Low-Intensity and Chemo-Free Treatments in Ph+ ALL: Progression-Free Survival Based on Indirect Comparisons
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Literature Search
2.3. Reconstruction of Individual Patient Data from Kaplan–Meier Survival Curves
2.4. Survival Statistics
2.5. Assessment of Heterogeneity in Studies Pooled Together
2.6. Data Sharing Statement
3. Results
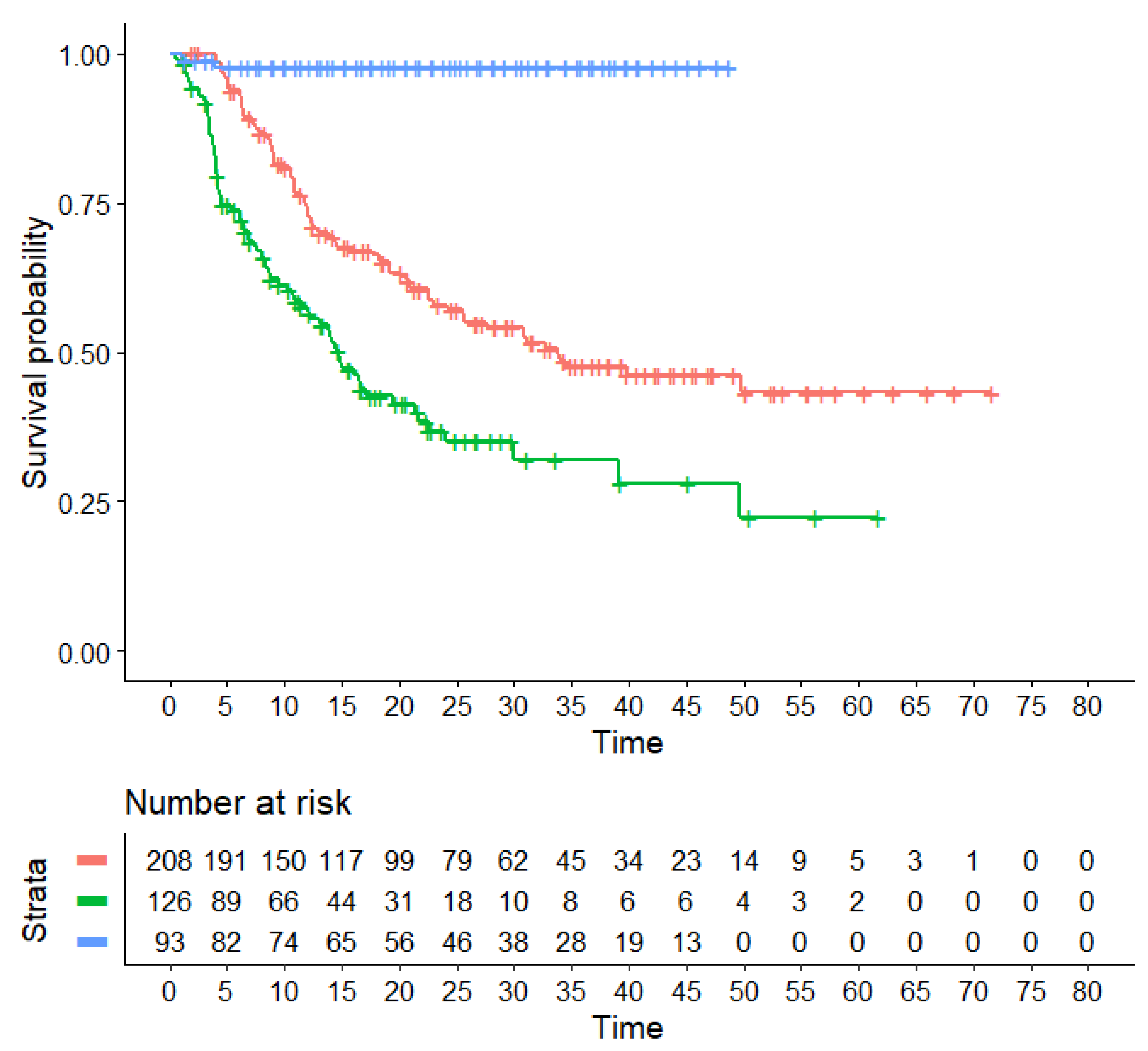
- (1)
- The regimen denoted TKICHE (i.e., TKI plus low-intensity chemotherapy), which includes four trials, namely dasatinib plus low-intensity chemotherapy in the trial by Rousselot et al. [19], nilotinib plus low-intensity chemotherapy in the GRAAPH-2014 Study by Rousselot et al. [20], and dasatinib plus steroids induction in the trial by Chiaretti et al. [21].
- (2)
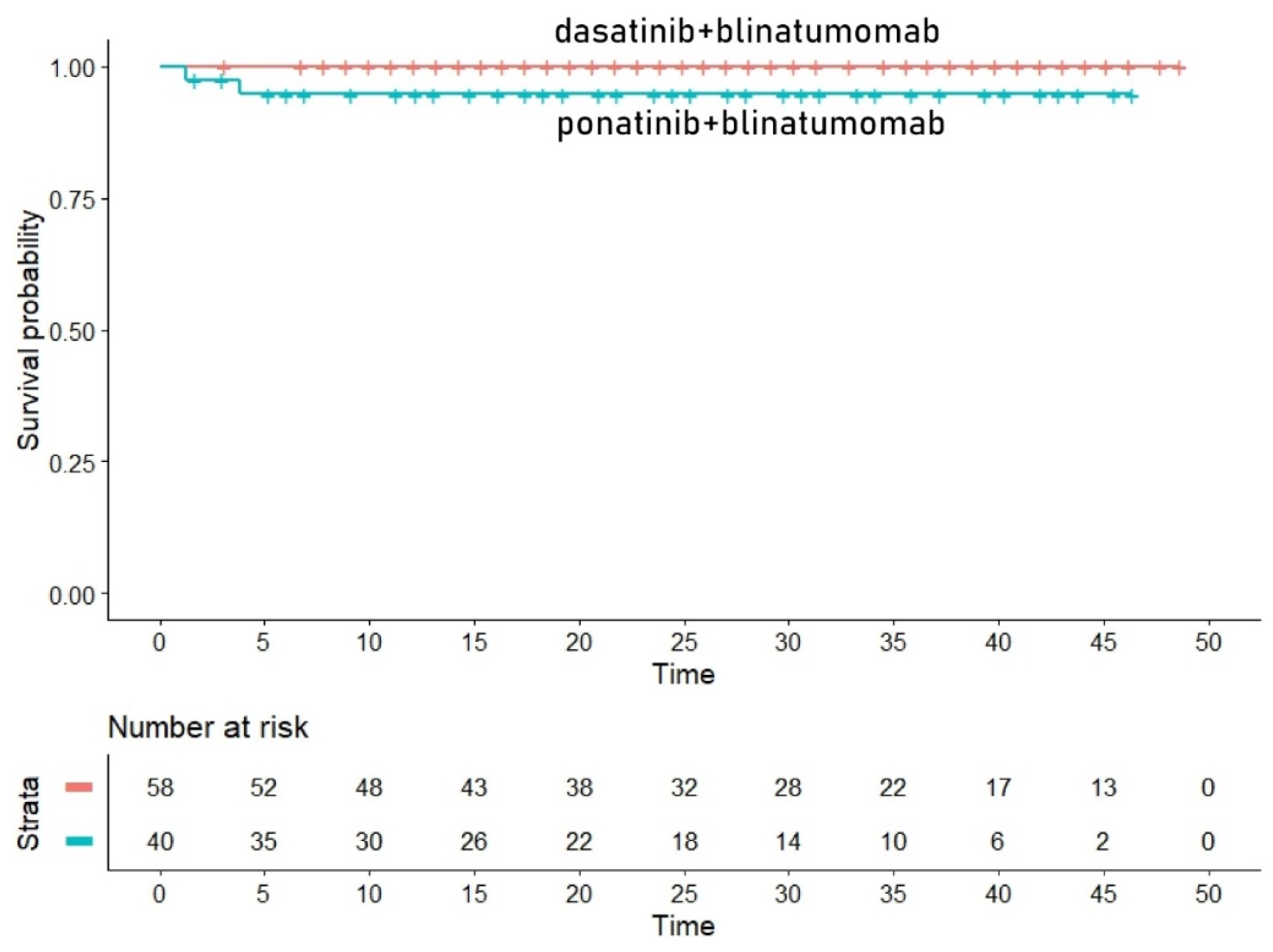
- (3)
- -
- HR for the comparison of TKICHE vs. TKISTE: 0.5066 (95% CI, 0.3705 to 0.6927; p = 0.00002);
- -
- HR for the comparison of TKIBLI vs. TKICHE: 0.448 (95% CI, 0.0110 to 0.182; p = 0.00014);
- -
- HR for the comparison of TKIBLI vs. TKISTE = 0.023 (95% CI, 0.0053 to 0.096; p < 0.001).
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Indirect Head-to-Head Efficacy Comparisons for the Three Regimens (TKICHE vs. TKISTE vs. TKIBLI)
Appendix A.2. Survival Analysis for the TKICHE Regimen (Three Trials)
- -
- Trial (b) vs. Trial (a): HR, 0.5693 (95% CI, 0.3411 to 0.9501; p = 0.031);
- -
- Trial (c) vs. Trial (a): HR, 0.470 (95% CI, 0.2856 to 0.7746; p = 0.003);
- -
- Trial (c) vs. Trial (b): HR, 0.826 (95% CI, 0.404 to 1.688; p > 0.05).
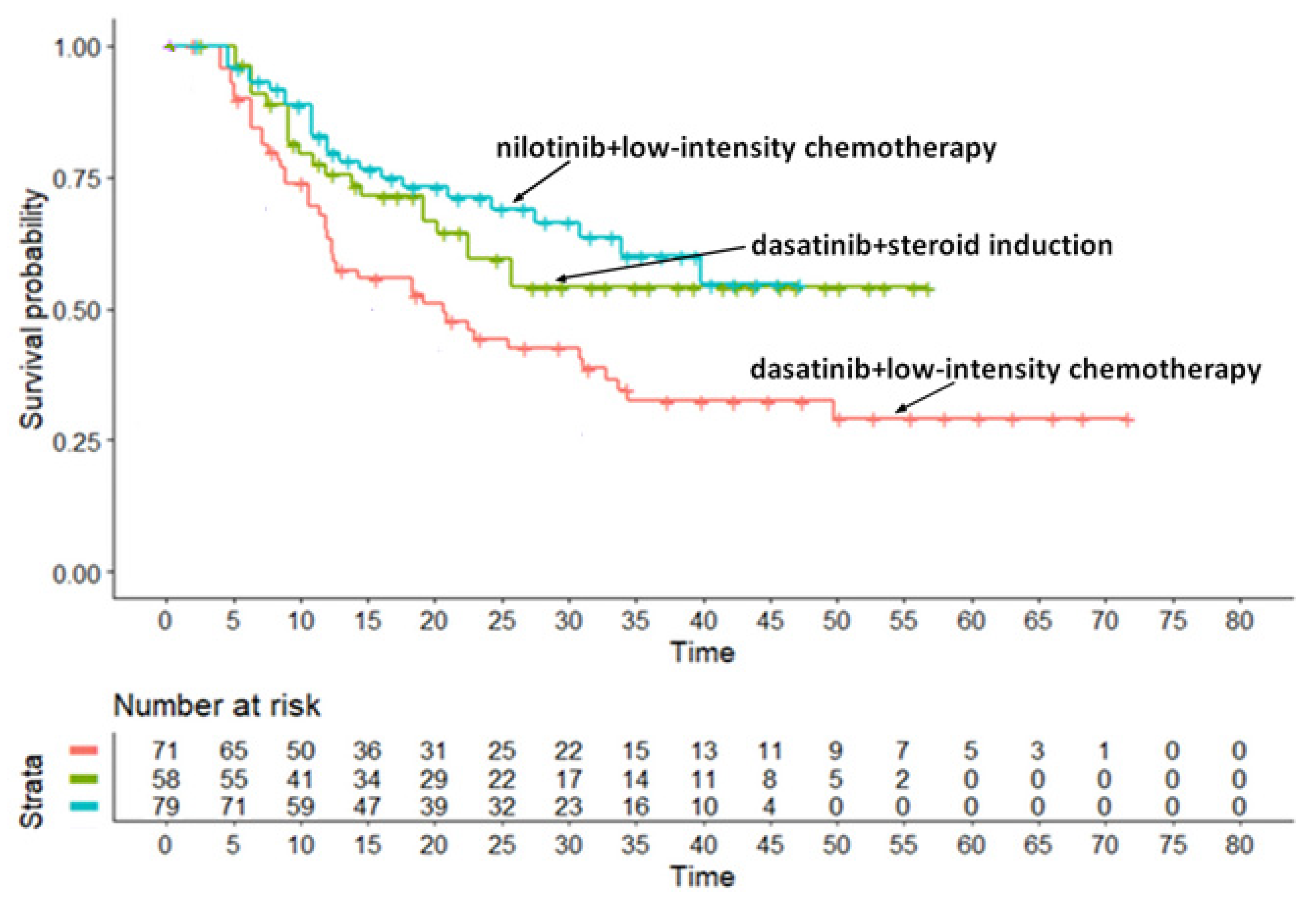
Appendix A.3. Survival Analysis for the TKISTE Regimen (Three Trials)

- -
- Trial (d): 21.67 months (95% CI, 11.97 to not computable);
- -
- Trial (e): 14.69 months (95% CI, 10.65 to 24.0);
- -
- Trial (f): 8.66 months (95% CI, 4.38 to not computable).
- -
- Trial (f) vs. Trial (d): HR= 1.22 (95% CI, 0.71 to 2.11);
- -
- Trial (e) vs. Trial (d): HR= 1.53 (95% CI, 0.82 to 2.88);
- -
- Trial (f) vs. Trial (e): HR = 1.26 (95% CI, 0.55 to 2.89).
Appendix A.4. Survival Analysis for the TKIBLI Regimen (Two Trials)
References
- Chiaretti, S.; Vitale, A.; Cazzaniga, G.; Orlando, S.M.; Silvestri, D.; Fazi, P.; Valsecchi, M.G.; Elia, L.; Testi, A.M.; Mancini, F.; et al. Clinico-biological features of 5202 patients with acute lymphoblastic leukemia enrolled in the Italian AIEOP and GIMEMA protocols and stratified in age cohorts. Haematologica 2013, 98, 1702–1710. [Google Scholar] [CrossRef]
- Ravandi, F.; Kebriaei, P. Philadelphia chromosome-positive acute lymphoblastic leukemia. Hematol. Oncol. Clin. N. Am. 2009, 23, 1043–1063. [Google Scholar] [CrossRef][Green Version]
- Burmeister, T.; Schwartz, S.; Bartram, C.R.; Gökbuget, N.; Hoelzer, D.; Thiel, E. Patients’ age and BCR–ABL frequency in adult B- precursor ALL: A retrospective analysis from the GMALL study group. Blood 2008, 112, 918–919. [Google Scholar] [CrossRef] [PubMed]
- Moorman, A.V.; Harrison, C.J.; Buck, G.A.; Richards, S.M.; Secker-Walker, L.M.; Martineau, M.; Vance, G.H.; Cherry, A.M.; Higgins, R.R.; Fielding, A.K.; et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): Analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/ Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood 2007, 109, 3189–3197. [Google Scholar] [CrossRef] [PubMed]
- Dombret, H.; Gabert, J.; Boiron, J.M.; Rigal-Huguet, F.; Blaise, D.; Thomas, X.; Delannoy, A.; Buzyn, A.; Bilhou-Nabera, C.; Cayuela, J.M.; et al. Outcome of treatment in adults Philadelphia chromosome-positive acute lymphoblastic leukemia-results of the prospective multi-center LALA-94 trial. Blood 2002, 100, 2357–2366. [Google Scholar] [CrossRef]
- Ottmann, O.G.; Wassmann, B.; Pfeifer, H.; Giagounidis, A.; Stelljes, M.; Dührsen, U.; Schmalzing, M.; Wunderle, L.; Binckebanck, A.; Hoelzer, D. Imatinib compared with chemotherapy as front-line treatment of elderly patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL). Cancer 2007, 109, 2068–2076. [Google Scholar] [CrossRef]
- Talpaz, M.; Shah, N.P.; Kantarjian, H.; Donato, N.; Nicoll, J.; Paquette, R.; Cortes, J.; O’Brien, S.; Nicaise, C.; Bleickardt, E.; et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 2006, 354, 2531–2541. [Google Scholar] [CrossRef] [PubMed]
- Bassan, R.; Rossi, G.; Pogliani, E.M.; Di Bona, E.; Angelucci, E.; Cavattoni, I.; Lambertenghi-Deliliers, G.; Mannelli, F.; Levis, A.; Ciceri, F.; et al. Chemotherapy-phased imatinib pulses improve long- term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J. Clin. Oncol. 2010, 28, 3644–3652. [Google Scholar] [CrossRef]
- Haddad, F.G.; Sawyers, J.; Short, N.J. Treatment de-escalation in Philadelphia chromosome-positive B-cell acute lymphoblastic leukemia: The emerging role of chemotherapy-free regimens. Ther. Adv. Hematol. 2023, 14, 20406207231151294. [Google Scholar] [CrossRef]
- Chiaretti, S.; Vitale, A.; Vignetti, M.; Piciocchi, A.; Fazi, P.; Elia, L.; Falini, B.; Ronco, F.; Ferrara, F.; De Fabritiis, P.; et al. A sequential approach with imatinib.; chemotherapy and transplant for adult Ph1 acute lymphoblastic leukemia: Final results of the GIMEMA LAL 0904 study. Haematologica 2016, 101, 1544–1552. [Google Scholar] [CrossRef]
- Jabbour, E.; Haddad, F.G.; Short, N.J.; Kantarjian, H. Treatment of Adults With Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia-From Intensive Chemotherapy Combinations to Chemotherapy-Free Regimens: A Review. JAMA Oncol. 2022, 8, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Assi, R.; Kantarjian, H.; Short, N.J.; Daver, N.; Takahashi, K.; Garcia-Manero, G.; DiNardo, C.; Burger, J.; Cortes, J.; Jain, N.; et al. Safety and Efficacy of Blinatumomab in Combination With a Tyrosine Kinase Inhibitor for the Treatment of Relapsed Philadelphia Chromosome-positive Leukemia. Clin. Lymphoma Myeloma Leuk. 2017, 17, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Foà, R.; Bassan, R.; Vitale, A.; Elia, L.; Piciocchi, A.; Puzzolo, M.C.; Canichella, M.; Viero, P.; Ferrara, F.; Lunghi, M.; et al. Dasatinib-Blinatumomab for Ph+ Acute Lymphoblastic Leukemia in Adults. N. Engl. J. Med. 2020, 383, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhou, Y.; Lee, J.J. IPDfromKM: Reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2021, 21, 11. [Google Scholar] [CrossRef]
- Messori, A.; Fadda, V.; Romeo, M.R.; Veneziano, S.; Trippoli, S. A Comparison of Statistical Analysis Between "Real" Patients Reported in Kaplan-Meier Curves and "Reconstructed" Patients Estimated Through the IPDfromKM Method: Analysis of Eight Trials Evaluating Catheter Ablation of Ventricular Tachycardia. Cureus 2023, 15, e47891. Available online: https://www.cureus.com/articles/198474-a-comparison-of-statistical-analysis-between-real-patients-reported-in-kaplan-meier-curves-and-reconstructed-patients-estimated-through-the-ipdfromkm-method-analysis-of-eight-trials-evaluating-catheter-ablation-of-ventricular-tachycardia.pdf (accessed on 15 October 2023). [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 2009, 151, W65–W94. [Google Scholar] [CrossRef]
- Ottmann, O.G.; Pfeifer, H.; Cayuela, J.M.; Spiekermann, K.; Jung, W.; Beck, J.; Raffoux, E.; Turlure, P.; Himberlin, C.; Huguet, F.; et al. Nilotinib (Tasigna®) and Low Intensity Chemotherapy for First-Line Treatment of Elderly Patients with BCR-ABL1-Positive Acute Lymphoblastic Leukemia: Final Results of a Prospective Multicenter Trial (EWALL-PH02). Blood 2018, 132 (Suppl. 1), 31. [Google Scholar] [CrossRef]
- Chalandon, Y.; Thomas, X.; Hayette, S.; Cayuela, J.M.; Abbal, C.; Huguet, F.; Raffoux, E.; Leguay, T.; Rousselot, P.; Lepretre, S.; et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph+ acute lymphoblastic leukemia. Blood 2015, 125, 3711–3719, Erratum in Blood 2015, 126, 1261. [Google Scholar] [CrossRef]
- Rousselot, P.; Coudé, M.M.; Gokbuget, N.; Gambacorti Passerini, C.; Hayette, S.; Cayuela, J.M.; Huguet, F.; Leguay, T.; Chevallier, P.; Salanoubat, C.; et al. Dasatinib and low-intensity chemotherapy in elderly patients with Philadelphia chromosome-positive ALL. Blood 2016, 128, 774–782. [Google Scholar] [CrossRef]
- Rousselot, P.; Chalandon, Y.; Chevret, S.; Cayuela, J.M.; Huguet, F.; Chevallier, P.; Graux, C.; Thiebaut-Bertrand, A.; Chantepie, S.; Thomas, X.; et al. The Omission of High-Dose Cytarabine during Consolidation Therapy of Ph+ ALL Patients Treated with Nilotinib and Low-Intensity Chemotherapy Results in an Increased Risk of Relapses Despite Non-Inferior Levels of Late BCR-ABL1 MRD Response. First Results of the Randomized Graaph-2014 Study. Blood 2021, 138 (Suppl. 1), 512. [Google Scholar]
- Chiaretti, S.; Ansuinelli, M.; Vitale, A.; Elia, L.; Matarazzo, M.; Piciocchi, A.; Fazi, P.; Di Raimondo, F.; Santoro, L.; Fabbiano, F.; et al. A multicenter total therapy strategy for de novo adult Philadelphia chromosome positive acute lymphoblastic leukemia patients: Final results of the GIMEMA LAL1509 protocol. Haematologica 2021, 106, 1828–1838. [Google Scholar] [CrossRef]
- Vignetti, M.; Fazi, P.; Cimino, G.; Martinelli, G.; Di Raimondo, F.; Ferrara, F.; Meloni, G.; Ambrosetti, A.; Quarta, G.; Pagano, L.; et al. Imatinib plus steroids induces complete remissions and prolonged survival in elderly Philadelphia chromosome-positive patients with acute lymphoblastic leukemia without additional chemotherapy: Results of the Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) LAL0201-B protocol. Blood 2007, 109, 3676–3678. [Google Scholar]
- Foà, R.; Vitale, A.; Vignetti, M.; Meloni, G.; Guarini, A.; De Propris, M.S.; Elia, L.; Paoloni, F.; Fazi, P.; Cimino, G.; et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood 2011, 118, 6521–6528. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, G.; Papayannidis, C.; Piciocchi, A.; Robustelli, V.; Soverini, S.; Terragna, C.; Marconi, G.; Lemoli, R.M.; Guolo, F.; Fornaro, A.; et al. INCB84344-201: Ponatinib and steroids in frontline therapy for unfit patients with Ph+ acute lymphoblastic leukemia. Blood Adv. 2022, 6, 1742–1753. [Google Scholar] [CrossRef] [PubMed]
- Chiaretti, S.; Bassan, R.; Vitale, A.; Elia, L.; Piciocchi, A.; Viero, P.; Ferrara, F.; Lunghi, M.; Fabbiano, F.; Bonifacio, M.; et al. Forty Months Update Of The Gimema Lal2116 (D-alba) Protocol And Ancillary Lal2217 Study For Newly Diagnosed Adult Ph+ All. HemaSphere 2022, 6, 253–254. [Google Scholar] [CrossRef]
- Jabbour, E.; Short, N.J.; Jain, N.; Huang, X.; Montalban-Bravo, G.; Banerjee, P.; Rezvani, K.; Jiang, X.; Kim, K.H.; Kanagal-Shamanna, R.; et al. Ponatinib and blinatumomab for Philadelphia chromosome-positive acute lymphoblastic leukaemia: A US.; single-centre.; single-arm.; phase 2 trial. Lancet Haematol. 2023, 10, e24–e34. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, G.; Boissel, N.; Chevallier, P.; Ottmann, O.; Gökbuget, N.; Topp, M.S.; Fielding, A.K.; Rambaldi, A.; Ritchie, E.K.; Papayannidis, C.; et al. Complete Hematologic and Molecular Response in Adult Patients With Relapsed/Refractory Philadelphia Chromosome-Positive B-Precursor Acute Lymphoblastic Leukemia Following Treatment With Blinatumomab: Results From a Phase II.; Single-Arm.; Multicenter Study. J. Clin. Oncol. 2017, 35, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Short, N.J.; Kantarjian, H.M.; Konopleva, M.; Bravo, G.M.; Ravandi, F.; Jain, N.; Kadia, T.M.; Alvarado Valero, Y.; Chien, K.S.; Daver, N.G.; et al. A phase II trial of a chemotherapy-free combination of ponatinib and blinatumomab in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL). J. Clin. Oncol. 2022, 40, 7009. [Google Scholar] [CrossRef]
- Steegmann, J.L.; Baccarani, M.; Breccia, M.; Casado, L.F.; García-Gutiérrez, V.; Hochhaus, A.; Kim, D.W.; Kim, T.D.; Khoury, H.J.; Le Coutre, P.; et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia 2016, 30, 1648–1671. [Google Scholar] [CrossRef]
- Messori, A.; Rivano, M.; Mengato, D.; Cancanelli, L.; Di Spazio, L.; Chiumente, M. A preliminary estimate of survival gain and cost-effectiveness of CAR-T in adult patients with acute lymphoblastic leukemia. Leuk. Lymphoma 2022, 63, 1261–1264. [Google Scholar] [CrossRef]
- Messori, A.; Chiumente, M.; Mengato, D. Chimeric Antigen Receptor T Cells in Large B-Cell Lymphoma: Analysis of Overall Survival Based on Reconstructed Patient-Level Data. Clin. Ther. 2022, 44, 1626–1632. [Google Scholar] [CrossRef]
- Messori, A.; Damuzzo, V.; Leonardi, L.; Agnoletto, L.; Chiumente, M.; Mengato, D. CAR-T Treatment: Determining the Survival Gain in Patients With Relapsed or Refractory Diffuse Large B-cell Lymphoma. Clin. Lymphoma Myeloma Leuk. 2020, 20, 490–491. [Google Scholar] [CrossRef]
- Messori, A. Long-term progression-free survival in patients with chronic lymphocytic leukemia treated with novel agents: An analysis based on indirect comparisons. Eur. J. Haematol. 2023, 110, 60–66. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guidelines for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
| First Author | Year | Trial | Treatment | Inclusion Criteria | Events | No of Patients | Notes | |
|---|---|---|---|---|---|---|---|---|
| TKICHE | Chaladon et al. [18] | 2015 | GRAAPH-2005 study | Imatinib combined with low-intensity chemotherapy | Patients aged 18 to 59 years with newly diagnosed Ph1 and/or BCR-ABL1-positive ALL were eligible | 65 | 135 | Excluded from our analysis because only induction was not intensive |
| Rousselot et al. [19] | 2016 | EWALL-PH-01 international study | Dasatinib in combination with low-intensity chemotherapy | Patients aged 55 years or older were eligible if they had newly diagnosed Ph1 and/or BCR-ABL ALL | 40 | 71 | Included | |
| Rousselot et al. [20] | 2021 | Graaph-2014 Study | Nilotinib combined with low-intensity chemotherapy | Ph-positive ALL patients aged 18–60 years old were randomized | 23 | 79 | Included | |
| Chiaretti et al. [21] | 2020 | GIMEMA LAL1509 | Dasatinib plus steroids induction followed by dasatinib alone | Adult Ph+ ALL patients (18–60 years). | 13 | 58 | Included | |
| TKISTE | Vignetti et al. [22] | 2007 | GIMEMA LAL0201-B | Imatinib combined with steroids | Patients with a diagnosis of ALL who were older than 60 years were eligible if they carried either the Ph chromosome or the BCR-ABL molecular translocation | 16 | 29 | Included |
| Foà et al. [23] | 2011 | GIMEMA LAL1205 | Dasatinib induction therapy combined with steroids | Patients 18 years of age or older (with no upper age limit) were eligible if they had been diagnosed with Ph/BCR-ABLALL | 23 | 53 | Included | |
| Martinelli et al. [24] | 2022 | GIMEMA LAL 1811 | Ponatinib plus prednisone | Patients had new-onset Ph+ ALL and were ≥60 years or were ≥18 years old but unfit for a program of intensive chemotherapy and SCT | 34 | 44 | Included | |
| TKIBLI | Chiaretti et al. [25] | 2022 | GIMEMA LAL2217 | Dasatinib + blinatumomab | Ph-positive ALL patients, median age was 54 years (24–82; no upper age limit) | 9 | 58 | Included |
| Jabbour et al. [26] | 2023 | NCT03263572 | Ponatinib + blinatumomab | Patients with newly diagnosed, relapsed/refractory Ph+ ALL or CML in lymphoid blast phase | 2 | 40 | Included |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivano, M.; Mengato, D.; Chiumente, M.; Messori, A. Low-Intensity and Chemo-Free Treatments in Ph+ ALL: Progression-Free Survival Based on Indirect Comparisons. Hematol. Rep. 2023, 15, 670-683. https://doi.org/10.3390/hematolrep15040068
Rivano M, Mengato D, Chiumente M, Messori A. Low-Intensity and Chemo-Free Treatments in Ph+ ALL: Progression-Free Survival Based on Indirect Comparisons. Hematology Reports. 2023; 15(4):670-683. https://doi.org/10.3390/hematolrep15040068
Chicago/Turabian StyleRivano, Melania, Daniele Mengato, Marco Chiumente, and Andrea Messori. 2023. "Low-Intensity and Chemo-Free Treatments in Ph+ ALL: Progression-Free Survival Based on Indirect Comparisons" Hematology Reports 15, no. 4: 670-683. https://doi.org/10.3390/hematolrep15040068
APA StyleRivano, M., Mengato, D., Chiumente, M., & Messori, A. (2023). Low-Intensity and Chemo-Free Treatments in Ph+ ALL: Progression-Free Survival Based on Indirect Comparisons. Hematology Reports, 15(4), 670-683. https://doi.org/10.3390/hematolrep15040068