Abstract
Post-transplant lymphoproliferative disease is a rare disorder with an annual incidence of 0.5% to 3.7%. Development of this disorder carries with it a poor prognosis. In this report, we describe a rare case of post-transplant primary cutaneous T-cell lymphoma (PT-CTCL) mycosis fungoides stage IIB in a patient following kidney transplantation, as well as a review of PT-CTCL reported in the literature. The treatment following diagnosis included bexarotene, cyclosporine, and prednisone. Currently, the patient is free from disease. This information aims to add to the knowledge of the prevalence and management of PT-CTCL.
1. Introduction
The success of solid organ transplantation is supported by a continuing progressive increase each year. In 2022, the Organ Procurement and Transplant Network (OPTN) reported 42,888 transplant procedures performed, with 25,499 of those consisting of kidney transplants. The mortality rate for first-time recipients of a kidney transplant is 20.5%, with the risk declining as the time since transplant increases. Cardiovascular disease is the main cause of death in kidney transplants, with cancer-related death comprising the second most common cause []. A rare complication from transplantation includes the development of post-transplant lymphoproliferative disease (PTLD), which has an annual incidence of 0.5% to 3.7% []. The incidence of PTLD varies and is dependent on the organ transplanted, with multiorgan/intestinal transplants being most frequent and kidney transplant being the least frequent [].
PTLDs have been grouped by the World Health Organization into four different subtypes, including early lesions, polymorphic PTLD, monomorphic PTLD, and classic Hodgkin lymphoma-type PTLD. PTLD originating from T-cell lineage is less common. Additionally, primary cutaneous PTLDs of both B- and T-cell origins comprise the least frequent subgroup of PTLDs, with fewer than 100 reports found in the literature [].
This report describes a rare case of post-transplant primary cutaneous T-cell lymphoma (PT-CTCL) of mycosis fungoides stage IIB development following kidney transplantation. Information regarding this subject contributes to the knowledge of treatments for patients with this condition.
2. Case Report
A 61-year-old male with a history of kidney transplantation in October of 2012 from stage two hypertension-induced end stage renal disease (ESRD) presented to the dermatology outpatient clinic with a concern of a greater than 2-year rash and tumor of the right leg. At presentation, the patient was 9 years post-transplant and his immunosuppression regimen included mycophenolate mofetil 250 mg twice daily, cyclosporine 100 mg twice daily, and prednisone 5 mg daily. Clinical examination showed numerous red to violaceous indurated plaques scattered on the flanks and proximal upper and lower extremities with scale and secondary impetiginization (Figure 1). Prominently, a large 5+ cm tumor with drainage was present on the right medial thigh. Prior to presentation, the patient had been self-treating the rash with over-the-counter (OTC) hydrocortisone that initially controlled the spread and itch. Initial biopsy of the rash showed robust spongiotic dermatitis with minimal epidermotropism and a mixed dermal inflammatory infiltrate, favored to represent an eczematous drug eruption (Figure 2A). Given the persistent clinical concern for CTCL, additional biopsies and immunophenotyping were performed a year later, which again showed similar histopathology with marked epidermal hyperplasia, prominent spongiosis, and serum crusting. The pathology also revealed focal T-lymphocytes in the epidermis, with CD4 subsets slightly predominating over CD8 (Figure 2F,G). CD30 was not detected in the neoplastic lymphocytes, and EBV in situ hybridization, performed due to the patient’s post-transplant status, was negative. A subsequent T-Cell receptor gene rearrangement assay was positive for clonality, supporting a diagnosis of spongiotic mycosis fungoides. Clinical staging to determine the presence of nodal or other organ involvement was assessed via PET-CT. The PET-CT results showed multiple areas of uptake along areas of skin, thickening on the bilateral lower extremities with maximal uptake in the right distal anteromedial leg, but did not identify nodal or visceral involvement (Figure 3). The tumor burden was consistent with ISCL stage IIB with tumor involvement (T3), a BSA of approximately 20%, and lack of involvement of nodes or other organs. These findings are consistent with primary cutaneous T-cell lymphoma without indication of metastasis.
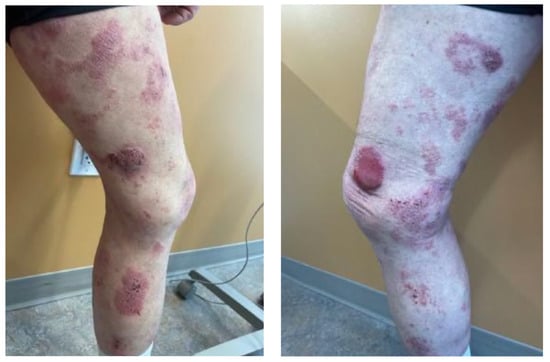
Figure 1.
Skin manifestation. Extensive involvement of CTCL with tumor, plaques and patches affecting bilateral lower legs. Thick plaques and patches (left panel) shown on the right leg. Thick eroded tumor present on the right anteromedial knee (right panel) showed maximal uptake on the PET-CT scan (Figure 3).
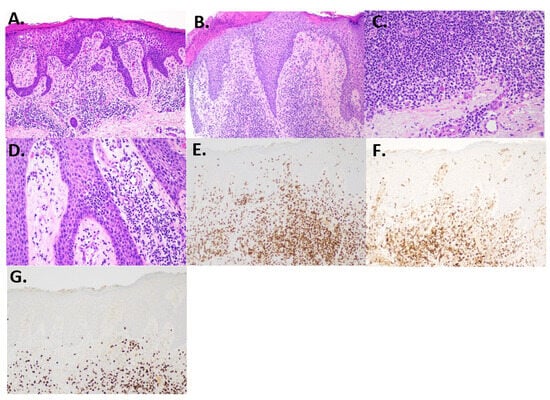
Figure 2.
Histology and immunohistochemistry. (A) Initial biopsy showing epidermal hyperplasia, spongiosis, and a mixed dermal inflammatory infiltrate (H and E, 100×). (B) Second biopsy showed acanthosis and spongiosis with some lymphocyte exocytosis (H and E, 100×). (C) Dermal infiltrate showed small lymphocytes, many plasma cells, and rare eosinophils (H and E, 200×). (D) Focal epidermotropism with slight lymphocyte atypia (H and E, 200×). (E) CD3-highlighted T-cells, including some within the epidermis (CD3, 100×). (F) CD4 shows predominance over CD8. (G) CD8 is reduced compared to CD4 (100×).
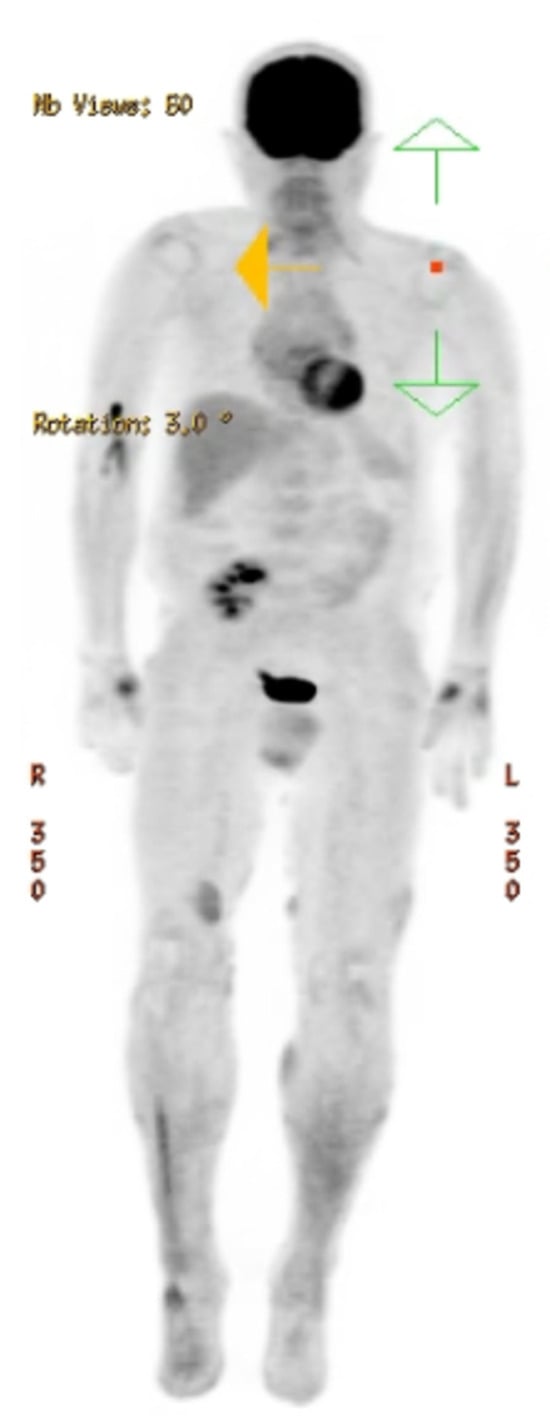
Figure 3.
Radiologic imaging. PET-CT vertex to feet showing multiple areas of cutaneous skin increased SUV signal enhancement in the bilateral lower extremities, particularly in the right distal anteromedial leg. No evidence of avid lymphadenopathy or abnormal signal enhancement in the spleen.
After diagnosis, the immunosuppressive regimen was revised. To treat his CTCL, the patient started on bexarotene 75 mg twice per day by mouth. Bexarotene was chosen due to its lack of immunosuppressive effects, FDA approval for prevention of CTCL progression, and reports of effectiveness in patients with PT-CTCL []. Cyclosporine 100 mg twice daily and prednisone 5 mg daily were continued to prevent graft rejection. Mycophenolate was discontinued. Additionally, topical corticosteroids (triamcinolone 0.1% ointment and fluocinonide 0.1% cream) were added for relief of pruritus. At the patients most recent follow-up, three years post diagnosis, the patient maintains good control of all skin lesions and has a well-controlled disease. Figure 4 can be used to best understand the overall timeline of clinical events.
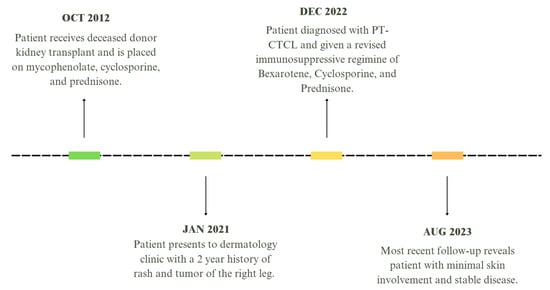
Figure 4.
Timeline of patient presentation and treatment.
3. Discussion
The risk of PTLD increases from altered immunity secondary to iatrogenically induced immune suppression associated with reduced tumor immune surveillance, which is necessary to the survival of the transplanted graft. Additionally, excessive immunosuppression increases the risk of serious infections. Thus, management of PTLDs is challenging due to the requirement of exquisitely balanced therapies that minimize the risk of graft rejection with undesired excessive lymphoproliferation. Some success has been achieved through a combination of reduction or alteration of immunosuppressive medication and local radiotherapy for improving graft survival, but the potential for rapidly progressing disease remains high [].
The clinical presentation of PTLD is highly heterogenous and is influenced by immunosuppression regimens and Epstein–Barr virus (EBV) status, which together affect the overall prognosis [,,,]. Those arising from B-cell-origin lymphomas comprise the majority of PTLDs. Of those from B-cells, EBV associations can be detected in 33–48%, which have poorer prognoses []. EBV-associated PTLD is also linked to an early onset of disease. EBV-positive patients often develop PTLD one year post transplant, while EBV-negative patients who develop PTLD often display a late onset, between 5 and 15 years post transplant [].
PTLD is an unfortunate complication of organ transplantation. The most common variant of PTLD originates from B-cells and develops most often early after transplant compared to PTLD originating from other immune cell types, and is often associated with EBV. In patients who develop PTLD after a prolonged post-transplant period, EBV is less frequently detected []. The expression of CD20 in B-cell PTLDs presents the ability for the use of targeted treatment with rituximab.
PTLDs arising from T-cells are less frequently observed. The spectrum of PTLD T-cell malignancies is heterogenous, and those that have been described are summarized in Table 1. Anaplastic large cell lymphoma (ALCL) is the most frequent variant reported in the literature (Table 1). Other less common variants include peripheral T-cell lymphoma (PTCL), which has a similar reported incidence as CTCL, as well as hepatosplenic gamma delta and rare T-cell variants (Table 1). The treatment of T-cell PTLD is challenging and the survival outcome, as summarized in Table 1, is dire for most patients.
PT-CTCL remains an infrequent malignancy, representing approximately 5% of PTLD cases involving the skin. Astute recognition of this variant is essential to comprehensively characterize the spectrum of PTLD and develop approaches for treatment. A meta-analysis describing the only 14 cases of PT-CTCL reported in the literature from 1992 to 2005 discovered the mean age of diagnosis to be 57.2 years old, with a mean time to diagnosis post transplant of 5.8 years. Of these 14 patients, 13/14 had received a renal transplant, with 1 patient receiving a heart transplant. Overall, the mortality rate was poor with six of the patients dying in less than a year and only four patients achieving complete remission. This same study looked specifically at skin findings related to MF and saw an increasing prevalence of erythroderma in the PT-CTCL patients. Frequent presentation with erythroderma and a high rate of mortality in PT-CTCL patients is uncharacteristic of MF, which more commonly includes patches, plaques, and an indolent disease course [].
This case represents a patient that developed PT-CTCL post kidney transplant that follows an uncommon clinical progression compared to those reported in the current literature. The development of symptoms was first noted in this patient 9 years post transplant, while the final diagnosis was not determined until 10 years post transplant. This time frame is greater than 3 years past the average diagnosis of other reports from patients with the same condition. Additionally, this patient presented with patches and plaques involving 20% BSA, which contrasts previously reported cases of predominant erythroderma. According to the patient, pruritis and skin discomfort was mild and was maintained with OTC topical corticosteroids for at least a year. The clinical presentation overall was indolent with a relatively slow development of new lesions. Indolent presentation of PT-CTCL is uncommon, with reports by Ravat et al. [] noting an average survival rate of only 10.2 months in a review of 23 patients.
In their series of eight patients from 1998 to 2013, Shimshak et.al. reported that the most common variant of PT-CTCL displays the folliculotropic variant, with positivity in CD3, CD4, and CD5, loss of CD7 expression, and negativity for CD30 and EBV []. The majority of patients in this study comprised liver transplant patients, which may explain the difference in presentation from previous reports of patients with erythroderma. A case series of 23 patients with PT-CTCL from Ravat et.al. reported the most common histological subtype as primary cutaneous anaplastic large cell lymphoma (PCALCL) []. This case series consisted of a majority of kidney transplant patients. Overall, cases of PT-CTCL exhibit a variety of clinical presentations and subtypes that may favor a specific organ transplant. A review of our case via biopsy revealed the histology of spongiotic MF in a renal transplant patient that presented with an indolent disease course. Immunohistochemistry was positive for CD3 and CD4, and negative for CD30 with negative CD7 staining.
Besides reduction of immunosuppression, no established treatment recommendations from the NCCN exist for CD30- cases of PTLD of T-cell origin. However, brentuximab with cyclophosphamide, doxorubicin, and prednisone has been suggested for CD30+ cases []. Due to this, the reduction, modification, or discontinuation of immunosuppressive agents is often the initial treatment approach for PT-CTCL. In addition to these therapeutic adjustments, patients with classical forms of MF are often treated with skin-directed therapies (topical corticosteroids, PUVA) []. Our patient’s treatment regimen consisted of reduction of immunosuppressors (cyclosporine, prednisone), discontinuation of mycophenolate, addition of bexarotene, and topical fluocinonide and triamcinolone. Bexarotene treatment was chosen as it is not immunosuppressing, is FDA-approved for CTCL to control the progression of the disease, and has been reported to be effective in patients with PT-CTCL []. In evaluations of our patient, PET-CT examination did not uncover systemic involvement and showed the PT-CTCL to be limited to the skin. Follow-up evaluation showed improvement in the skin lesions with bexarotene and topical treatment.
Although rare, PT-CTCL may develop during long-term treatment of transplant patients. Cutaneous lesions and rashes may be the initial presentation of this disease and can often lead to serious complications in patients, as reported among others in the literature. Skin biopsy analysis with immunohistochemistry is critical for accurate diagnoses. In this case, we presented a patient who developed spongiotic MF stage IIB nine years post kidney transplant with an indolent course, skin-limited disease and response to bexarotene and topical treatment. The management and treatment of PT-CTCL can vary, and this report contributes to the heterogenous presentation and understanding of these diseases to improve patient outcomes (Table 1).

Table 1.
Literature review of PT-CTCL.
Table 1.
Literature review of PT-CTCL.
| Author | Lymphoma Subtype | Organ Transplant | Onset Post-Transplant | Survival | Clinical Presentation | Management |
|---|---|---|---|---|---|---|
| Singavi [] | CTCL mycosis fungoides type | Allogenic HSCT | 6 years | 1 year after Dx | Asymptomatic eczema-like cutaneous lesions | No change in therapy |
| Santos-Briz [] | CTCL mycosis fungoides type | Renal | Preceded transplant | Indolent 6 years post transplant | Nodular skin lesion | Narrow band UVB (nbUVB) phototherapy |
| Griffin [] | Folliculotropic mycosis fungoides | Cardiac | N/A | N/A | Inflamed and pruritic skin lesions | Oral bexarotene, nbUVB, topical retinoid |
| Rogers [] | Mycosis fungoides | Small bowel and liver | 10 years | DOD 1 year | Pruritic scaly plaque | Topical steroids, UVB, EPOCH chemotherapy **** |
| Lewis [] | MF stage IB | Liver | 5 years | Complete remission at 16 years | Annular skin lesion | Topical nitrogen mustard, bexarotene, fenofibrate, levothyroxine, ROI |
| Al Airoush [] | Sezary syndrome | Liver | 13 years | Not mentioned | Pruritic eczematous dermatitis | ROI |
| Rajakariar [] | ALCL, ALK(-) | Renal | 10 years | DOD 6 weeks | Pyrexia of unknown origin (POU) and nodular skin lesion | Methylprednisolone |
| Coyne [] | ALCL, ALK (-) | Renal | 2 years | DOD 18 months | Nodular skin lesions, fever | ROI; increased prednisolone and acyclovir |
| Coyne [] | ALCL, ALK (-) | Renal | 6 years | DOD at 15 months | Ulcerating skin lesions | VAPEC-B chemotherapy followed by radiotherapy ** |
| Coyne [] | ALCL, ALK (+) | Renal | 9 years | DOD “several months post Dx” | Multinodular skin lesion | Discontinuation of cyclosporin; CHOP chemotherapy |
| Lucioni [] | ALCL, ALK (-) | Cardiac | 9 years | Complete remission at 49 mo f/u | Nodular skin lesions | External radiotherapy; ROI; IgG; IFN-alpha; CHOP |
| Magro [] | ALCL, ALK(?) | Liver | 15 years | DOD shortly after Dx | Lower extremity edema | Death before change of Tx |
| Magro [] | ALCL, ALK(-) | Liver | 7 years | Remission at 3-year f/u | Nodular skin lesions | ROI |
| Balachandran [] | ALCL, ALK(-) | Renal | 10 years | Complete remission at 2-year f/u | Posterior mediastinum | CHOP |
| Treaba [] | ALCL, ALK(-) | Renal | 6 years | Alive at 5 mo f/u with residual disease | Nausea, fever, diarrhea, vomiting, LE pitting edema | CHOP |
| Salama [] | ALCL, ALK(-) | Renal | 2 years | Death 22 months after Dx | Nodular skin lesions | ROI; radiation; chlorambucil |
| Salama [] | ALCL, ALK(-) | Renal | 8 years | DOD at 3 years | Skin nodules | ROI, herbal remedies, local radiation, etoposide |
| Belloni-Fortina [] | ALCL, CD30+ ALK(ND) | Cardiac | 11 years | Complete remission at 2 years | Nodular skin lesion | Surgical removal; radiotherapy |
| Ward [] | ALCL, CD30+ | Renal | 56 months | DOD | Erythema with papules and hyperpigmented nodules | Prednisone, cyclosporin A, azathioprine |
| Seckin D [] | ALCL, CD30+ | Renal | 10 months | Remission at 5 months | Nodule | Prednisone, cyclosporin, azathioprine |
| Cooper SM [] | ALCL, CD30+ | Renal | 66 months | DOD | Nodules | Mycophenolate mofetil, prednisone, cyclosporin A |
| Kim HK [] | ALCL, CD30+ | Renal | 16 years | Complete remission at 10 months | Polypoid mass | Prednisone, cyclosporin A, azathioprine |
| De Nisi MC [] | ALCL, CD30+ | Cardiac | 60 months | Complete remission | Nodule | Mycophenolate mofetil, tacrolimus, prednisone |
| Albrecht [] | PTCL-NOS | Autologous stem cell | 3 months | “Good response” | Nodular skin lesions | Gemcitabine chemotherapy |
| Kajimoto [] | PTCL-NOS (with pre-existing DLBCL) | Autologous HSCT | 4 years | Not mentioned | Multiple lung nodules on CT | Not mentioned |
| Rajakarjar [] | Hepato-splenic gamma delta type | Renal | 7 years | DOD 2 weeks | POU and jaundice | Death before start of chemotherapy |
| Rajakarjar [] | Hepato-splenic gamma delta type | Renal | 8 years | DOD 4 months | AIHA | ROI |
| Draoua [] | Hepato-splenic gamma delta type | Cardiac | 9 years | DOD 9 months | N/A | ROI; CHOP x4 ESHAP; mitoxantrone/Navelbine/MTX/leukovorin |
| Lin [] | ƴδ T-cell lymphoma | Kidney | 5 years | Rapidly following Dx | Cough, sinus congestion, fever, night sweats | ROI, ganciclovir, 2-chlorodeoxyadenosine, G-CSF |
| Tey [] | Hepato-splenic | Cardiac | 15 years | Alive at 8-year f/u | Isolated neutropenia | Reduction of immunosuppression (ROI) HyperCVAD and MTX/HiDAC chemotherapy * |
| Elstrom [] | Widespread T-cell PTLD | Renal Pancreas | 2 years | Controlled at 23-month f/u | Abdominal pain, fever, weight loss, HA | ROI; CHOP; bexarotene |
| Haldas [] | T-cell PTLD | Cardiac | 8 years | Rapidly following Dx | Fever and chills | Death before start of Tx |
| Cardwell [] | PTCL-NOS | Cardiac | 21 months | Alive at 3 years, with relapse | Fevers and progressive dermatitis | ROI, chemo (unspecified), radiation, HSCT |
| Rajakarjar [] | Extranodal NK | Renal | 6 years | DOD 2 months | POU | Death before start of chemotherapy |
| Dalal [] | T-cell lymphoma | Kidney | 15 years | DOD 2 days after Dx | Thrombocytopenia and neutropenia | Comfort care |
| Draoua [] | T-cell lymphoblastic | Cardiac | 4 years | Alive at 48-month f/u | N/A | SWOG 9400 protocol |
| Kaminska [] | T-cell lymphoma | Renal | 4 years | DOD 14 months | Malaise, night sweats, fever, abdominal pain, weight loss | Switch to sirolimus and prednisone then CHOP |
| Kaminska [] | T-cell lymphoma | Renal | 4 years | Resolved in 12 months | Skin tumors | Switch to sirolimus and prednisone then surgery |
| Azhir [] | Diffuse large T-cell lymphoma | Renal | 4 years | Remission at 11-month f/u | Fever | ROI; gancyclovir; adriamycin, cyclophosphamide, vindesine, and prednisone. |
| Wang [] | Diffuse large T-cell lymphoma | Bone marrow | 2 years | Controlled 19 months after Dx | Bilateral cervical lymphadenopathy | All treatment stopped for personal reasons |
| Bregman [] | Subcutaneous panniculitic-like T-cell lymphoma | Cardiac | 3 years | DOD at 7.5 months | Nodular skin lesions | No change in Tx |
* HyperCVAD consisted of cyclo-phosphamide 300 mg/m2 every 12 h for six doses (days 1–3), mesna 1800 mg/m2 over 3 days (days 1–3), vincristine 2 mg (days 4 and 11), doxorubicin 50 mg/m2 (day 4), and dexamethasone 40 mg/day (days 1–4 and 11–14). MTX/HiDAC cycle consisted of methotrexate 1 g/m2 over 24 h with leucovorin rescue, and Ara-C 3 g/m2 every 12 h for four doses (days 2–3). Both cycles included intrathecal methotrexate 12 mg on day 2 and Ara-C 100 mg on day 8. ** Chemotherapy regimen consisting of vincristine, doxorubicin (adriamycin), prednisolone, etoposide, cyclophosphamide, and bleomycin. **** Etoposide, prednisone, vincristine, and doxorubicin hydrochloride.
Author Contributions
Conceptualization, J.P. and H.K.W.; writing—original draft preparation, J.P.; writing—review and editing, H.K.W. and S.S.; visualization, J.P., H.K.W. and S.S.; supervision, H.K.W.; project administration, H.K.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the UAMS IRB deemed that case report is not human research. No intervention nor study proposed. UAMS #276254.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ying, T.; Shi, B.; Kelly, P.J.; Pilmore, H.; Clayton, P.A.; Chadban, S.J. Death after Kidney Transplantation: An Analysis by Era and Time Post-Transplant. JASN 2020, 31, 2887–2899. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.; Viseux, V.; Denoeux, J.P.; Bagot, M. Post-transplant cutaneous T-cell lymphomas. Crit. Rev. Oncol./Hematol. 2005, 56, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Singavi, A.K.; Harrington, A.M.; Fenske, T.S. Post-transplant Lymphoproliferative Disorders. In Non-Hodgkin Lymphoma. Cancer Treatment and Research; Springer: Cham, Germany, 2015; pp. 305–327. [Google Scholar] [CrossRef]
- Seçkin, D.; Barete, S.; Euvrard, S.; Francès, C.; Kanitakis, J.; Geusau, A.; Del Marmol, V.; Harwood, C.A.; Proby, C.M.; Ali, I.; et al. Primary cutaneous posttransplant lymphoproliferative disorders in solid organ transplant recipients: A multicenter European case series. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2013, 13, 2146–2153. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.J.; Huang, S.; Duvic, M. Oral bexarotene for post-transplant cutaneous T-cell lymphoma. Dermatol. Ther. 2017, 30, e12524. [Google Scholar] [CrossRef] [PubMed]
- Ravat, F.E.; Spittle, M.F.; Russell-Jones, R. Primary cutaneous T-cell lymphoma occurring after organ transplantation. J. Am. Acad. Dermatol. 2006, 54, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Opelz, G.; Dohler, B. Lymphomas after solid organ € transplantation: A collaborative transplant study report. Am. J. Transplant. 2004, 4, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Opelz, G.; Henderson, R. Incidence of non-Hodgkin lymphoma in kidney and heart transplant recipients. Lancet 1993, 342, 1514–1516. [Google Scholar] [CrossRef]
- Dierickx, D.; Habermann, T.M. Post-transplantation lymphoproliferative disorders in adults. N. Engl. J. Med. 2018, 378, 549–562. [Google Scholar] [CrossRef]
- Sprangers, B.; Riella, L.V.; Dierickx, D. Posttransplant Lymphoproliferative Disorder Following Kidney Transplantation: A Review. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2021, 78, 272–281. [Google Scholar] [CrossRef]
- Alabdulbaqi, M.; Toupin, M.; Berardi, P.; McCurdy, A. Anaplastic large cell lymphoma as a posttransplant lymphoproliferative disorder in a renal transplant patient: A case report. EJHaem 2020, 1, 364–367. [Google Scholar] [CrossRef]
- Elstrom, R.L.; Andreadis, C.; Aqui, N.A.; Ahya, V.N.; Bloom, R.D.; Brozena, S.C.; Olthoff, K.M.; Schuster, S.J.; Nasta, S.D.; Stadtmauer, E.A.; et al. Treatment of PTLD with rituximab or chemotherapy. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2006, 6, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Shimshak, S.; Dai, C.; Comfere, N.; Sokumbi, O. Characterization of primary cutaneous T-cell lymphoma following solid organ transplantation. Int. J. Dermatol. 2023, 62, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.; O’Connor, O.A.; Pro, B.; Illidge, T.; Fanale, M.; Advani, R.; Bartlett, N.L.; Christensen, J.H.; Morschhauser, F.; Domingo-Domenech, E.; et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): A global, double-blind, randomised, phase 3 trial. Lancet 2019, 393, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Santos-Briz, A.; Romo, A.; Antúnez, P.; Román, C.; Alcoceba, M.; Garcia, J.L.; Vazquez, L.; González, M.; Unamuno, P. Primary cutaneous T-cell lymphoproliferative disorder of donor origin after allogeneic haematopoietic stem-cell transplantation. Clin. Exp. Dermatol. 2009, 34, e778–e781. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.B.; Hajar, T.; Simpson, E.; White, K.P.; Shinohara, M.M. Management of Cutaneous T-Cell Lymphoma/Mycosis Fungoides Occurring in the Setting of Solid Organ Transplantation: Report of 2 Cases. Clin. Lymphoma Myeloma Leuk. 2020, 20, e39–e42. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.S.; McGevna, L.; Cook, D.L. Pediatric cutaneous T-cell post-transplant lymphoproliferative disorder: Case report and review of the literature. J. Cutan. Pathol. 2018, 45, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Al Ajroush, N.; Rafique Sheikh, K.; Kadry, R.; Almutawa, A.; Alfadley, A. T-cell lymphoma/Sézary syndrome in a liver transplant recipient. J. Cutan. Med. Surg. 2012, 16, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Rajakariar, R.; Bhattacharyya, M.; Norton, A.; Sheaff, M.; Cavenagh, J.; Raftery, M.J.; Yaqoob, M.M. Post transplant T-cell lymphoma: A case series of four patients from a single unit and review of the literature. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2004, 4, 1534–1538. [Google Scholar] [CrossRef]
- Coyne, J.D.; Banerjee, S.S.; Bromley, M.; Mills, S.; Diss, T.C.; Harris, M. Post-transplant T-cell lymphoproliferative disorder/T-cell lymphoma: A report of three cases of T-anaplastic large-cell lymphoma with cutaneous presentation and a review of the literature. Histopathology 2004, 44, 387–393. [Google Scholar] [CrossRef]
- Lucioni, M.; Ippoliti, G.; Campana, C.; Cavallini, D.; Incardona, P.; Viglio, A.; Riboni, R.; Viganò, M.; Magrini, U.; Paulli, M. EBV positive primary cutaneous CD30+ large T-cell lymphoma in a heart transplanted patient: Case report. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2004, 4, 1915–1920. [Google Scholar] [CrossRef]
- Magro, C.M.; Weinerman, D.J.; Porcu, P.L.; Morrison, C.D. Post-transplant EBV-negative anaplastic large-cell lymphoma with dual rearrangement: A propos of two cases and review of the literature. J. Cutan. Pathol. 2007, 34 (Suppl. S1), 1–8. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, I.; Walker, J.W., Jr.; Broman, J. Fine needle aspiration cytology of ALK1(-), CD30+ anaplastic large cell lymphoma post renal transplantation: A case report and literature review. Diagn. Cytopathol. 2010, 38, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Treaba, D.; Assad, L.; Goldberg, C.; Loew, J.; Reddy, V.B.; Kluskens, L.; Gattuso, P. Anaplastic T large cell lymphoma diagnosed by exfoliative cytology in a post renal transplant patient. Diagn. Cytopathol. 2002, 27, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Salama, S.; Todd, S.; Cina, D.P.; Margetts, P. Cutaneous presentation of post-renal transplant lymphoproliferative disorder: A series of four cases. J. Cutan. Pathol. 2010, 37, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Salama, S. Primary “cutaneous” T-cell anaplastic large cell lymphoma, CD30+, neutrophil-rich variant with subcutaneous panniculitic lesions, in a post-renal transplant patient: Report of unusual case and literature review. Am. J. Dermatopathol. 2005, 27, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Belloni-Fortina, A.; Montesco, M.C.; Piaserico, S.; Bordignon, M.; Tona, F.; Feltrin, G.; Alaibac, M. Primary cutaneous CD30+ anaplastic large cell lymphoma in a heart transplant patient: Case report and literature review. Acta Derm.-Venereol. 2009, 89, 74–77. [Google Scholar] [CrossRef]
- Ward, H.A.; Russo, G.G.; McBurney, E.; Millikan, L.E.; Boh, E.E. Posttransplant primary cutaneous T-cell lymphoma. J. Am. Acad. Dermatol. 2001, 44, 675–680. [Google Scholar] [CrossRef]
- Seckin, D.; Demirhan, B.; Oguz Gulec, T.; Arikan, U.; Haberal, M. Posttransplantation primary cutaneous CD30 (Ki-1)-positive large-cell lymphoma. J. Am. Acad. Dermatol. 2001, 45, S197–S199. [Google Scholar] [CrossRef]
- Cooper, S.M.; Turner, G.D.H.; Hollowood, K.; Gatter, K.; Hatton, C.; Gray, D.; Russell-Jones, R.; Wojnarowska, F. Primary cutaneous large cell CD30+ lympho-ma in a renal transplant recipient. Br. J. Dermatol. 2003, 149, 426–428. [Google Scholar] [CrossRef]
- Kim, H.K.; Jin, S.Y.; Lee, N.S.; Won, J.H.; Park, H.S.; Yang, W.I. Posttransplant primary cutaneous Ki-1 (CD30)+/CD56+ anaplastic large cell lymphoma. Arch. Pathol. Lab. Med. 2004, 128, 96–99. [Google Scholar] [CrossRef]
- De Nisi, M.C.; D’Amuri, A.; Lalinga, A.V.; Occhini, R.; Biagioli, M.; Miracco, C. Posttransplant primary cutaneous CD30 (Ki-1)-positive anaplastic large T-cell lymphoma. A case report. Br. J. Dermatol. 2005, 152, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, H.; Woodroof, J.M.; Reyes, R.; Powers, B.C.; Fraga, G.R. CD30 expression in cutaneous B-cell and post-transplant peripheral T-cell lymphoma: Report of 2 cases. Dermatol. Online J. 2014, 20. [Google Scholar] [CrossRef]
- Kajimoto, Y.; Terasaki, Y.; Terasaki, M.; Kunugi, S.; Okabe, Y.; Wakita, S.; Inokuchi, K.; Shimizu, A. T-cell lymphoma with a granulomatous lesion of the lungs after autologous hematopoietic stem cell transplantation for Epstein-Barr virus-positive diffuse large B-cell lymphoma: A unique rare case of metachronous B-cell and T-cell lymphoma. Diagn. Pathol. 2020, 15, 125. [Google Scholar] [CrossRef] [PubMed]
- Draoua, H.Y.; Tsao, L.; Mancini, D.M.; Addonizio, L.J.; Bhagat, G.; Alobeid, B. T-cell post-transplantation lymphoproliferative disorders after cardiac transplantation: A single institutional experience. Br. J. Haematol. 2004, 127, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Moore, J.O.; Mann, K.P.; Traweek, S.T.; Smith, C. Post transplant CD8+ gammadelta T-cell lymphoma associated with human herpes virus-6 infection. Leuk. Lymphoma 1999, 33, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Tey, S.K.; Marlton, P.V.; Hawley, C.M.; Norris, D.; Gill, D.S. Post-transplant hepatosplenic T-cell lymphoma successfully treated with HyperCVAD regimen. Am. J. Hematol. 2008, 83, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Haldas, J.; Wang, W.; Lazarchick, J. Post-transplant lymphoproliferative disorders: T-cell lymphoma following cardiac transplant. Leuk. Lymphoma 2002, 43, 447–450. [Google Scholar] [CrossRef]
- Cardwell, L.A.; Majerowski, J.; Chiu, Y.E.; Harrington, A.M.; Sokumbi, O. Post-transplant primary cutaneous peripheral T-cell lymphoma not otherwise specified in a pediatric patient. J. Cutan. Pathol. 2021, 48, 706–712. [Google Scholar] [CrossRef]
- Dalal, P.; Bichu, P.; Dhawan, V.; Ariyamuthu, V.; Malhotra, K.; Misra, M.; Khanna, R. Post-transplant Lymphoproliferative Disorder--a case of late-onset T-cell lymphoma after failed renal transplant. Adv. Perit. Dial. Conf. Perit. Dial. 2012, 28, 94–96. [Google Scholar]
- Kamińska, D.; Krajewska, M.; Mazanowska, O.; Poznański, P.; Boratyńska, M.; Klinger, M. Post-transplant lymphoproliferative disorder in adult renal transplant recipients: Case series and review of literature. Cent.-Eur. J. Immunol. 2020, 45, 498–506. [Google Scholar] [CrossRef]
- Azhir, A.; Reisi, N.; Taheri, D.; Adibi, A. Post transplant anaplastic large T-cell lymphoma. Saudi J. Kidney Dis. Transplant. Off. Publ. Saudi Cent. Organ Transplant. Saudi Arab. 2009, 20, 646–651. [Google Scholar]
- Wang, L.C.; Lu, M.Y.; Yu, J.; Jou, S.T.; Chiang, I.P.; Lin, K.H.; Lin, D.T. T cell lymphoproliferative disorder following bone marrow transplantation for severe aplastic anemia. Bone Marrow Transplant. 2000, 26, 893–897. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bregman, S.G.; Yeaney, G.A.; Greig, B.W.; Vnencak-Jones, C.L.; Hamilton, K.S. Subcutaneous panniculitic T-cell lymphoma in a cardiac allograft recipient. J. Cutan. Pathol. 2005, 32, 366–370. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).