Abstract
Background: Second- and third-generation tyrosine kinase inhibitors (TKIs) are now available to treat chronic-phase chronic myeloid leukemia (CP-CML) in the first and second line. However, vascular adverse events (VAEs) have been reported for patients with CML treated with some TKIs. Methods: We retrospectively evaluated the cumulative incidence (CI) and cardiovascular risk for 210 patients included in the Canarian Registry of CML. Result: With a mean follow up of 6 years, 19/210 (9.1%) patients developed VAEs, all of whom presented at least one cardiovascular risk factor at diagnosis. The mean time to VAE presentation was 54 months from the start of TKI treatment. We found a statistically significant difference between the CI for nilotinib-naïve vs. nilotinib-treated patients (p = 0.005), between dasatinib-naïve and dasatinib-treated patients (p = 0.039), and for patients who received three lines of treatment with first-line imatinib vs. first-line imatinib (p < 0.001). From the multivariable logistic regression analyses, the Framingham risk score (FRS) and patients with three lines of TKI with first-line imatinib were the only variables with statistically significant hazard ratios for VAE development. Significant increases in HDL-C and total cholesterol may also be predictive for VAE. Conclusions: In conclusion, it is important to estimate the cardiovascular risk at the diagnosis of CML as it can help determine whether a patient is likely to develop a VAE during TKI treatment.
1. Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm characterized by the expression of the BCR::ABL1 fusion protein, which exhibits constitutive tyrosine kinase activity [1]. In addition to defining the diagnosis, the detection of BCR::ABL1 transcripts is essential for the molecular monitoring of patients with CML and assessing their response to tyrosine kinase inhibitor (TKI) treatment [2].
The introduction of second-generation TKIs (2G-TKIs)—nilotinib, dasatinib, and bosutinib—has significantly improved the treatment of chronic-phase CML (CP-CML). These 2G-TKIs have demonstrated superior response rates, leading to earlier cytogenetic and molecular responses compared to imatinib [3,4,5,6]. Moreover, when used as second-line therapy, 2G-TKIs can rescue a significant percentage of patients from imatinib treatment failure [7,8,9]. With the approval of the third-generation TKIs ponatinib, for patients in the chronic, accelerated, or blast phase who are resistant to dasatinib or nilotinib, and asciminib, for patients with CP-CML previously treated with two or more TKIs (with both also being approved for patients with the T315I kinase domain mutation), physicians now have a total of six TKIs available for the treatment of CP-CML.
During the follow up of patients with CML on TKI, it is important to periodically assess the appearance of treatment-related toxicities [10]. Each approved TKI has a unique off-target spectrum, meaning that physicians should consider patient comorbidities in order to choose the most appropriate treatment [11,12]. While different TKIs display diverse toxicities, some adverse events (AEs) are common, including facial and peripheral edema, diarrhea, skin rash, muscle cramps or myalgia, myelosuppressive effects, and glycometabolic alterations [13]. In the majority of cases, these AEs are not severe and can be reversed through dose interruptions or reductions [2,10].
Although imatinib has a well-documented and favorable safety profile, over the past decade, more serious toxicities have been reported in patients with CML treated with second- and third-generation TKIs, particularly vascular adverse events (VAEs) [14]. Subsequent clinical trials and retrospective studies have confirmed the risks of morbidity and mortality [3,6,15,16,17]. This is particularly important given that CML is typically diagnosed in older adults, with age as a CV risk factor for VAE [18,19]. Moreover, comorbidities such as arterial hypertension or diabetes mellitus are frequent among older patients. Thus, vascular safety is an important issue for patients with CML undergoing TKI treatment.
Reported VAEs associated with the use of second- and third-generation TKIs include severe occlusive arterial events, such as peripheral artery occlusive disease (PAOD), coronary artery disease, and ischemic cerebrovascular events. For instance, the 5-year follow-up study of ENESTnd indicated that among patients receiving nilotinib, 2.5% experienced PAOD, 3.9% experienced ischemic heart disease, and 1.4% experienced ischemic cerebrovascular events with the 300 mg dose, while 2.5%, 8.5%, and 3.2% experienced these events with the 400 mg dose. In comparison, the corresponding rates for patients receiving imatinib were 0%, 1.8%, and 0.4%, respectively [20]. In the initial results of the PACE clinical trial, cardiovascular, cerebrovascular, and peripheral vascular events that could possibly be related to treatment were observed in 2.2%, 0.7%, and 1.6% of patients receiving ponatinib, respectively [17]. In the DASISION trial, any-cause arterial ischemic events were reported in 5% of patients receiving dasatinib, compared to 2% of those receiving imatinib [6]. Similarly, in the BELA study, cardiac AEs were experienced by 4% of patients receiving bosutinib vs. 3% of patients receiving imatinib, with a prolonged QT interval being the most commonly reported VAE [21].
Outside of clinical trials, the actual incidence of VAEs in patients treated with TKIs is not extensively documented. In this retrospective real-life study, we aimed to assess the occurrence of VAEs in patients with CP-CML included in the Canarian CML Registry and examine the risk factors associated with VAE during TKI treatment.
2. Materials and Methods
2.1. Patient Cohort
The medical records of patients with CP-CML diagnosed and treated between 2009 and 2017 in the 7 hospitals of the Canary Islands, Spain, and included in the Canarian CML Registry (Registro Canario de Leucemia Mieloide Crónica, https://rclmc.es/rclmc/ (accessed on 1 December 2022)) were reviewed. Data were retrospectively collected on TKI treatment, concomitant medication, VAE occurrence, and cardiovascular risk factors (CVRFs), including age, gender, body mass index (BMI), smoking, arterial hypertension (AHT), and diabetes mellitus (DM).
Fasting lipid profiles in protein serum were collected from routine blood biochemistry analyses for total cholesterol (TC), low- and high-density lipoprotein cholesterol (LDL-C and HDL-C), and triglycerides (TG). Patients receiving lipid-lowering therapy (such as statins) at the TKI start were excluded from this study due to the possible effects on lipid profiles.
BMI was classified according to the World Health Organization classification as normal weight (BMI < 24.99), overweight (25 < BMI < 29.99), or obese (BMI ≥ 30) [22,23,24].
2.2. Statistical Analysis
The difference in the incidence of VAE between nilotinib-naïve patients (had received a TKI other than nilotinib) and patients who had received nilotinib as well as dasatinib-naïve patients (had received a TKI other than dasatinib) and patients who had received dasatinib for at least 98 months was estimated using Kaplan–Meier curves. The log-rank or Breslow test was applied to quantify differences in the time to the event (VAE) between groups.
To analyze the impact of the different TKIs according to the treatment line, (I) first-line imatinib (reference group) was compared against first-line nilotinib (II), first-line dasatinib (III), second-line nilotinib after first-line imatinib (IV), second-line dasatinib after first-line imatinib during at least 55 months (V), patients who received 2 lines of 2G-TKI (VI), second-line imatinib after a first-line 2G-TKI (VII), and patients who received 3 lines of treatment with imatinib as the first line during at least 30 months (VIII).
To estimate the 10-year cardiovascular risk, the Framingham risk score (FRS) was assessed at diagnosis and used to categorize patients into three groups: ≤10% likelihood of developing a VAE, 10–20% likelihood of developing a VAE, and ≥20% likelihood of developing a VAE [22].
A multivariable logistic regression analysis was applied to investigate the impact of covariates (risk factors, exposure to TKI, or previous VAE event) on the occurrence of the VAE as the response variable. The following risk factors at diagnosis were included in the model selection: FRS, cohort (I-VIII), and the presence of a previous VAE.
For comparison of the eight treatment groups, the differences in biochemical levels of lipids (total cholesterol, HDL-C, LDL-C, and triglycerides) between the time of diagnosis and after each treatment were analyzed using ANOVA with a Bonferroni post hoc test.
Statistical analyses were performed with IBM SPSS v.23 and the results were considered significant at p < 0.05.
3. Results
3.1. Patient Characteristics
Data were included from the Canarian CML Registry for 210 patients diagnosed with CP-CML and treated with a TKI. The median age at diagnosis was 52 years (range of 13–84 years) and 106 patients (50.5%) were male. In terms of CVRF, 92 patients (43.8%) received concomitant medication for AHT, 50 patients (23.8%) had DM (all of whom were receiving hypoglycemic medication), 19 patients (9.1%) were smokers, and 42 patients (20%) were ex-smokers. According to the BMI, 37 patients were classified as possessing a normal weight, 39 patients were overweight, and 55 were obese; 79 were unclassified due to insufficient information. According to the Framingham risk score (FRS), at the time of diagnosis, 147 patients (70%) were classified as possessing a low-risk FRS (<10% chance of suffering an atherosclerotic event), 38 patients (18.1%) as intermediate-risk (10–20% chance of suffering an atherosclerotic event), and 25 patients (11.9%) as high-risk (>20% chance of suffering an atherosclerotic event).
A total of 19 patients (9.1%) had a VAE while on TKI treatment, with the median age at the event of 70 years. Of these, 19 (100%) were known to have an underlying CVRF and 10 (52.6%) had received nilotinib as first- or second-line treatment (Table 1). The mean range of time to present a VAE from the start of the TKI treatment line was 53.9 months (range of 0.3–171 months).

Table 1.
VAE patient cases.
3.2. Incidence Analysis
When the patients who presented VAE were distributed according to cohorts, the highest incidence of VAE was seen for the cohort of patients who received three lines of treatment with first-line imatinib (30%, cohort VIII, Table 2). The cumulative incidence for first-line dasatinib (cohort III) and first-line imatinib with second-line nilotinib (cohort IV), both at 12.5%, was higher than for first-line nilotinib (8.1%) or first-line imatinib (7.3%), although only one patient who received dasatinib in the first line had a VAE. No VAEs were registered in the cohorts of patients who received first-line imatinib with second-line dasatinib (cohort V), two 2G-TKI lines (cohort VI), or first-line 2G-TKI with second-line imatinib (cohort VII).

Table 2.
Distribution of patients who developed a vascular adverse event (VAE) across the cohorts.
Analyzing the time to VAE according to first-line treatment with the Kaplan–Meier analysis, the VAE risk at 55 months was 3.8% for imatinib, 5.7% for nilotinib, and 25% for dasatinib (p = 0.06, log-rank; p = 0.025, Breslow).
The Kaplan–Meier analysis of the time to VAE showed a statistically significant difference in the risk of VAE at 98 months between the nilotinib-naïve patients (had received a TKI other than nilotinib, 5.5%) and patients who had received nilotinib (25.8%, p = 0.029, Figure 1a). The risk of VAE at 98 months was also significantly different for the dasatinib-naïve patients (8.7%) vs. patients who had received dasatinib (25.6%, p = 0.039, Figure 1b).
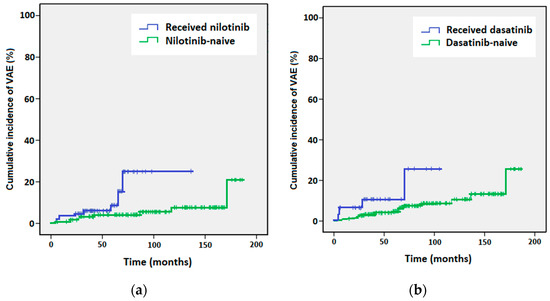
Figure 1.
Kaplan–Meier curves of cumulative incidence of vascular adverse events (VAEs) for (a) patients who received nilotinib (blue line) versus nilotinib-naïve patients (green line), and (b) patients who received dasatinib (blue line) versus dasatinib-naïve patients (green line).
Using an incidence pairwise comparison between the imatinib cohort (I) and the rest of the cohorts, only patients on second-line nilotinib after first-line imatinib (cohort IV) and patients with three lines of treatment with imatinib as the first line (cohort VIII) showed a statistically significant increase in the VAE risk (p = 0.029 and p < 0.0001, respectively, log-rank test). Comparison with the cohort of patients who received three lines of treatment with imatinib as the first line (VIII) was also statistically significant when the Breslow test was applied (p < 0.001).
3.3. Multivariable Logistic Regression
The evaluation of the impact of covariates (CVRF, exposure to TKI, or previous VAE) on the occurrence of VAEs as the response variable was carried out using multivariable logistic regression. The multivariable analysis showed that previous VAE was not significant for any of the cohorts. For the nilotinib-naïve/nilotinib and dasatinib-naïve/dasatinib cohorts, only FRS had a statistically significant association with VAE (Table 3). Considering the treatment cohorts, only the cohort of patients who received three lines of treatment with imatinib as the first line was significantly associated with occurrence of VAE (HR: 13.42, 95% CI: 2.15–83.78) when compared to first-line imatinib as a reference (cohort I).

Table 3.
Multivariable logistic regression.
Interestingly, FRS was associated with the occurrence of VAE for all the cohorts. Specifically, when comparing an FRS of 10–20 with ≤10, and ≥20 FRS with ≤10, the hazard ratios were 9.73 (95% CI: 2.43–38.99) and 7.40 (95% CI: 1.56–35.12), respectively.
3.4. Biochemical Lipid Analysis
A statistically significant elevation in TC and HDL-C and decrease in TG were observed for nilotinib-treated compared to nilotinib-naïve patients. However, only a statistically significant increase in HDL-C and a decrease in TG were observed for nilotinib-naïve patients before vs. after TKI treatment.
When comparing the lipid panels before and after treatment for the eight treatment cohorts, a statistically significant elevation in HDL-C (p < 0.05) and decline in TG (p < 0.01) were observed for first-line imatinib (cohort I), an elevation in HDL-C (p < 0.01) and a decrease in TG (p < 0.05) for first-line nilotinib (cohort II), an elevation in TC (p < 0.05) for first-line dasatinib (cohort III), an increase in TC (p < 0.01) and HDL-C (p < 0.01) for second-line nilotinib after first-line imatinib (cohort IV), an increase in LDL-C (p < 0.01) and decline in TG (p < 0.01) for second-line dasatinib after first-line imatinib (cohort V), an elevation in HDL-C (p < 0.01) for second-line imatinib after a first-line 2G-TKI (cohort VII), and a rise in TC (p < 0.05) for three lines of treatment with first-line imatinib (cohort VIII). In contrast, no significant differences in lipid levels were observed before and after treatment for two second-generation TKI treatment lines (cohort VI) (Table 4).

Table 4.
Biochemical risk factors for VAEs in patients under tyrosine kinase inhibitor treatment.
4. Discussion
In this retrospective study, the mean follow-up interval was 6 years (ranging from 3 months to 16 years). This extended follow-up period allowed late toxicities to be captured, such as the development of atherosclerotic lesions. This was one advantage of our study, since prior reports of VAE for patients with CML on TKI have predominantly been based on clinical trials with follow-up periods of less than 5 years. For example, one patient developed a VAE after 14.3 years of first-line imatinib treatment. However, it is important to acknowledge that the sample size for some of the treatment cohorts included in the analyses was relatively small. This limitation reduces the statistical power of this study.
The incidence of VAEs in patients with CP-CML is as yet unknown and it remains unclear if the incidence differs from that of the age-matched general population. Indeed, in our study, we observed a similar incidence of VAEs in our cohort of 210 patients with CP-CML who were treated with TKIs (9.1%) compared to the age-matched general population (10–20% for adults aged 50–70 years [25]), even though the patients in our cohort had CML and many had one or more CVRFs.
Nevertheless, consistent with prior reports, our analysis confirmed a higher incidence of VAEs in patients who were treated with nilotinib compared to those receiving other TKIs [15,16,18,26]. Specifically, a higher incidence of VAEs was observed for second-line nilotinib after first-line imatinib vs. first-line nilotinib (12.5% vs. 8.1%, respectively), and statistically significant differences in VAE were also observed when comparing nilotinib-naïve versus nilotinib-treated patients, and patients who received three lines of treatment with first-line imatinib versus first-line imatinib. When compared with patients on first-line imatinib, a statistically significant difference was only observed for patients who received nilotinib as second- or third-line TKI treatment, as previously reported [20]. Moreover, the multivariable logistic regression analysis did not identify treatment with nilotinib as a significant risk factor for developing a VAE. These results may suggest a latency period of nilotinib treatment before VAEs develop [14]. Indeed, the mean time to VAE from the TKI start was 54 months (for the whole cohort).
The results of the multivariable logistic regression analysis revealed that only one variable, the FRS, obtained a statistically significant hazard ratio for the development of a VAE. Interestingly, the hazard ratio for the 10–20 FRS group was higher than the hazard ratio for the ≥20 FRS group when compared to the ≤10 FRS group. This difference may be due to the closer follow up of patients with a higher FRS and/or use of prophylaxis (such as aspirin or statins). This hypothesis might also explain why previous VAE was not found to be a risk factor for any of the treatment cohorts. However, no significant difference in VAE incidence was observed between the 10–20 FRS and ≥20 FRS groups regardless of the treatment cohort (data not shown). Similarly, other studies have shown that the FRS or other CV risk stratification tools, such as the HFA/ICOS or the Systematic Coronary Risk Evaluation (SCORE), may be useful for predicting the risk of VAE for patients with CML on TKI treatment [27,28,29]. It is noteworthy that all patients with VAEs presented at least one CVRF at diagnosis. This observation underscores the importance of considering CV risk factors when choosing an appropriate TKI.
Consistent with previous studies [20,30,31], changes to lipid metabolism were observed with the TKI treatment start. Indeed, the first study of TKI effects on lipid profiles reported that of six patients with CML and hypercholesteremia (two of whom also had hypertriglyceridemia), all six had a long-lasting normalization of lipid levels while receiving imatinib [31]. For patients who received first-line imatinib in our study (cohort I), a decline in TG was also observed, although HDL-C levels increased. Meanwhile, a significant rise in TC and HDL-C and a decrease in TG were observed for patients who received nilotinib; contrary to previous reports, modification in LDL-C levels was not observed [30]. A significant increase in LDL-C levels was only identified for patients who received first-line imatinib with second-line dasatinib (cohort V), although only three patients received this treatment combination. Nevertheless, it remains to be determined if these changes in lipid metabolism were responsible for the development of a VAE.
Taken together, our observations are consistent with prior reports [18,20,32,33] that suggest patients at risk of developing VAEs during TKI therapy might be identifiable at the time of diagnosis. Our results support the estimation of the cardiovascular risk at the time of diagnosis in order to choose the most appropriate first-line treatment to prevent the appearance of VAEs [32]. For patients with intermediate and high cardiovascular risk, physicians should establish a prevention plan to diminish the cardiovascular risks throughout the TKI treatment [34]. The plan may include anti-ischemic/hypertensive drugs, statins, optimum control of DM (as needed), and increased patient monitoring, including perhaps lipid profiles. However, it is also important to monitor patients with low cardiovascular risk during TKI treatment to re-adapt strategies when required (i.e., if the cardiovascular risk increases to intermediate or high).
Finally, a potential strategy to reduce VAE risk among patients with CML could be the discontinuation of TKI treatment [35]. The global time of TKI treatment is an important variable when considering if a patient is eligible for TKI discontinuation, as is the duration of a deep molecular response, both associated with a higher probability of treatment-free remission [36].
5. Conclusions
A higher incidence of VAEs was observed for patients treated with nilotinib compared to patients treated with other TKIs and for patients who received three lines of TKI treatment.
We consider that the estimation of the cardiovascular risk at diagnosis is a powerful tool to determine if a patient with CML is likely to develop a VAE during TKI treatment. To this end, our results reveal that the FRS is useful. Changes in HDL-C and total cholesterol levels with TKI treatment may also be predictive for VAE. The assessment of cardiovascular risk at the time of diagnosis would allow hematologists and other specialists, as part of a multidisciplinary team, to develop a prevention plan to minimize the incidence and severity of VAEs.
Author Contributions
Conceptualization, M.T.G.-C., C.B.-S., A.S.-D., and M.N.S.P.; methodology, M.N.S.P., E.G.-P., and C.B.-S.; contributed data, S.L.L., J.D.G.S.M., N.H.S., M.G., G.G.B., M.T.-T., A.R., A.S.-D., and H.L.; formal analysis, M.N.S.P., R.S., and E.G.-P.; resources, M.N.S.P., R.S., S.S.-S., C.B.-S., and M.T.G.-C.; data curation, M.N.S.P., E.G.-P., S.S.-S., and P.E.-C.; writing—original draft preparation, M.N.S.P. and R.S.; writing—review and editing, M.N.S.P., R.S., C.B.-S., and M.T.G.-C.; funding acquisition, C.B.-S. and M.T.G.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Fundación DISA (OA18/016) and Incyte (OA19/010). The Canarian CML Registry was supported by Novartis and Bristol Myers Squibb.
Institutional Review Board Statement
All procedures were followed in accordance with the Helsinki Declaration and were approved on 17 July 2017 by the local ethics committee (ref. 170104).
Informed Consent Statement
Patients gave written informed consent for inclusion of their anonymized data in the registry and its retrospective analysis.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
R.S.’s travel expenses: Celgene, Gilead, Novartis. A.S.D., speaker: Novartis, Sanofi Genzyme. M.T.G.C., speaker bureau: BMS, Incyte, Novartis; research funding: Amgen, Astellas, Incyte, Novartis; training support: AstraZeneca, Gilead, Janssen. Other authors: no conflicts of interest to disclose. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bartram, C.R.; de Klein, A.; Hagemeijer, A.; van Agthoven, T.; Geurts van Kessel, A.; Bootsma, D.; Grosveld, G.; Ferguson-Smith, M.A.; Davies, T.; Stone, M. Translocation of c-ab1 oncogene correlates with the presence of a Philadelphia chromosome in chronic myelocytic leukaemia. Nature 1983, 306, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Hochhaus, A.; Baccarani, M.; Silver, R.T.; Schiffer, C.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Deininger, M.W.; Guilhot, F.; et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 2020, 34, 966–984. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.A.; Hochhaus, A.; Hughes, T.P.; Clark, R.E.; Etienne, G.; Kim, D.W.; Flinn, I.W.; Kurokawa, M.; Moiraghi, B.; Yu, R.; et al. Nilotinib vs. imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia 2012, 26, 2197–2203. [Google Scholar] [CrossRef] [PubMed]
- Saglio, G.; Kim, D.W.; Issaragrisil, S.; le Coutre, P.; Etienne, G.; Lobo, C.; Pasquini, R.; Clark, R.E.; Hochhaus, A.; Hughes, T.P.; et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N. Engl. J. Med. 2010, 362, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Shah, N.P.; Hochhaus, A.; Cortes, J.; Shah, S.; Ayala, M.; Moiraghi, B.; Shen, Z.; Mayer, J.; Pasquini, R.; et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N. Engl. J. Med. 2010, 362, 2260–2270. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Saglio, G.; Kantarjian, H.M.; Baccarani, M.; Mayer, J.; Boqué, C.; Shah, N.P.; Chuah, C.; Casanova, L.; Bradley-Garelik, B.; et al. Final 5-Year Study Results of DASISION: The Dasatinib Versus Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients Trial. J. Clin. Oncol. 2016, 34, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Giles, F.J.; le Coutre, P.D.; Pinilla-Ibarz, J.; Larson, R.A.; Gattermann, N.; Ottmann, O.G.; Hochhaus, A.; Radich, J.P.; Saglio, G.; Hughes, T.P.; et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia 2013, 27, 107–112. [Google Scholar] [CrossRef]
- Shah, N.P.; Guilhot, F.; Cortes, J.E.; Schiffer, C.A.; le Coutre, P.; Brümmendorf, T.H.; Kantarjian, H.M.; Hochhaus, A.; Rousselot, P.; Mohamed, H.; et al. Long-term outcome with dasatinib after imatinib failure in chronic-phase chronic myeloid leukemia: Follow-up of a phase 3 study. Blood 2014, 123, 2317–2324. [Google Scholar] [CrossRef]
- Douglas Smith, B.; Brummendorf, T.H.; Roboz, G.J.; Gambacorti-Passerini, C.; Charbonnier, A.; Viquiera, A.; Leip, A.; Giles, F.; Ernst, T.; Hochhaus, A.; et al. Efficacy of bosutinib in imatinib-resistant vs. dasatinib/nilotinib-resistant chronic phase chronic myeloid leukemia: Results from the Phase 4 BYOND Study. Blood 2019, 134, 1650. [Google Scholar] [CrossRef]
- Grupo Español de Leucemia Mieloide Crónica (GELMC). Manual Para el Control y el Tratamiento de los Pacientes con Leucemia Mieloide Crónica [Manual for the Control and Treatment of Patients with Chronic Myeloid Leukemia] (Spanish); Edición 2020; MFAR, Ed.; GELMC: Barcelona, Brazil, 2020. [Google Scholar]
- Hantschel, O.; Rix, U.; Superti-Furga, G. Target spectrum of the BCR-ABL inhibitors imatinib, nilotinib and dasatinib. Leuk. Lymphoma 2008, 49, 615–619. [Google Scholar] [CrossRef]
- Steegmann, J.L.; Baccarani, M.; Breccia, M.; Casado, L.F.; García-Gutiérrez, V.; Hochhaus, A.; Kim, D.W.; Kim, T.D.; Khoury, H.J.; Le Coutre, P.; et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia 2016, 30, 1648–1671. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.A.; Garcia Gonzalez, A.G.; Ault, P.; Mendoza, T.R.; Sailors, M.L.; Williams, J.L.; Huang, F.; Nazha, A.; Kantarjian, H.M.; Cleeland, C.S.; et al. Measuring the symptom burden associated with the treatment of chronic myeloid leukemia. Blood 2013, 122, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Hadzijusufovic, E.; Schernthaner, G.H.; Wolf, D.; Rea, D.; le Coutre, P. Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors. Blood 2015, 125, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.D.; Rea, D.; Schwarz, M.; Grille, P.; Nicolini, F.E.; Rosti, G.; Levato, L.; Giles, F.J.; Dombret, H.; Mirault, T.; et al. Peripheral artery occlusive disease in chronic phase chronic myeloid leukemia patients treated with nilotinib or imatinib. Leukemia 2013, 27, 1316–1321. [Google Scholar] [CrossRef]
- Le Coutre, P.; Rea, D.; Abruzzese, E.; Dombret, H.; Trawinska, M.M.; Herndlhofer, S.; Dörken, B.; Valent, P. Severe peripheral arterial disease during nilotinib therapy. J. Natl. Cancer Inst. 2011, 103, 1347–1348. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Kim, D.W.; Pinilla-Ibarz, J.; le Coutre, P.; Paquette, R.; Chuah, C.; Nicolini, F.E.; Apperley, J.F.; Khoury, H.J.; Talpaz, M.; et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 2013, 369, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Stuckey, R.; Segura-Díaz, A.; Sáez Perdomo, M.N.; Pérez Encinas, M.M.; González San Miguel, J.D.; Florido, Y.; Sánchez-Sosa, S.; López-Rodríguez, J.F.; Bilbao-Sieyro, C.; Gómez-Casares, M.T. Presence of Myeloid Mutations in Patients with Chronic Myeloid Leukemia Increases Risk of Cardiovascular Event on Tyrosine Kinase Inhibitor Treatment. Cancers 2023, 15, 3384. [Google Scholar] [CrossRef]
- Giles, F.J.; Rea, D.; Rosti, G.; Cross, N.C.P.; Steegmann, J.L.; Griskevicius, L.; le Coutre, P.; Coriu, D.; Petrov, L.; Ossenkoppele, G.J.; et al. Impact of age on efficacy and toxicity of nilotinib in patients with chronic myeloid leukemia in chronic phase: ENEST1st subanalysis. J. Cancer Res. Clin. Oncol. 2017, 143, 1585–1596. [Google Scholar] [CrossRef]
- Hochhaus, A.; Saglio, G.; Hughes, T.P.; Larson, R.A.; Kim, D.W.; Issaragrisil, S.; le Coutre, P.D.; Etienne, G.; Dorlhiac-Llacer, P.E.; Clark, R.E.; et al. Long-term benefits and risks of frontline nilotinib vs. imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia 2016, 30, 1044–1054. [Google Scholar] [CrossRef]
- Cortes, J.E.; Kim, D.W.; Kantarjian, H.M.; Brümmendorf, T.H.; Dyagil, I.; Griskevicius, L.; Malhotra, H.; Powell, C.; Gogat, K.; Countouriotis, A.M.; et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: Results from the BELA trial. J. Clin. Oncol. 2012, 30, 3486–3492. [Google Scholar] [CrossRef]
- World Health Organization. WHO Consultation on Obesity; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Erlinger, T.P. Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation 2004, 110, 738–743. [Google Scholar] [CrossRef]
- Aichberger, K.J.; Herndlhofer, S.; Schernthaner, G.H.; Schillinger, M.; Mitterbauer-Hohendanner, G.; Sillaber, C.; Valent, P. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. Am. J. Hematol. 2011, 86, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Caocci, G.; Mulas, O.; Abruzzese, E.; Luciano, L.; Iurlo, A.; Attolico, I.; Castagnetti, F.; Galimberti, S.; Sgherza, N.; Bonifacio, M.; et al. Arterial occlusive events in chronic myeloid leukemia patients treated with ponatinib in the real-life practice are predicted by the Systematic Coronary Risk Evaluation (SCORE) chart. Hematol. Oncol. 2019, 37, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Di Lisi, D.; Madaudo, C.; Alagna, G.; Santoro, M.; Rossetto, L.; Siragusa, S.; Novo, G. The new HFA/ICOS risk assessment tool to identify patients with chronic myeloid leukaemia at high risk of cardiotoxicity. ESC Heart Fail. 2022, 9, 1914–1919. [Google Scholar] [CrossRef]
- Fujioka, I.; Takaku, T.; Iriyama, N.; Tokuhira, M.; Kimura, Y.; Sato, E.; Ishikawa, M.; Nakazato, T.; Sugimoto, K.J.; Fujita, H.; et al. Features of vascular adverse events in Japanese patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors: A retrospective study of the CML Cooperative Study Group database. Ann. Hematol. 2018, 97, 2081–2088. [Google Scholar] [CrossRef]
- Rea, D.; Mirault, T.; Cluzeau, T.; Gautier, J.F.; Guilhot, F.; Dombret, H.; Messas, E. Early onset hypercholesterolemia induced by the 2nd-generation tyrosine kinase inhibitor nilotinib in patients with chronic phase-chronic myeloid leukemia. Haematologica 2014, 99, 1197–1203. [Google Scholar] [CrossRef]
- Gottardi, M.; Manzato, E.; Gherlinzoni, F. Imatinib and hyperlipidemia. N. Engl. J. Med. 2005, 353, 2722–2723. [Google Scholar] [CrossRef]
- Breccia, M.; Colafigli, G.; Molica, M.; Alimena, G. Cardiovascular risk assessments in chronic myeloid leukemia allow identification of patients at high risk of cardiovascular events during treatment with nilotinib. Am. J. Hematol. 2015, 90, E100–E101. [Google Scholar] [CrossRef]
- Rea, D.; Mirault, T.; Raffoux, E.; Boissel, N.; Andreoli, A.L.; Rousselot, P.; Dombret, H.; Messas, E. Usefulness of the 2012 European CVD risk assessment model to identify patients at high risk of cardiovascular events during nilotinib therapy in chronic myeloid leukemia. Leukemia 2015, 29, 1206–1209. [Google Scholar] [CrossRef]
- García-Gutiérrez, V.; Jiménez-Velasco, A.; Gómez-Casares, M.T.; Sánchez-Guijo, F.; López-Sendón, J.L.; Steegmann Olmedillas, J.L. Cardiovascular management of patients with chronic myeloid leukemia from a multidisciplinary per-spective, and proposing action protocol by consensus meeting. Med. Clin. 2016, 146, 561.e1–561.e8. [Google Scholar]
- Mauro, M.J. Lifelong TKI therapy: How to manage cardiovascular and other risks. Hematol. Am. Soc. Hematol. Educ. Program 2021, 2021, 113–121. [Google Scholar]
- Stuckey, R.; López-Rodríguez, J.F.; Sánchez-Sosa, S.; Segura-Díaz, A.; Sánchez-Farías, N.; Bilbao-Sieyro, C.; Gómez-Casares, M.T. Predictive indicators of successful tyrosine kinase inhibitor discontinuation in patients with chronic myeloid leukemia. World J. Clin. Oncol. 2020, 11, 996–1007. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).