod. Pathol. 2008, 21, S2–S7. [Google Scholar] [CrossRef]
- Santos, F.P.S.; Kantarjian, H.; Quintás-Cardama, A.; Cortes, J. Evolution of Therapies for Chronic Myelogenous Leukemia. Cancer J. 2011, 17, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Bewersdorf, J.P.; Shallis, R.M.; Derkach, A.; Goldberg, A.D.; Stein, A.; Stein, E.M.; Marcucci, G.; Zeidan, A.M.; Shimony, S.; DeAngelo, D.J.; et al. Efficacy of FLT3 and IDH1/2 Inhibitors in Patients with Acute Myeloid Leukemia Previously Treated with Venetoclax. Leuk. Res. 2022, 122, 106942. [Google Scholar] [CrossRef] [PubMed]
- Megías-Vericat, J.E.; Martínez-Cuadrón, D.; Solana-Altabella, A.; Montesinos, P. Precision Medicine in Acute Myeloid Leukemia: Where Are We Now and What Does the Future Hold? Expert Rev. Hematol. 2020, 13, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Li, W. Measurable Residual Disease Testing in Acute Leukemia: Technology and Clinical Significance. In Leukemia; Li, W., Ed.; Exon Publications: Brisbane, Australia, 2022; ISBN 978-0-645-33207-0. [Google Scholar]
- Della Starza, I.; De Novi, L.A.; Elia, L.; Bellomarino, V.; Beldinanzi, M.; Soscia, R.; Cardinali, D.; Chiaretti, S.; Guarini, A.; Foà, R. Optimizing Molecular Minimal Residual Disease Analysis in Adult Acute Lymphoblastic Leukemia. Cancers 2023, 15, 374. [Google Scholar] [CrossRef]
- Pulsipher, M.A.; Han, X.; Maude, S.L.; Laetsch, T.W.; Qayed, M.; Rives, S.; Boyer, M.W.; Hiramatsu, H.; Yanik, G.A.; Driscoll, T.; et al. Next-Generation Sequencing of Minimal Residual Disease for Predicting Relapse after Tisagenlecleucel in Children and Young Adults with Acute Lymphoblastic Leukemia. Blood Cancer Discov. 2022, 3, 66–81. [Google Scholar] [CrossRef]
- Medeiros, B.C.; Chan, S.M.; Daver, N.G.; Jonas, B.A.; Pollyea, D.A. Optimizing Survival Outcomes with Post-remission Therapy in Acute Myeloid Leukemia. Am. J. Hematol. 2019, 94, 803–811. [Google Scholar] [CrossRef]
- Duncavage, E.J.; Bagg, A.; Hasserjian, R.P.; DiNardo, C.D.; Godley, L.A.; Iacobucci, I.; Jaiswal, S.; Malcovati, L.; Vannucchi, A.M.; Patel, K.P.; et al. Genomic Profiling for Clinical Decision Making in Myeloid Neoplasms and Acute Leukemia. Blood 2022, 140, 2228–2247. [Google Scholar] [CrossRef]
- Sahoo, O.S.; Aidasani, H.; Nayek, A.; Tripathi, S.; Talukdar, J.; Gul, A.; Kumar, D.; Dhar, R.; Karmakar, S. Role of Next-Generation Sequencing in Revolutionizing Healthcare for Cancer Management. MedComm—Future Med. 2024, 3, e70001. [Google Scholar] [CrossRef]
- Sagniez, M.; Simpson, S.M.; Caron, M.; Rozendaal, M.; Paré, B.; Sontag, T.; Langlois, S.; Rouette, A.; Lavallée, V.-P.; Cellot, S.; et al. Real-Time Molecular Classification of Leukemias. medRxiv 2022. [Google Scholar] [CrossRef]
- Rindy, L.J.; Chambers, A.R. Bone Marrow Aspiration and Biopsy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Jain, S.; Sharma, R. Laboratory Evaluation of Bone Marrow. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Percival, M.-E.; Lai, C.; Estey, E.; Hourigan, C.S. Bone Marrow Evaluation for Diagnosis and Monitoring of Acute Myeloid Leukemia. Blood Rev. 2017, 31, 185–192. [Google Scholar] [CrossRef]
- Humphries, J.E. Dry Tap Bone Marrow Aspiration: Clinical Significance. Am. J. Hematol. 1990, 35, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Astle, J.M.; Xu, M.L.; Friedman, T.; Brown, E. Limitations of Poor Bone Marrow Aspirations (for an Accurate Diagnosis) despite the Multimodal Analytical Era: A Longitudinal Retrospective Study. Am. J. Hematol. 2017, 92, E600–E602. [Google Scholar] [CrossRef] [PubMed]
- Donald, S.; Kakkar, N. Dry Tap on Bone Marrow Aspiration: A Red Flag. J. Hematop. 2021, 14, 125–130. [Google Scholar] [CrossRef]
- Azharuddin, M.; Alvi, U.; Rao, S.; Fatima, A.; Uplaonkar, S. Comparative Evaluation of Simultaneous Bone Marrow Aspiration and Bone Marrow Biopsy in Diagnosis of Leukemic Patients—An Institutional Study. Glob. J. Res. Anal. 2023, 12, 54–56. [Google Scholar] [CrossRef]
- Helbig, D.; Quesada, A.E.; Xiao, W.; Roshal, M.; Tallman, M.S.; Knorr, D.A. Spontaneous Remission in a Patient With Acute Myeloid Leukemia Leading to Undetectable Minimal Residual Disease. J. Hematol. 2020, 9, 18–22. [Google Scholar]
- Balciuniene, J.; Ning, Y.; Lazarus, H.M.; Aikawa, V.; Sherpa, S.; Zhang, Y.; Morrissette, J.J.D. Cancer Cytogenetics in a Genomics World: Wedding the Old with the New. Blood Rev. 2024, 66, 101209. [Google Scholar] [CrossRef]
- Jain, H.; Shetty, D. Role of Cytogenetics and Fluorescence In Situ Hybridization in the Laboratory Workup of Acute Myeloid Leukemias. Indian J. Med. Paediatr. Oncol. 2023, 44, 543–553. [Google Scholar] [CrossRef]
- Ozkan, E.; Lacerda, M.P. Genetics, Cytogenetic Testing And Conventional Karyotype. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kang, Z.-J.; Liu, Y.-F.; Xu, L.-Z.; Long, Z.-J.; Huang, D.; Yang, Y.; Liu, B.; Feng, J.-X.; Pan, Y.-J.; Yan, J.-S.; et al. The Philadelphia Chromosome in Leukemogenesis. Chin. J. Cancer 2016, 35, 48. [Google Scholar] [CrossRef]
- Luquet, I.; Bidet, A.; Cuccuini, W.; Lafage-Pochitaloff, M.; Mozziconacci, M.-J.; Terré, C. Cytogenetics in the Management of Acute Myeloid Leukemia: An Update by the Groupe Francophone de Cytogénétique Hématologique (GFCH). Ann. Biol. Clin. 2016, 74, 535–546. [Google Scholar] [CrossRef]
- Mrózek, K. Acute Myeloid Leukemia with a Complex Karyotype. Semin. Oncol. 2008, 35, 365–377. [Google Scholar] [CrossRef]
- Hou, H.A.; Lin, C.C.; Chou, W.C.; Liu, C.Y.; Chen, C.Y.; Tang, J.L.; Lai, Y.J.; Tseng, M.H.; Huang, C.F.; Chiang, Y.C.; et al. Integration of Cytogenetic and Molecular Alterations in Risk Stratification of 318 Patients with de Novo Non-M3 Acute Myeloid Leukemia. Leukemia 2014, 28, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Boscaro, E.; Urbino, I.; Catania, F.M.; Arrigo, G.; Secreto, C.; Olivi, M.; D’Ardia, S.; Frairia, C.; Giai, V.; Freilone, R.; et al. Modern Risk Stratification of Acute Myeloid Leukemia in 2023: Integrating Established and Emerging Prognostic Factors. Cancers 2023, 15, 3512. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Chang, L.; Zhu, X. Pathogenesis of ETV6/RUNX1-Positive Childhood Acute Lymphoblastic Leukemia and Mechanisms Underlying Its Relapse. Oncotarget 2017, 8, 35445–35459. [Google Scholar] [CrossRef] [PubMed]
- Baranger, L.; Cuccuini, W.; Lefebvre, C.; Luquet, I.; Perot, C.; Radford, I.; Lafage-Pochitaloff, M. Cytogenetics in the Management of Children and Adult Acute Lymphoblastic Leukemia (ALL): An Update by the Groupe Francophone de Cytogénétique Hématologique (GFCH). Ann. Biol. Clin. 2016, 74, 547–560. [Google Scholar] [CrossRef]
- Bridge, J.A. Advantages and Limitations of Cytogenetic, Molecular Cytogenetic, and Molecular Diagnostic Testing in Mesenchymal Neoplasms. J. Orthop. Sci. 2008, 13, 273–282. [Google Scholar] [CrossRef]
- He, R.; Wiktor, A.E.; Hanson, C.A.; Ketterling, R.P.; Kurtin, P.J.; Van Dyke, D.L.; Litzow, M.R.; Howard, M.H.; Reichard, K.K. Conventional Karyotyping and Fluorescence In Situ Hybridization. Am. J. Clin. Pathol. 2015, 143, 873–878. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Zaman, S.B.; Mehta, V.; Haidere, M.F.; Runa, N.J.; Akter, N. Application of Fluorescence In Situ Hybridization (FISH) Technique for the Detection of Genetic Aberration in Medical Science. Cureus 2017, 9, e1325. [Google Scholar] [CrossRef]
- Wolff, D.J.; Bagg, A.; Cooley, L.D.; Dewald, G.W.; Hirsch, B.A.; Jacky, P.B.; Rao, K.W.; Rao, P.N. Guidance for Fluorescence in Situ Hybridization Testing in Hematologic Disorders. J. Mol. Diagn. 2007, 9, 134–143. [Google Scholar] [CrossRef]
- Mark, H.F.L.; Sokolic, R.A.; Mark, Y. Conventional Cytogenetics and FISH in the Detection of BCR/ABL Fusion in Chronic Myeloid Leukemia (CML). Exp. Mol. Pathol. 2006, 81, 1–7. [Google Scholar] [CrossRef]
- UZ, B.; Eliaçık, E.; Işık, A.; Aksu, S.; Büyükaşık, Y.; Haznedaroğlu, İ.C.; Göker, H.; Sayınalp, N.; Özcebe, O.İ. Co-Expression of t(15;17) and t(8;21) in a Case of Acute Promyelocytic Leukemia: Review of the Literature. Turk. J. Haematol. 2013, 30, 400–404. [Google Scholar] [CrossRef]
- Abdool, A.; Donahue, A.C.; Wohlgemuth, J.G.; Yeh, C.-H. Detection, Analysis and Clinical Validation of Chromosomal Aberrations by Multiplex Ligation-Dependent Probe Amplification in Chronic Leukemia. PLoS ONE 2010, 5, e15407. [Google Scholar] [CrossRef] [PubMed]
- Quijano, S.; López, A.; Rasillo, A.; Sayagués, J.M.; Barrena, S.; Sánchez, M.L.; Teodosio, C.; Giraldo, P.; Giralt, M.; Pérez, M.C.; et al. Impact of Trisomy 12, Del(13q), Del(17p), and Del(11q) on the Immunophenotype, DNA Ploidy Status, and Proliferative Rate of Leukemic B-Cells in Chronic Lymphocytic Leukemia. Cytom. Part B Clin. Cytom. 2008, 74B, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.G.; Van Dyke, D.L. Analysis of Common Abnormalities Seen in Chronic Lymphocytic Leukemia Using Fluorescence In Situ Hybridization. In Chronic Lymphocytic Leukemia; Malek, S.N., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1881, pp. 35–49. ISBN 978-1-4939-8875-4. [Google Scholar]
- Jing, Y.; Lin, S.; Wang, F.-T.; Yu, J.-W.; Jiang, F. Detection of Gene Abnormalities in 43 Cases of Chronic Lymphocytic Leukemia by Fluorescence in Situ Hybridization. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2018, 26, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.C.S.; Egan, D.; Radich, J.P. Molecular Monitoring of Chronic Myeloid Leukemia: Present and Future. Expert Rev. Mol. Diagn. 2016, 16, 1083–1091. [Google Scholar] [CrossRef]
- Ali, J.; Khan, S.A.; Rauf, S.-E.-; Ayyub, M.; Ali, N.; Afridi, N.K. Comparative Analysis of Fluorescence In Situ Hybridization and Real Time Polymerase Chain Reaction in Diagnosis of Chronic Myeloid Leukemia. J. Coll. Physicians Surg. Pak. 2017, 27, 26–29. [Google Scholar]
- Shakoori, A.R. Fluorescence In Situ Hybridization (FISH) and Its Applications. In Chromosome Structure and Aberrations; Bhat, T.A., Wani, A.A., Eds.; Springer: New Delhi, India, 2017; pp. 343–367. ISBN 978-81-322-3671-9. [Google Scholar]
- Qin, D. Molecular Testing for Acute Myeloid Leukemia. Cancer Biol. Med. 2022, 19, 4–13. [Google Scholar] [CrossRef]
- Taylor, J.; Xiao, W.; Abdel-Wahab, O. Diagnosis and Classification of Hematologic Malignancies on the Basis of Genetics. Blood 2017, 130, 410–423. [Google Scholar] [CrossRef]
- Quattrocchi, A.; Cappelli, L.V.; De Simone, G.; De Marinis, E.; Gentile, M.; Gasperi, T.; Pulsoni, A.; Ascenzi, P.; Nervi, C. Biomarkers in Acute Myeloid Leukemia: From State of the Art in Risk Classification to Future Challenges of RNA Editing as Disease Predictor and Therapy Target. Asp. Mol. Med. 2023, 2, 100023. [Google Scholar] [CrossRef]
- Khehra, N.; Padda, I.S.; Swift, C.J. Polymerase Chain Reaction (PCR). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Testa, U.; Pelosi, E. Function of PML-RARA in Acute Promyelocytic Leukemia. Adv. Exp. Med. Biol. 2024, 1459, 321–339. [Google Scholar] [CrossRef]
- Shigeto, S.; Matsuda, K.; Yamaguchi, A.; Sueki, A.; Uehara, M.; Sugano, M.; Uehara, T.; Honda, T. Rapid Diagnosis of Acute Promyelocytic Leukemia with the PML-RARA Fusion Gene Using a Combination of Droplet-Reverse Transcription-Polymerase Chain Reaction and Instant-Quality Fluorescence in Situ Hybridization. Clin. Chim. Acta 2016, 453, 38–41. [Google Scholar] [CrossRef]
- Press, R.D.; Kamel-Reid, S.; Ang, D. BCR-ABL1 RT-qPCR for Monitoring the Molecular Response to Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia. J. Mol. Diagn. 2013, 15, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Salmon, M.; White, H.E.; Zizkova, H.; Gottschalk, A.; Motlova, E.; Cerveira, N.; Colomer, D.; Coriu, D.; Franke, G.N.; Gottardi, E.; et al. Impact of BCR::ABL1 Transcript Type on RT-qPCR Amplification Performance and Molecular Response to Therapy. Leukemia 2022, 36, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, T.; Gökbuget, N.; Schwartz, S.; Fischer, L.; Hubert, D.; Sindram, A.; Hoelzer, D.; Thiel, E. Clinical Features and Prognostic Implications of TCF3-PBX1 and ETV6-RUNX1 in Adult Acute Lymphoblastic Leukemia. Haematologica 2010, 95, 241–246. [Google Scholar] [CrossRef]
- Guo, J.Q.; Lin, H.; Kantarjian, H.; Talpaz, M.; Champlin, R.; Andreeff, M.; Glassman, A.; Arlinghaus, R.B. Comparison of Competitive-Nested PCR and Real-Time PCR in Detecting BCR-ABL Fusion Transcripts in Chronic Myeloid Leukemia Patients. Leukemia 2002, 16, 2447–2453. [Google Scholar] [CrossRef]
- Frenoy, N.; Chabli, A.; Sol, O.; Goldschmit, E.; Lemonnier, M.P.; Misset, J.L.; Debuire, B. Application of a New Protocol for Nested PCR to the Detection of Minimal Residual Bcr/Abl Transcripts. Leukemia 1994, 8, 1411–1414. [Google Scholar]
- Pessoa, F.M.C.d.P.; de Oliveira, M.B.; Barreto, I.V.; Machado, A.K.d.C.; de Oliveira, D.S.; Ribeiro, R.M.; Medeiros, J.C.; Maciel, A.d.R.; Silva, F.A.C.; Gurgel, L.A.; et al. Nested-PCR vs. RT-qPCR: A Sensitivity Comparison in the Detection of Genetic Alterations in Patients with Acute Leukemias. DNA 2024, 4, 285–299. [Google Scholar] [CrossRef]
- Ip, B.B.K.; Wong, A.T.C.; Law, J.H.Y.; Au, C.H.; Ma, S.Y.; Chim, J.C.S.; Liang, R.H.S.; Leung, A.Y.H.; Wan, T.S.K.; Ma, E.S.K. Application of Droplet Digital PCR in Minimal Residual Disease Monitoring of Rare Fusion Transcripts and Mutations in Haematological Malignancies. Sci. Rep. 2024, 14, 6400. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Zhang, A.; Yi, M.; Lan, Y.; Zheng, Z.; Zhang, L.; Liu, X.; Chang, L.; Zou, Y.; et al. Droplet Digital PCR for Genetic Mutations Monitoring Predicts Relapse Risk in Pediatric Acute Myeloid Leukemia. Int. J. Hematol. 2022, 116, 669–677. [Google Scholar] [CrossRef]
- Bilous, N.I.; Abramenko, I.V.; Chumak, A.A.; Dyagil, I.S.; Martina, Z.V. Detection of notch1 C.7544_7545delct mutation in chronic lymphocytic leukemia using conventional and real-time polymerase chain reaction. Exp. Oncol. 2016, 38, 112–116. Available online: http://dspace.nbuv.gov.ua/handle/123456789/138001 (accessed on 15 March 2025).
- Garibyan, L.; Avashia, N. Research Techniques Made Simple: Polymerase Chain Reaction (PCR). J. Investig. Dermatol. 2013, 133, e6. [Google Scholar] [CrossRef]
- Ghannam, M.G.; Varacallo, M.A. Biochemistry, Polymerase Chain Reaction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Li, W. Flow Cytometry in the Diagnosis of Leukemias. In Leukemia; Li, W., Ed.; Exon Publications: Brisbane, Australia, 2022; ISBN 978-0-645-33207-0. [Google Scholar]
- Cai, Q.; Lan, H.; Yi, D.; Xian, B.; Zidan, L.; Li, J.; Liao, Z. Flow Cytometry in Acute Myeloid Leukemia and Detection of Minimal Residual Disease. Clin. Chim. Acta 2025, 564, 119945. [Google Scholar] [CrossRef]
- Ally, F.; Chen, X. Acute Myeloid Leukemia: Diagnosis and Evaluation by Flow Cytometry. Cancers 2024, 16, 3855. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, R.; Nair, R.A.; Jacob, P.M.; Nair Anila, K.A.R.; Prem, S.; Binitha, R.; Kusumakumary, P. Flow Cytometric Analysis of Mixed Phenotype Acute Leukemia: Experience from a Tertiary Oncology Center. Indian J. Pathol. Microbiol. 2015, 58, 181. [Google Scholar] [CrossRef]
- Kruse, A.; Abdel-Azim, N.; Kim, H.N.; Ruan, Y.; Phan, V.; Ogana, H.; Wang, W.; Lee, R.; Gang, E.J.; Khazal, S.; et al. Minimal Residual Disease Detection in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2020, 21, 1054. [Google Scholar] [CrossRef]
- Chen, X.; Cherian, S. Acute Myeloid Leukemia Immunophenotyping by Flow Cytometric Analysis. Clin. Lab. Med. 2017, 37, 753–769. [Google Scholar] [CrossRef]
- Wang, X.M. Advances and Issues in Flow Cytometric Detection of Immunophenotypic Changes and Genomic Rearrangements in Acute Pediatric Leukemia. Transl. Pediatr. 2014, 3, 149–155. [Google Scholar] [CrossRef]
- Kala, P.S.; Zubair, M. Flow Cytometry Blood Cell Identification. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Smith, A.C.; Hoischen, A.; Raca, G. Cytogenetics Is a Science, Not a Technique! Why Optical Genome Mapping Is So Important to Clinical Genetic Laboratories. Cancers 2023, 15, 5470. [Google Scholar] [CrossRef]
- Wiedmeier-Nutor, J.E.; McCabe, C.E.; O’Brien, D.R.; Jessen, E.; Bonolo de Campos, C.; Boddicker, N.J.; Griffin, R.; Allmer, C.; Rabe, K.G.; Cerhan, J.R.; et al. Utility of Targeted Sequencing Compared to FISH for Detection of Chronic Lymphocytic Leukemia Copy Number Alterations. Cancers 2024, 16, 2450. [Google Scholar] [CrossRef]
- Koutsi, A.; Vervesou, E.-C. Diagnostic Molecular Techniques in Haematology: Recent Advances. Ann. Transl. Med. 2018, 6, 242. [Google Scholar] [CrossRef]
- Oduro, K.A.; Spivey, T.; Moore, E.M.; Meyerson, H.; Yoest, J.; Tomlinson, B.; Beck, R.; Alouani, D.; Sadri, N. Clonal Dynamics and Relapse Risk Revealed by High-Sensitivity FLT3-Internal Tandem Duplication Detection in Acute Myeloid Leukemia. Mod. Pathol. 2024, 37, 100534. [Google Scholar] [CrossRef]
- Mareschal, S.; Palau, A.; Lindberg, J.; Ruminy, P.; Nilsson, C.; Bengtzén, S.; Engvall, M.; Eriksson, A.; Neddermeyer, A.; Marchand, V.; et al. Challenging Conventional Karyotyping by Next-Generation Karyotyping in 281 Intensively Treated Patients with AML. Blood Adv. 2021, 5, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Vosberg, S.; Greif, P.A. Clonal Evolution of Acute Myeloid Leukemia from Diagnosis to Relapse. Genes Chromosomes Cancer 2019, 58, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vicente, A.E.; Bikos, V.; Hernández-Sánchez, M.; Malcikova, J.; Hernández-Rivas, J.-M.; Pospisilova, S. Next-Generation Sequencing in Chronic Lymphocytic Leukemia: Recent Findings and New Horizons. Oncotarget 2017, 8, 71234–71248. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.M.; Llop, M.; Sargas, C.; Pedrola, L.; Panadero, J.; Hervás, D.; Cervera, J.; Such, E.; Ibáñez, M.; Ayala, R.; et al. Clinical Utility of a Next-Generation Sequencing Panel for Acute Myeloid Leukemia Diagnostics. J. Mol. Diagn. 2019, 21, 228–240. [Google Scholar] [CrossRef]
- Madaci, L.; Farnault, L.; Abbou, N.; Gabert, J.; Venton, G.; Costello, R. Impact of Next-Generation Sequencing in Diagnosis, Prognosis and Therapeutic Management of Acute Myeloid Leukemia/Myelodysplastic Neoplasms. Cancers 2023, 15, 3280. [Google Scholar] [CrossRef]
- Prieto-Conde, M.I.; Corchete, L.A.; García-Álvarez, M.; Jiménez, C.; Medina, A.; Balanzategui, A.; Hernández-Ruano, M.; Maldonado, R.; Sarasquete, M.E.; Alcoceba, M.; et al. A New Next-Generation Sequencing Strategy for the Simultaneous Analysis of Mutations and Chromosomal Rearrangements at DNA Level in Acute Myeloid Leukemia Patients. J. Mol. Diagn. 2020, 22, 60–71. [Google Scholar] [CrossRef]
- Bacher, U.; Shumilov, E.; Flach, J.; Porret, N.; Joncourt, R.; Wiedemann, G.; Fiedler, M.; Novak, U.; Amstutz, U.; Pabst, T. Challenges in the Introduction of Next-Generation Sequencing (NGS) for Diagnostics of Myeloid Malignancies into Clinical Routine Use. Blood Cancer J. 2018, 8, 113. [Google Scholar] [CrossRef]
- Soverini, S.; Abruzzese, E.; Bocchia, M.; Bonifacio, M.; Galimberti, S.; Gozzini, A.; Iurlo, A.; Luciano, L.; Pregno, P.; Rosti, G.; et al. Next-Generation Sequencing for BCR-ABL1 Kinase Domain Mutation Testing in Patients with Chronic Myeloid Leukemia: A Position Paper. J. Hematol. Oncol. 2019, 12, 131. [Google Scholar] [CrossRef]
- Haferlach, T. Advancing Leukemia Diagnostics: Role of Next Generation Sequencing (NGS) in Acute Myeloid Leukemia. Hematol. Rep. 2020, 12, 8957. [Google Scholar] [CrossRef]
- Coccaro, N.; Anelli, L.; Zagaria, A.; Specchia, G.; Albano, F. Next-Generation Sequencing in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2019, 20, 2929. [Google Scholar] [CrossRef]
- Chen, W.-L.; Guo, Z.; Hou, Z.-Z.; Chen, Y.-J. Research Progress of Next Generation Sequencing in Acute Leukemia—Review. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2023, 31, 612–615. Available online: https://pubmed.ncbi.nlm.nih.gov/37096544/#:~:text=NGS%20reveals%20the%20genetic%20characteristics,predict%20disease%20recurrence%20by%20detecting (accessed on 23 March 2025). [PubMed]
- Bourbon, E.; Chabane, K.; Mosnier, I.; Bouvard, A.; Thonier, F.; Ferrant, E.; Michallet, A.-S.; Poulain, S.; Hayette, S.; Sujobert, P.; et al. Next-CLL, a New Next-Generation Sequencing–Based Method for Assessment of IGHV Gene Mutational Status in Chronic Lymphoid Leukemia. J. Mol. Diagn. 2023, 25, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Brash, D.E. Next-Generation Sequencing Methodologies To Detect Low-Frequency Mutations: Catch Me If You Can. Mutat. Res. Rev. Mutat. Res. 2023, 792, 108471. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, H.; Zhang, V.W.; Tian, X.; Feng, Y.; Wang, G.; Gorman, E.; Wang, H.; Lutz, R.E.; Schmitt, E.S.; et al. Capture-Based High-Coverage NGS: A Powerful Tool to Uncover a Wide Spectrum of Mutation Types. Genet. Med. 2016, 18, 513–521. [Google Scholar] [CrossRef]
- Levine, R.L.; Valk, P.J.M. Next-Generation Sequencing in the Diagnosis and Minimal Residual Disease Assessment of Acute Myeloid Leukemia. Haematologica 2019, 104, 868–871. [Google Scholar] [CrossRef]
- Chaudhary, P.; Chaudhary, S.; Patel, F.; Patel, S.; Vaishnani, T.; Trivedi, N.; Patel, D.; Sonagara, T.; Hirapara, A.; Vyas, K.; et al. Validation of a Novel NGS Based BCR::ABL1 Kinase Domain Mutation Detection Assay in Indian Cohort. Sci. Rep. 2024, 14, 15745. [Google Scholar] [CrossRef]
- Bhatwadekar, S.S.; Shah, P. Mutational Phasing: Clinical Relevance in Tyrosine Kinase Domain Mutations Using Next Generation Sequencing in Chronic Myeloid Leukemia. Blood 2018, 132, 4269. [Google Scholar] [CrossRef]
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G.; et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef]
- Ryan, S.L.; Peden, J.F.; Kingsbury, Z.; Schwab, C.J.; James, T.; Polonen, P.; Mijuskovic, M.; Becq, J.; Yim, R.; Cranston, R.E.; et al. Whole Genome Sequencing Provides Comprehensive Genetic Testing in Childhood B-Cell Acute Lymphoblastic Leukaemia. Leukemia 2023, 37, 518–528. [Google Scholar] [CrossRef]
- Nannini, M.; Pantaleo, M.A. Next Generation Sequencing (NGS) in Oncology: Lights and Shadows. CBN 2016, 4, 17–19. [Google Scholar] [CrossRef]
- Ljungström, V.; Cortese, D.; Young, E.; Pandzic, T.; Mansouri, L.; Plevova, K.; Ntoufa, S.; Baliakas, P.; Clifford, R.; Sutton, L.-A.; et al. Whole-Exome Sequencing in Relapsing Chronic Lymphocytic Leukemia: Clinical Impact of Recurrent RPS15 Mutations. Blood 2016, 127, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, B.; Tekin, M.; Mahdieh, N. The Promise of Whole-Exome Sequencing in Medical Genetics. J. Hum. Genet. 2014, 59, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Debarri, H.; Lebon, D.; Roumier, C.; Cheok, M.; Marceau-Renaut, A.; Nibourel, O.; Geffroy, S.; Helevaut, N.; Rousselot, P.; Gruson, B.; et al. IDH1/2 but Not DNMT3A Mutations Are Suitable Targets for Minimal Residual Disease Monitoring in Acute Myeloid Leukemia Patients: A Study by the Acute Leukemia French Association. Oncotarget 2015, 6, 42345–42353. [Google Scholar] [CrossRef]
- Miller, E.M.; Patterson, N.E.; Zechmeister, J.M.; Bejerano-Sagie, M.; Delio, M.; Patel, K.; Ravi, N.; Quispe-Tintaya, W.; Maslov, A.; Simmons, N.; et al. Development and Validation of a Targeted next Generation DNA Sequencing Panel Outperforming Whole Exome Sequencing for the Identification of Clinically Relevant Genetic Variants. Oncotarget 2017, 8, 102033–102045. [Google Scholar] [CrossRef]
- Ahmed, F.; Zhong, J. Advances in DNA/RNA Sequencing and Their Applications in Acute Myeloid Leukemia (AML). Int. J. Mol. Sci. 2024, 26, 71. [Google Scholar] [CrossRef]
- Petiti, J.; Pignochino, Y.; Schiavon, A.; Giugliano, E.; Berrino, E.; Giordano, G.; Itri, F.; Dragani, M.; Cilloni, D.; Lo Iacono, M. Comprehensive Molecular Profiling of NPM1-Mutated Acute Myeloid Leukemia Using RNAseq Approach. Int. J. Mol. Sci. 2024, 25, 3631. [Google Scholar] [CrossRef]
- Kim, J.C.; Chan-Seng-Yue, M.; Ge, S.; Zeng, A.G.X.; Ng, K.; Gan, O.I.; Garcia-Prat, L.; Flores-Figueroa, E.; Woo, T.; Zhang, A.X.W.; et al. Transcriptomic Classes of BCR-ABL1 Lymphoblastic Leukemia. Nat. Genet. 2023, 55, 1186–1197. [Google Scholar] [CrossRef]
- Borad, M.J.; Egan, J.; Champion, M.; Hunt, K.; McWilliams, R.; McCullough, A.; Aldrich, J.; Nasser, S.; Liang, W.; Barrett, M.; et al. Abstract CT112: Implementation of CLIA Enabled Integrated Whole Genome (WGS)/Exome (WES)/Transcriptome (RNAseq) next-Gen Sequencing to Identify Therapeutically Relevant Targets in Advanced Cancer Patients. Cancer Res. 2015, 75, CT112. [Google Scholar] [CrossRef]
- Ramarao-Milne, P.; Kondrashova, O.; Patch, A.-M.; Nones, K.; Koufariotis, L.T.; Newell, F.; Addala, V.; Lakis, V.; Holmes, O.; Leonard, C.; et al. Comparison of Actionable Events Detected in Cancer Genomes by Whole-Genome Sequencing, in Silico Whole-Exome and Mutation Panels. ESMO Open 2022, 7, 100540. [Google Scholar] [CrossRef]
- Li, J.; Batcha, A.M.N.; Grüning, B.; Mansmann, U.R. An NGS Workflow Blueprint for DNA Sequencing Data and Its Application in Individualized Molecular Oncology. Cancer Inform. 2016, 14, 87–107. [Google Scholar] [CrossRef]
- Vicente-Garcés, C.; Esperanza-Cebollada, E.; Montesdeoca, S.; Torrebadell, M.; Rives, S.; Dapena, J.L.; Català, A.; Conde, N.; Camós, M.; Vega-García, N. Technical Validation and Clinical Utility of an NGS Targeted Panel to Improve Molecular Characterization of Pediatric Acute Leukemia. Front. Mol. Biosci. 2022, 9, 854098. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.R. Target Enrichment Approaches for Next-Generation Sequencing Applications in Oncology. Diagnosis 2022, 12, 1539. [Google Scholar] [CrossRef]
- Espinosa, E.; Bautista, R.; Larrosa, R.; Plata, O. Advancements in Long-Read Genome Sequencing Technologies and Algorithms. Genomics 2024, 116, 110842. [Google Scholar] [CrossRef]
- Ramesh, A.; Koo, S.; Kang, S.J.; Ghosal, A.; Alarcon, F.; Gyuris, T.; Jung, S.C.; Magnan, C.; Nam, H.; Thomas, B.B.; et al. A Novel NGS-Based Simultaneous Detection of DNA and RNA Biomarkers Using Total Nucleic Acid (TNA) for Acute Lymphocytic Leukemia (ALL). Blood 2021, 138, 1875. [Google Scholar] [CrossRef]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. COSMIC: The Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res. 2019, 47, D941–D947. [Google Scholar] [CrossRef]
- Sondka, Z.; Dhir, N.B.; Carvalho-Silva, D.; Jupe, S.; Madhumita, N.; McLaren, K.; Starkey, M.; Ward, S.; Wilding, J.; Ahmed, M.; et al. COSMIC: A Curated Database of Somatic Variants and Clinical Data for Cancer. Nucleic Acids Res. 2024, 52, D1210–D1217. [Google Scholar] [CrossRef]
- Gil, J.V.; Such, E.; Sargas, C.; Simarro, J.; Miralles, A.; Pérez, G.; De Juan, I.; Palanca, S.; Avetisyan, G.; Santiago, M.; et al. Design and Validation of a Custom Next-Generation Sequencing Panel in Pediatric Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2023, 24, 4440. [Google Scholar] [CrossRef]
- Inaba, H.; Azzato, E.M.; Mullighan, C.G. Integration of Next-Generation Sequencing to Treat Acute Lymphoblastic Leukemia with Targetable Lesions: The St. Jude Children’s Research Hospital Approach. Front. Pediatr. 2017, 5, 258. [Google Scholar] [CrossRef]
- Zaliova, M.; Kotrova, M.; Bresolin, S.; Stuchly, J.; Stary, J.; Hrusak, O.; Te Kronnie, G.; Trka, J.; Zuna, J.; Vaskova, M. ETV6/RUNX1-like Acute Lymphoblastic Leukemia: A Novel B-Cell Precursor Leukemia Subtype Associated with the CD27/CD44 Immunophenotype. Genes Chromosomes Cancer 2017, 56, 608–616. [Google Scholar] [CrossRef]
- Paolino, J.; Tsai, H.K.; Harris, M.H.; Pikman, Y. IKZF1 Alterations and Therapeutic Targeting in B-Cell Acute Lymphoblastic Leukemia. Biomedicines 2024, 12, 89. [Google Scholar] [CrossRef]
- Gupta, D.G.; Varma, N.; Sreedharanunni, S.; Abdulkadir, S.A.; Naseem, S.; Sachdeva, M.U.S.; Binota, J.; Bose, P.; Malhotra, P.; Khadwal, A.; et al. ‘Evaluation of Adverse Prognostic Gene Alterations & MRD Positivity in BCR::ABL1-like B-Lineage Acute Lymphoblastic Leukaemia Patients, in a Resource-Constrained Setting. Br. J. Cancer 2023, 129, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Alghandour, R.; Sakr, D.H.; Shaaban, Y. Philadelphia-like Acute Lymphoblastic Leukemia: The Journey from Molecular Background to the Role of Bone Marrow Transplant—Review Article. Ann. Hematol. 2023, 102, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- Dörge, P.; Meissner, B.; Zimmermann, M.; Möricke, A.; Schrauder, A.; Bouquin, J.-P.; Schewe, D.; Harbott, J.; Teigler-Schlegel, A.; Ratei, R.; et al. IKZF1 Deletion Is an Independent Predictor of Outcome in Pediatric Acute Lymphoblastic Leukemia Treated According to the ALL-BFM 2000 Protocol. Haematologica 2013, 98, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, R.P.; Waanders, E.; van der Velden, V.H.J.; van Reijmersdal, S.V.; Venkatachalam, R.; Scheijen, B.; Sonneveld, E.; van Dongen, J.J.M.; Veerman, A.J.P.; van Leeuwen, F.N.; et al. IKZF1 Deletions Predict Relapse in Uniformly Treated Pediatric Precursor B-ALL. Leukemia 2010, 24, 1258–1264. [Google Scholar] [CrossRef]
- Stanulla, M.; Cavé, H.; Moorman, A.V. IKZF1 Deletions in Pediatric Acute Lymphoblastic Leukemia: Still a Poor Prognostic Marker? Blood 2020, 135, 252–260. [Google Scholar] [CrossRef]
- Huang, Z.; Jia, Y.; Ruan, G.; Zuo, Y.; Wu, J.; Lu, A.; Xue, Y.; Cheng, Y.; Zhang, L. Quantitative Analysis of IKZF1 Gene Deletions in Pediatric B-Cell Precursor Acute Lymphoblastic Leukemia: Higher Levels Are Associated with a Poorer Prognosis. Pediatr. Hematol. Oncol. 2022, 39, 243–253. [Google Scholar] [CrossRef]
- Clappier, E.; Grardel, N.; Bakkus, M.; Rapion, J.; Moerloose, B.; Kastner, P.; Caye, A.; Vivent, J.; Costa, V.; Ferster, A.; et al. IKZF1 Deletion Is an Independent Prognostic Marker in Childhood B-Cell Precursor Acute Lymphoblastic Leukemia, and Distinguishes Patients Benefiting from Pulses during Maintenance Therapy: Results of the EORTC Children’s Leukemia Group Study 58951. Leukemia 2015, 29, 2154–2161. [Google Scholar] [CrossRef]
- Vervoort, B.M.T.; Butler, M.; Grünewald, K.J.T.; van Ingen Schenau, D.S.; Tee, T.M.; Lucas, L.; Huitema, A.D.R.; Boer, J.M.; Bornhauser, B.C.; Bourquin, J.; et al. IKZF1 Gene Deletions Drive Resistance to Cytarabine in B-Cell Precursor Acute Lymphoblastic Leukemia. Haematologica 2024, 109, 3904–3917. [Google Scholar] [CrossRef]
- von Goessel, H.; Jacobs, U.; Semper, S.; Krumbholz, M.; Langer, T.; Keller, T.; Schrauder, A.; van der Velden, V.H.J.; van Dongen, J.J.M.; Harbott, J.; et al. Cluster Analysis of Genomic ETV6–RUNX1 (TEL–AML1) Fusion Sites in Childhood Acute Lymphoblastic Leukemia. Leuk. Res. 2009, 33, 1082–1088. [Google Scholar] [CrossRef]
- Schäfer, D.; Olsen, M.; Lähnemann, D.; Stanulla, M.; Slany, R.; Schmiegelow, K.; Borkhardt, A.; Fischer, U. Five Percent of Healthy Newborns Have an ETV6-RUNX1 Fusion as Revealed by DNA-Based GIPFEL Screening. Blood 2018, 131, 821–826. [Google Scholar] [CrossRef]
- van der Weyden, L.; Giotopoulos, G.; Rust, A.G.; Matheson, L.S.; van Delft, F.W.; Kong, J.; Corcoran, A.E.; Greaves, M.F.; Mullighan, C.G.; Huntly, B.J.; et al. Modeling the Evolution of ETV6-RUNX1-Induced B-Cell Precursor Acute Lymphoblastic Leukemia in Mice. Blood 2011, 118, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, G.; Hauer, J.; Martín-Lorenzo, A.; Schäfer, D.; Bartenhagen, C.; García-Ramírez, I.; Auer, F.; González-Herrero, I.; Ruiz-Roca, L.; Gombert, M.; et al. Infection Exposure Promotes ETV6-RUNX1 Precursor B-Cell Leukemia via Impaired H3K4 Demethylases. Cancer Res. 2017, 77, 4365–4377. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.S.; Francis, A.; Turkistany, S.; Shukla, D.; Wong, A.; Batista, C.R.; DeKoter, R.P. ETV6-RUNX1 Interacts with a Region in SPIB Intron 1 to Regulate Gene Expression in Pre-B-Cell Acute Lymphoblastic Leukemia. Exp. Hematol. 2019, 73, 50–63.e2. [Google Scholar] [CrossRef]
- Zerella, J.R.; Homan, C.C.; Arts, P.; Brown, A.L.; Scott, H.S.; Hahn, C.N. Transcription Factor Genetics and Biology in Predisposition to Bone Marrow Failure and Hematological Malignancy. Front. Oncol. 2023, 13, 1183318. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S.; Jang, P.-S.; Chung, N.-G.; Jeong, D.-C.; Kim, M.; Cho, B.; Kim, H.-K. Outcome and Prognostic Factors for ETV6/RUNX1 Positive Pediatric Acute Lymphoblastic Leukemia Treated at a Single Institution in Korea. Cancer Res. Treat. 2017, 49, 446–453. [Google Scholar] [CrossRef]
- Meyer, L.K.; Delgado-Martin, C.; Maude, S.L.; Shannon, K.M.; Teachey, D.T.; Hermiston, M.L. CRLF2 Rearrangement in Ph-like Acute Lymphoblastic Leukemia Predicts Relative Glucocorticoid Resistance That Is Overcome with MEK or Akt Inhibition. PLoS ONE 2019, 14, e0220026. [Google Scholar] [CrossRef]
- Tasian, S.K.; Loh, M.L. Understanding the Biology of CRLF2-Overexpressing Acute Lymphoblastic Leukemia. Crit. Rev. Oncog. 2011, 16, 13–24. [Google Scholar]
- Harvey, R.C.; Mullighan, C.G.; Chen, I.-M.; Wharton, W.; Mikhail, F.M.; Carroll, A.J.; Kang, H.; Liu, W.; Dobbin, K.K.; Smith, M.A.; et al. Rearrangement of CRLF2 Is Associated with Mutation of JAK Kinases, Alteration of IKZF1, Hispanic/Latino Ethnicity, and a Poor Outcome in Pediatric B-Progenitor Acute Lymphoblastic Leukemia. Blood 2010, 115, 5312–5321. [Google Scholar] [CrossRef]
- Tasian, S.K.; Doral, M.Y.; Borowitz, M.J.; Wood, B.L.; Chen, I.-M.; Harvey, R.C.; Gastier-Foster, J.M.; Willman, C.L.; Hunger, S.P.; Mullighan, C.G.; et al. Aberrant STAT5 and PI3K/mTOR Pathway Signaling Occurs in Human CRLF2-Rearranged B-Precursor Acute Lymphoblastic Leukemia. Blood 2012, 120, 833–842. [Google Scholar] [CrossRef]
- Péterffy, B.; Krizsán, S.; Egyed, B.; Bedics, G.; Benard-Slagter, A.; Palit, S.; Erdélyi, D.J.; Müller, J.; Nagy, T.; Hegyi, L.L.; et al. Molecular Profiling Reveals Novel Gene Fusions and Genetic Markers for Refined Patient Stratification in Pediatric Acute Lymphoblastic Leukemia. Mod. Pathol. 2025, 38, 100741. [Google Scholar] [CrossRef]
- Simonin, M.; Vasseur, L.; Lengliné, E.; Lhermitte, L.; Cabannes-Hamy, A.; Balsat, M.; Schmidt, A.; Dourthe, M.-E.; Touzart, A.; Graux, C.; et al. NGS-Based Stratification Refines the Risk Stratification in T-ALL and Identifies a Very-High-Risk Subgroup of Patients. Blood 2024, 144, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Sherali, N.; Hamadneh, T.; Aftab, S.; Alfonso, M.S.; Tsouklidis, N. Integration of Next-Generation Sequencing in Diagnosing and Minimal Residual Disease Detection in Patients With Philadelphia Chromosome-Like Acute Lymphoblastic Leukemia. Cureus 2020, 12, e10696. [Google Scholar] [CrossRef] [PubMed]
- Ashouri, K.; Nittur, V.; Ginosyan, A.A.; Hwang, J.; Adnani, B.; Chen, D.; Savitala-Damerla, L.; Schiff, K.; Chaudhary, P.; Kovach, A.E.; et al. Concordance of Next-Generation Sequencing and Multiparametric Flow Cytometry Methods for Detecting Measurable Residual Disease in Adult Acute Lymphoblastic Leukemia: Optimizing Prediction of Clinical Outcomes From a Single-Center Study. Clin. Lymphoma Myeloma Leuk. 2024, 24, e59–e66.e2. [Google Scholar] [CrossRef] [PubMed]
- Paolino, J.D.; Harris, M.H.; Stevenson, K.E.; Koch, V.; Cole, P.D.; Gennarini, L.M.; Kahn, J.M.; Kelly, K.M.; Kirsch, I.; Michon, B.; et al. Performance of Next Generation Sequencing for Minimal Residual Disease Detection for Pediatric Patients with Acute Lymphoblastic Leukemia: Results from the Prospective Clinical Trial DFCI 16-001. Blood 2021, 138, 3485. [Google Scholar] [CrossRef]
- Krstevska Bozhinovikj, E.; Matevska-Geshkovska, N.; Staninova Stojovska, M.; Gjorgievska, E.; Jovanovska, A.; Ridova, N.; Panovska Stavridis, I.; Kocheva, S.; Dimovski, A. Presence of Minimal Residual Disease Determined by Next-Generation Sequencing Is Not a Reliable Prognostic Biomarker in Children with Acute Lymphoblastic Leukemia. Leuk. Lymphoma 2025, 1–8. [Google Scholar] [CrossRef]
- Woolbright, W.C.; Wong, V.; Severson, E.; Kuo, D.J. The Application of Next-Generation Sequencing Tumor Molecular Profiling in the Diagnosis and Management of a Case of Acute Myelogenous Leukemia With MLL-PTD in a Pediatric Heart Transplant Recipient. J. Pediatr. Hematol./Oncol. 2021, 43, e246–e249. [Google Scholar] [CrossRef]
- Mosquera Orgueira, A.; Peleteiro Raíndo, A.; Cid López, M.; Antelo Rodríguez, B.; Díaz Arias, J.Á.; Ferreiro Ferro, R.; Alonso Vence, N.; Bendaña López, Á.; Abuín Blanco, A.; Bao Pérez, L.; et al. Gene Expression Profiling Identifies FLT3 Mutation-like Cases in Wild-Type FLT3 Acute Myeloid Leukemia. PLoS ONE 2021, 16, e0247093. [Google Scholar] [CrossRef]
- Tao, S.; Wang, C.; Chen, Y.; Deng, Y.; Song, L.; Shi, Y.; Ling, L.; Ding, B.; He, Z.; Yu, L. Prognosis and Outcome of Patients with Acute Myeloid Leukemia Based on FLT3-ITD Mutation with or without Additional Abnormal Cytogenetics. Oncol. Lett. 2019, 18, 6766–6774. [Google Scholar] [CrossRef]
- Jain, P.; Kantarjian, H.; Patel, K.; Faderl, S.; Garcia-Manero, G.; Benjamini, O.; Borthakur, G.; Pemmaraju, N.; Kadia, T.; Daver, N.; et al. Mutated Nucleophosmin-1 (NPM1) in Patients with Acute Myeloid Leukemia (AML) in Remission and Relapse. Leuk. Lymphoma 2014, 55, 1337–1344. [Google Scholar] [CrossRef]
- Becker, H.; Marcucci, G.; Maharry, K.; Radmacher, M.D.; Mrózek, K.; Margeson, D.; Whitman, S.P.; Wu, Y.-Z.; Schwind, S.; Paschka, P.; et al. Favorable Prognostic Impact of NPM1 Mutations in Older Patients With Cytogenetically Normal De Novo Acute Myeloid Leukemia and Associated Gene- and MicroRNA-Expression Signatures: A Cancer and Leukemia Group B Study. J. Clin. Oncol. 2010, 28, 596–604. [Google Scholar] [CrossRef]
- Baek, D.W.; Lee, J.M.; Kim, J.-H.; Cho, H.J.; Ham, J.-Y.; Suh, J.-S.; Sohn, S.-K.; Moon, J.H. Favorable Long-Term Survival Using Consolidation Chemotherapy without Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia with Wild-Type NPM1 without FLT3-ITD. Blood Res. 2019, 54, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Issa, G.C.; Bidikian, A.; Venugopal, S.; Konopleva, M.; DiNardo, C.D.; Kadia, T.M.; Borthakur, G.; Jabbour, E.; Pemmaraju, N.; Yilmaz, M.; et al. Clinical Outcomes Associated with NPM1 Mutations in Patients with Relapsed or Refractory AML. Blood Adv. 2022, 7, 933–942. [Google Scholar] [CrossRef]
- Sallmyr, A.; Fan, J.; Datta, K.; Kim, K.-T.; Grosu, D.; Shapiro, P.; Small, D.; Rassool, F. Internal Tandem Duplication of FLT3 (FLT3/ITD) Induces Increased ROS Production, DNA Damage, and Misrepair: Implications for Poor Prognosis in AML. Blood 2008, 111, 3173–3182. [Google Scholar] [CrossRef] [PubMed]
- Dunna, N.R.; Rajappa, S.; Digumarti, R.; Vure, S.; Kagita, S.; Damineni, S.; Rao, V.R.; Yadav, S.K.; Ravuri, R.R.; Satti, V. Fms like Tyrosine Kinase (FLT3) and Nucleophosmin 1 (NPM1) Mutations in de Novo Normal Karyotype Acute Myeloid Leukemia (AML). Asian Pac. J. Cancer Prev. 2010, 11, 1811–1816. [Google Scholar]
- Lazana, I.; Tsirigotis, P.; Konstantellos, I.; Chondropoulos, S.; Stamouli, M.; Gkirkas, K.; Baltadakis, I.; Theodorou, E.; Karaolidou, F.; Tzenou, T.; et al. The Prognostic Impact of Other Somatic Mutations and Cytogenetic Abnormalities in Patients with NPM1 and/or FLT3-ITD Mutated AML in the Era of FLT3-Inhibitors: Real-Life Data from the Greek AML Registry. Blood 2024, 144, 5956. [Google Scholar] [CrossRef]
- Yang, H.; Ye, D.; Guan, K.-L.; Xiong, Y. IDH1 and IDH2 Mutations in Tumorigenesis: Mechanistic Insights and Clinical Perspectives. Clin. Cancer Res. 2012, 18, 5562–5571. [Google Scholar] [CrossRef]
- Rakheja, D.; Konoplev, S.; Medeiros, L.J.; Chen, W. IDH Mutations in Acute Myeloid Leukemia. Human Pathol. 2012, 43, 1541–1551. [Google Scholar] [CrossRef]
- Lachowiez, C.A.; Reville, P.K.; Kantarjian, H.; Jabbour, E.; Borthakur, G.; Daver, N.; Issa, G.; Furudate, K.; Tanaka, T.; Pierce, S.; et al. Contemporary Outcomes in -Mutated Acute Myeloid Leukemia: The Impact of Co-Occurring Mutations and Venetoclax-Based Treatment. Am. J. Hematol. 2022, 97, 1443–1452. [Google Scholar] [CrossRef]
- Salem, D.; El-Aziz, S.A.; El-Menshawy, N.; Abouzeid, T.; Ebrahim, M. Prevalence and Prognostic Value of IDH1 R132 Mutation in Newly Diagnosed AML Egyptian Patients with Normal Karyotype. Indian J. Hematol. Blood Transfus. 2017, 33, 49–55. [Google Scholar] [CrossRef]
- Abbas, S.; Lugthart, S.; Kavelaars, F.G.; Schelen, A.; Koenders, J.E.; Zeilemaker, A.; van Putten, W.J.L.; Rijneveld, A.W.; Löwenberg, B.; Valk, P.J.M. Acquired Mutations in the Genes Encoding IDH1 and IDH2 Both Are Recurrent Aberrations in Acute Myeloid Leukemia: Prevalence and Prognostic Value. Blood 2010, 116, 2122–2126. [Google Scholar] [CrossRef]
- Issa, G.C.; DiNardo, C.D. Acute Myeloid Leukemia with IDH1 and IDH2 Mutations: 2021 Treatment Algorithm. Blood Cancer J. 2021, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Morita, K.; DiNardo, C.D.; Furudate, K.; Tanaka, T.; Yan, Y.; Patel, K.P.; MacBeth, K.J.; Wu, B.; Liu, G.; et al. Leukemia Stemness and Co-Occurring Mutations Drive Resistance to IDH Inhibitors in Acute Myeloid Leukemia. Nat. Commun. 2021, 12, 2607. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Zarychta, J.; Lejman, M.; Latoch, E.; Zawitkowska, J. Clinical Implications of Isocitrate Dehydrogenase Mutations and Targeted Treatment of Acute Myeloid Leukemia with Mutant Isocitrate Dehydrogenase Inhibitors—Recent Advances, Challenges and Future Prospects. Int. J. Mol. Sci. 2024, 25, 7916. [Google Scholar] [CrossRef] [PubMed]
- Castelli, G.; Pelosi, E.; Testa, U. Targeted Therapies in the Treatment of Adult Acute Myeloid Leukemias: Current Status and Future Perspectives. Int. J. Hematol. Oncol. 2016, 5, 143–164. [Google Scholar] [CrossRef]
- Yang, F.; Anekpuritanang, T.; Press, R.D. Clinical Utility of Next-Generation Sequencing in Acute Myeloid Leukemia. Mol. Diagn. Ther. 2020, 24, 1–13. [Google Scholar] [CrossRef]
- Fedorov, K.; Maiti, A.; Konopleva, M. Targeting FLT3 Mutation in Acute Myeloid Leukemia: Current Strategies and Future Directions. Cancers 2023, 15, 2312. [Google Scholar] [CrossRef]
- Ok, C.Y.; Loghavi, S.; Sui, D.; Wei, P.; Kanagal-Shamanna, R.; Yin, C.C.; Zuo, Z.; Routbort, M.J.; Tang, G.; Tang, Z.; et al. Persistent IDH1/2 Mutations in Remission Can Predict Relapse in Patients with Acute Myeloid Leukemia. Haematologica 2019, 104, 305–311. [Google Scholar] [CrossRef]
- Jain, G.; Thakral, D.; Sahoo, R.K.; Kumar, I.; Vashishtha, S.; Verma, P.; Gupta, R. Next Generation Sequencing Guided Treatment Modulation and Prognosis in Acute Myeloid Leukemia: Case Vignettes. Am. J. Blood Res. 2020, 10, 134–144. [Google Scholar]
- Kim, J.J.; Jang, J.E.; Lee, H.A.; Park, M.R.; Kook, H.W.; Lee, S.-T.; Choi, J.R.; Min, Y.H.; Shin, S.; Cheong, J.-W. Development of a Next-Generation Sequencing-Based Gene Panel Test to Detect Measurable Residual Disease in Acute Myeloid Leukemia. Ann. Lab. Med. 2023, 43, 328–336. [Google Scholar] [CrossRef]
- Chiaretti, S.; Marinelli, M.; Del Giudice, I.; Bonina, S.; Piciocchi, A.; Messina, M.; Vignetti, M.; Rossi, D.; Di Maio, V.; Mauro, F.R.; et al. NOTCH1, SF3B1, BIRC3 and TP53 Mutations in Patients with Chronic Lymphocytic Leukemia Undergoing First-Line Treatment: Correlation with Biological Parameters and Response to Treatment. Leuk. Lymphoma 2014, 55, 2785–2792. [Google Scholar] [CrossRef]
- Lazarian, G.; Tausch, E.; Eclache, V.; Sebaa, A.; Bianchi, V.; Letestu, R.; Collon, J.-F.; Lefebvre, V.; Gardano, L.; Varin-Blank, N.; et al. TP53 Mutations Are Early Events in Chronic Lymphocytic Leukemia Disease Progression and Precede Evolution to Complex Karyotypes. Int. J. Cancer 2016, 139, 1759–1763. [Google Scholar] [CrossRef] [PubMed]
- Zenz, T.; Vollmer, D.; Trbusek, M.; Smardova, J.; Benner, A.; Soussi, T.; Helfrich, H.; Heuberger, M.; Hoth, P.; Fuge, M.; et al. TP53 Mutation Profile in Chronic Lymphocytic Leukemia: Evidence for a Disease Specific Profile from a Comprehensive Analysis of 268 Mutations. Leukemia 2010, 24, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
- Brieghel, C.; Aarup, K.; Torp, M.H.; Andersen, M.A.; Yde, C.W.; Tian, X.; Wiestner, A.; Ahn, I.E.; Niemann, C.U. Clinical Outcomes in Patients with Multi-Hit TP53 Chronic Lymphocytic Leukemia Treated with Ibrutinib. Clin. Cancer Res. 2021, 27, 4531–4538. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.; Martinez, P.; Wade, R.; Hockley, S.; Oscier, D.; Matutes, E.; Dearden, C.E.; Richards, S.M.; Catovsky, D.; Morgan, G.J. Mutational Status of the TP53 Gene as a Predictor of Response and Survival in Patients with Chronic Lymphocytic Leukemia: Results from the LRF CLL4 Trial. J. Clin. Oncol. 2011, 29, 2223–2229. [Google Scholar] [CrossRef]
- Robak, T. TP53 Mutations in Chronic Lymphocytic Leukemia. Acta Haematol. Pol. 2021, 52, 75–76. [Google Scholar] [CrossRef]
- Fürstenau, M.; Eichhorst, B. Novel Agents in Chronic Lymphocytic Leukemia: New Combination Therapies and Strategies to Overcome Resistance. Cancers 2021, 13, 1336. [Google Scholar] [CrossRef]
- De Luca, G.; Cerruti, G.; Lastraioli, S.; Conte, R.; Ibatici, A.; Di Felice, N.; Morabito, F.; Monti, P.; Fronza, G.; Matis, S.; et al. The Spectrum of Subclonal TP53 Mutations in Chronic Lymphocytic Leukemia: A next Generation Sequencing Retrospective Study. Hematol. Oncol. 2022, 40, 962–975. [Google Scholar] [CrossRef]
- Malcikova, J.; Tausch, E.; Rossi, D.; Sutton, L.A.; Soussi, T.; Zenz, T.; Kater, A.P.; Niemann, C.U.; Gonzalez, D.; Davi, F.; et al. ERIC Recommendations for TP53 Mutation Analysis in Chronic Lymphocytic Leukemia—Update on Methodological Approaches and Results Interpretation. Leukemia 2018, 32, 1070–1080. [Google Scholar] [CrossRef]
- Campo, E.; Cymbalista, F.; Ghia, P.; Jäger, U.; Pospisilova, S.; Rosenquist, R.; Schuh, A.; Stilgenbauer, S. TP53 Aberrations in Chronic Lymphocytic Leukemia: An Overview of the Clinical Implications of Improved Diagnostics. Haematologica 2018, 103, 1956–1968. [Google Scholar] [CrossRef]
- Hoofd, C.; Huang, S.J.; Gusscott, S.; Lam, S.; Wong, R.; Johnston, A.; Ben-Neriah, S.; Steidl, C.; Scott, D.W.; Bruyere, H.; et al. Ultrasensitive Detection of NOTCH1 c.7544_7545delCT Mutations in Chronic Lymphocytic Leukemia by Droplet Digital PCR Reveals High Frequency of Subclonal Mutations and Predicts Clinical Outcome in Cases with Trisomy 12. J. Mol. Diagn. 2020, 22, 571–578. [Google Scholar] [CrossRef]
- Willander, K.; Dutta, R.K.; Ungerbäck, J.; Gunnarsson, R.; Juliusson, G.; Fredrikson, M.; Linderholm, M.; Söderkvist, P. NOTCH1 Mutations Influence Survival in Chronic Lymphocytic Leukemia Patients. BMC Cancer 2013, 13, 274. [Google Scholar] [CrossRef]
- Jelloul, F.Z.; Yang, R.; Garces, S.; Kanagal-Shamanna, R.; Ok, C.Y.; Loghavi, S.; Routbort, M.J.; Zuo, Z.; Yin, C.C.; Floyd, K.; et al. Landscape of NOTCH1 Mutations and Co-Occurring Biomarker Alterations in Chronic Lymphocytic Leukemia. Leuk. Res. 2022, 116, 106827. [Google Scholar] [CrossRef] [PubMed]
- Villamor, N.; Conde, L.; Martínez-Trillos, A.; Cazorla, M.; Navarro, A.; Beà, S.; López, C.; Colomer, D.; Pinyol, M.; Aymerich, M.; et al. NOTCH1 Mutations Identify a Genetic Subgroup of Chronic Lymphocytic Leukemia Patients with High Risk of Transformation and Poor Outcome. Leukemia 2013, 27, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Rasi, S.; Fabbri, G.; Spina, V.; Fangazio, M.; Forconi, F.; Marasca, R.; Laurenti, L.; Bruscaggin, A.; Cerri, M.; et al. Mutations of NOTCH1 Are an Independent Predictor of Survival in Chronic Lymphocytic Leukemia. Blood 2012, 119, 521–529. [Google Scholar] [CrossRef]
- Pozzo, F.; Bittolo, T.; Tissino, E.; Zucchetto, A.; Bomben, R.; Polcik, L.; Dannewitz Prosseda, S.; Hartmann, T.N.; Gattei, V. Multiple Mechanisms of NOTCH1 Activation in Chronic Lymphocytic Leukemia: NOTCH1 Mutations and Beyond. Cancers 2022, 14, 2997. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, S.; Chen, S.; Chen, G.; Zhang, R.; Li, J.; Qu, J. SF3B1 Mutation Is a Prognostic Factor in Chronic Lymphocytic Leukemia: A Meta-Analysis. Oncotarget 2017, 8, 69916–69923. [Google Scholar] [CrossRef]
- Cazzola, M.; Rossi, M.; Malcovati, L. Biologic and Clinical Significance of Somatic Mutations of SF3B1 in Myeloid and Lymphoid Neoplasms. Blood 2013, 121, 260–269. [Google Scholar] [CrossRef]
- Wan, Y.; Wu, C.J. SF3B1 Mutations in Chronic Lymphocytic Leukemia. Blood 2013, 121, 4627–4634. [Google Scholar] [CrossRef]
- Wang, L.; Brooks, A.N.; Fan, J.; Wan, Y.; Gambe, R.; Li, S.; Hergert, S.; Yin, S.; Freeman, S.S.; Levin, J.Z.; et al. Transcriptomic Characterization of SF3B1 Mutation Reveals Its Pleiotropic Effects in Chronic Lymphocytic Leukemia. Cancer Cell 2016, 30, 750–763. [Google Scholar] [CrossRef]
- Rossi, D.; Bruscaggin, A.; Spina, V.; Rasi, S.; Khiabanian, H.; Messina, M.; Fangazio, M.; Vaisitti, T.; Monti, S.; Chiaretti, S.; et al. Mutations of the SF3B1 Splicing Factor in Chronic Lymphocytic Leukemia: Association with Progression and Fludarabine-Refractoriness. Blood 2011, 118, 6904–6908. [Google Scholar] [CrossRef]
- Wang, L.; Lawrence, M.S.; Wan, Y.; Stojanov, P.; Sougnez, C.; Stevenson, K.; Werner, L.; Sivachenko, A.; DeLuca, D.S.; Zhang, L.; et al. SF3B1 and Other Novel Cancer Genes in Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2011, 365, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Sud, A.; Parry, E.M.; Wu, C.J. The Molecular Map of CLL and Richter’s Syndrome. Semin. Hematol. 2024, 61, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Zou, Y.-X.; Gu, D.-L.; Zhu, H.-C.; Zhu, H.-Y.; Wang, L.; Liang, J.-H.; Xia, Y.; Wu, J.-Z.; Shao, C.-L.; et al. SF3B1 Mutation Predicts Unfavorable Treatment-Free Survival in Chinese Chronic Lymphocytic Leukemia Patients. Ann. Transl. Med. 2019, 7, 176. [Google Scholar] [CrossRef]
- Alanazi, M.A.; Kwa, F.A.A.; Omar, M.M.A.; Antonipillai, J.; Jackson, D.E. Efficacy and Challenges Involving Combination Therapies in CLL. Drug Discov. Today 2024, 29, 104243. [Google Scholar] [CrossRef]
- Nadeu, F.; Delgado, J.; Royo, C.; Baumann, T.; Stankovic, T.; Pinyol, M.; Jares, P.; Navarro, A.; Martín-García, D.; Beà, S.; et al. Clinical Impact of Clonal and Subclonal TP53, SF3B1, BIRC3, NOTCH1, and ATM Mutations in Chronic Lymphocytic Leukemia. Blood 2016, 127, 2122–2130. [Google Scholar] [CrossRef]
- Tam, C.S.; Balendran, S.; Blombery, P. Novel Mechanisms of Resistance in CLL: Variant BTK Mutations in Second-Generation and Noncovalent BTK Inhibitors. Blood 2025, 145, 1005–1009. [Google Scholar] [CrossRef]
- Lovell, A.R.; Sawyers, J.; Bose, P. An Update on the Efficacy of Venetoclax for Chronic Lymphocytic Leukemia. Expert Opin. Pharmacother. 2023, 24, 1307–1316. [Google Scholar] [CrossRef]
- Machnicki, M.M.; Górniak, P.; Pepek, M.; Szymczyk, A.; Iskierka-Jażdżewska, E.; Steckiewicz, P.; Bluszcz, A.; Rydzanicz, M.; Hus, M.; Ploski, R.; et al. Predictive Significance of Selected Gene Mutations Identified Using Next Generation Sequencing in Relapsed and Refractory Chronic Lymphocytic Leukemia Patients Treated with Ibrutinib. Blood 2019, 134, 5456. [Google Scholar] [CrossRef]
- Brown, J.; Mashima, K.; Fernandes, S.; Naeem, A.; Shupe, S.; Fardoun, R.; Davids, M. Mutations Detected in Real World Clinical Sequencing during BTK Inhibitor Treatment in CLL. Res. Sq. 2024, rs.3.rs-3837426. [Google Scholar] [CrossRef]
- Sun, C.; Mali, R.; Kositsky, R.; Tian, X.; Tomczak, H.; Nuttall, B.; White, R.; Rule, S.; Munugalavadla, V.; Wiestner, A. Extended Follow-up and Resistance Mutations in CLL Patients Treated with Acalabrutinib. Blood 2023, 142, 1891. [Google Scholar] [CrossRef]
- Soverini, S.; Bavaro, L.; De Benedittis, C.; Martelli, M.; Iurlo, A.; Orofino, N.; Sica, S.; Sorà, F.; Lunghi, F.; Ciceri, F.; et al. Prospective Assessment of NGS-Detectable Mutations in CML Patients with Nonoptimal Response: The NEXT-in-CML Study. Blood 2020, 135, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Branford, S.; Shanmuganathan, N. NGS in CML—New Standard Diagnostic Procedure? Hemasphere 2019, 3, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Jean, J.; Sukhanova, M.; Dittmann, D.; Gao, J.; Jennings, L.J. A Novel BCR::ABL1 Variant Detected with Multiple Testing Modalities. Case Rep. Hematol. 2024, 2024, 8486267. [Google Scholar] [CrossRef]
- Cross, N.C.P.; Ernst, T.; Branford, S.; Cayuela, J.-M.; Deininger, M.; Fabarius, A.; Kim, D.D.H.; Machova Polakova, K.; Radich, J.P.; Hehlmann, R.; et al. European LeukemiaNet Laboratory Recommendations for the Diagnosis and Management of Chronic Myeloid Leukemia. Leukemia 2023, 37, 2150–2167. [Google Scholar] [CrossRef]
- Sahoo, T.; Hastie, A.; Ortega, A.; Mylavarapu, A.; Matthews, B.; Hauenstein, J.; Chaubey, A. 91. Atypical BCR::ABL1 Rearrangements Identified by Optical Genome Mapping in Patients with Chronic Myeloid Leukemia. Cancer Genet. 2023, 278–279, 28. [Google Scholar] [CrossRef]
- Crampe, M.; Haslam, K.; Groarke, E.; Kelleher, E.; O’Shea, D.; Conneally, E.; Langabeer, S.E. Chronic Myeloid Leukemia with an E6a2 BCR-ABL1 Fusion Transcript: Cooperating Mutations at Blast Crisis and Molecular Monitoring. Case Rep. Hematol. 2017, 2017, 9071702. [Google Scholar] [CrossRef]
- Molica, M.; Abruzzese, E.; Breccia, M. Prognostic Significance of Transcript-Type BCR—ABL1 in Chronic Myeloid Leukemia. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020062. [Google Scholar] [CrossRef]
- Pretzsch, T.; Progscha, S.; Burmeister, T. Diagnostic Ambiguity Caused by an Atypical E18a2 BCR::ABL1 Transcript in a Chronic Myeloid Leukemia Patient. Case Rep. Hematol. 2024, 2024, 9439134. [Google Scholar] [CrossRef]
- Hanfstein, B.; Lauseker, M.; Hehlmann, R.; Saussele, S.; Erben, P.; Dietz, C.; Fabarius, A.; Proetel, U.; Schnittger, S.; Haferlach, C.; et al. Distinct Characteristics of E13a2 versus E14a2 BCR-ABL1 Driven Chronic Myeloid Leukemia under First-Line Therapy with Imatinib. Haematologica 2014, 99, 1441–1447. [Google Scholar] [CrossRef]
- Jinawath, N.; Norris-Kirby, A.; Smith, B.D.; Gocke, C.D.; Batista, D.A.; Griffin, C.A.; Murphy, K.M. A Rare E14a3 (B3a3) BCR-ABL Fusion Transcript in Chronic Myeloid Leukemia: Diagnostic Challenges in Clinical Laboratory Practice. J. Mol. Diagn. 2009, 11, 359–363. [Google Scholar] [CrossRef]
- Leske, I.B.; Hantschel, O. The E13a3 (B2a3) and E14a3 (B3a3) BCR::ABL1 Isoforms Are Resistant to Asciminib. Leukemia 2024, 38, 2041–2045. [Google Scholar] [CrossRef] [PubMed]
- Langabeer, S.E.; McCarron, S.L.; Kelly, J.; Krawczyk, J.; McPherson, S.; Perera, K.; Murphy, P.T. Chronic Myeloid Leukemia with E19a2 BCR-ABL1 Transcripts and Marked Thrombocytosis: The Role of Molecular Monitoring. Case Rep. Hematol. 2012, 2012, 458716. [Google Scholar] [CrossRef] [PubMed]
- Tutulan-Cunita, A.C.; Chirieac, S.M.; Mocanu, G.; Luca, C.; Costache, M.; Lungeanu, A.; Arghir, A. Variant E19a2 BCR-ABL1 Fusion Transcript in Typical Chronic Myeloid Leukemia. Clin. Lab. 2011, 57, 785–788. [Google Scholar] [PubMed]
- Limsuwanachot, N.; Rerkamnuaychoke, B.; Niparuck, P.; Singdong, R.; Kongruang, A.; Hirunpatrawong, P.; Siriyakorn, T.; Yenchitsomanus, P.; Siriboonpiputtana, T. A Customized Mass Array Panel for BCR::ABL1 Tyrosine Kinase Domain Mutation Screening in Chronic Myeloid Leukemia. J. Mass Spectrom. Adv. Clin. Lab 2023, 28, 122–132. [Google Scholar] [CrossRef]
- Tanneeru, K.; Guruprasad, L. Ponatinib Is a Pan-BCR-ABL Kinase Inhibitor: MD Simulations and SIE Study. PLoS ONE 2013, 8, e78556. [Google Scholar] [CrossRef]
- Dmytrenko, I.; Minchenko, J.; Dyagil, I. Characteristics of BCR/ABL1 Kinase Domain Mutations in the Patients with Chronic Myeloid Leukemia Who Are Primary Resistant to the Imatinib Therapy. Biosystems 2019, 11, 27–33. [Google Scholar] [CrossRef]
- Jabbour, E.; Parikh, S.A.; Kantarjian, H.; Cortes, J. Chronic Myeloid Leukemia—Mechanisms of Resistance and Treatment. Hematol. Oncol. Clin. N. Am. 2011, 25, 981–995. [Google Scholar] [CrossRef]
- Alves, R.; Gonçalves, A.C.; Rutella, S.; Almeida, A.M.; De Las Rivas, J.; Trougakos, I.P.; Sarmento Ribeiro, A.B. Resistance to Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia—From Molecular Mechanisms to Clinical Relevance. Cancers 2021, 13, 4820. [Google Scholar] [CrossRef]
- Manley, P.W.; Barys, L.; Cowan-Jacob, S.W. The Specificity of Asciminib, a Potential Treatment for Chronic Myeloid Leukemia, as a Myristate-Pocket Binding ABL Inhibitor and Analysis of Its Interactions with Mutant Forms of BCR-ABL1 Kinase. Leuk. Res. 2020, 98, 106458. [Google Scholar] [CrossRef]
- Yan, Z.; Shi, L.; Li, W.; Liu, W.; Galderisi, C.; Spittle, C.; Li, J. A Novel Next-Generation Sequencing Assay for the Identification of BCR::ABL1 Transcript Type and Accurate and Sensitive Detection of TKI-Resistant Mutations. J. Appl. Lab. Med. 2024, 9, 886–900. [Google Scholar] [CrossRef]
- Khorashad, J.S.; Kelley, T.W.; Szankasi, P.; Mason, C.C.; Soverini, S.; Adrian, L.T.; Eide, C.A.; Zabriskie, M.S.; Lange, T.; Estrada, J.C.; et al. BCR-ABL1 Compound Mutations in Tyrosine Kinase Inhibitor–Resistant CML: Frequency and Clonal Relationships. Blood 2013, 121, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Vannuffel, P.; Bavaro, L.; Nollet, F.; Aynaci, A.; Martelli, M.; Devos, H.; De Rop, C.; Soverini, S. Droplet Digital PCR Phasing (DROP-PHASE): A Novel Method for Straightforward Detection of BCR-ABL1 Compound Mutations in Tyrosine Kinase Inhibitors Resistant Chronic Myeloid Leukemia (CML) and Acute Lymphoblastic Leukemia (ALL). Blood 2019, 134, 4660. [Google Scholar] [CrossRef]
- Iqbal, Z.; Akram, A.M.; Akhtar, T.; Khalid, M.; Aziz, Z.; Aleem, A.; Gill, A.T.; Khalid, A.M.; Alanazi, A.; Shah, I.H.; et al. High Frequencies of Compound BCR-ABL Mutations and Their Association with Imatinib Resistant, Disease Progression and Late Chronic Phase Disease in Pakistani Chronic Myeloid Leukemia Patients Necessitate the Inclusion of Molecular Testing in Routine Clinical Settings. Blood 2015, 126, 5167. [Google Scholar] [CrossRef]
- Machova Polakova, K.; Kulvait, V.; Benesova, A.; Linhartova, J.; Klamova, H.; Jaruskova, M.; De Benedittis, C.; Haferlach, T.; Baccarani, M.; Martinelli, G.; et al. Next-Generation Deep Sequencing Improves Detection of BCR-ABL1 Kinase Domain Mutations Emerging under Tyrosine Kinase Inhibitor Treatment of Chronic Myeloid Leukemia Patients in Chronic Phase. J. Cancer Res. Clin. Oncol. 2015, 141, 887–899. [Google Scholar] [CrossRef]
- Branford, S.; Apperley, J.F. Measurable Residual Disease in Chronic Myeloid Leukemia. Haematologica 2022, 107, 2794–2809. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, X.; Zhou, X.; Cheng, Z.; Hu, Y. Exploration of Treatment-free Remission in CML, Based on Molecular Monitoring. Cancer Med. 2023, 13, e6849. [Google Scholar] [CrossRef]
- Bernardi, S.; Cavalleri, A.; Mutti, S.; Garuffo, L.; Farina, M.; Leoni, A.; Iurlo, A.; Bucelli, C.; Toffoletti, E.; Di Giusto, S.; et al. Digital PCR (dPCR) Is Able to Anticipate the Achievement of Stable Deep Molecular Response in Adult Chronic Myeloid Leukemia Patients: Results of the DEMONSTRATE Study. Ann. Hematol. 2025, 104, 207–217. [Google Scholar] [CrossRef]
- Abruzzese, E.; Bocchia, M.; Trawinska, M.M.; Raspadori, D.; Bondanini, F.; Sicuranza, A.; Pacelli, P.; Re, F.; Cavalleri, A.; Farina, M.; et al. Minimal Residual Disease Detection at RNA and Leukemic Stem Cell (LSC) Levels: Comparison of RT-qPCR, d-PCR and CD26+ Stem Cell Measurements in Chronic Myeloid Leukemia (CML) Patients in Deep Molecular Response (DMR). Cancers 2023, 15, 4112. [Google Scholar] [CrossRef]
- Park, H.; Kim, I.; Kim, H.-J.; Shin, D.-Y.; Lee, S.-Y.; Kwon, O.-H.; Kim, D.-Y.; Lee, K.-H.; Ahn, J.-S.; Park, J.; et al. Ultra-Deep Sequencing Mutation Analysis of the BCR/ABL1 Kinase Domain in Newly Diagnosed Chronic Myeloid Leukemia Patients. Leuk. Res. 2021, 111, 106728. [Google Scholar] [CrossRef]
- Grossmann, V.; Wild, M.; Schnittger, S.; Kern, W.; Haferlach, T.; Haferlach, C.; Kohlmann, A. Ultra-Deep Next-Generation Sequencing (NGS) Detects BCR-ABL1 Kinase Domain Mutations with High Sensitivity and Allows to Monitor the Composition of Distinct Subclones During Tyrosine Kinase Inhibitor Treatment. Blood 2010, 116, 2268. [Google Scholar] [CrossRef]
- Talati, C.; Isenalumhe, L.; Kuykendall, A.; Shah, B.D.; Chavez, J.C.; Hussaini, M.O.; Pinilla-Ibarz, J.; Sweet, K.L. Role of Somatic Mutations in Clonal Evolution of Chronic Myeloid Leukemia from Chronic Phase to Blast Phase. Blood 2017, 130, 1587. Available online: https://ashpublications.org/blood/article/130/Supplement%201/1587/79741/Role-of-Somatic-Mutations-in-Clonal-Evolution-of (accessed on 23 March 2025).
- Gotlib, J. How I Treat Atypical Chronic Myeloid Leukemia. Blood 2017, 129, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Piazza, R.; Valletta, S.; Winkelmann, N.; Redaelli, S.; Spinelli, R.; Pirola, A.; Antolini, L.; Mologni, L.; Donadoni, C.; Papaemmanuil, E.; et al. Recurrent SETBP1 Mutations in Atypical Chronic Myeloid Leukemia. Nat. Genet. 2013, 45, 18–24. [Google Scholar] [CrossRef]
- Makishima, H.; Yoshida, K.; Nguyen, N.; Przychodzen, B.; Sanada, M.; Okuno, Y.; Ng, K.P.; Gudmundsson, K.O.; Vishwakarma, B.A.; Jerez, A.; et al. Somatic SETBP1 Mutations in Myeloid Malignancies. Nat. Genet. 2013, 45, 942–946. [Google Scholar] [CrossRef]
- Wang, L.; Du, F.; Zhang, H.-M.; Wang, H.-X. Evaluation of a Father and Son with Atypical Chronic Myeloid Leukemia with SETBP1 Mutations and a Review of the Literature. Braz. J. Med. Biol. Res. 2015, 48, 583–587. [Google Scholar] [CrossRef]
- Makishima, H. Somatic SETBP1 Mutations in Myeloid Neoplasms. Int. J. Hematol. 2017, 105, 732–742. [Google Scholar] [CrossRef]
- Kojima, K.; Ishizawa, J.; Andreeff, M. Pharmacological Activation of Wild-Type P53 in the Therapy of Leukemia. Exp. Hematol. 2016, 44, 791–798. [Google Scholar] [CrossRef]
- Neubauer, A.; He, M.; Schmidt, C.A.; Huhn, D.; Liu, E.T. Genetic Alterations in the P53 Gene in the Blast Crisis of Chronic Myelogenous Leukemia: Analysis by Polymerase Chain Reaction Based Techniques. Leukemia 1993, 7, 593–600. [Google Scholar]
- Ahmadi, S.E.; Rahimian, E.; Rahimi, S.; Zarandi, B.; Bahraini, M.; Soleymani, M.; Safdari, S.M.; Shabannezhad, A.; Jaafari, N.; Safa, M. From Regulation to Deregulation of P53 in Hematologic Malignancies: Implications for Diagnosis, Prognosis and Therapy. Biomark. Res. 2024, 12, 137. [Google Scholar] [CrossRef]
- Sobczyńska-Konefał, A.; Jasek, M.; Karabon, L.; Jaskuła, E. Insights into Genetic Aberrations and Signalling Pathway Interactions in Chronic Lymphocytic Leukemia: From Pathogenesis to Treatment Strategies. Biomark. Res. 2024, 12, 162. [Google Scholar] [CrossRef]
- Zenz, T.; Häbe, S.; Denzel, T.; Mohr, J.; Winkler, D.; Bühler, A.; Sarno, A.; Groner, S.; Mertens, D.; Busch, R.; et al. Detailed Analysis of P53 Pathway Defects in Fludarabine-Refractory Chronic Lymphocytic Leukemia (CLL): Dissecting the Contribution of 17p Deletion, TP53 Mutation, P53-P21 Dysfunction, and miR34a in a Prospective Clinical Trial. Blood 2009, 114, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Dufies, M.; Jacquel, A.; Belhacene, N.; Robert, G.; Cluzeau, T.; Luciano, F.; Cassuto, J.P.; Raynaud, S.; Auberger, P. Mechanisms of AXL Overexpression and Function in Imatinib-Resistant Chronic Myeloid Leukemia Cells. Oncotarget 2011, 2, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Sharma, A.; Patne, K.; Tabasum, S.; Suryavanshi, J.; Rawat, L.; Machaalani, M.; Eid, M.; Singh, R.P.; Choueiri, T.K.; et al. AXL Signaling in Cancer: From Molecular Insights to Targeted Therapies. Signal Transduct. Target. Ther. 2025, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Ben-Batalla, I.; Erdmann, R.; Jørgensen, H.; Mitchell, R.; Ernst, T.; von Amsberg, G.; Schafhausen, P.; Velthaus, J.L.; Rankin, S.; Clark, R.E.; et al. Axl Blockade by BGB324 Inhibits BCR-ABL Tyrosine Kinase Inhibitor–Sensitive and -Resistant Chronic Myeloid Leukemia. Clin. Cancer Res. 2017, 23, 2289–2300. [Google Scholar] [CrossRef]
- Neubauer, A.; Burchert, A.; Maiwald, C.; Gruss, H.-J.; Serke, S.; Huhn, D.; Wittig, B.; Liu, E. Recent Progress On the Role of Axl, A Receptor Tyrosine Kinase, in Malignant Transformation of Myeloid Leukemias. Leuk. Lymphoma 1997, 25, 91–96. [Google Scholar] [CrossRef]
- Deininger, M.W.N.; Goldman, J.M.; Melo, J.V. The Molecular Biology of Chronic Myeloid Leukemia. Blood 2000, 96, 3343–3356. [Google Scholar] [CrossRef]
- Morgan, M.A.; Dolp, O.; Reuter, C.W. Cell-Cycle-Dependent Activation of Mitogen-Activated Protein Kinase Kinase (MEK-1/2) in Myeloid Leukemia Cell Lines and Induction of Growth Inhibition and Apoptosis by Inhibitors of RAS Signaling. Blood 2001, 97, 1823–1834. [Google Scholar] [CrossRef]
- Shang, Z.-C.; Sun, B.-Z.; Chen, Z.-N.; Wang, W.; Feng, Q.; Wang, S.; Zhang, T. Effects of mitogen-activated protein kinase(MAPK) in cell signal transduction of chronic myelogenous leukemia cell line K562. Ai Zheng 2003, 22, 140–142. [Google Scholar]
- Reuter, C.W.M.; Morgan, M.A.; Bergmann, L. Effect of Mutationally Activated Ras on the Ras to MAP Kinase Signaling Pathway and Growth Inhibition of Myeloid Leukemia Cells by Inhibitors of the MAP Kinase Cascade. In Acute Leukemias VIII; Büchner, T., Hiddemann, W., Wörmann, B., Schellong, G., Ritter, J., Creutzig, U., Eds.; Haematology and Blood Transfusion/Hämatologie und Bluttransfusion; Springer: Berlin/Heidelberg, Germany, 2001; Volume 40, pp. 100–108. ISBN 978-3-642-62109-3. [Google Scholar]
- LeMaistre, A.; Lee, M.; Talpaz, M.; Kantarjian, H.; Freireich, E.; Deisseroth, A.; Trujillo, J.; Stass, S. Ras Oncogene Mutations Are Rare Late Stage Events in Chronic Myelogenous Leukemia. Blood 1989, 73, 889–891. [Google Scholar] [CrossRef]
- Li, N.; Chen, M.; Yin, C.C. Advances in Molecular Evaluation of Myeloproliferative Neoplasms. Semin. Diagn. Pathol. 2023, 40, 187–194. [Google Scholar] [CrossRef]
- Parikh, C.; Subrahmanyam, R.; Ren, R. Oncogenic NRAS, KRAS, and HRAS Exhibit Different Leukemogenic Potentials in Mice. Cancer Res. 2007, 67, 7139–7146. [Google Scholar] [CrossRef] [PubMed]
- Tyner, J.W.; Erickson, H.; Deininger, M.W.N.; Willis, S.G.; Eide, C.A.; Levine, R.L.; Heinrich, M.C.; Gattermann, N.; Gilliland, D.G.; Druker, B.J.; et al. High-Throughput Sequencing Screen Reveals Novel, Transforming RAS Mutations in Myeloid Leukemia Patients. Blood 2009, 113, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Basu, S.; Shah, S.; Bhattacharyya, D.; Gupta, P.P.; Acharjee, M.; Roychoudhury, S.; Nath, S. Deep Sequencing Reveals the Spectrum of BCR-ABL1 Mutations upon Front-Line Therapy Resistance in Chronic Myeloid Leukemia: An Eastern-Indian Cohort Study. Cancer Treat. Res. Commun. 2022, 33, 100635. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Moon, J.H.; Ahn, J.-S.; Kim, Y.-K.; Lee, S.-S.; Ahn, S.-Y.; Jung, S.-H.; Yang, D.-H.; Lee, J.-J.; Choi, S.H.; et al. Next-Generation Sequencing–Based Posttransplant Monitoring of Acute Myeloid Leukemia Identifies Patients at High Risk of Relapse. Blood 2018, 132, 1604–1613. [Google Scholar] [CrossRef]
- Langabeer, S.E.; Haslam, K.; Kelly, J.; Quinn, J.; Morrell, R.; Conneally, E. Targeted Next-Generation Sequencing Identifies Clinically Relevant Mutations in Patients with Chronic Neutrophilic Leukemia at Diagnosis and Blast Crisis. Clin. Transl. Oncol. 2018, 20, 420–423. [Google Scholar] [CrossRef]
- Endrullat, C.; Glökler, J.; Franke, P.; Frohme, M. Standardization and Quality Management in Next-Generation Sequencing. Appl. Transl. Genom. 2016, 10, 2–9. [Google Scholar] [CrossRef]
- Federici, G.; Soddu, S. Variants of Uncertain Significance in the Era of High-Throughput Genome Sequencing: A Lesson from Breast and Ovary Cancers. J. Exp. Clin. Cancer Res. 2020, 39, 46. [Google Scholar] [CrossRef]
- Landrum, M.J.; Chitipiralla, S.; Brown, G.R.; Chen, C.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; Kaur, K.; Liu, C.; et al. ClinVar: Improvements to Accessing Data. Nucleic Acids Res. 2020, 48, D835–D844. [Google Scholar] [CrossRef]
- Khotskaya, Y.B.; Mills, G.B.; Mills Shaw, K.R. Next-Generation Sequencing and Result Interpretation in Clinical Oncology: Challenges of Personalized Cancer Therapy. Annu. Rev. Med. 2017, 68, 113–125. [Google Scholar] [CrossRef]
- Zhong, J.; Stucky, A.; Sun, F.; Huang, J. PB1800: DEEPDECON: A DEEP-LEARNING METHOD FOR DETECTING MINIMAL RESIDUAL DISEASE OF ACUTE MYELOID LEUKEMIA. Hemasphere 2022, 6, 1680–1681. [Google Scholar] [CrossRef]
- Luh, F.; Yen, Y. FDA Guidance for next Generation Sequencing-Based Testing: Balancing Regulation and Innovation in Precision Medicine. NPJ Genom. Med. 2018, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves NGS-Based Test for Patients With ALL or MM|ASH Clinical News|American Society of Hematology. Available online: https://multiplemyeloma.org.nz/trial-represents-bold-move-for-treatment-of-newly-diagnosed-myeloma-2-2-2-2/ (accessed on 23 March 2025).
- Evans, B.J. The Limits of FDA’s Authority to Regulate Clinical Research Involving High-Throughput DNA Sequencing. Food Drug Law J. 2015, 70, 259–287. [Google Scholar] [PubMed]
- CMS Finalizes Coverage of Next Generation Sequencing Tests, Ensuring Enhanced Access for Cancer Patients|CMS. Available online: https://www.cms.gov/newsroom/press-releases/cms-finalizes-coverage-next-generation-sequencing-tests-ensuring-enhanced-access-cancer-patients (accessed on 23 March 2025).
- Hedblom, A.H.; Pruneri, G.; Quagliata, L.; Costa, J.L.; Dumanois, R.; Rolando, C.; Saunders, R. Cancer Patient Management: Current Use of next-Generation Sequencing in the EU TOP4. J Cancer Policy 2023, 35, 100376. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and Management of AML in Adults: 2022 Recommendations from an International Expert Panel on Behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Béné, M.C.; Grimwade, D.; Haferlach, C.; Haferlach, T.; Zini, G.; European LeukemiaNet. Leukemia Diagnosis: Today and Tomorrow. Eur. J. Haematol. 2015, 95, 365–373. [Google Scholar] [CrossRef]
- Jennings, L.J.; Arcila, M.E.; Corless, C.; Kamel-Reid, S.; Lubin, I.M.; Pfeifer, J.; Temple-Smolkin, R.L.; Voelkerding, K.V.; Nikiforova, M.N. Guidelines for Validation of Next-Generation Sequencing–Based Oncology Panels. J. Mol. Diagn. 2017, 19, 341–365. [Google Scholar] [CrossRef]
- Horgan, D.; Curigliano, G.; Rieß, O.; Hofman, P.; Büttner, R.; Conte, P.; Cufer, T.; Gallagher, W.M.; Georges, N.; Kerr, K.; et al. Identifying the Steps Required to Effectively Implement Next-Generation Sequencing in Oncology at a National Level in Europe. JPM 2022, 12, 72. [Google Scholar] [CrossRef]
- Kaminski, A.; Szamreta, E.A.; Shah, R.; Ning, N.; Aggarwal, J.; Hussain, A.; Adeboyeje, G. Barriers to Next-Generation Sequencing despite Increased Utilization: U.S. Physician Survey Results. JCO 2021, 39, e18754. [Google Scholar] [CrossRef]
- Dhawale, T.; Nardi, V.; Wang, C.; Bard, A.; Essigmann, N.; Leonard, D.; Graubert, T.; Fathi, A.T. Impact of Ultra Rapid Molecular Profiling on Treatment Delays and Healthcare Utilization of Patients Hospitalized for Acute Leukemia. Blood 2024, 144, 5058. [Google Scholar] [CrossRef]
- Llop, M.; Sargas, C.; Barragán, E. The Role of Next-Generation Sequencing in Acute Myeloid Leukemia. Curr. Opin. Oncol. 2022, 34, 723–728. [Google Scholar] [CrossRef]
- Shah, N.P.; Bhatia, R.; Altman, J.K.; Amaya, M.; Begna, K.H.; Berman, E.; Chan, O.; Clements, J.; Collins, R.H.; Curtin, P.T.; et al. Chronic Myeloid Leukemia, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024, 22, 43–69. [Google Scholar] [CrossRef]
- Said, B.; Gilles, S.; Weisdorf, D.; Rashidi, A. The Role of Bone Marrow Morphology in the Diagnosis of Relapsed Acute Myeloid Leukemia. Leukemia 2018, 32, 1052. [Google Scholar] [CrossRef]
- Szamreta, E.A.; Kaminski, A.; Shah, R.; Ning, N.; Aggarwal, J.; Hussain, A.; Adeboyeje, G. Survey Study of Barriers to Evidence-Based Decision-Making in Oncology Care Using next-Generation Sequencing. JCO 2021, 39, e18757. [Google Scholar] [CrossRef]
- Akhoundova, D.; Rubin, M.A. Clinical Application of Advanced Multi-Omics Tumor Profiling: Shaping Precision Oncology of the Future. Cancer Cell 2022, 40, 920–938. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.C.; Lalonde, E.; Li, L.; Cavallone, L.; Natrajan, R.; Lambros, M.B.; Mitsopoulos, C.; Hakas, J.; Kozarewa, I.; Fenwick, K.; et al. Identification of Gene Fusion Transcripts by Transcriptome Sequencing in BRCA1-Mutated Breast Cancers and Cell Lines. BMC Med. Genom. 2011, 4, 75. [Google Scholar] [CrossRef]
- Lilljebjörn, H.; Orsmark-Pietras, C.; Mitelman, F.; Hagström-Andersson, A.; Fioretos, T. Transcriptomics Paving the Way for Improved Diagnostics and Precision Medicine of Acute Leukemia. Semin. Cancer Biol. 2022, 84, 40–49. [Google Scholar] [CrossRef]
- Hou, S.; Liu, J.; Zhu, Y. Multi-Omics Advances for Molecular Characterization, Precision Medicine, and Prognostic Implications in Leukemia. Cell Investig. 2025, 1, 100007. [Google Scholar] [CrossRef]
- Casado, P.; Cutillas, P.R. Proteomic Characterization of Acute Myeloid Leukemia for Precision Medicine. Mol. Cell. Proteom. 2023, 22, 100517. [Google Scholar] [CrossRef]
- Casado, P.; Wilkes, E.H.; Miraki-Moud, F.; Hadi, M.M.; Rio-Machin, A.; Rajeeve, V.; Pike, R.; Iqbal, S.; Marfa, S.; Lea, N.; et al. Proteomic and Genomic Integration Identifies Kinase and Differentiation Determinants of Kinase Inhibitor Sensitivity in Leukemia Cells. Leukemia 2018, 32, 1818–1822. [Google Scholar] [CrossRef]
- Bao, Y.; Qiao, J.; Gong, W.; Zhang, R.; Zhou, Y.; Xie, Y.; Xie, Y.; He, J.; Yin, T. Spatial Metabolomics Highlights Metabolic Reprogramming in Acute Myeloid Leukemia Mice through Creatine Pathway. Acta Pharm. Sin. B 2024, 14, 4461–4477. [Google Scholar] [CrossRef]
- Shafiei, F.S.; Abroun, S.; Vahdat, S.; Rafiee, M. Omics Approaches: Role in Acute Myeloid Leukemia Biomarker Discovery and Therapy. Cancer Genet. 2025, 292–293, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhao, H.; Wang, R.; Chen, Y.; Ouyang, X.; Li, W.; Sun, Y.; Peng, A. Cancer Epigenetics: From Laboratory Studies and Clinical Trials to Precision Medicine. Cell Death Discov. 2024, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Metzeler, K.H.; Walker, A.; Geyer, S.; Garzon, R.; Klisovic, R.B.; Bloomfield, C.D.; Blum, W.; Marcucci, G. DNMT3A Mutations and Response to the Hypomethylating Agent Decitabine in Acute Myeloid Leukemia. Leukemia 2012, 26, 1106–1107. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Shen, K.; Xiao, M. TET2 Mutation in Acute Myeloid Leukemia: Biology, Clinical Significance, and Therapeutic Insights. Clin. Epigenet. 2024, 16, 155. [Google Scholar] [CrossRef]
- Wang, T.; Cui, S.; Lyu, C.; Wang, Z.; Li, Z.; Han, C.; Liu, W.; Wang, Y.; Xu, R. Molecular Precision Medicine: Multi-Omics-Based Stratification Model for Acute Myeloid Leukemia. Heliyon 2024, 10, e36155. [Google Scholar] [CrossRef]
- Kosvyra, A.; Karadimitris, A.; Papaioannou, Μ.; Chouvarda, I. Machine Learning and Integrative Multi-Omics Network Analysis for Survival Prediction in Acute Myeloid Leukemia. Comput. Biol. Med. 2024, 178, 108735. [Google Scholar] [CrossRef]
- Mohr, A.E.; Ortega-Santos, C.P.; Whisner, C.M.; Klein-Seetharaman, J.; Jasbi, P. Navigating Challenges and Opportunities in Multi-Omics Integration for Personalized Healthcare. Biomedicines 2024, 12, 1496. [Google Scholar] [CrossRef]
- Tsagiopoulou, M.; Gut, I.G. Machine Learning and Multi-Omics Data in Chronic Lymphocytic Leukemia: The Future of Precision Medicine? Front. Genet. 2024, 14, 1304661. [Google Scholar] [CrossRef]
- Abbasi, E.Y.; Deng, Z.; Ali, Q.; Khan, A.; Shaikh, A.; Reshan, M.S.A.; Sulaiman, A.; Alshahrani, H. A Machine Learning and Deep Learning-Based Integrated Multi-Omics Technique for Leukemia Prediction. Heliyon 2024, 10, e25369. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Bollas, A.; Wang, Y.; Au, K.F. Nanopore Sequencing Technology, Bioinformatics and Applications. Nat. Biotechnol. 2021, 39, 1348–1365. [Google Scholar] [CrossRef]
- Flach, J.; Shumilov, E.; Wiedemann, G.; Porret, N.; Shakhanova, I.; Bürki, S.; Legros, M.; Joncourt, R.; Pabst, T.; Bacher, U. Clinical Potential of Introducing Next-Generation Sequencing in Patients at Relapse of Acute Myeloid Leukemia. Hematol. Oncol. 2020, 38, 425–431. [Google Scholar] [CrossRef]
- Chanan-Khan, A.A.; Challagulla, S.; Kaushiva, A.; Miller, V.; Wang, Y.; Smith, H.; Yang, K. Impact of Real-World Treatment Sequencing Patterns on Time to Next Treatment among Patients with Chronic Lymphocytic Leukemia in the United States. Blood 2023, 142, 5143. [Google Scholar] [CrossRef]
- McGowan, P.F.; Hyter, S.D.; Cui, W.; Plummer, R.M.; Godwin, A.K.; Zhang, D. Comparison of Flow Cytometry and Next-Generation Sequencing in Minimal Residual Disease Monitoring of Acute Myeloid Leukemia: One Institute’s Practical Clinical Experience. Int. J. Lab. Hematol. 2022, 44, 118–126. [Google Scholar] [CrossRef]
- Zhang, T.; Li, H.; Jiang, M.; Hou, H.; Gao, Y.; Li, Y.; Wang, F.; Wang, J.; Peng, K.; Liu, Y.-X. Nanopore Sequencing: Flourishing in Its Teenage Years. J. Genet. Genom. 2024, 51, 1361–1374. [Google Scholar] [CrossRef]
- Wagner, U.; Wong, C.; Camenisch, U.; Zimmermann, K.; Rechsteiner, M.; Valtcheva, N.; Theocharides, A.; Widmer, C.C.; Manz, M.G.; Moch, H.; et al. Comprehensive Validation of Diagnostic Next-Generation Sequencing Panels for Acute Myeloid Leukemia Patients. J. Mol. Diagn. 2022, 24, 935–954. [Google Scholar] [CrossRef]
- Doostparast Torshizi, A.; Wang, K. Next-Generation Sequencing in Drug Development: Target Identification and Genetically Stratified Clinical Trials. Drug Discov. Today 2018, 23, 1776–1783. [Google Scholar] [CrossRef]
- Hux, A.; Lewis, A.; Sachwitz, D.; Gregory, T. Clinical Utility of Next-Generation Sequencing in Precision Oncology. JAAPA 2019, 32, 35–39. [Google Scholar] [CrossRef]
- Duan, X.-P.; Qin, B.-D.; Jiao, X.-D.; Liu, K.; Wang, Z.; Zang, Y.-S. New Clinical Trial Design in Precision Medicine: Discovery, Development and Direction. Signal Transduct. Target. Ther. 2024, 9, 57. [Google Scholar] [CrossRef]
- Venugopal, S.; Watts, J. The Future Paradigm of HMA + VEN or Targeted Inhibitor Approaches: Sequencing or Triplet Combinations in AML Therapy. Hematology 2023, 2023, 192–197. [Google Scholar] [CrossRef]
- Dhawan, D. Clinical Next-Generation Sequencing. In Progress and Challenges in Precision Medicine; Elsevier: Amsterdam, The Netherlands, 2017; pp. 35–54. ISBN 978-0-12-809411-2. [Google Scholar]
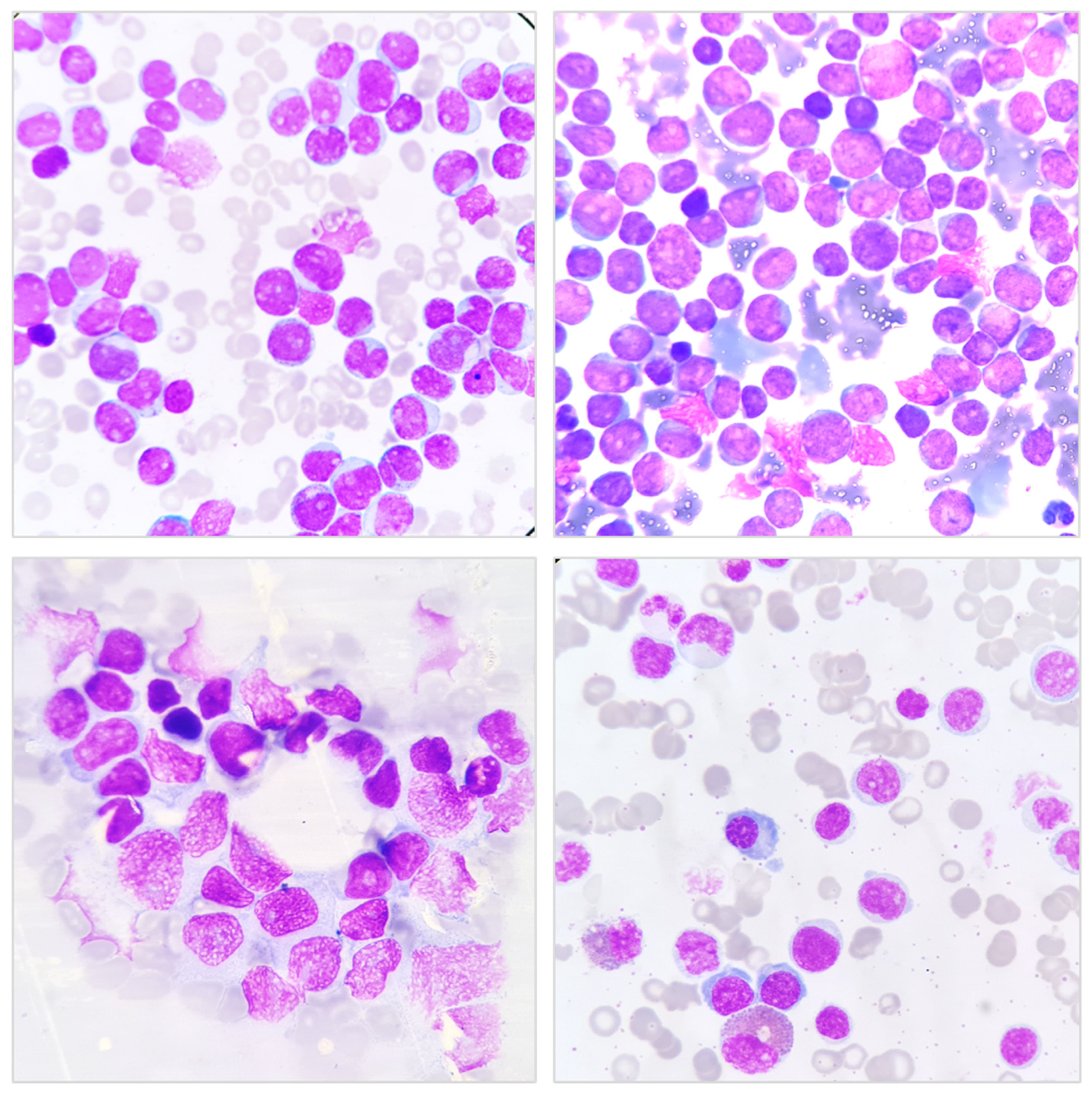
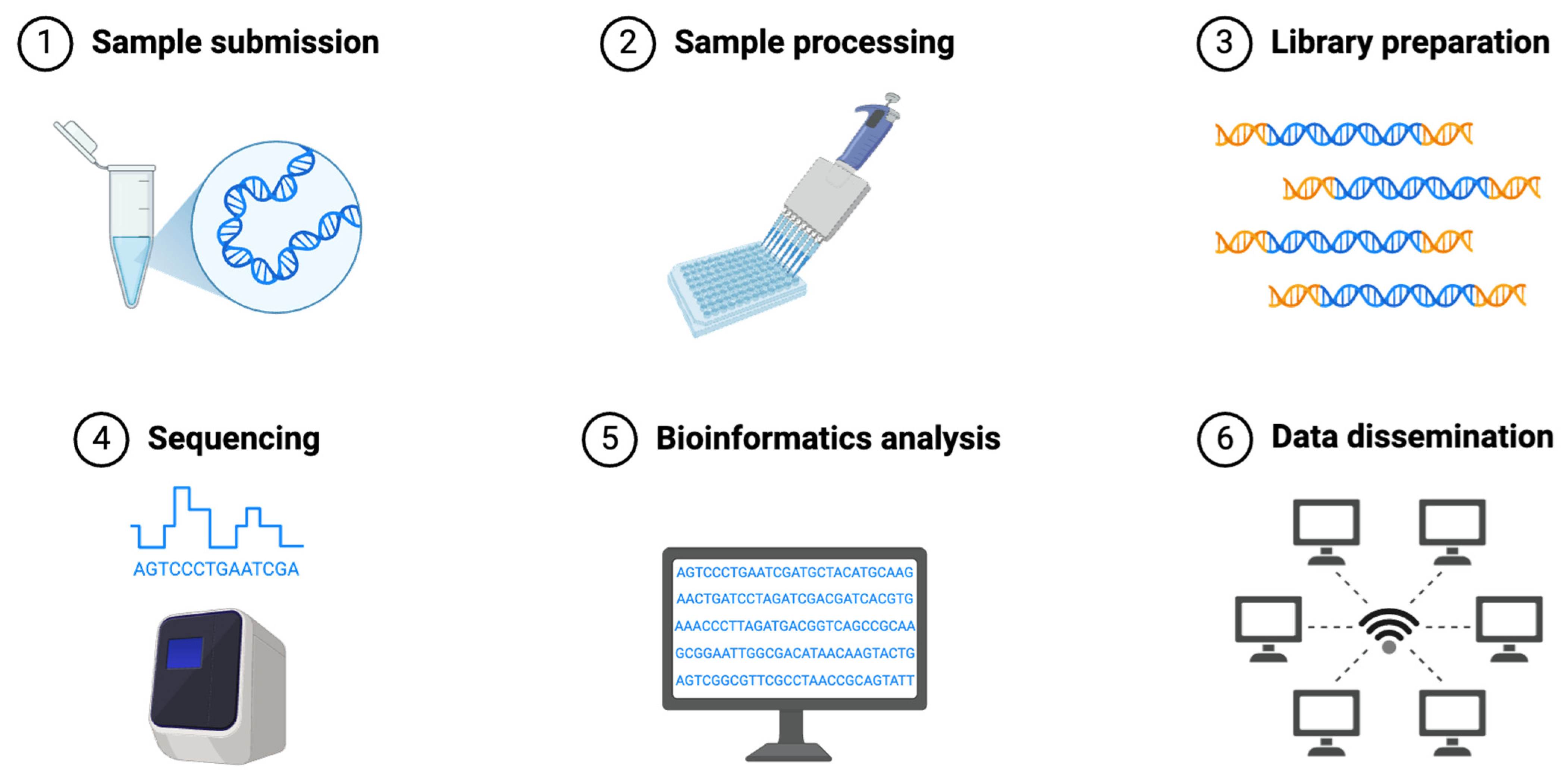
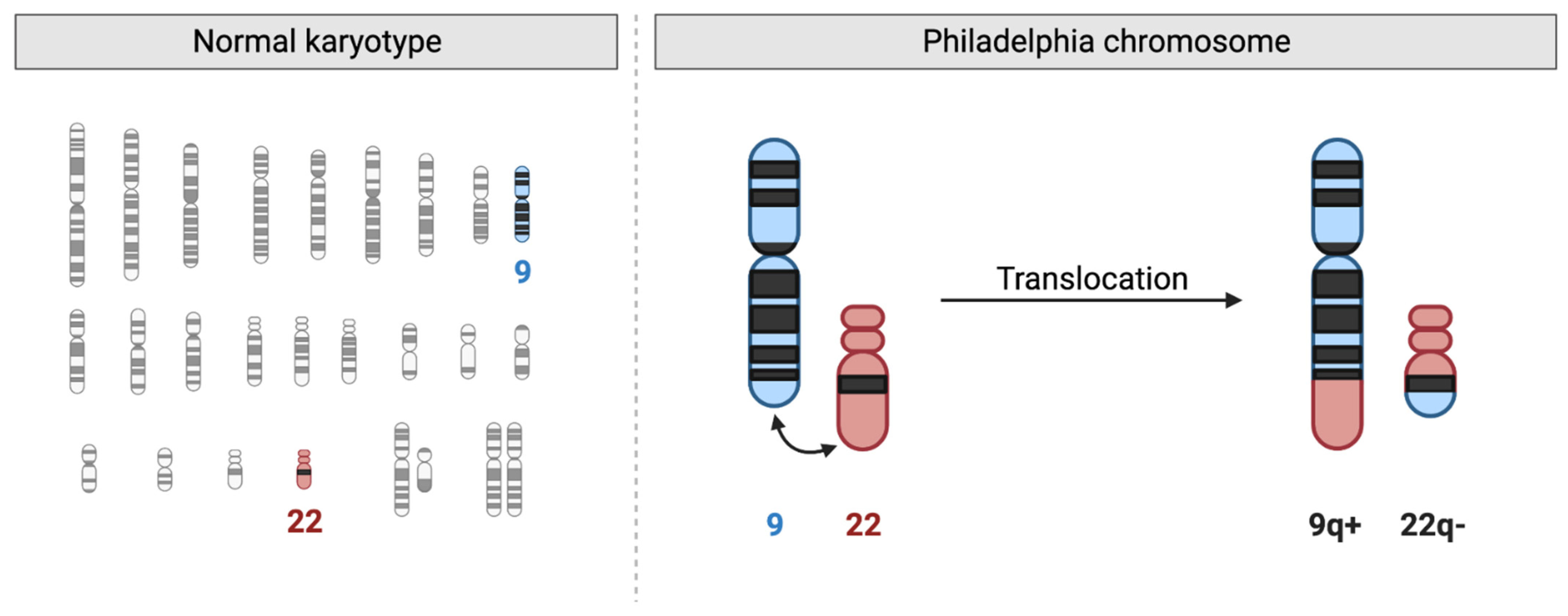
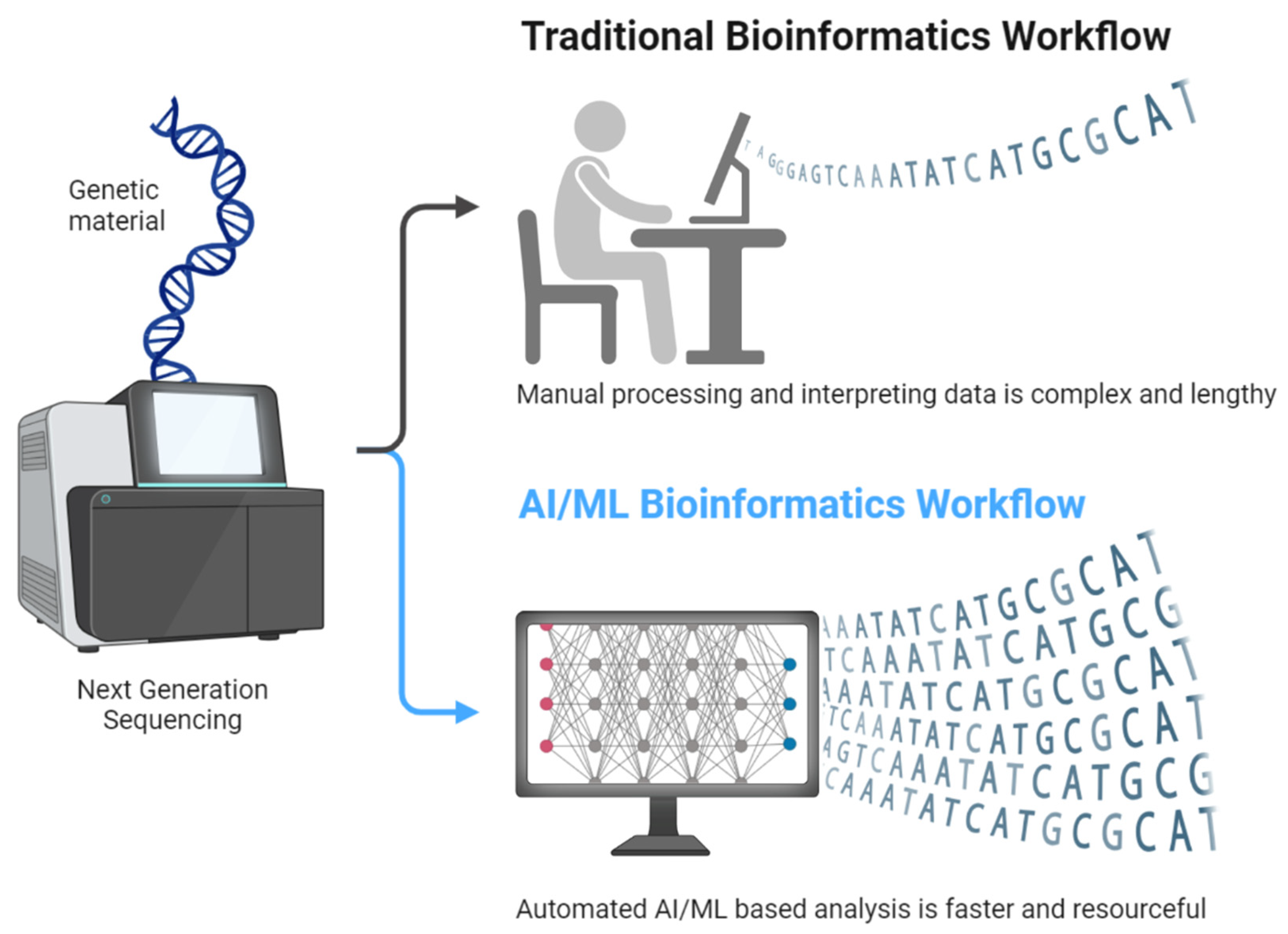
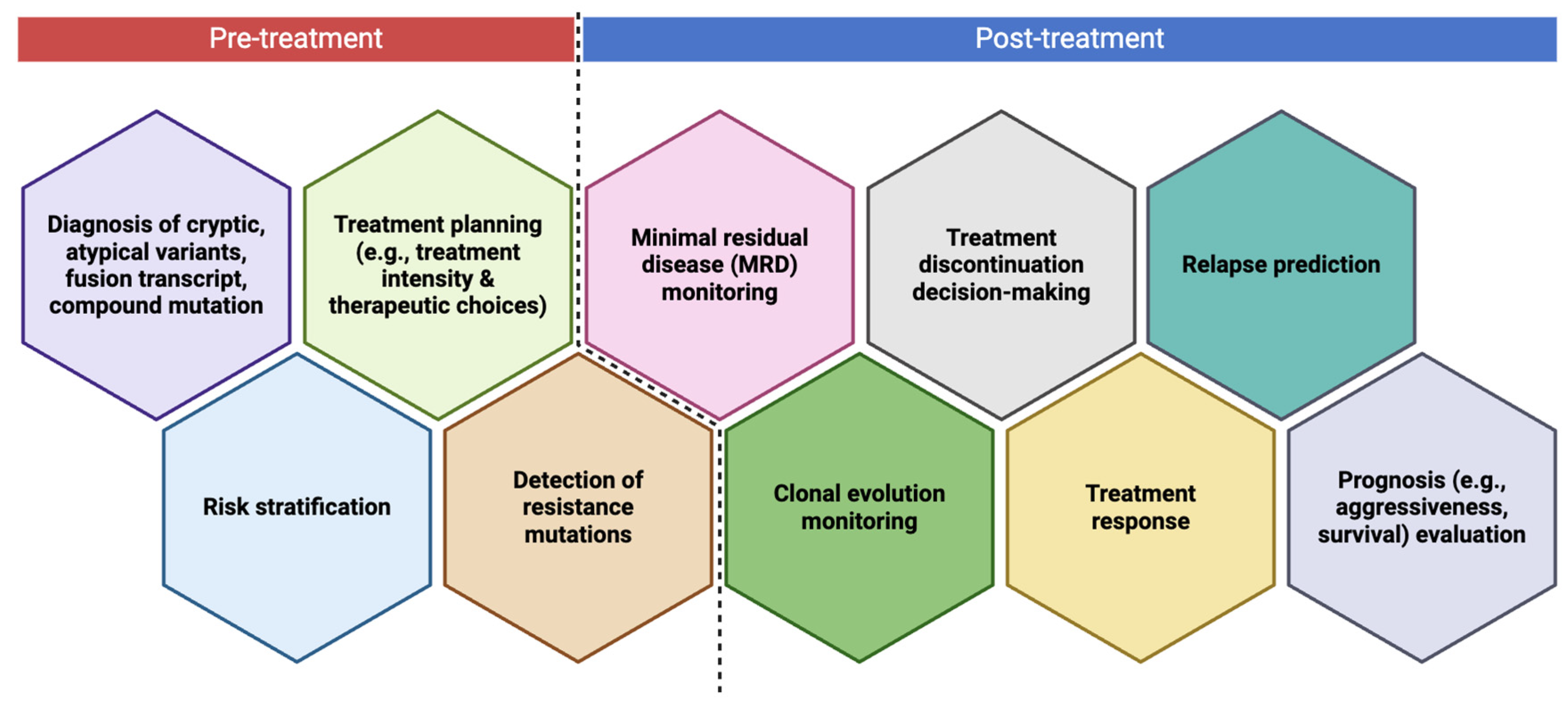
| Feature | Acute Lymphoblastic Leukemia (ALL) | Acute Myeloid Leukemia (AML) | Chronic Lymphocytic Leukemia (CLL) | Chronic Myeloid Leukemia (CML) |
|---|---|---|---|---|
| Cell of Origin | Lymphoid progenitor cells (B or T cells) | Myeloid progenitor cells | Mature B lymphocytes | Myeloid stem cells |
| Age Group Affected | Most common in children, but also occurs in adults | More common in adults, especially older than 60 years | Primarily affects older adults (>60 years) | Typically affects middle-aged adults (40–60 years) |
| Onset and Progression | Rapid onset and aggressive course | Rapid onset and aggressive course | Slow progression, often asymptomatic initially | Gradual progression, can transition to a blast crisis |
| Common Genetic Abnormalities | ETV6::RUNX1, IKZF1 deletions, CRLF2 rearrangements | FLT3, NPM1, TP53, IDH1/2, RUNX1 mutations | TP53, NOTCH1, SF3B1 mutations, del(13q14), trisomy 12 | BCR::ABL1 fusion (Philadelphia chromosome) |
| Symptoms | Fatigue, fever, bone pain, lymphadenopathy, bleeding | Fatigue, anemia, recurrent infections, bleeding | Lymphadenopathy, fatigue, hepatosplenomegaly | Fatigue, weight loss, splenomegaly |
| Diagnostic Methods | Bone marrow biopsy, flow cytometry, cytogenetics, PCR, NGS | Bone marrow biopsy, flow cytometry, cytogenetics, PCR, NGS | Peripheral blood smear, flow cytometry, FISH, NGS | PCR for BCR::ABL1, FISH, cytogenetics, NGS |
| Treatment Approaches | Chemotherapy, targeted therapy (TKIs for Ph+ ALL), immunotherapy, bone marrow transplant | Chemotherapy, targeted therapy (FLT3 inhibitors, IDH inhibitors), bone marrow transplant | BTK inhibitors (ibrutinib, acalabrutinib), BCL2 inhibitors (venetoclax), monoclonal antibodies (rituximab) | Tyrosine kinase inhibitors (TKIs) such as imatinib, nilotinib, and dasatinib |
| Prognosis | Highly variable; better in children, worse in adults | Prognosis depends on mutations and risk group; high-risk mutations have a poor prognosis | Generally good with targeted therapies, but incurable | Excellent long-term prognosis with TKIs; risk of transformation to blast crisis |
| Diagnostic Method | Acute Lymphoblastic Leukemia (ALL) | Acute Myeloid Leukemia (AML) | Chronic Lymphocytic Leukemia (CLL) | Chronic Myeloid Leukemia (CML) |
|---|---|---|---|---|
| Peripheral Blood Smear | Increased lymphoblasts, anemia, thrombocytopenia | Increased myeloblasts, Auer rods present | Increased mature lymphocytes, smudge cells | Increased myeloid precursors, left-shifted granulocytes |
| Bone Marrow Aspiration and Biopsy | Hypercellular marrow with >20% lymphoblasts | Hypercellular marrow with >20% myeloblasts | Not always required, but shows lymphocytic infiltration | Hypercellular marrow with granulocytic hyperplasia |
| Flow Cytometry | Identifies B-cell (CD19, CD22) or T-cell (CD3, CD7) markers | Identifies myeloid markers (CD13, CD33, CD117) | Confirms monoclonal B-cell population (CD5, CD19, CD23) | Not routinely needed, but can confirm granulocytic lineage |
| Cytogenetics and Karyotyping | Detects chromosomal translocations (ETV6::RUNX1, BCR::ABL1) | Identifies abnormalities like t(8;21), inv(16), or complex karyotypes | Detects chromosomal abnormalities like del(13q), trisomy 12 | Detects Philadelphia chromosome (t(9;22)) |
| Fluorescence In Situ Hybridization (FISH) | Confirms BCR::ABL1, MLL rearrangements | Detects RUNX1-RUNX1T1, PML::RARA fusions | Identifies TP53 deletion, ATM deletion | Detects BCR::ABL1 fusion gene in non-dividing cells using fluorescent probes. |
| Polymerase Chain Reaction (PCR) | Detects BCR::ABL1, ETV6::RUNX1, IKZF1 deletions | Identifies FLT3, NPM1, IDH1/2 mutations | Detects IGHV mutation, NOTCH1 mutations | Quantifies BCR::ABL1 transcript levels for monitoring minimal residual disease. |
| Next-Generation Sequencing (NGS) | Identifies risk-associated mutations (CRLF2, PAX5, IKZF1) | Detects multiple mutations for risk stratification (FLT3, TP53, DNMT3A) | Provides full mutational landscape (TP53, NOTCH1, SF3B1) | Comprehensively sequences genes to detect mutations, fusions, and clonal evolution. |
| Minimal Residual Disease (MRD) Monitoring * | Flow cytometry (10−4–10−5), PCR (10−5–10−6), NGS (10−6, targeted or error-corrected ultra-deep sequencing) | Flow cytometry (10−3–10−4), PCR (10−4–10−5), NGS (10−5–10−6, targeted deep sequencing) | PCR (10−4–10−5), NGS (10−5–10−6, error-corrected ultra-deep sequencing) | qPCR (10−5), NGS (10−5–10−6, targeted deep sequencing for BCR::ABL1 transcript monitoring) |
| NGS Type | Description | Coverage | Sensitivity | Advantages | Limitations | Common Applications in Leukemia |
|---|---|---|---|---|---|---|
| Whole-Genome Sequencing (WGS) | Sequences the entire genome, including coding and non-coding regions | Comprehensive | Moderate (10−3–10−5) | Detects all genetic variations, including structural variants and non-coding mutations | High cost, large data volume, complex interpretation | Research, discovery of novel mutations, understanding clonal evolution |
| Whole-Exome Sequencing (WES) | Sequences only the protein-coding regions (exome), which comprise ~1–2% of the genome | Protein-coding regions | High (10−4–10−5) | Cost-effective compared to WGS, detects clinically relevant mutations | Misses non-coding variants and structural abnormalities | Identifying actionable mutations in leukemia, risk stratification |
| Targeted Gene Panel Sequencing | Focuses on a preselected set of leukemia-associated genes | Limited to selected genes | Very high (10−5–10−6) | Cost-effective, high-depth, detects low-frequency mutations | Limited to known genes, cannot detect novel mutations | Routine clinical diagnostics, mutation-driven therapy selection, minimal residual disease (MRD) monitoring |
| RNA Sequencing (RNA-Seq) | Analyzes the transcriptome to measure gene expression and detect fusion genes | Coding and non-coding RNA | Moderate (10−3–10−5) | Detects gene fusions, alternative splicing, and expression changes | Requires high-quality RNA, can be influenced by degradation | Identifying leukemia-specific fusion genes (BCR::ABL1, ETV6::RUNX1), transcriptomic profiling |
| Single-Cell Sequencing (scRNA-Seq) | Analyzes genetic information from individual cells instead of bulk tissue | Single-cell resolution | High (10−4–10−5) | Identifies rare subclones, provides insight into tumor heterogeneity | Expensive, complex data interpretation requires specialized bioinformatics | Studying clonal evolution, therapy resistance, leukemia stem cell characterization |
| Error-Corrected Ultra-Deep Sequencing (Duplex Sequencing, UMI-Based Methods) | Uses unique molecular identifiers (UMIs) to correct sequencing errors, improving accuracy | Focused on selected mutations | Ultra-high (10−6–10−7) | Detects ultra-low-frequency mutations, useful for MRD detection | High cost, requires advanced bioinformatics | Highly sensitive MRD monitoring, tracking clonal evolution, resistance detection |
| Feature | Illumina | Oxford Nanopore | PacBio (SMRT Sequencing) |
|---|---|---|---|
| Sequencing Technology | Short-read sequencing (SBS—Sequencing by Synthesis) | Long-read sequencing using nanopores | Long-read sequencing using Single Molecule Real-Time (SMRT) technology |
| Read Length | Short reads (50–600 bp) | Ultra-long reads (up to >2 Mb) | Long reads (10–100 kb) |
| Accuracy | High (>99.9%) | Moderate (~90–98%) | High (~99.9%) |
| Error Profile | Low error rate, but struggles with structural variants and complex regions | Higher error rate, especially with homopolymers | Low error rate after consensus correction |
| Throughput | High (billions of reads per run) | Moderate (variable based on platform) | Moderate (lower than Illumina but higher than Nanopore) |
| Turnaround Time | Hours to days (depending on coverage) | Real-time sequencing (minutes to hours) | Hours to days |
| Cost per Base | Low ($) | Moderate ($$) | Higher than Illumina, but improving ($$$) |
| Best Applications in Leukemia | Targeted gene panels, whole-exome sequencing (WES), whole-genome sequencing (WGS), minimal residual disease (MRD) monitoring | Rapid real-time sequencing, structural variant detection, BCR::ABL1 fusion identification | Structural variant detection, full-length transcript sequencing, clonal evolution studies |
| Strengths | High accuracy, cost-effective for large-scale sequencing, widely used in clinical applications | Real-time data output, ultra-long reads enable full-length fusion gene detection | High accuracy for long reads, superior for phasing and detecting epigenetic modifications |
| Limitations | Short reads may miss large structural variants, complex rearrangements | Higher error rate requires error correction, lower throughput than Illumina | Expensive, lower throughput than Illumina, requires high DNA quality |
| Gene | Type of Mutation/Variant | Functional Impact | Clinical Significance |
|---|---|---|---|
| ETV6::RUNX1 | Chromosomal translocation t(12;21) | Aberrant transcriptional regulation | Associated with a favorable prognosis, common in pediatric ALL |
| BCR::ABL1 | Chromosomal translocation t(9;22) (Philadelphia chromosome) | Constitutive tyrosine kinase activation | Poor prognosis, treated with tyrosine kinase inhibitors (TKIs) |
| CRLF2 | Overexpression due to translocations or mutations | Activates JAK-STAT signaling | Enriched in Ph-like ALL, associated with high risk |
| IKZF1 | Deletions or point mutations | Loss of function in lymphoid differentiation | Poor prognosis, often co-occurs with Ph-like ALL |
| PAX5 | Point mutations, deletions, translocations | Disrupts B-cell differentiation | Frequently mutated in B-ALL |
| JAK1/JAK2 | Activating mutations | Constitutive cytokine signaling | Common in Ph-like ALL, potential target for JAK inhibitors |
| FLT3 | Internal tandem duplication (ITD) or point mutations | Increased tyrosine kinase activity | Associated with high-risk ALL, possible target for FLT3 inhibitors |
| RAS Pathway (NRAS, KRAS, PTPN11) | Point mutations | Hyperactive RAS signaling | Contributes to chemoresistance and relapse risk |
| NOTCH1 | Activating mutations | Dysregulation of T-cell development | Common in T-ALL, potential therapeutic target |
| FBXW7 | Loss-of-function mutations | Increased NOTCH1 signaling | Associated with T-ALL, linked to resistance to therapy |
| TP53 | Point mutations, deletions | Loss of tumor suppression | Poor prognosis, associated with therapy resistance |
| CDKN2A/CDKN2B | Deletions | Loss of cell cycle regulation | Common in both B-ALL and T-ALL, linked to poor prognosis |
| EP300, CREBBP | Loss-of-function mutations | Impaired histone acetylation and transcriptional regulation | Associated with resistance to corticosteroids and chemotherapy |
| Gene | Type of Mutation/Variant | Functional Impact | Clinical Significance |
|---|---|---|---|
| FLT3 | Internal tandem duplication (ITD) or tyrosine kinase domain (TKD) mutations | Increased tyrosine kinase activity | Poor prognosis, associated with high relapse risk, targetable with FLT3 inhibitors (midostaurin, gilteritinib) |
| NPM1 | Frameshift or insertion mutations | Aberrant cytoplasmic localization of nucleophosmin | Favorable prognosis in the absence of FLT3-ITD, common in younger AML patients |
| IDH1/IDH2 | Point mutations | Alters DNA methylation and metabolism via 2-HG production | Targetable with IDH inhibitors (ivosidenib for IDH1, enasidenib for IDH2) |
| DNMT3A | Loss-of-function mutations | Aberrant DNA methylation and epigenetic dysregulation | Poor prognosis, associated with clonal hematopoiesis and therapy resistance |
| TP53 | Point mutations, deletions | Loss of tumor suppression | Very poor prognosis, linked to therapy resistance and high relapse risk |
| RUNX1 | Loss-of-function mutations | Disrupts hematopoietic differentiation | Associated with therapy-related AML and poor prognosis |
| CEBPA | Biallelic mutations | Impaired myeloid differentiation | Favorable prognosis if present in biallelic form |
| KIT | Activating mutations | Increased proliferation via RAS/MAPK signaling | Common in core-binding factor AML, worsens prognosis despite favorable karyotype |
| TET2 | Loss-of-function mutations | Epigenetic dysregulation | Linked to clonal hematopoiesis, may contribute to resistance |
| ASXL1 | Truncating mutations | Disrupts chromatin remodeling | Poor prognosis, frequently found in secondary AML |
| WT1 | Frameshift or missense mutations | Defective tumor suppression | Associated with high relapse risk and poor prognosis |
| RAD21 | Loss-of-function mutations | Impaired cohesin complex function | Contributes to genomic instability and therapy resistance |
| SRSF2 | Splicing mutations | Abnormal RNA splicing | Common in secondary AML, often co-occurs with RUNX1 and ASXL1 mutations |
| BCOR/BCORL1 | Loss-of-function mutations | Disrupts transcriptional repression | Associated with poor prognosis and resistance to therapy |
| Gene | Type of Mutation/Variant | Functional Impact | Clinical Significance |
|---|---|---|---|
| TP53 | Point mutations, deletions (del(17p)) | Loss of tumor suppression, genomic instability | Poor prognosis, resistance to chemoimmunotherapy, requires BTK or BCL2 inhibitors |
| NOTCH1 | Frameshift or nonsense mutations | Constitutive activation of NOTCH signaling | Associated with Richter’s transformation, poor response to anti-CD20 therapy |
| SF3B1 | Splicing mutations | Aberrant RNA splicing, altered gene expression | Linked to poor prognosis, resistance to fludarabine |
| ATM | Deletions (del(11q)) or point mutations | Impaired DNA repair | Associated with disease progression, sensitivity to PARP inhibitors |
| BIRC3 | Loss-of-function mutations | Deregulated NF-κB signaling | Linked to resistance to chemoimmunotherapy, poor prognosis |
| MYD88 | Activating mutations (L265P) | Hyperactive B-cell receptor (BCR) signaling | More common in atypical CLL, associated with better prognosis |
| IGHV | Hypermutation status (mutated or unmutated) | Affects BCR signaling dependency | Unmutated IGHV associated with more aggressive disease and poor response to chemotherapy |
| XPO1 | Point mutations | Altered nuclear export of tumor suppressors | Associated with poor prognosis and therapy resistance |
| EGR2 | Missense mutations | Impaired B-cell differentiation | Enriched in aggressive and relapsed CLL cases |
| KRAS/NRAS | Activating mutations | Increased RAS/MAPK signaling | Rare in CLL but linked to Richter’s transformation |
| CARD11 | Gain-of-function mutations | Enhances BCR signaling | Associated with aggressive disease phenotypes |
| FBXW7 | Loss-of-function mutations | Disrupts ubiquitin-mediated degradation of oncogenic proteins | Contributes to therapy resistance and disease progression |
| DLEU2 | Deletions (part of del(13q)) | Loss of tumor suppressor miRNAs | Associated with favorable prognosis in isolated del(13q) cases |
| Gene/Fusion | Type of Mutation/Variant | Functional Impact | Clinical Significance |
|---|---|---|---|
| BCR::ABL1 (Canonical) | Chromosomal translocation t(9;22) (Philadelphia chromosome) | Constitutive tyrosine kinase activation | Hallmark of CML, targetable with tyrosine kinase inhibitors (TKIs) |
| BCR::ABL1 Kinase Domain Mutations | Point mutations (e.g., T315I, E255K, Y253H, F317L) | Resistance to TKIs | T315I mutation confers resistance to first- and second-generation TKIs, requiring ponatinib |
| Atypical BCR::ABL1 Fusions | Alternative breakpoints in BCR or ABL1 (e.g., e19a2, e1a2, e6a2) | Altered protein function and signaling activity | May affect TKI sensitivity and disease progression |
| Complex BCR::ABL1 Fusion Variants | Involvement of third or additional partner genes (e.g., BCR::ABL1-ETV6, BCR::ABL1-LAMP1) | Additional oncogenic effects | May confer aggressive disease behavior or altered treatment responses |
| ASXL1 | Loss-of-function mutations | Epigenetic dysregulation | Associated with disease progression and transformation to blast crisis |
| RUNX1 | Point mutations, deletions | Impaired hematopoietic differentiation | Frequently mutated in blast phase CML, poor prognosis |
| TET2 | Loss-of-function mutations | Alters DNA methylation and hematopoietic differentiation | May contribute to clonal evolution in advanced CML |
| SETBP1 | Gain-of-function mutations | Enhances leukemic cell proliferation | Associated with progression to blast crisis and poor outcomes |
| EZH2 | Loss-of-function mutations | Disrupts chromatin remodeling and gene silencing | Frequently mutated in advanced-phase CML, linked to aggressive disease |
| IKZF1 | Deletions or loss-of-function mutations | Loss of lymphoid differentiation control | Predicts transformation to the lymphoid blast phase of CML |
| NRAS/KRAS | Activating mutations | Increases RAS/MAPK signaling, promoting leukemic cell survival, proliferation, and resistance to apoptosis. | Associated with TKI resistance and progression to blast crisis |
| TP53 | Point mutations, deletions | Loss of tumor suppressor function | Poor prognosis, linked to disease progression and therapy resistance |
| DNMT3A | Loss-of-function mutations | Alters DNA methylation patterns | Implicated in clonal hematopoiesis and blast crisis transformation |
| EVI1 (MECOM) | Overexpression due to chromosomal rearrangements | Disrupts myeloid differentiation | Strongly associated with blast crisis and poor prognosis |
| Molecular Response (MR) * | BCR::ABL1 Transcript Level (IS) | Clinical Significance |
|---|---|---|
| MR1 | ≤10% | Indicates early response; goal at 3 months to predict long-term outcomes |
| MR2 | ≤1% | Optimal response at 6 months; associated with improved survival |
| MR3 (Major Molecular Response, MMR) | ≤0.1% | Standard treatment goal; associated with reduced risk of progression |
| MR4 | ≤0.01% | Indicates deep molecular remission (DMR); considered for treatment-free remission (TFR) evaluation |
| MR4.5 | ≤0.0032% | Deeper molecular response; increases the likelihood of successful TFR |
| MR5 | ≤0.001% | Undetectable disease by qPCR; associated with long-term remission and possible cure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pratiwi, L.; Mashudi, F.H.; Ningtyas, M.C.; Sutanto, H.; Romadhon, P.Z. Genetic Profiling of Acute and Chronic Leukemia via Next-Generation Sequencing: Current Insights and Future Perspectives. Hematol. Rep. 2025, 17, 18. https://doi.org/10.3390/hematolrep17020018
Pratiwi L, Mashudi FH, Ningtyas MC, Sutanto H, Romadhon PZ. Genetic Profiling of Acute and Chronic Leukemia via Next-Generation Sequencing: Current Insights and Future Perspectives. Hematology Reports. 2025; 17(2):18. https://doi.org/10.3390/hematolrep17020018
Chicago/Turabian StylePratiwi, Laras, Fawzia Hanum Mashudi, Mukti Citra Ningtyas, Henry Sutanto, and Pradana Zaky Romadhon. 2025. "Genetic Profiling of Acute and Chronic Leukemia via Next-Generation Sequencing: Current Insights and Future Perspectives" Hematology Reports 17, no. 2: 18. https://doi.org/10.3390/hematolrep17020018
APA StylePratiwi, L., Mashudi, F. H., Ningtyas, M. C., Sutanto, H., & Romadhon, P. Z. (2025). Genetic Profiling of Acute and Chronic Leukemia via Next-Generation Sequencing: Current Insights and Future Perspectives. Hematology Reports, 17(2), 18. https://doi.org/10.3390/hematolrep17020018






