EBV-Positive Nodal T- and NK-Cell Lymphoma Mimicking Anaplastic Large Cell Lymphoma: A Case Report
Abstract
1. Introduction
2. Case Presentation
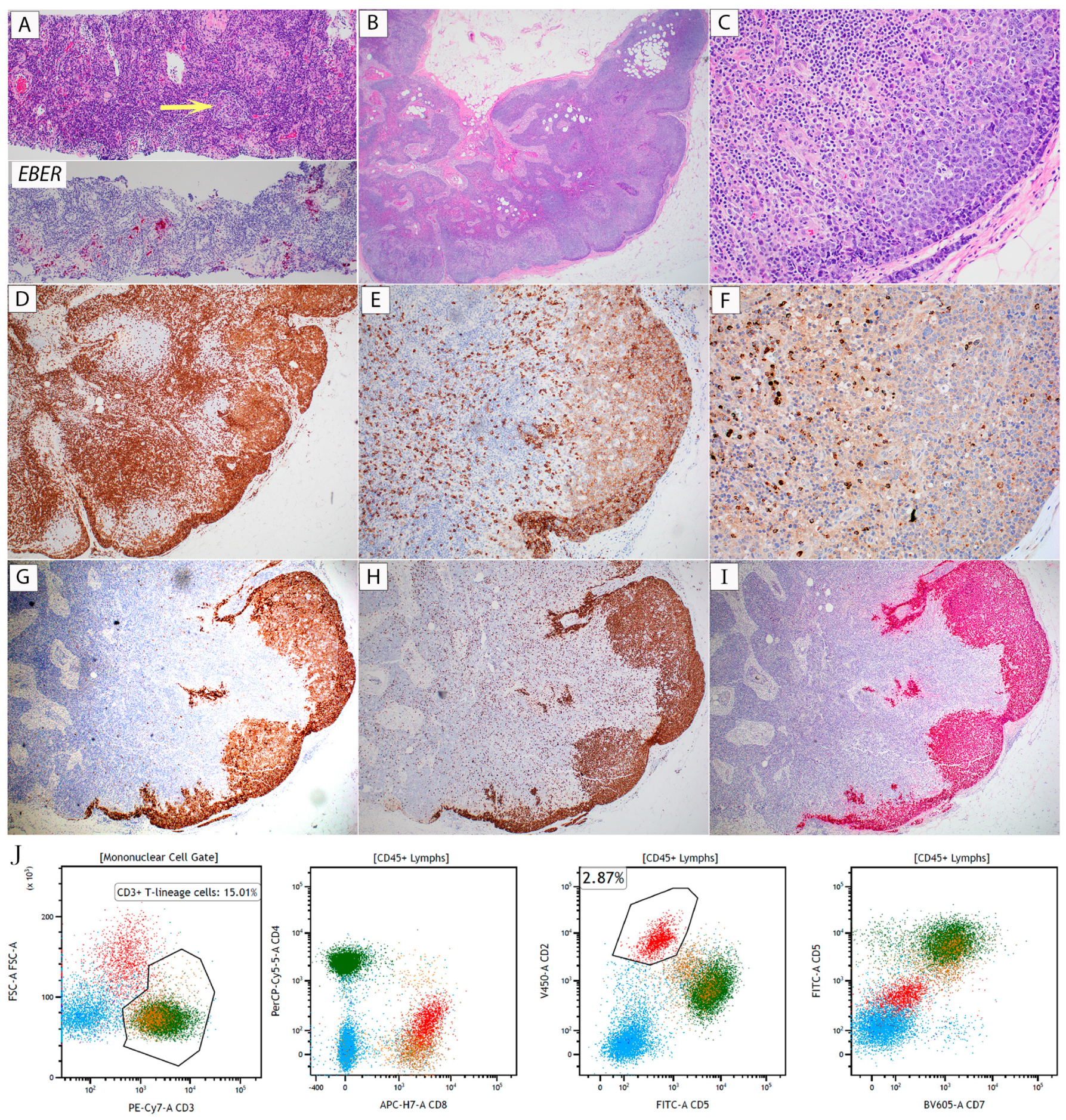
2.1. Case 1 (EBV+ NT/NKCL)
2.2. Case 2 (ALK-Negative ALCL)
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.d.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Campo, E.; Jaffe, E.S.; Cook, J.R.; Quintanilla-Martinez, L.; Swerdlow, S.H.; Anderson, K.C.; Brousset, P.; Cerroni, L.; de Leval, L.; Dirnhofer, S.; et al. The International Consensus Classification of Mature Lymphoid Neoplasms: A report from the Clinical Advisory Committee. Blood 2022, 140, 1229–1253. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Takahashi, E.; Asano, N.; Tanaka, T.; Megahed, N.; Kinoshita, T.; Nakamura, S. Nodal cytotoxic molecule (CM)-positive Epstein–Barr virus (EBV)-associated peripheral T cell lymphoma (PTCL): A clinicopathological study of 26 cases. Histopathology 2012, 61, 186–199. [Google Scholar] [CrossRef]
- Karaarslan, S.; Hekimgil, M.; Soydan, S.; Ertan, Y.; Doğanavşargil, B. Evaluation of the role of Epstein-Barr virus in cases of nodal or extranodal T- and NK-cell lymphoma using eber in situ hybridization. Pol. J. Pathol. 2015, 2, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Went, P.; Agostinelli, C.; Gallamini, A.; Piccaluga, P.P.; Ascani, S.; Sabattini, E.; Bacci, F.; Falini, B.; Motta, T.; Paulli, M.; et al. Marker expression in peripheral T-cell lymphoma: A proposed clinical-pathologic prognostic score. J. Clin. Oncol. 2006, 24, 2472–2479. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, D.; Shimada, K.; Takata, K.; Miyata-Takata, T.; Kohno, K.; Satou, A.; Sakakibara, A.; Nakamura, S.; Asano, N.; Kato, S. Reappraisal of nodal Epstein-Barr Virus-negative cytotoxic T-cell lymphoma: Identification of indolent CD5+ diseases. Cancer Sci. 2018, 109, 2599–2610. [Google Scholar] [CrossRef]
- Ma, L.; Katz, Y.; Sharan, K.P.; Schwarting, R.; Kim, A.S. Epstein-Barr virus positive anaplastic large cell lymphoma: Myth or reality? Int. J. Clin. Exp. Pathol. 2010, 4, 100–110. [Google Scholar]
- Amador, C.; Feldman, A.L. How I Diagnose anaplastic large cell lymphoma. Am. J. Clin. Pathol. 2021, 155, 479–497. [Google Scholar] [CrossRef]
- Feldman, A.L.; Laurent, C.; Narbaitz, M.; Nakamura, S.; Chan, W.C.; de Leval, L.; Gaulard, P. Classification and diagnostic evaluation of nodal T- and NK-cell lymphomas. Virchows Arch. 2023, 482, 265–279. [Google Scholar] [CrossRef]
- Fitzpatrick, M.J.; Massoth, L.R.; Marcus, C.; Vergilio, J.-A.; Severson, E.; Duncan, D.; Ramkissoon, S.H.; Hasserjian, R.P.; Kim, A.S.; Sohani, A.R.; et al. JAK2 rearrangements are a recurrent alteration in CD30+ systemic T-cell lymphomas with anaplastic morphology. Am. J. Surg. Pathol. 2021, 45, 895–904. [Google Scholar] [CrossRef]
- Wei, W.; Wu, P.; Li, L.; Zhang, Z.-H. Effectiveness of pegaspargase, gemcitabine, and oxaliplatin (P-GEMOX) chemotherapy combined with radiotherapy in newly diagnosed, stage IE to IIE, nasal-type, extranodal natural killer/T-cell lymphoma. Hematology 2017, 22, 320–329. [Google Scholar] [CrossRef]
- Kato, S.; Yamashita, D.; Nakamura, S. Nodal EBV+ cytotoxic T-cell lymphoma: A literature review based on the 2017 WHO classification. J. Clin. Exp. Hematop. 2020, 60, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Gru, A.A.; Williams, E.; Junkins-Hopkins, J.M. An Immune Suppression-associated EBV-positive Anaplastic Large Cell Lymphoma With a BRAF V600E Mutation. Am. J. Surg. Pathol. 2019, 43, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Campuzano-Zuluaga, G.; Pimentel, A.; Chapman-Fredricks, J.R.; Ramos, J.C. Differential CD30 expression in adult T-cell leukemia-lymphoma subtypes. Retrovirology 2014, 11, P129. [Google Scholar] [CrossRef]
- Yao, J.; Gottesman, S.R.; Ayalew, G.; Braverman, A.S.; Axiotis, C.A. Loss of Foxp3 is associated with CD30 expression in the anaplastic large cell subtype of adult T-cell leukemia/lymphoma (ATLL) in US/Caribbean patients: Potential therapeutic implications for CD30 antibody-mediated therapy. Am. J. Surg. Pathol. 2013, 37, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Pan, Z.; Chen, W.; Shi, Y.; Wang, W.; Yuan, J.; Wang, E.; Zhang, S.; Kurt, H.; Mai, B.; et al. Primary Effusion Lymphoma: A Clinicopathological Study of 70 Cases. Cancers 2021, 13, 878. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.-B.; Chung, T.-H.; Kato, S.; Nakamura, S.; Takahashi, E.; Ko, Y.-H.; Khoury, J.D.; Yin, C.C.; Soong, R.; Jeyasekharan, A.D.; et al. Epstein-Barr virus-associated primary nodal T/NK-cell lymphoma shows a distinct molecular signature and copy number changes. Haematologica 2018, 103, 278–287. [Google Scholar] [CrossRef]
- Wai, C.M.M.; Chen, S.; Phyu, T.; Fan, S.; Leong, S.M.; Zheng, W.; Low, L.C.Y.; Choo, S.-N.; Lee, C.-K.; Chung, T.-H.; et al. Immune pathway upregulation and lower genomic instability distinguish EBV-positive nodal T/NK-cell lymphoma from ENKTL and PTCL-NOS. Haematologica 2022, 107, 1864–1879. [Google Scholar] [CrossRef]
- Nicolae, A.; Bouilly, J.; Lara, D.; Fataccioli, V.; Lemonnier, F.; Drieux, F.; Parrens, M.; Robe, C.; Poullot, E.; Bisig, B.; et al. Nodal cytotoxic peripheral T-cell lymphoma occurs frequently in the clinical setting of immunodysregulation and is associated with recurrent epigenetic alterations. Mod. Pathol. 2022, 35, 1126–1136. [Google Scholar] [CrossRef]
- Pina-Oviedo, S.; Ortiz-Hidalgo, C.; Carballo-Zarate, A.A.; Zarate-Osorno, A. ALK-Negative Anaplastic Large Cell Lymphoma: Current Concepts and Molecular Pathogenesis of a Heterogeneous Group of Large T-Cell Lymphomas. Cancers 2021, 13, 4667. [Google Scholar] [CrossRef]
- Agnelli, L.; Mereu, E.; Pellegrino, E.; Limongi, T.; Kwee, I.; Bergaggio, E.; Ponzoni, M.; Zamò, A.; Iqbal, J.; Piccaluga, P.P.; et al. Identification of a 3-gene model as a powerful diagnostic tool for the recognition of ALK-negative anaplastic large-cell lymphoma. Blood 2012, 120, 1274–1281. [Google Scholar] [CrossRef]
- Savage, K.J.; Harris, N.L.; Vose, J.M.; Ullrich, F.; Jaffe, E.S.; Connors, J.M.; Rimsza, L.; Pileri, S.A.; Chhanabhai, M.; Gascoyne, R.D.; et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: Report from the International Peripheral T-Cell Lymphoma Project. Blood 2008, 111, 5496–5504. [Google Scholar] [CrossRef] [PubMed]
- Shustov, A.; Cabrera, M.E.; Civallero, M.; Bellei, M.; Ko, Y.H.; Manni, M.; Skrypets, T.; Horwitz, S.M.; De Souza, C.A.; Radford, J.A.; et al. ALK-negative anaplastic large cell lymphoma: Features and outcomes of 235 patients from the International T-Cell Project. Blood Adv. 2021, 5, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Asano, N.; Miyata-Takata, T.; Takata, K.; Elsayed, A.A.; Satou, A.; Takahashi, E.; Kinoshita, T.; Nakamura, S. T-cell receptor (TCR) phenotype of nodal Epstein-Barr virus (EBV)-positive cytotoxic T-cell lymphoma (CTL): A clinicopathologic study of 39 cases. Am. J. Surg. Pathol. 2015, 39, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Suzuki, R.; Oguchi, M.; Asano, N.; Amaki, J.; Akiba, T.; Maeda, T.; Itasaka, S.; Kubota, N.; Saito, Y.; et al. Treatments and Outcomes of Patients with Extranodal Natural Killer/T-Cell Lymphoma Diagnosed Between 2000 and 2013: A Cooperative Study in Japan. J. Clin. Oncol. 2017, 35, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Q.; Huang, D.; Tang, T.; Tan, D.; Laurensia, Y.; Peng, R.-J.; Wong, E.K.Y.; Cheah, D.M.Z.; Chia, B.K.H.; Iqbal, J.; et al. Whole-genome sequencing identifies responders to Pembrolizumab in relapse/refractory natural-killer/T cell lymphoma. Leukemia 2020, 34, 3413–3419. [Google Scholar] [CrossRef]
- Kim, S.J.; Lim, J.Q.; Laurensia, Y.; Cho, J.; Yoon, S.E.; Lee, J.Y.; Ryu, K.J.; Ko, Y.H.; Koh, Y.; Cho, D.; et al. Avelumab for the treatment of relapsed or refractory extranodal NK/T-cell lymphoma: An open-label phase 2 study. Blood 2020, 136, 2754–2763. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abro, B.; Allen, P.; Asakrah, S.; Bradley, K.; Zhang, L. EBV-Positive Nodal T- and NK-Cell Lymphoma Mimicking Anaplastic Large Cell Lymphoma: A Case Report. Hematol. Rep. 2024, 16, 308-316. https://doi.org/10.3390/hematolrep16020031
Abro B, Allen P, Asakrah S, Bradley K, Zhang L. EBV-Positive Nodal T- and NK-Cell Lymphoma Mimicking Anaplastic Large Cell Lymphoma: A Case Report. Hematology Reports. 2024; 16(2):308-316. https://doi.org/10.3390/hematolrep16020031
Chicago/Turabian StyleAbro, Brooj, Pamela Allen, Saja Asakrah, Kyle Bradley, and Linsheng Zhang. 2024. "EBV-Positive Nodal T- and NK-Cell Lymphoma Mimicking Anaplastic Large Cell Lymphoma: A Case Report" Hematology Reports 16, no. 2: 308-316. https://doi.org/10.3390/hematolrep16020031
APA StyleAbro, B., Allen, P., Asakrah, S., Bradley, K., & Zhang, L. (2024). EBV-Positive Nodal T- and NK-Cell Lymphoma Mimicking Anaplastic Large Cell Lymphoma: A Case Report. Hematology Reports, 16(2), 308-316. https://doi.org/10.3390/hematolrep16020031






