Risk Factors for Impaired Glucose Metabolism in Transfusion-Dependent Patients with β-Thalassemia: A Single-Center Retrospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carsote, M.; Vasiliu, C.; Trandafir, A.I.; Albu, S.E.; Dumitrascu, M.C.; Popa, A.; Mehedintu, C.; Petca, R.C.; Petca, A.; Sandru, F. New Entity—Thalassemic Endocrine Disease: Major Beta-Thalassemia and Endocrine Involvement. Diagnostics 2022, 12, 1921. [Google Scholar] [CrossRef]
- Nienhuis, A.W.; Nathan, D.G. Pathophysiology and Clinical Manifestations of the β-Thalassemias. Cold Spring Harb. Perspect. Med. 2012, 2, a011726. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, V.; Soliman, A.; Daar, S.; Tzoulis, P.; Yassin, M.A.; Di Maio, S.; Kattamis, C. Insulin-like Growth Factor-1 (IGF-1) and Glucose Dysregulation in Young Adult Patients with β-Thalassemia Major: Causality or Potential Link? Acta Biomed. 2022, 93, e2022331. [Google Scholar] [CrossRef]
- Viprakasit, V.; Ekwattanakit, S. Clinical Classification, Screening and Diagnosis for Thalassemia. Hematol. Oncol. Clin. N. Am. 2018, 32, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Farmakis, D.; Porter, J.; Taher, A.; Cappellini, M.D.; Angastiniotis, M.; Eleftheriou, A.; Alassaf, A.; Angastiniotis, M.; Angelucci, E.; Aydinok, Y.; et al. 2021 Thalassaemia International Federation Guidelines for the Management of Transfusion-Dependent Thalassemia. Hemasphere 2022, 6, e732. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.-D.; Cohen, A.; Eleftheriou, A.; Piga, A.; Porter, J.; Taher, A. Chapter 2—Blood Transfusion Therapy in β-Thalassaemia Major. In Guidelines for the Clinical Management of Thalassaemia, 2nd Revised ed.; Thalassaemia International Federation: Nicosia, Cyprus, 2008. Available online: https://www.ncbi.nlm.nih.gov/books/nbk173967/ (accessed on 10 December 2023).
- De Sanctis, V.; Soliman, A.T.; Elsedfy, H.; Al Yaarubi, S.; Skordis, N.; Khater, D.; El Kholy, M.; Stoeva, I.; Fiscina, B.; Angastiniotis, M.; et al. The ICET-A Recommendations for the Diagnosis and Management of Disturbances of Glucose Homeostasis in Thalassemia Major Patients. Mediterr. J. Hematol. Infect. Dis. 2016, 8, e2016058. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, V.; Daar, S.; Soliman, A.T.; Tzoulis, P.; Karimi, M.; Di Maio, S.; Kattamis, C. Screening for Glucose Dysregulation in β-Thalassemia Major (β-TM): An Update of Current Evidences and Personal Experience. Acta Biomed. 2022, 93, e2022158. [Google Scholar] [CrossRef]
- Ricchi, P.; Meloni, A.; Pistoia, L.; Gamberini, M.R.; Cuccia, L.; Allo, M.; Putti, M.C.; Spasiano, A.; Rosso, R.; Cecinati, V.; et al. Longitudinal Prospective Comparison of Pancreatic Iron by Magnetic Resonance in Thalassemia Patients Transfusion-Dependent since Early Childhood Treated with Combination Deferiprone- Desferrioxamine vs Deferiprone or Deferasirox Monotherapy. Blood Transfus. 2024, 22. [Google Scholar] [CrossRef]
- Noetzli, L.J.; Coates, T.D.; Wood, J.C. Pancreatic Iron Loading in Chronically Transfused Sickle Cell Disease Is Lower than in Thalassaemia Major. Br. J. Haematol. 2011, 152, 229–233. [Google Scholar] [CrossRef] [PubMed]
- de Sanctis, V.; Soliman, A.; Tzoulis, P.; Daar, S.; Pozzobon, G.C.; Kattamis, C. A Study of Isolated Hyperglycemia (Blood Glucose ≥155 Mg/Dl) at 1-Hour of Oral Glucose Tolerance Test (OGTT) in Patients with β-Transfusion Dependent Thalassemia (β-TDT) Followed for 12 Years. Acta Biomed. 2021, 92, e2021322. [Google Scholar] [CrossRef]
- Karamanou, M. Milestones in the History of Diabetes Mellitus: The Main Contributors. World J. Diabetes 2016, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Skoczek, D.; Dulak, J.; Kachamakova-Trojanowska, N. Maturity Onset Diabetes of the Young—New Approaches for Disease Modelling. Int. J. Mol. Sci. 2021, 22, 7553. [Google Scholar] [CrossRef] [PubMed]
- Kaul, K.; Tarr, J.M.; Ahmad, S.I.; Kohner, E.M.; Chibber, R. Introduction to Diabetes Mellitus. In Diabetes: An Old Disease, a New Insight; Ahmad, S.I., Ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Caro, J.J.; Huybrechts, K.F.; Green, T.C. Estimates of the Effect on Hepatic Iron of Oral Deferiprone Compared with Subcutaneous Desferrioxamine for Treatment of Iron Overload in Thalassemia Major: A Systematic Review. BMC Hematol. 2002, 2, 4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meloni, A.; Pistoia, L.; Gamberini, M.R.; Ricchi, P.; Cecinati, V.; Sorrentino, F.; Cuccia, L.; Allò, M.; Righi, R.; Fina, P.; et al. The Link of Pancreatic Iron with Glucose Metabolism and Cardiac Iron in Thalassemia Intermedia: A Large, Multicenter Observational Study. J. Clin. Med. 2021, 10, 5561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Meng, Z.; Jiang, Z.; Liu, Z.; Hou, L.; Cai, G.; Ou, H.; Huang, S.; Song, Q.; Fang, J.; et al. Indicators of Glucose Dysregulation and the Relationship with Iron Overload in Chinese Children with Beta Thalassemia Major. Pediatr. Diabetes 2022, 23, 562–568. [Google Scholar] [CrossRef]
- El-Samahy, M.H.; Tantawy, A.A.; Adly, A.A.; Abdelmaksoud, A.A.; Ismail, E.A.; Salah, N.Y. Evaluation of Continuous Glucose Monitoring System for Detection of Alterations in Glucose Homeostasis in Pediatric Patients with β-Thalassemia Major. Pediatr. Diabetes 2019, 20, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Bazi, A.; Sharifi-Rad, J.; Rostami, D.; Sargazi-Aval, O.; Safa, A. Diabetes Mellitus in Thalassaemia Major Patients: A Report from the Southeast of Iran. J. Clin. Diagn. Res. 2017, 11, BC01–BC04. [Google Scholar] [CrossRef] [PubMed]
- Hafez, M.; Youssry, L.; El-Hamed, F.A.; Ibrahim, A. Abnormal Glucose Tolerance in Βthalassemia: Assessment of Risk Factors. Hemoglobin 2009, 33, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; Abd El-Fatah, A.H.; Abd El-Halim, A.F.; Mohamed, F.F. Serum Ferritin Levels and Other Associated Parameters with Diabetes Mellitus in Adult Patients Suffering from Beta Thalassemia Major. J. Blood Med. 2023, 14, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Chern, J.P.S.; Lin, K.-H.; Lu, M.-Y.; Lin, D.-T.; Lin, K.-S.; Chen, J.-D.; Fu, C.-C. Abnormal Glucose Tolerance in Transfusion-Dependent-Thalassemic Patients. Diabetes Care 2001, 24, 850–854. [Google Scholar] [CrossRef]
- Jaruratanasirikul, S.; Chareonmuang, R.; Wongcharnchailert, M.; Laosombat, V.; Sangsupavanich, P.; Leetanaporn, K. Prevalence of Impaired Glucose Metabolism in β-Thalassemic Children Receiving Hypertransfusions with a Suboptimal Dosage of Iron-Chelating Therapy. Eur. J. Pediatr. 2008, 167, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Farmaki, K.; Angelopoulos, N.; Anagnostopoulos, G.; Gotsis, E.; Rombopoulos, G.; Tolis, G. Effect of Enhanced Iron Chelation Therapy on Glucose Metabolism in Patients with β-Thalassaemia Major. Br. J. Haematol. 2006, 134, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Mashhadi, M.A.; Sepehri, Z.; Heidari, Z.; Kaykhaei, M.A.; Sargazi, A.; Kohan, F.; Heidari, H. A Cross-Sectional Study of Glycemic Status and Zinc Level in Patients with Beta-Thalassemia Major. Int. J. Hematol.-Oncol. Stem Cell Res. 2017, 11, 273. [Google Scholar] [PubMed]
- Suvarna, J.; Ingle, H.; Deshmukh, C.T. Insulin Resistance and Beta Cell Function in Chronically Transfused Patients of Thalassemia Major. Indian Pediatr. 2006, 43, 393. [Google Scholar] [PubMed]
- Chatterjee, R.; Bajoria, R. New Concept in Natural History and Management of Diabetes Mellitus in Thalassemia Major Diabetes and Thalassaemia. Hemoglobin 2009, 33 (Suppl. S1), S127–S130. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, V.; Soliman, A.T.; Daar, S.; Di Maio, S.; Elsedfy, H.; Kattamis, C. A Transfusion Dependent Thalassemia and HbA1c Assessment and Evaluation. Pediatr. Endocrinol. 2020, 17, 26–234. [Google Scholar] [CrossRef]
- Ke, P.; Liu, J.; Chao, Y.; Wu, X.; Xiong, Y.; Lin, L.; Wan, Z.; Wu, X.; Xu, J.; Zhuang, J.; et al. Measurement of HbA1c and HbA2 by Capillarys 2 Flex Piercing HbA1c Programme for Simultaneous Management of Diabetes and Screening for Thalassemia. Biochem. Med. 2017, 27, 030704. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.A.C.; Ehlert, L.R.; Camargo, J.L. Glycated Albumin: A Potential Biomarker in Diabetes. Arch. Endocrinol. Metab. 2017, 61, 296–304. [Google Scholar] [CrossRef]
- Candrarukmi, D.; Moelyo, A.G.; Riza, M. Glycated Albumin as Marker for Early Hyperglycemia Detection in Adolescent with β Thalassemia Major. Indones. Biomed. J. 2021, 13, 281–288. [Google Scholar] [CrossRef]
- Christoforidis, A.; Perifanis, V.; Athanassiou-Metaxa, M. Combined Chelation Therapy Improves Glucose Metabolism in Patients with β-Thalassaemia Major. Br. J. Haematol. 2006, 135, 271–272. [Google Scholar] [CrossRef] [PubMed]
- Pinto, V.M.; Bacigalupo, L.; Gianesin, B.; Balocco, M.; De Franceschi, L.; Malagò, R.; Wood, J.; Forni, G.L. Lack of Correlation between Heart, Liver and Pancreas MRI-R2*: Results from Long-Term Follow-up in a Cohort of Adult β-Thalassemia Major Patients. Am. J. Hematol. 2018, 93, E79–E82. [Google Scholar] [CrossRef]
- Farmaki, K.; Tzoumari, I.; Pappa, C.; Chouliaras, G.; Berdoukas, V. Normalisation of Total Body Iron Load with Very Intensive Combined Chelation Reverses Cardiac and Endocrine Complications of Thalassaemia Major. Br. J. Haematol. 2010, 148, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Paulo, M.S.; Abdo, N.M.; Bettencourt-Silva, R.; Al-Rifai, R.H. Gestational Diabetes Mellitus in Europe: A Systematic Review and Meta-Analysis of Prevalence Studies. Front. Endocrinol. 2021, 12, 691033. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Abbasi, A.; Adler, A.I. Gamma-Glutamyl Transferase and Risk of Type II Diabetes: An Updated Systematic Review and Dose-Response Meta-Analysis. Ann. Epidemiol. 2014, 24, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Nano, J.; Muka, T.; Ligthart, S.; Hofman, A.; Murad, S.D.; Janssen, H.L.A.; Franco, O.H.; Dehghan, A. Gamma-Glutamyltransferase Levels, Prediabetes and Type 2 Diabetes: A Mendelian Randomization Study. Int. J. Epidemiol. 2017, 46, 1400–1409. [Google Scholar] [CrossRef]
- Fraser, A.; Harris, R.; Sattar, N.; Ebrahim, S.; Smith, G.D.; Lawlor, D.A. Alanine Aminotransferase, γ-Glutamyltransferase, and Incident Diabetes. Diabetes Care 2009, 32, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.Y.; Fawzi, M.; Al-Maloul, S.R.; El-Banna, N.; Tayyem, R.F.; Ahmad, I.M. Increased Oxidative Stress and Iron Overload in Jordanian β-Thalassemic Children. Hemoglobin 2011, 35, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Fibach, E.; Dana, M. Oxidative Stress in β-Thalassemia. Mol. Diagn. Ther. 2019, 23, 245–261. [Google Scholar] [CrossRef]
- Zhang, P.; Li, H.; Tan, X.; Chen, L.; Wang, S. Association of Metformin Use with Cancer Incidence and Mortality: A Meta-Analysis. Cancer Epidemiol. 2013, 37, 207–218. [Google Scholar] [CrossRef]
- Casale, M.; Cinque, P.; Ricchi, P.; Costantini, S.; Spasiano, A.; Prossomariti, L.; Minelli, S.; Frega, V.; Filosa, A. Effect of Splenectomy on Iron Balance in Patients with β-Thalassemia Major: A Long-Term Follow-Up. Eur. J. Haematol. 2013, 91, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Kosaryan, M.; Rahimi, M.; Darvishi-Khezri, H.; Gholizadeh, N.; Akbarzadeh, R.; Aliasgharian, A. Correlation of Pancreatic Iron Overload Measured by T2*-Weighted Magnetic Resonance Imaging in Diabetic Patients with β-Thalassemia Major. Hemoglobin 2017, 41, 151–156. [Google Scholar] [CrossRef]
- Hashemieh, M.; Radfar, M.; Azarkeivan, A.; Noghabaei, G.; Sheibani, K. T2* Magnetic Resonance Imaging Study of Pancreatic Iron Overload and Its Relation With the Diabetic State in Thalassemic Patients. J. Pediatr. Hematol./Oncol. 2017, 39, 337–340. [Google Scholar] [CrossRef]
- Pepe, A.; Pistoia, L.; Gamberini, M.R.; Cuccia, L.; Peluso, A.; Messina, G.; Spasiano, A.; Allò, M.; Bisconte, M.G.; Putti, M.C.; et al. The Close Link of Pancreatic Iron with Glucose Metabolism and with Cardiac Complications in Thalassemia Major: A Large, Multicenter Observational Study. Diabetes Care 2020, 43, 2830–2839. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.T.; Cappellini, M.D. How I Treat Sickle Cell Disease and Thalassemia: How I Manage Medical Complications of B-Thalassemia in Adults. Blood 2018, 132, 1781–1791. [Google Scholar] [CrossRef]
- Fularska, K.; Oleszko, M.; Wąsiewicz, E.; Kuźniar, A.; Szawica, D. Beta-Blockers Used in Cardiac Failure and Blood Glucose Level Impairment—A Literature Review. J. Educ. Health Sport 2023, 23, 40–51. [Google Scholar] [CrossRef]


| SF (ng/mL) | Cardiac MRI T2* (ms) | Hepatic MRI T2* (ms) | |
|---|---|---|---|
| Normal | <1000 | >20 | >8 |
| Mild | 1000–2000 | 14–20 | 4–8 |
| Moderate | 2000–4000 | 10–14 | 2–4 |
| Severe | >4000 | <10 | <2 |
| Groups | ||
|---|---|---|
| Group 1 | 46/64 (71.8%) | |
| Group 2 | 18/64 (28.1%) | Diabetes Mellitus: 8/64 (12.5%) |
| Impaired Glucose Tolerance: 10/64 (15.6%) | ||
| Normal Distribution: Mean (SD) Non-Normal Distribution: Media (IQR) | p-Value | ||
|---|---|---|---|
| Group 1 | Group 2 | ||
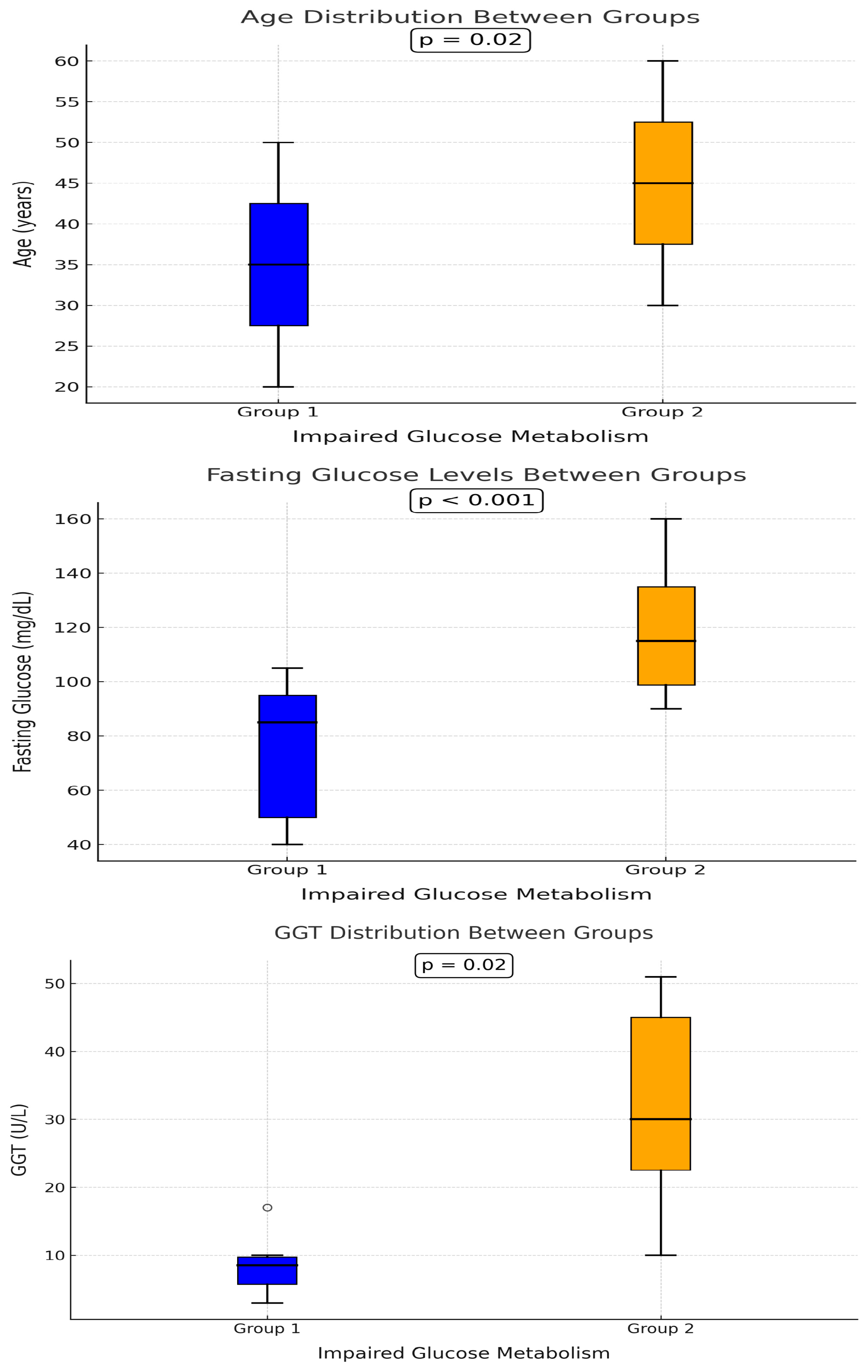
| Age | 39 (9) | 45 (10) | 0.02 |
| Body weight | 65 (18) | 72 (20) | 0.9 |
| Hemoglobin | 9.8 (0.5) | 9.8 (0.7) | 0.82 |
| Annual transfused Blood volume | 10,453 (3336) | 9793 (2342) | 0.48 |
| WBC | 9300 (7900) | 12,300 (7770) | 0.38 |
| NEU | 5258 (2713) | 5822 (3675) | 0.74 |
| LYM | 2896 (1871) | 3233 (1843) | 0.4 |
| MON | 770 (860) | 690 (635) | 0.9 |
| PLT | 421,520 (235,700) | 455,100 (237,210) | 0.5 |
| GLU | 88 (10) | 104 (42) | <0.001 |
| Urea | 39.3 (9) | 39.8 (10) | 0.93 |
| Serum creatinine | 0.8 (0.16) | 0.83 (0.2) | 0.6 |
| LDH | 233 (111) | 238 (62) | 0.99 |
| Total bil | 1.93 (1.17) | 1.63 (1.6) | 0.34 |
| Direct bil | 0.4 (0.32) | 0.27 (0.23) | 0.63 |
| Indirect bil | 1.55 (1.1) | 1.3 (1.25) | 0.2 |
| GGT | 14 (8) | 22 (18) | 0.02 |
| AST | 19 (19) | 17 (36) | 0.6 |
| ALT | 21 (13) | 20 (17) | 0.7 |
| Amylase | 54 (24) | 45 (28) | 0.98 |
| CRP | 1.7 (2.8) | 1.9 (3.8) | 0.4 |
| MRI—liver | 11.2 (9.6) | 8 (9) | 0.7 |
| LIC | 2.4 (2.35) | 3.3 (4.5) | 0.82 |
| MRI—heart | 35.3 (5) | 33 (7) | 0.41 |
| EF | 62.3 (5.6) | 61.9 (4.5) | 0.96 |
| Serum ferritin | 720 (506) | 620 (620) | 0.36 |
| Treatment | YES | NO | |
|---|---|---|---|
| Hormonal replacement therapy | 16 (25%) | 48 (75%) | 0.75 |
| Corticosteroids | 2 (3.1%) | 62 (96.9%) | 0.49 |
| Beta-blocker | 13 (20.3%) Group 1:6 Group 2:7 | 51 (79.7%) Group 1:40 Group 2:11 | 0.02 |
| Zinc | 14 (21.9%) | 50 (78.1%) | 0.96 |
| n | 64 | 64 |
| Hypothyroidism | HCV Infection | Splenectomy | |
|---|---|---|---|
| Group 1 | Present: 7 (15.2%) Absent: 39 (84.7%) | Present: 8 (17.3%) Absent: 38 (82.6%) | Positive: 18 (39.1%) Negative: 28 (60.8%) |
| Group 2 | Present: 6 (33.3%) Absent: 12 (66.6%) | Present: 5 (27.7%) Absent: 13 (72.2%) | Positive: 10 (55.5%) Negative: 8 (44.4%) |
| p | 0.1 | 0.35 | 0.23 |
| Variables (Univariate Logistic Regression Analysis) | p-Value |
|---|---|
| Age | 0.03 |
| Gender | 0.63 |
| Blood volume (per year) | 0.45 |
| Blood type | 0.66 |
| Hemoglobin | 0.86 |
| Urea | 0.85 |
| Creatinine | 0.53 |
| Neutrophils | 0.71 |
| Lymphocytes | 0.51 |
| Monocytes | 0.9 |
| Glu | <0.01 |
| LDH | 0.52 |
| Total bilirubin | 0.32 |
| Direct bilirubin | 0.62 |
| Indirect bilirubin | 0.36 |
| GGT | 0.05 |
| AST | 0.11 |
| ALT | 0.11 |
| Amylase | 0.8 |
| CPK | 0.21 |
| CRP | 0.2 |
| Type of iron chelation | 0.33 |
| Liver MRI T2* | 0.48 |
| Heart MRI T2* | 0.15 |
| LIC | 0.72 |
| EF | 0.76 |
| Hormonal replacement therapy | 0.74 |
| Beta-blocker | 0.03 |
| Corticosteroids | 0.5 |
| Zinc | 0.96 |
| HCV infection | 0.35 |
| Splenectomy | 0.23 |
| Serum Ferritin | 0.43 |
| Variables (Multivariate Logistic Regres-Sion Analysis) | p-Value | OR | 95% CI |
|---|---|---|---|
| Age | 0.16 | 1 | 0.97–1.1 |
| ALT | 0.71 | 0.98 | 0.91–1.06 |
| AST | 0.64 | 1 | 0.96–1.05 |
| GGT | 0.14 | 1 | 0.99–1.07 |
| Heart MRI T2* | 0.19 | 0.9 | 0.81–1.04 |
| Beta-blocker | 0.18 | 2.4 | 0.63–1.7 |
| Pancreatic MRI T2* | Pearson Coefficient | p-Value |
|---|---|---|
| Liver MRI T2* | −0.27 | 0.24 |
| Heart MRI T2* | 0.45 | 0.04 |
| LIC | 0.16 | 0.49 |
| Fasting serum glucose (mg/dl) | −0.33 | 0.15 |
| Serum ferritin (μg/L) | −0.05 | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venou, T.M.; Kyriakidis, F.; Barmpageorgopoulou, F.; Theodoridou, S.; Vyzantiadis, A.; Klonizakis, P.; Gavriilaki, E.; Vlachaki, E. Risk Factors for Impaired Glucose Metabolism in Transfusion-Dependent Patients with β-Thalassemia: A Single-Center Retrospective Observational Study. Hematol. Rep. 2025, 17, 6. https://doi.org/10.3390/hematolrep17010006
Venou TM, Kyriakidis F, Barmpageorgopoulou F, Theodoridou S, Vyzantiadis A, Klonizakis P, Gavriilaki E, Vlachaki E. Risk Factors for Impaired Glucose Metabolism in Transfusion-Dependent Patients with β-Thalassemia: A Single-Center Retrospective Observational Study. Hematology Reports. 2025; 17(1):6. https://doi.org/10.3390/hematolrep17010006
Chicago/Turabian StyleVenou, Theodora Maria, Filippos Kyriakidis, Fani Barmpageorgopoulou, Stamatia Theodoridou, Athanasios Vyzantiadis, Philippos Klonizakis, Eleni Gavriilaki, and Efthymia Vlachaki. 2025. "Risk Factors for Impaired Glucose Metabolism in Transfusion-Dependent Patients with β-Thalassemia: A Single-Center Retrospective Observational Study" Hematology Reports 17, no. 1: 6. https://doi.org/10.3390/hematolrep17010006
APA StyleVenou, T. M., Kyriakidis, F., Barmpageorgopoulou, F., Theodoridou, S., Vyzantiadis, A., Klonizakis, P., Gavriilaki, E., & Vlachaki, E. (2025). Risk Factors for Impaired Glucose Metabolism in Transfusion-Dependent Patients with β-Thalassemia: A Single-Center Retrospective Observational Study. Hematology Reports, 17(1), 6. https://doi.org/10.3390/hematolrep17010006







