The Role of Ferritin and Folate in Determining Stem Cell Collection for Autologous Stem Cell Transplant in Multiple Myeloma
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Mobilization
2.3. Clinical Laboratory Values
2.4. Statistical Analysis
3. Results
3.1. Baseline Demographics, Platelet Counts, Anemia, and B12
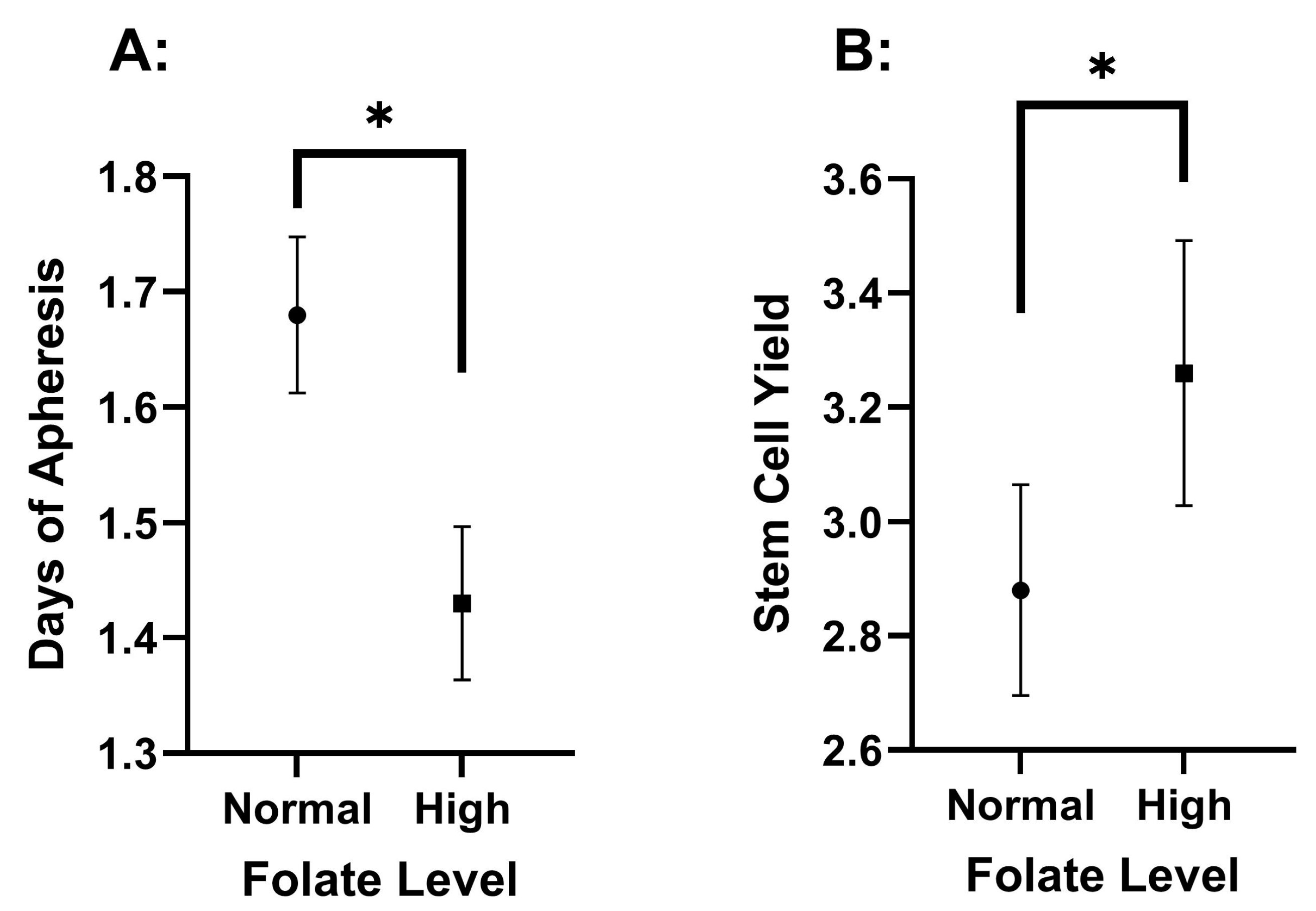
3.2. Differences Among Pre-Collection Folate Serum Concentrations
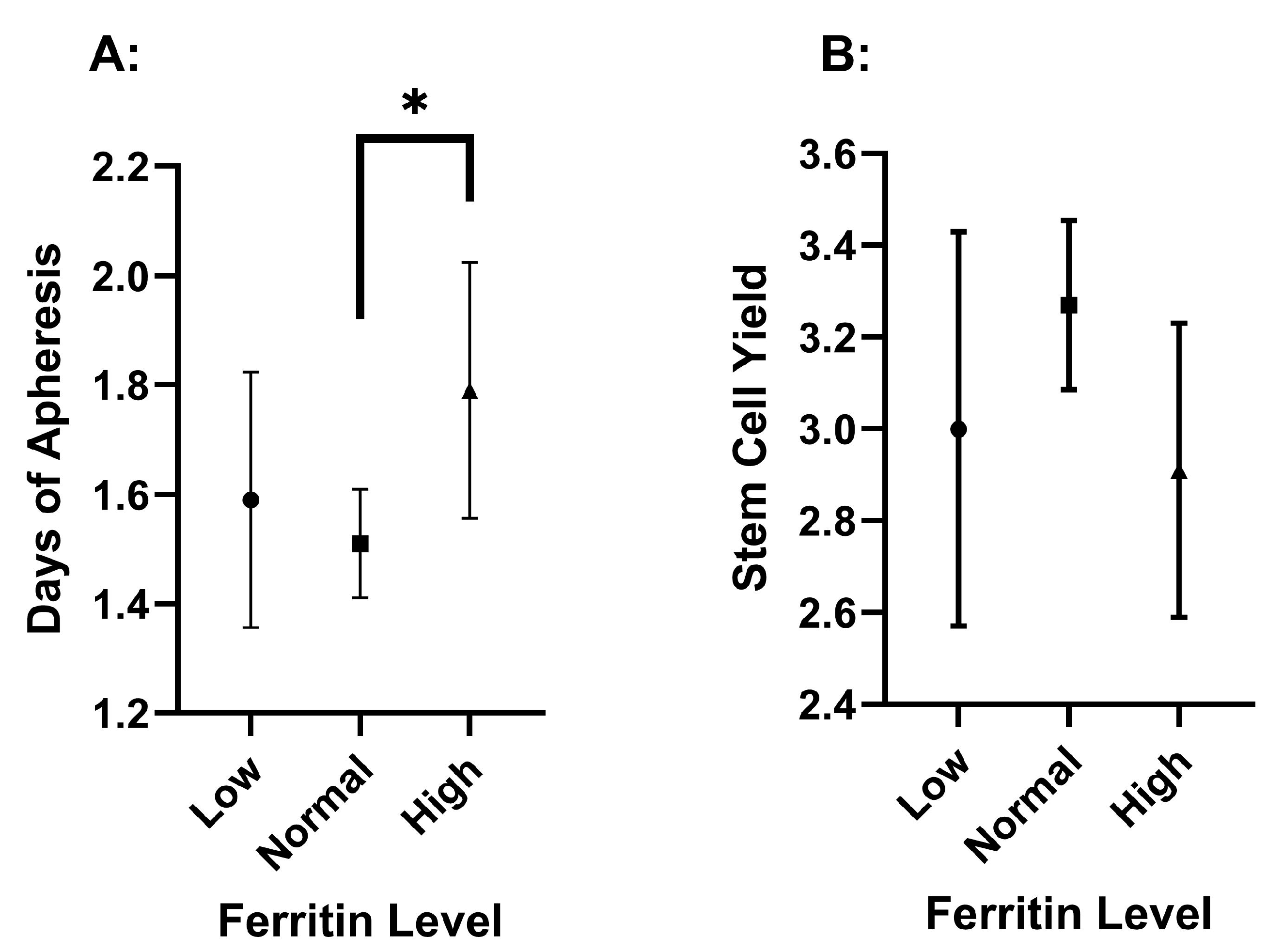
3.3. Differences Among Pre-Collection Ferritin Serum Concentrations
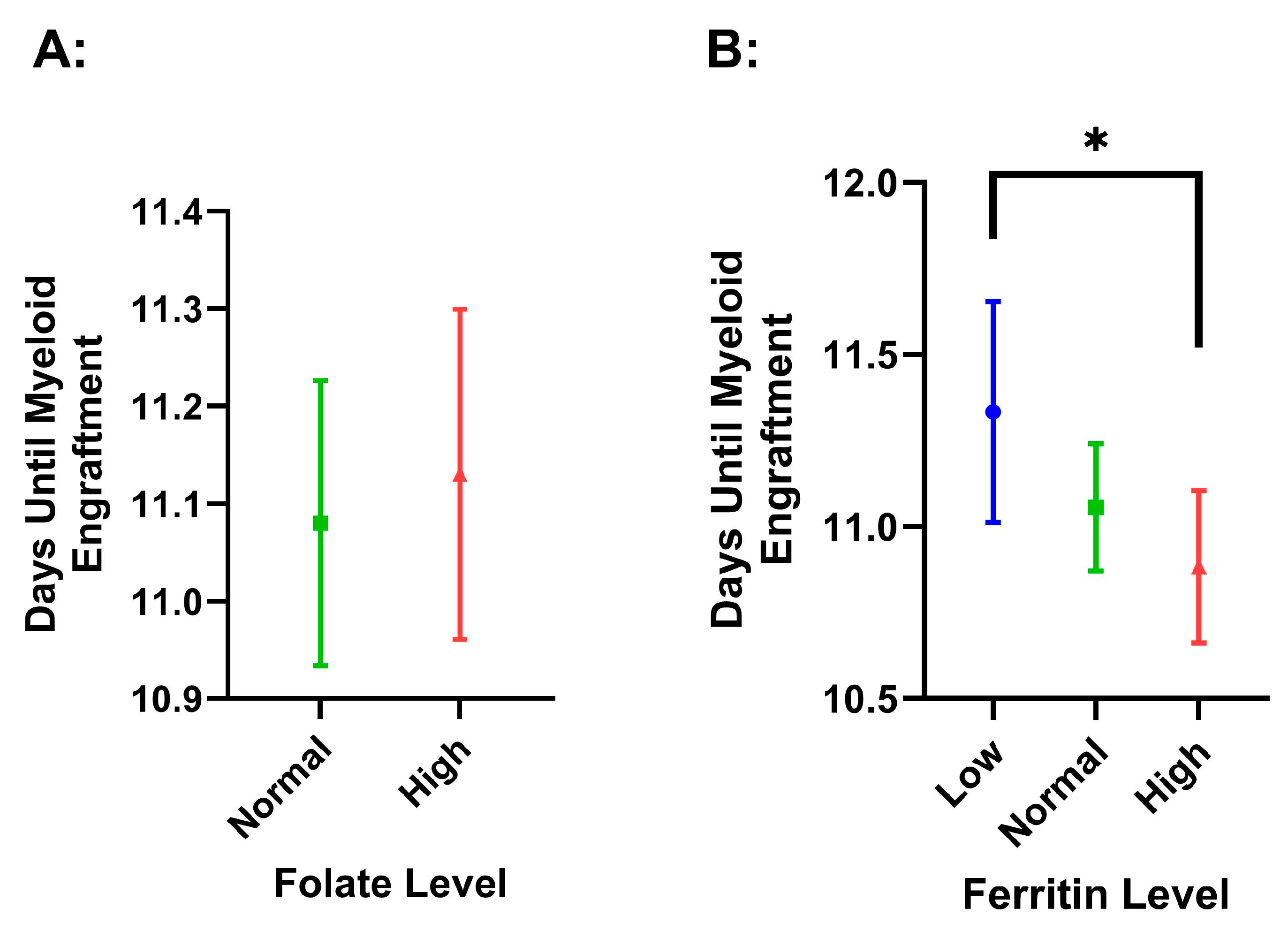
3.4. Survival and Engraftment Differences Among Pre-Collection Ferritin and Folate Concentrations
4. Discussion
4.1. The Association of Elevated Folate with Improved Mobilization
4.2. The Association of Abnormal Ferritin with Poorer Mobilization
4.3. Other Factors That May Influence Mobilization
4.4. Engraftment and Survival
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The International Myeloma Working Group. Criteria for the Classification of Monoclonal Gammopathies, Multiple Myeloma and Related Disorders: A Report of the International Myeloma Working Group. Br. J. Haematol. 2003, 121, 749–757. [Google Scholar] [CrossRef]
- Kyle, R.A.; Rajkumar, S.V. Multiple Myeloma. N. Engl. J. Med. 2004, 351, 1860–1873. [Google Scholar] [CrossRef] [PubMed]
- Myeloma—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/mulmy.html (accessed on 2 November 2024).
- Rajkumar, S.V. Multiple Myeloma: 2022 Update on Diagnosis, Risk Stratification, and Management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef] [PubMed]
- Bühler, S.; Akhoundova, D.; Jeker, B.; Legros, M.; Seipel, K.; Daskalakis, M.; Bacher, U.; Pabst, T. Stem Cell Mobilization with Ixazomib and G-CSF in Patients with Multiple Myeloma. Cancers 2023, 15, 430. [Google Scholar] [CrossRef]
- Özkurt, Z.N.; Batmaz, L.; Yeğin, Z.A.; İlhan, Ç. Factors Affecting Hematopoietic Stem Cell Mobilization and Apheresis in Allogeneic Donors: The Role of Iron Status. Transfus. Apher. Sci. Off. J. World Apher. Assoc. Off. J. Eur. Soc. Haemapheresis 2017, 56, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, S.; Wang, Y.; Lu, M.; Chu, B.; Shi, L.; Xiang, Q.; Fang, L.; Ding, Y.; Wang, M.; et al. Pre-Mobilization Platelet Count Predicts Stem Cell Yield during Mobilization in Patients with Multiple Myeloma. Cancer Pathog. Ther. 2022, 1, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Kamezaki, K.; Miyamoto, T.; Henzan, T.; Numata, A.; Iwasaki, H.; Nagafuji, K.; Harada, M.; Teshima, T.; Akashi, K. Collection of Mobilized Peripheral Blood Stem Cells from a Donor with Severe Iron Deficient Anemia. J. Clin. Apher. 2007, 22, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Yiğenoğlu, T.N.; Başcı, S.; Bakırtaş, M.; Ulu, B.U.; Kılınç, A.; Şahin, D.; Darçın, T.; Yıldız, J.; Merdin, A.; Baysal, N.A.; et al. The Effect of Serum Vitamin B12, Folate, Ferritin Levels and Transferrin Saturation on Stem Cell Mobilization in Allogeneic Donors. Transfus. Apher. Sci. 2020, 59, 102726. [Google Scholar] [CrossRef]
- Hopman, R.K.; DiPersio, J.F. Advances in Stem Cell Mobilization. Blood Rev. 2014, 28, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Salman, H.; Lazarus, H.M. Plerixafor for Autologous CD34+ Cell Mobilization. Core Evid. 2011, 6, 23–29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koulaouzidis, A.; Cottier, R.; Bhat, S.; Said, E.; Linaker, B.D.; Saeed, A.A. A Ferritin Level > 50 Μg/L Is Frequently Consistent with Iron Deficiency. Eur. J. Intern. Med. 2009, 20, 168–170. [Google Scholar] [CrossRef]
- Yoh, K.A.; Lee, H.S.; Park, L.C.; Lee, E.M.; Shin, S.H.; Park, D.J.; Ye, B.J.; Kim, Y.S. The Prognostic Significance of Elevated Levels of Serum Ferritin before Chemotherapy in Patients with Non-Hodgkin Lymphoma. Clin. Lymphoma Myeloma Leuk. 2014, 14, 43–49. [Google Scholar] [CrossRef]
- Yokus, O.; Sunger, E.; Cinli, T.A.; Goze, H.; Serin, I. Serum Albumin and Ferritin Levels: A Practical Indicator of Prognosis in Acute Myeloid Leukemia over 50 Years of Age? Am. J. Blood Res. 2022, 12, 97–104. [Google Scholar] [PubMed]
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.C.; Ivashkin, V. The Concept of Folic Acid in Health and Disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef] [PubMed]
- Hanna, S.; Lachover, L.; Rajarethinam, R.P. Vitamin B12 Deficiency and Depression in the Elderly: Review and Case Report. Prim. Care Companion J. Clin. Psychiatry 2009, 11, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Wolters, M.; Ströhle, A.; Hahn, A. Cobalamin: A Critical Vitamin in the Elderly. Prev. Med. 2004, 39, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Giles, C. The Platelet Count and Mean Platelet Volume. Br. J. Haematol. 1981, 48, 31–37. [Google Scholar] [CrossRef]
- Warner, M.A.; Hanson, A.C.; Frank, R.D.; Schulte, P.J.; Go, R.S.; Storlie, C.B.; Kor, D.J. Prevalence of and Recovery From Anemia Following Hospitalization for Critical Illness Among Adults. JAMA Netw. Open 2020, 3, e2017843. [Google Scholar] [CrossRef] [PubMed]
- Anemia. Available online: https://www.hematology.org/education/patients/anemia (accessed on 3 January 2025).
- Zarou, M.M.; Vazquez, A.; Vignir Helgason, G. Folate Metabolism: A Re-Emerging Therapeutic Target in Haematological Cancers. Leukemia 2021, 35, 1539–1551. [Google Scholar] [CrossRef]
- Bills, N.; Koury, M.; Clifford, A.; Dessypris, E. Ineffective Hematopoiesis in Folate-Deficient Mice. Blood 1992, 79, 2273–2280. [Google Scholar] [CrossRef]
- Berardi, A.; Geraci, L.; Quaglietta, A.M.; Di Bartolomeo, G.; Dragani, A. Pharmacological Mobilization of Haemopoietic Progenitor Cells in Human Peripheral Blood. Haematologica 1990, 75 (Suppl. S1), 15–17. [Google Scholar] [PubMed]
- Zabun, M.M.; Köksal, Y.; Çelik, B.; Özgüner, M.; Özbek, N.Y. Effects of Iron and Vitamin B12 Deficiencies on Peripheral Blood Colony-Forming Unit Capacity. Pediatr. Transplant. 2018, 22, e13144. [Google Scholar] [CrossRef] [PubMed]
- Koreth, J.; Antin, J.H. Iron Overload in Hematologic Malignancies and Outcome of Allogeneic Hematopoietic Stem Cell Transplantation. Haematologica 2010, 95, 364–366. [Google Scholar] [CrossRef]
- Maradei, S.C.; Maiolino, A.; de Azevedo, A.M.; Colares, M.; Bouzas, L.F.; Nucci, M. Serum Ferritin as Risk Factor for Sinusoidal Obstruction Syndrome of the Liver in Patients Undergoing Hematopoietic Stem Cell Transplantation. Blood 2009, 114, 1270–1275. [Google Scholar] [CrossRef]
- Pullarkat, V.; Blanchard, S.; Tegtmeier, B.; Dagis, A.; Patane, K.; Ito, J.; Forman, S.J. Iron Overload Adversely Affects Outcome of Allogeneic Hematopoietic Cell Transplantation. Bone Marrow Transplant. 2008, 42, 799–805. [Google Scholar] [CrossRef]
- Mahindra, A.; Sobecks, R.; Rybicki, L.; Pohlman, B.; Dean, R.; Andresen, S.; Kalaycio, M.; Sweetenham, J.; Bolwell, B.; Copelan, E. Elevated Pretransplant Serum Ferritin Is Associated with Inferior Survival Following Nonmyeloablative Allogeneic Transplantation. Bone Marrow Transplant. 2009, 44, 767–768. [Google Scholar] [CrossRef]
- Park, I.H.; Kim, Y.; Kim, J.S.; Cheong, J.-W.; Song, J.W.; Min, Y.H. Transfusion-Associated Iron Overload as a Predictive Factor for Poor Stem Cell Mobilization in Patients with Haematological Malignancies. Transfus. Med. Oxf. Engl. 2008, 18, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Alva, L.C.; Bacher, U.; Seipel, K.; Mansouri Taleghani, B.; Mueller, B.U.; Novak, U.; Pabst, T. Iron Overload Is Correlated with Impaired Autologous Stem Cell Mobilization and Survival in Acute Myeloid Leukemia. Transfusion 2018, 58, 2365–2373. [Google Scholar] [CrossRef]
- Sevilla, J.; Navarro, S.; Rio, P.; Sánchez-Domínguez, R.; Zubicaray, J.; Gálvez, E.; Merino, E.; Sebastián, E.; Azqueta, C.; Casado, J.A.; et al. Improved Collection of Hematopoietic Stem Cells and Progenitors from Fanconi Anemia Patients for Gene Therapy Purposes. Mol. Ther. Methods Clin. Dev. 2021, 22, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Baertsch, M.-A.; Kriegsmann, K.; Pavel, P.; Bruckner, T.; Hundemer, M.; Kriegsmann, M.; Ho, A.D.; Goldschmidt, H.; Wuchter, P. Platelet Count before Peripheral Blood Stem Cell Mobilization Is Associated with the Need for Plerixafor But Not with the Collection Result. Transfus. Med. Hemotherapy 2018, 45, 24–31. [Google Scholar] [CrossRef]
- Zhu, J.; Emerson, S.G. Hematopoietic Cytokines, Transcription Factors and Lineage Commitment. Oncogene 2002, 21, 3295–3313. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.L.; Siegel, E.; Barlogie, B.; Cottler-Fox, M.; Lin, P.; Fassas, A.; Zangari, M.; Anaissie, E.; Tricot, G. Mobilization of CD34+ Cells in Elderly Patients (>/= 70 Years) with Multiple Myeloma: Influence of Age, Prior Therapy, Platelet Count and Mobilization Regimen. Br. J. Haematol. 2003, 120, 413–423. [Google Scholar] [CrossRef]
- Sancho, J.-M.; Morgades, M.; Grifols, J.-R.; Juncà, J.; Guardia, R.; Vives, S.; Ferrà, C.; Batlle, M.; Ester, A.; Gallardo, D.; et al. Predictive Factors for Poor Peripheral Blood Stem Cell Mobilization and Peak CD34(+) Cell Count to Guide Pre-Emptive or Immediate Rescue Mobilization. Cytotherapy 2012, 14, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, X.; Rosenbaum, E.; Tyler, L.; Sawyer, J.; Heuck, C.; Barlogie, B.; Cottler-Fox, M. Hematopoietic Progenitor Cell Collection after Autologous Transplant for Multiple Myeloma: Low Platelet Count Predicts for Poor Collection and Sole Use of Resulting Graft Enhances Risk of Myelodysplasia. Leukemia 2014, 28, 888–893. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nishimura, K.K.; Barlogie, B.; van Rhee, F.; Zangari, M.; Walker, B.A.; Rosenthal, A.; Schinke, C.; Thanendrarajan, S.; Davies, F.E.; Hoering, A.; et al. Long-Term Outcomes after Autologous Stem Cell Transplantation for Multiple Myeloma. Blood Adv. 2020, 4, 422–431. [Google Scholar] [CrossRef]


| Low | Normal | High | p-Value | |
|---|---|---|---|---|
| Folate | n = 0 | n = 146 | n = 84 | |
| DOA | 1.68 ± 0.82 | 1.43 ± 0.61 | 0.013 * | |
| FC | 2.88 ± 1.13 | 3.26 ± 1.07 | 0.013 * | |
| B12 | n = 23 | n = 184 | n = 45 | |
| DOA | 1.48 ± 0.73 | 1.61 ± 0.76 | 1.58 ± 0.78 | 0.735 |
| FC | 2.84 ± 1.29 | 3.41 ± 1.66 | 3.41 ± 1.66 | 0.151 |
| Platelet | n = 38 | n = 246 | n = 8 | |
| DOA | 1.68 ± 0.70 | 1.56 ± 0.74 | 1.50 ± 0.76 | 0.605 |
| FC | 2.89 ± 1.19 | 3.20 ± 1.30 | 3.31 ± 1.63 | 0.364 |
| Ferritin | n = 41 | n = 193 | n = 58 | |
| DOA | 1.59 ± 0.74 | 1.51 ± 0.70 | 1.79 ± 0.89 | 0.034 * |
| FC | 3.00 ± 1.36 | 3.27 ± 1.30 | 2.91 ± 1.22 | 0.131 |
| Anemia Grade | Normal | Mild | Moderate | p-Value |
| n = 83 | n = 167 | n = 40 | ||
| DOA | 1.57 ± 0.74 | 1.51 ± 0.69 | 1.85 ± 0.83 | 0.030 * |
| FC | 3.38 ± 1.51 | 3.17 ± 1.18 | 2.70 ± 1.17 | 0.023 * |
| Normal | High | p-Value | |
|---|---|---|---|
| n = 146 | n = 84 | ||
| Sex | 0.676 | ||
| Male | 81 (55.5%) | 49 (58.3%) | |
| Female | 65 (44.5%) | 35 (41.7%) | |
| Race | 0.306 | ||
| White | 55 (37.7%) | 42 (50.0%) | |
| Black | 90 (61.6%) | 40 (47.6%) | |
| Other | 1 (0.7%) | 2 (2.4%) | |
| Age | 60.9 ± 9.5 | 63.6 ± 9.7 | 0.039 * |
| Ferritin | 341 ± 378 | 288 ± 350 | 0.288 |
| B12 | 696 ± 1069 | 683 ± 754 | 0.928 |
| Hgb | 11.6 ± 1.8 | 12.1 ± 1.6 | 0.019 * |
| Plerixafor | 1.12 ± 0.73 | 0.95 ± 0.74 | 0.082 |
| Platelet | 228 ± 76 | 228 ± 73 | 0.997 |
| Low | Normal | High | p-Value | |
|---|---|---|---|---|
| n = 41 | n = 193 | n = 58 | ||
| Sex | 0.206 | |||
| Male | 21 (51.2%) | 114 (59.1%) | 27 (46.6.0%) | |
| Female | 20 (48.8%) | 79 (40.9%) | 31 (53.4%) | |
| Race | 0.002 * | |||
| White | 18 (43.9%) | 90 (46.6%) | 16 (27.6%) | |
| Black | 22 (53.7%) | 103 (53.4%) | 40 (69.0%) | |
| Other | 1 (2.4%) | 0 (0.0%) | 2 (3.4%) | |
| Age | 64.5 ± 11.5 | 60.7 ± 10.0 | 60.57 ± 9.4 | 0.066 |
| Ferritin | 17.9 ± 9.5 | 17.0 ± 10.6 | 14.8 ± 10.3 | 0.363 |
| B12 | 668 ± 724 | 661 ± 954 | 871 ± 1301 | 0.426 |
| Hgb | 11.9 ± 1.5 | 12.1 ± 1.6 | 10.9 ± 1.8 | <0.001 * |
| Plerixafor | 1.17 ± 0.97 | 1.18 ± 0.75 | 1.14 ± 0.81 | 0.949 |
| Platelet | 241 ± 71 | 229 ± 75 | 213 ± 71 | 0.169 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weeks, C.J.; Mian, M.; Stokes, M.; Gold, M.; Shah, A.; Vuppala, R.; Kim, K.J.; Simon, A.B.; Cortes, J.; Jillela, A.; et al. The Role of Ferritin and Folate in Determining Stem Cell Collection for Autologous Stem Cell Transplant in Multiple Myeloma. Hematol. Rep. 2025, 17, 5. https://doi.org/10.3390/hematolrep17010005
Weeks CJ, Mian M, Stokes M, Gold M, Shah A, Vuppala R, Kim KJ, Simon AB, Cortes J, Jillela A, et al. The Role of Ferritin and Folate in Determining Stem Cell Collection for Autologous Stem Cell Transplant in Multiple Myeloma. Hematology Reports. 2025; 17(1):5. https://doi.org/10.3390/hematolrep17010005
Chicago/Turabian StyleWeeks, Charles J., Mohammad Mian, Michael Stokes, Matthew Gold, Anvay Shah, Rohan Vuppala, Katherine J. Kim, Abigayle B. Simon, Jorge Cortes, Anand Jillela, and et al. 2025. "The Role of Ferritin and Folate in Determining Stem Cell Collection for Autologous Stem Cell Transplant in Multiple Myeloma" Hematology Reports 17, no. 1: 5. https://doi.org/10.3390/hematolrep17010005
APA StyleWeeks, C. J., Mian, M., Stokes, M., Gold, M., Shah, A., Vuppala, R., Kim, K. J., Simon, A. B., Cortes, J., Jillela, A., & Kota, V. (2025). The Role of Ferritin and Folate in Determining Stem Cell Collection for Autologous Stem Cell Transplant in Multiple Myeloma. Hematology Reports, 17(1), 5. https://doi.org/10.3390/hematolrep17010005






