Survival Outcomes of Patients with Mantle Cell Lymphoma: A Retrospective, 15-Year, Real-Life Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Treatment Regimens
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Patients
3.2. Treatment Regimens
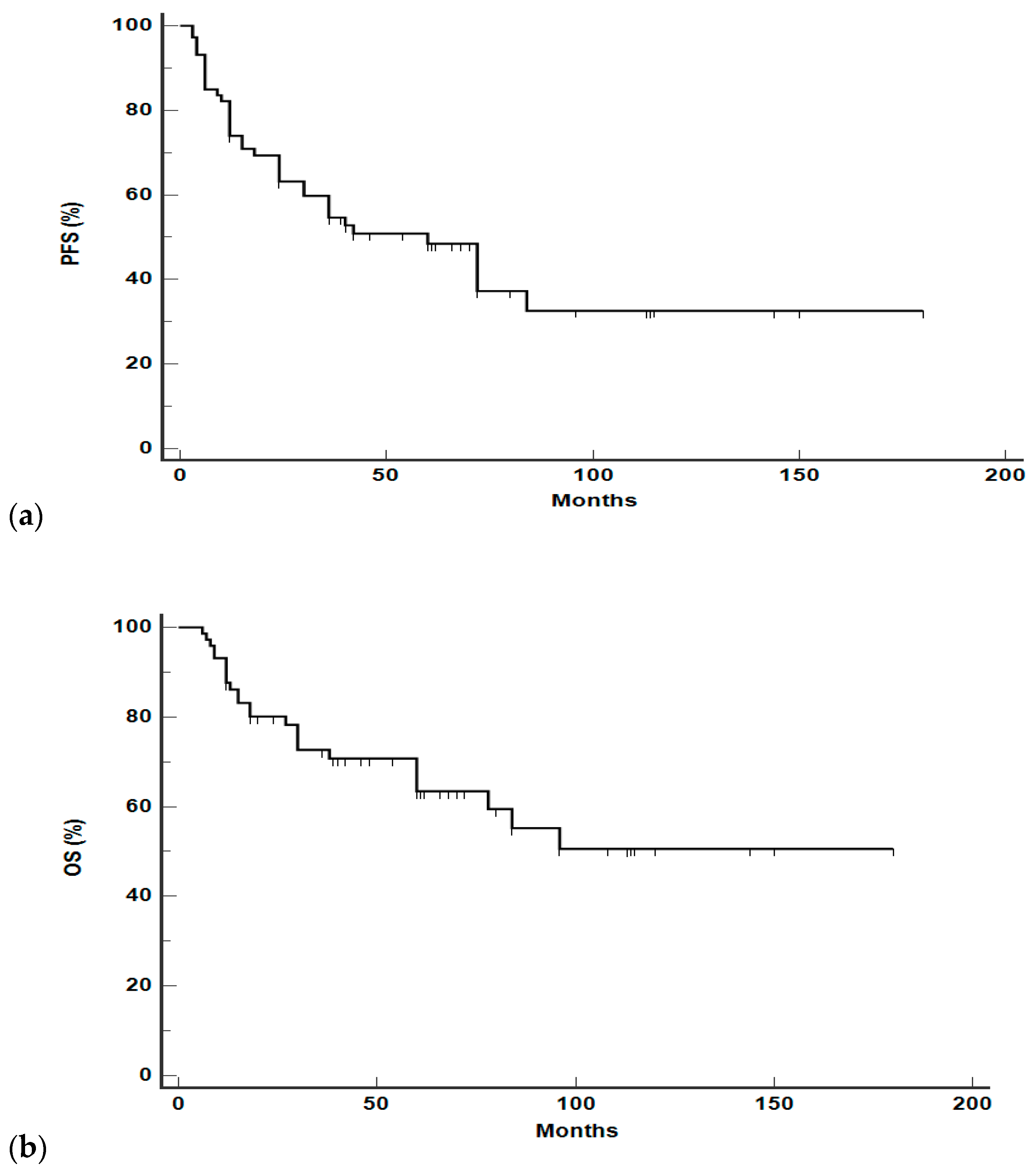
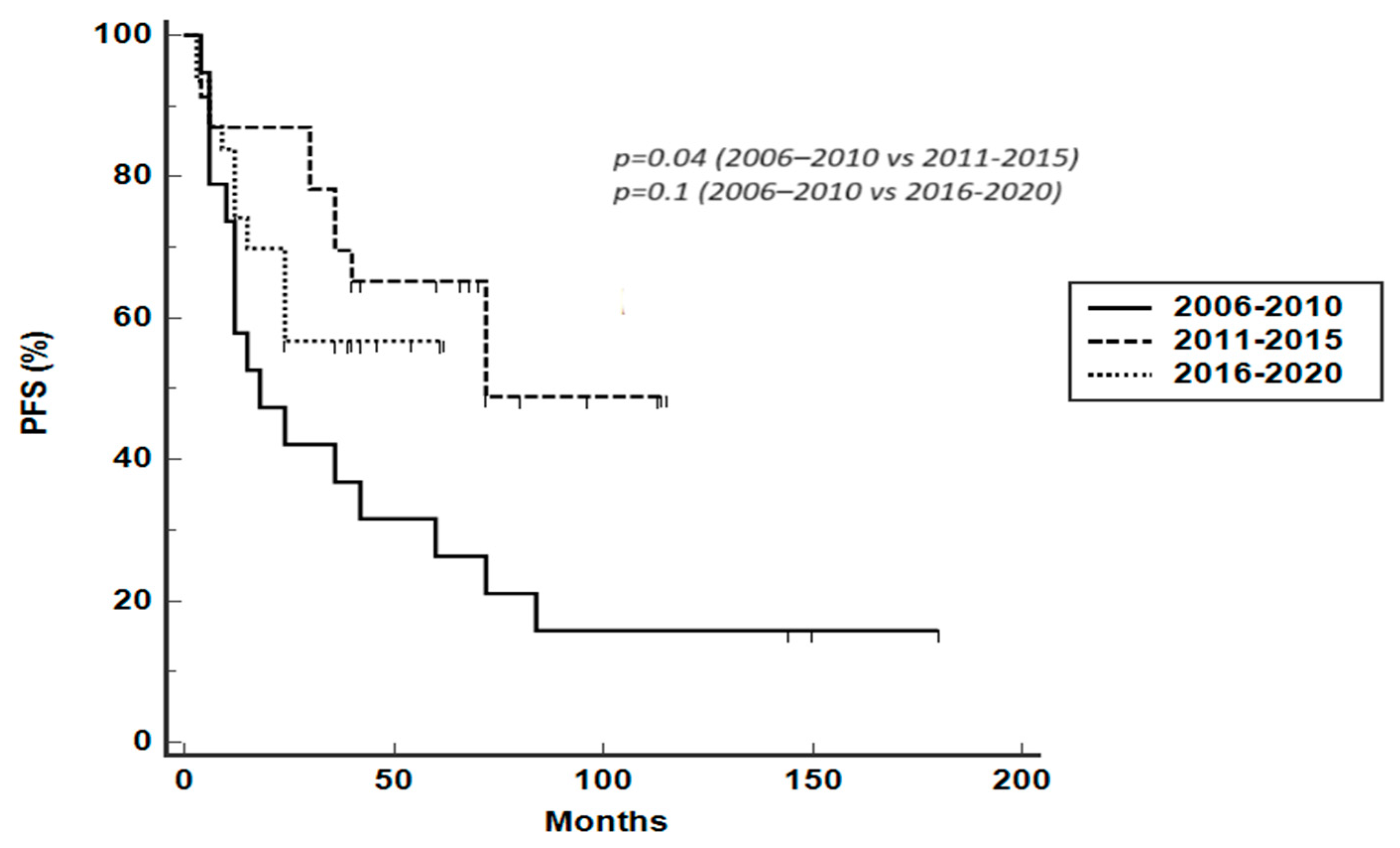
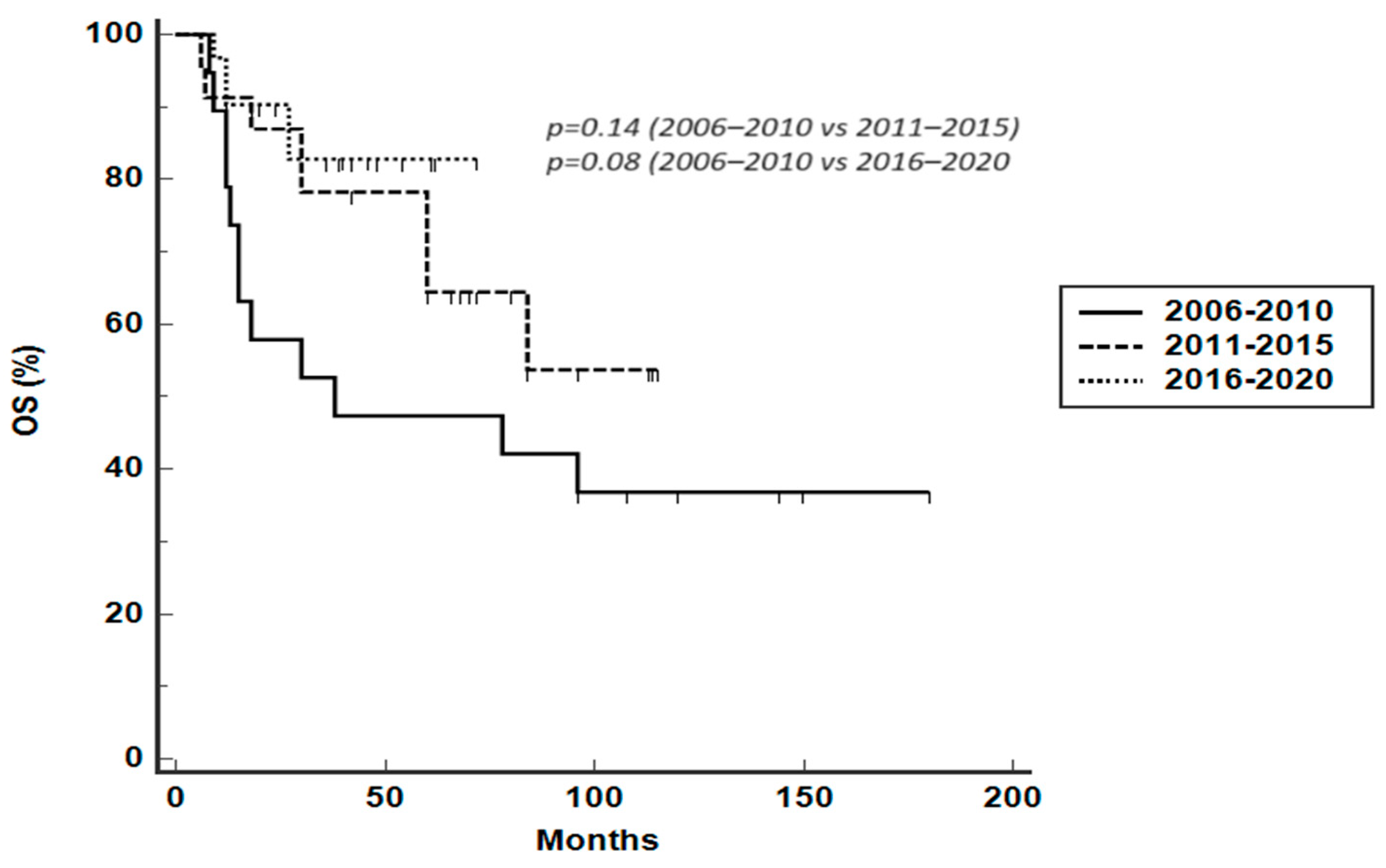
3.3. Survival Analysis
3.4. Long-Term Toxicity, Second Malignancies, and Causes of Death
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silkenstedt, E.; Dreyling, M. Mantle cell lymphoma-Update on molecular biology, prognostication and treatment approaches. Hematol. Oncol. 2023, 41, 36–42. [Google Scholar] [CrossRef]
- Rozental, A.; Jim, H.S.L.; Extermann, M. Treatment of older patients with mantle cell lymphoma in the era of novel agents. Leuk. Lymphoma 2023, 64, 1514–1526. [Google Scholar] [CrossRef]
- Patel, D.; Kahl, B. SOHO State of the Art Updates and Next Questions: Tailoring Upfront Therapy in Mantle Cell Lymphoma. Clin. Lymphoma Myeloma Leuk. 2023, 23, 633–641. [Google Scholar] [CrossRef]
- Dreyling, M.; Campo, E.; Hermine, O.; Jerkeman, M.; Le Gouill, S.; Rule, S.; Shpilberg, O.; Walewski, J.; Ladetto, M.; ESMO Guidelines Committee. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv62–iv71. [Google Scholar] [CrossRef] [PubMed]
- Isaac, K.M.; Portell, C.A.; Williams, M.E. Leukemic Variant of Mantle Cell Lymphoma: Clinical Presentation and Management. Curr. Oncol. Rep. 2021, 23, 102. [Google Scholar] [CrossRef]
- Ye, H.; Desai, A.; Zeng, D.; Nomie, K.; Romaguera, J.; Ahmed, M.; Wang, M.L. Smoldering mantle cell lymphoma. J. Exp. Clin. Cancer Res. 2017, 36, 185. [Google Scholar] [PubMed]
- He, J.S.; Chen, X.; Wei, G.Q.; Sun, J.; Zheng, W.Y.; Shi, J.M.; Wu, W.J.; Zhao, Y.; Zheng, G.F.; Huang, H.; et al. Simplified MIPI-B prognostic stratification method can predict the outcome well—Retrospective analysis of clinical characteristics and management of newly-diagnosed mantle cell lymphoma patients from China. Medicine 2019, 98, e13741. [Google Scholar] [CrossRef]
- Scheubeck, G.; Jiang, L.; Hermine, O.; Kluin-Nelemans, H.C.; Schmidt, C.; Unterhalt, M.; Rosenwald, A.; Klapper, W.; Evangelista, A.; Ladetto, M.; et al. Clinical outcome of Mantle Cell Lymphoma patients with high-risk disease (high-risk MIPI-c or high p53 expression). Leukemia 2023, 37, 1887–1894. [Google Scholar]
- Hermine, O.; Hoster, E.; Walewski, J.; Bosly, A.; Stilgenbauer, S.; Thieblemont, C.; Szymczyk, M.; Bouabdallah, R.; Kneba, M.; Hallek, M.; et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): A randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet 2016, 388, 565–575. [Google Scholar]
- Le Gouill, S.; Thieblemont, C.; Oberic, L.; Moreau, A.; Bouabdallah, K.; Dartigeas, C.; Damaj, G.; Gastinne, T.; Ribrag, V.; Feugier, P.; et al. Rituximab after Autologous Stem-Cell Transplantation in Mantle-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 1250–1260. [Google Scholar] [PubMed]
- Tisi, M.C.; Moia, R.; Patti, C.; Evangelista, A.; Ferrero, S.; Spina, M.; Tani, M.; Botto, B.; Celli, M.; Puccini, B.; et al. Long-term follow-up of rituximab plus bendamustine and cytarabine in older patients with newly diagnosed MCL. Blood Adv. 2023, 7, 3916–3924. [Google Scholar] [CrossRef] [PubMed]
- Flinn, I.W.; van der Jagt, R.; Kahl, B.; Wood, P.; Hawkins, T.; MacDonald, D.; Simpson, D.; Kolibaba, K.; Issa, S.; Chang, J.; et al. First-Line Treatment of Patients with Indolent Non-Hodgkin Lymphoma or Mantle Cell Lymphoma with Bendamustine Plus Rituximab Versus R-CHOP or R-CVP: Results of the BRIGHT 5-Year Follow-Up Study. J. Clin. Oncol. 2019, 37, 984–991. [Google Scholar] [CrossRef]
- Cencini, E.; Sicuranza, A.; Fabbri, A.; Ferrigno, I.; Rigacci, L.; Cox, M.C.; Raspadori, D.; Bocchia, M. Study of gene polymorphisms as predictors of treatment efficacy and toxicity in patients with indolent non-hodgkin lymphomas and mantle cell lymphoma receiving bendamustine and rituximab. Br. J. Haematol. 2019, 184, 223–231. [Google Scholar] [CrossRef]
- Robak, T.; Jin, J.; Pylypenko, H.; Verhoef, G.; Siritanaratkul, N.; Drach, J.; Raderer, M.; Mayer, J.; Pereira, J.; Tumyan, G.; et al. Frontline bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in transplantation-ineligible patients with newly diagnosed mantle cell lymphoma: Final overall survival results of a randomised, open-label, phase 3 study. Lancet Oncol. 2018, 19, 1449–1458. [Google Scholar] [PubMed]
- Eskelund, C.W.; Kolstad, A.; Jerkeman, M.; Räty, R.; Laurell, A.; Eloranta, S.; Smedby, K.E.; Husby, S.; Pedersen, L.B.; Andersen, N.S.; et al. 15-year follow-up of the Second Nordic Mantle Cell Lymphoma trial (MCL2): Prolonged remissions without survival plateau. Br. J. Haematol. 2016, 175, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.I.; Bernstein, S.H.; Kahl, B.S.; Djulbegovic, B.; Robertson, M.J.; de Vos, S.; Epner, E.; Krishnan, A.; Leonard, J.P.; Lonial, S.; et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J. Clin. Oncol. 2006, 24, 4867–4874. [Google Scholar]
- Morabito, F.; Skafi, M.; Recchia, A.G.; Kashkeesh, A.; Hindiyeh, M.; Sabatleen, A.; Morabito, L.; Alijanazreh, H.; Hamamreh, Y.; Gentile, M. Lenalidomide for the treatment of mantle cell lymphoma. Expert Opin. Pharmacother. 2019, 20, 487–494. [Google Scholar] [PubMed]
- Hess, G.; Herbrecht, R.; Romaguera, J.; Verhoef, G.; Crump, M.; Gisselbrecht, C.; Laurell, A.; Offner, F.; Strahs, A.; Berkenblit, A.; et al. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J. Clin. Oncol. 2009, 27, 3822–3829. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Rule, S.; Martin, P.; Goy, A.; Auer, R.; Kahl, B.S.; Jurczak, W.; Advani, R.H.; Romaguera, J.E.; Williams, M.E.; et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 2013, 369, 507–516. [Google Scholar] [CrossRef]
- Tucker, D.; Morley, N.; MacLean, P.; Vandenberghe, E.; Booth, S.; Parisi, L.; Rule, S. The 5-year follow-up of a real-world observational study of patients in the United Kingdom and Ireland receiving ibrutinib for relapsed/refractory mantle cell lymphoma. Br. J. Haematol. 2021, 192, 1035–1038. [Google Scholar] [CrossRef]
- Rusconi, C.; Cheah, C.Y.; Eyre, T.A.; Tucker, D.; Klener, P.; Giné, E.; Crucitti, L.; Muzi, C.; Iadecola, S.; Infante, G.; et al. Ibrutinib improves survival compared with chemotherapy in mantle cell lymphoma with central nervous system relapse. Blood 2022, 140, 1907–1916. [Google Scholar]
- Zinzani, P.L.; Martelli, M.; Ferrero, S.; Gentile, M.; Laurenti, L.; Mauro, F.R.; Sportoletti, P.; Tedeschi, A.; Varettoni, M.; Visco, C. Use of BTK inhibitors with focus on ibrutinib in mantle cell lymphoma: An expert panel opinion statement. Hematol. Oncol. 2022, 40, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Rule, S.; Dreyling, M.; Goy, A.; Hess, G.; Auer, R.; Kahl, B.; Cavazos, N.; Liu, B.; Yang, S.; Clow, F.; et al. Outcomes in 370 patients with mantle cell lymphoma treated with ibrutinib: A pooled analysis from three open-label studies. Br. J. Haematol. 2017, 179, 430–438. [Google Scholar] [PubMed]
- Visco, C.; Di Rocco, A.; Evangelista, A.; Quaglia, F.M.; Tisi, M.C.; Morello, L.; Zilioli, V.R.; Rusconi, C.; Hohaus, S.; Sciarra, R.; et al. Outcomes in first relapsed-refractory younger patients with mantle cell lymphoma: Results from the MANTLE-FIRST study. Leukemia 2021, 35, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.L.; Pararajalingam, P.; Jin, L.; Balasubramanian, S.; Jiang, A.; Xu, W.; Grau, M.; Zapukhlyak, M.; Boyle, M.; Hodkinson, B.; et al. Molecular determinants of outcomes in relapsed or refractory mantle cell lymphoma treated with ibrutinib or temsirolimus in the MCL3001 (RAY) trial. Leukemia 2022, 36, 2479–2487. [Google Scholar]
- Martin, P.; Maddocks, K.; Leonard, J.P.; Ruan, J.; Goy, A.; Wagner-Johnston, N.; Rule, S.; Advani, R.; Iberri, D.; Phillips, T.; et al. Postibrutinib outcomes in patients with mantle cell lymphoma. Blood 2016, 127, 1559–1563. [Google Scholar] [PubMed]
- Huang, Z.; Chavda, V.P.; Bezbaruah, R.; Dhamne, H.; Yang, D.H.; Zhao, H.B. CAR T-Cell therapy for the management of mantle cell lymphoma. Mol. Cancer 2023, 22, 67. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jain, P.; Locke, F.L.; Maurer, M.J.; Frank, M.J.; Munoz, J.L.; Dahiya, S.; Beitinjaneh, A.M.; Jacobs, M.T.; Mcguirk, J.P.; et al. Brexucabtagene Autoleucel for Relapsed or Refractory Mantle Cell Lymphoma in Standard-of-Care Practice: Results from the US Lymphoma CAR T Consortium. J. Clin. Oncol. 2023, 41, 2594–2606. [Google Scholar]
- Harmanen, M.; Hujo, M.; Sund, R.; Sorigue, M.; Khan, M.; Prusila, R.; Klaavuniemi, T.; Kari, E.; Jantunen, E.; Sunela, K.; et al. Survival of patients with mantle cell lymphoma in the rituximab era: Retrospective binational analysis between 2000 and 2020. Br. J. Haematol. 2023, 201, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, J.; Zhang, X.; Yang, H.; Wang, Y.; Sun, P.; Cai, Q.; Xia, Y.; Liu, P. Survival trends in patients under age 65 years with mantle cell lymphoma, 1995-2016: A SEER-based analysis. Front. Oncol. 2020, 10, 588314. [Google Scholar] [CrossRef]
- Yang, P.; Cai, Q.Q.; Zhang, W.; Liu, S.Z.; Liu, H.; Sun, X.H.; Dong, Y.J.; Xiao, X.B.; Wang, J.W.; Li, Z.L.; et al. Real-world treatment and outcome patterns of patients with mantle cell lymphoma in China: A large, multicenter retrospective analysis. Cancer Med. 2023, 12, 13204–13216. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar]
- Dreyling, M.; Thieblemont, C.; Gallamini, A.; Arcaini, L.; Campo, E.; Hermine, O.; Kluin-Nelemans, J.C.; Ladetto, M.; Le Gouill, S.; Iannitto, E.; et al. ESMO Consensus conferences: Guidelines on malignant lymphoma. part 2: Marginal zone lymphoma, mantle cell lymphoma, peripheral T-cell lymphoma. Ann. Oncol. 2013, 24, 857–877. [Google Scholar] [PubMed]
- Ghielmini, M.; Zucca, E. How I treat mantle cell lymphoma. Blood. 2009, 114, 1469–1476. [Google Scholar] [CrossRef]
- Merli, M.; Marino, D.; Cencini, E.; Rattotti, S.; Fraenza, C.; Grossi, P.; Bianchi, B.; Mora, B.; Sciarra, R.; Finotto, S.; et al. Direct-acting antivirals in hepatitis C virus-positive mantle cell lymphomas. Hematol. Oncol. 2021, 39, 263–266. [Google Scholar] [CrossRef]
- Kluin-Nelemans, H.C.; Hoster, E.; Hermine, O.; Walewski, J.; Geisler, C.H.; Trneny, M.; Stilgenbauer, S.; Kaiser, F.; Doorduijn, J.K.; Salles, G.; et al. Treatment of Older Patients with Mantle Cell Lymphoma (MCL): Long-Term Follow-Up of the Randomized European MCL Elderly Trial. J. Clin. Oncol. 2020, 38, 248–256. [Google Scholar] [PubMed]
- Rule, S.; Dreyling, M.; Goy, A.; Hess, G.; Auer, R.; Kahl, B.; Hernández-Rivas, J.Á.; Qi, K.; Deshpande, S.; Parisi, L.; et al. Ibrutinib for the treatment of relapsed/refractory mantle cell lymphoma: Extended 3.5-year follow up from a pooled analysis. Haematologica 2019, 104, e211–e214. [Google Scholar] [PubMed]
- Cencini, E.; Sicuranza, A.; Fabbri, A.; Marzano, C.; Pacelli, P.; Caroni, F.; Raspadori, D.; Bocchia, M. The prognostic role of gene polymorphisms in patients with indolent non-Hodgkin lymphomas and mantle-cell lymphoma receiving bendamustine and rituximab: Results of the 5-year follow-up study. Leuk. Lymphoma 2023, 64, 1634–1642. [Google Scholar] [CrossRef]
- Kotchetkov, R.; Drennan, I.R.; Susman, D.; DiMaria, E.; Gerard, L.; Nay, D.; Prica, A. Bendamustine and rituximab is well-tolerated and efficient in the treatment of indolent non-Hodgkin’s lymphoma and mantle cell lymphoma in elderly: A single center observational study. Int. J. Cancer 2023, 152, 1884–1893. [Google Scholar] [PubMed]
- Yi, J.H.; Kim, S.J.; Lee, J.O.; Lee, G.W.; Kwak, J.Y.; Eom, H.S.; Jo, J.C.; Choi, Y.S.; Oh, S.Y.; Kim, W.S. Bendamustine Plus Rituximab for Mantle Cell Lymphoma: A Korean, Multicenter Retrospective Analysis. Anticancer Res. 2022, 42, 6083–6089. [Google Scholar] [CrossRef] [PubMed]
- Alnassfan, T.; Cox-Pridmore, M.J.; Taktak, A.; Till, K.J. Mantle cell lymphoma treatment options for elderly/unfit patients: A systematic review. EJHaem 2021, 3, 276–290. [Google Scholar] [CrossRef]
- Bega, G.; Olivieri, J.; Riva, M.; Scapinello, G.; Paolini, R.; Finotto, S.; Sartori, R.; Lucchini, E.; Guandalini, G.; Facchinelli, D.; et al. Rituximab and Bendamustine (BR) Compared with Rituximab, Bendamustine, and Cytarabine (R-BAC) in Previously Untreated Elderly Patients with Mantle Cell Lymphoma. Cancers 2021, 13, 6089. [Google Scholar] [CrossRef] [PubMed]
- Dreyling, M.; Goy, A.; Hess, G.; Kahl, B.S.; Hernández-Rivas, J.Á.; Schuier, N.; Qi, K.; Deshpande, S.; Zhu, A.; Parisi, L.; et al. Long-term Outcomes with Ibrutinib Treatment for Patients with Relapsed/Refractory Mantle Cell Lymphoma: A Pooled Analysis of 3 Clinical Trials with Nearly 10 Years of Follow-up. Hemasphere 2022, 6, e712. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, P.; Cai, J.; Jing, H.; Zou, L.; Huang, H.; Wu, Y.; Li, W.; Zhong, L.; Jin, X.; et al. Ibrutinib as monotherapy versus combination therapy in Chinese patients with relapsed/refractory mantle cell lymphoma: A multicenter study. Cancer Med. 2022, 11, 4134–4145. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Wang, M.L. Mantle cell lymphoma in 2022-A comprehensive update on molecular pathogenesis, risk stratification, clinical approach, and current and novel treatments. Am. J. Hematol. 2022, 97, 638–656. [Google Scholar] [CrossRef] [PubMed]
- Arun Kumar, S.; Gao, J.; Patel, S.A. The shifting therapeutic paradigm for relapsed/refractory mantle cell lymphoma. Leuk. Res. 2023, 134, 107385. [Google Scholar] [CrossRef] [PubMed]
- Di, M.; Long, J.B.; Kothari, S.K.; Sethi, T.; Zeidan, A.M.; Podoltsev, N.A.; Shallis, R.M.; Wang, R.; Ma, X.; Huntington, S.F. Treatment patterns and real-world effectiveness of rituximab maintenance in older patients with mantle cell lymphoma: A population-based analysis. Haematologica 2023, 108, 2218–2223. [Google Scholar] [CrossRef] [PubMed]

| Treatment Era | Entire Cohort n = 73 | 2006–2010 n = 19 | 2011–2015 n = 23 | 2016–2020 n = 31 |
|---|---|---|---|---|
| Age: median [range] | 70 [34–92] | |||
| <65 | 27/73 (37%) | 6/19 (31.6%) | 8/23 (34.8%) | 13/31 (41.9%) |
| 65–74 | 20/73 (27.4%) | 5/19 (26.3%) | 7/23 (30.4%) | 8/31 (25.8%) |
| ≥75 | 26/73 (35.6%) | 8/19 (42.1%) | 8/23 (34.8%) | 10/31 (32.3%) |
| Male | 51/73 (69.9%) | 13/19 (68.4%) | 16/23 (69.6%) | 22/31 (71%) |
| Blastoid/pleomorphic | 9/73 (12.3%) | 1/19 (5.3%) | 3/23 (13%) | 5/31 (16.1%) |
| Stage | ||||
| I–II | 11/73 (15.1%) | 2/19 (10.5%) | 4/23 (17.4%) | 5/31 (16.1%) |
| III | 9/73 (12.3%) | 2/19 (10.5%) | 3/23 (13%) | 4/31 (12.9%) |
| IV | 53/73 (72.6%) | 15/19 (79%) | 16/23 (69.6% | 22/31 (71%) |
| B-symptoms | 14/73 (19.2%) | 3/19 (15.8%) | 4/23 (17.4%) | 7/31 (22.6%) |
| sMIPI score | ||||
| low | 10/73 (13.7%) | 2/19 (10.5%) | 3/23 (13%) | 5/31 (16.1%) |
| intermediate | 21/73 (28.8%) | 6/19 (31.6%) | 7/23 (30.4%) | 8/31 (25.8%) |
| high | 42/73 (57.5%) | 11/19 (57.9%) | 13/23 (56.6%) | 18/31 (58.1%) |
| Elevated LDH | 28/73 (38.4%) | 4/19 (21%) | 10/23 (43.5%) | 14/31 (45.2%) |
| ECOG PS 0–1 | 62/73 (84.9%) | 16/19 (84.2%) | 19/23 (82.6%) | 27/31 (87.1%) |
| Treatment | Number of Patients (%) | 2006–2010 n = 19 | 2011–2015 n = 23 | 2016–2020 n = 31 |
|---|---|---|---|---|
| Rituximab-bendamustine | 26/73 (35.6%) | 0 | 9/23 (39.1%) | 17/31 (54.8%) |
| High-dose cytarabine-based therapies | 24/73 (32.9%) | 6/19 (31.6%) | 8/23 (34.8%) | 10/31 (32.3%) |
| Autologous stem-cell transplantation | 21/73 (28.8%) | 6/19 (31.6%) | 6/23 (26%) | 9/31 (29%) |
| Rituximab-bendamustine-cytarabine (R-BAC) | 9/73 (12.3%) | 0 | 5/23 (21.7%) | 4/31 (12.9%) |
| Rituximab-CHOP or CHOP-like regimens | 6/73 (8.2%) | 5/19 (26.3%) | 1/23 (4.4%) | 0 |
| Fludarabine-containing regimens | 5/73 (6.8%) | 5/19 (26.3%) | 0 | 0 |
| Rituximab-alkylating agents | 2/73 (2.8%) | 2/19 (10.5%) | 0 | 0 |
| Alkylating agents | 1/73 (1.4%) | 1/19 (5.3%) | 0 | 0 |
| Number of Patients (%) | |
|---|---|
| Causes of death | 25/73 (34.2%) |
| Progressive disease | 12/25 (48%) |
| Second primary malignancies | 5/25 (20%) |
| Infections | 4/25 (16%) |
| Unknown origin | 3/25 (12%) |
| Thrombotic thrombocytopenic purpura | 1/25 (4%) |
| Second primary malignancies | 5/73 (6.8%) |
| Lung cancer | 1/5 (20%) |
| Acute myeloid leukemia | 1/5 (20%) |
| Melanoma | 1/5 (20%) |
| Non-melanoma skin cancer | 1/5 (20%) |
| Biliary tract carcinoma | 1/5 (20%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cencini, E.; Calomino, N.; Franceschini, M.; Dragomir, A.; Fredducci, S.; Esposito Vangone, B.; Lucco Navei, G.; Fabbri, A.; Bocchia, M. Survival Outcomes of Patients with Mantle Cell Lymphoma: A Retrospective, 15-Year, Real-Life Study. Hematol. Rep. 2024, 16, 50-62. https://doi.org/10.3390/hematolrep16010006
Cencini E, Calomino N, Franceschini M, Dragomir A, Fredducci S, Esposito Vangone B, Lucco Navei G, Fabbri A, Bocchia M. Survival Outcomes of Patients with Mantle Cell Lymphoma: A Retrospective, 15-Year, Real-Life Study. Hematology Reports. 2024; 16(1):50-62. https://doi.org/10.3390/hematolrep16010006
Chicago/Turabian StyleCencini, Emanuele, Natale Calomino, Marta Franceschini, Andreea Dragomir, Sara Fredducci, Beatrice Esposito Vangone, Giulia Lucco Navei, Alberto Fabbri, and Monica Bocchia. 2024. "Survival Outcomes of Patients with Mantle Cell Lymphoma: A Retrospective, 15-Year, Real-Life Study" Hematology Reports 16, no. 1: 50-62. https://doi.org/10.3390/hematolrep16010006
APA StyleCencini, E., Calomino, N., Franceschini, M., Dragomir, A., Fredducci, S., Esposito Vangone, B., Lucco Navei, G., Fabbri, A., & Bocchia, M. (2024). Survival Outcomes of Patients with Mantle Cell Lymphoma: A Retrospective, 15-Year, Real-Life Study. Hematology Reports, 16(1), 50-62. https://doi.org/10.3390/hematolrep16010006






