Management and Outcomes of Invasive Procedures in Individuals with Hemophilia A on Emicizumab Prophylaxis: A Single Center Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Patients and Data Collection
2.3. Laboratory Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
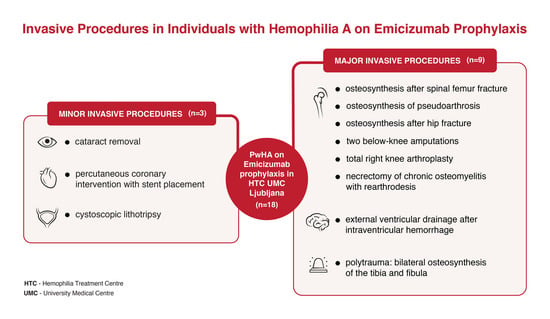
3.2. Management and Outcomes of Minor Invasive Procedures
- PwHA case #1: Cataract procedure
- PwHA case #2: Cystoscopic lithotripsy
- PwHA case #3: Urgent percutaneous coronary interventions (PCI) after STEMI
3.3. Management and Outcomes of Major Surgical Procedures
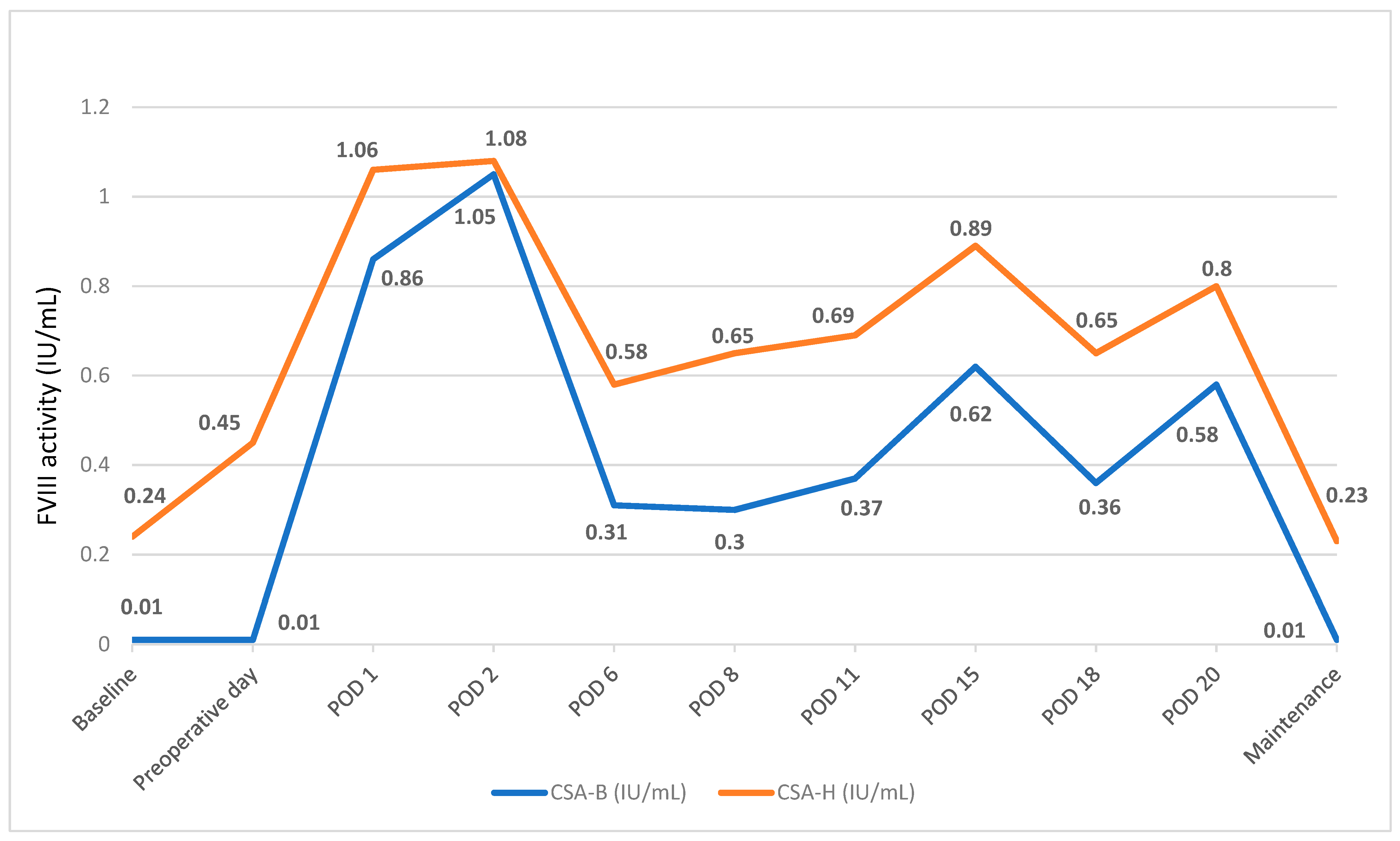
- PwHA case #4 with inhibitors: Spiral fracture and surgery followed by osteosynthesis of pseudoarthrosis of the right femur
- PwHA case #5: Complex re-arthrodesis of the left ankle
- PwHA case #6: Bilateral osteosynthesis of the tibia and fibula and below-knee amputation
- PwHA case #7: External ventricular drainage after intraventricular hemorrhage and osteosynthesis after hip fracture
- PwHA case #8: Total knee arthroplasty
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shapiro, S.; Makris, M. Haemophilia and ageing. Br. J. Haematol. 2019, 184, 712–720. [Google Scholar] [CrossRef]
- Srivastava, A.; Santagostino, E.; Dougall, A.; Kitchen, S.; Sutherland, M.; Pipe, S.W.; Carcao, M.; Mahlangu, J.; Ragni, M.V.; Windyga, J.; et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia 2020, 26 (Suppl. S6), 1–158. [Google Scholar] [CrossRef]
- Uchida, N.; Sambe, T.; Yoneyama, K.; Fukazawa, N.; Kawanishi, T.; Kobayashi, S.; Shima, M. A first-in-human phase 1 study of ACE910, a novel factor VIII–mimetic bispecific antibody, in healthy subjects. Blood 2016, 127, 1633–1641. [Google Scholar] [CrossRef]
- Krumb, E.; Fijnvandraat, K.; Makris, M.; Peyvandi, F.; Ryan, A.; Athanasopoulos, A.; Hermans, C. Adoption of emicizumab (Hemlibra®) for hemophilia A in Europe: Data from the 2020 European Association for Haemophilia and Allied Disorders survey. Haemophilia 2021, 27, 736–743. [Google Scholar] [CrossRef]
- Collins, P.W.; Liesner, R.; Makris, M.; Talks, K.; Chowdary, P.; Chalmers, E.; Hall, G.; Riddell, A.; Percy, C.L.; Hay, C.R.; et al. Treatment of bleeding episodes in haemophilia A complicated by a factor VIII inhibitor in patients receiving Emicizumab. Interim guidance from UKHCDO Inhibitor Working Party and Executive Committee. Haemophilia 2018, 24, 344–347. [Google Scholar] [CrossRef]
- Coppola, A.; Castaman, G.; Santoro, R.C.; Mancuso, M.E.; Franchini, M.; Marino, R.; Rivolta, G.F.; Santoro, C.; Zanon, E.; Sciacovelli, L.; et al. Management of patients with severe haemophilia a without inhibitors on prophylaxis with emicizumab: AICE recommendations with focus on emergency in collaboration with SIBioC, SIMEU, SIMEUP, SIPMeL and SISET. Haemophilia 2020, 26, 937–945. [Google Scholar] [CrossRef]
- Holstein, K.; Albisetti, M.; Bidlingmaier, C.; Halimeh, S.; Heine, S.; Klamroth, R.; Königs, C.; Kurnik, K.; Male, C.; Oldenburg, J.; et al. Practical Guidance of the GTH Haemophilia Board on the Use of Emicizumab in Patients with Haemophilia A. Hamostaseologie 2020, 40, 561–571. [Google Scholar] [CrossRef]
- Fontana, P.; Alberio, L.; Albisetti, M.; Angelillo-Scherrer, A.; Asmis, L.M.; Casini, A.; Gerber, B.; Graf, L.; Hegemann, I.; Korte, W.; et al. Management of bleeding events and invasive procedures in patients with haemophilia A without inhibitors treated with emicizumab. Swiss Med. Wkly. 2020, 150, w20422. [Google Scholar] [CrossRef]
- National Hemophilia Foundation. Masac Document 268—Recommendation on the Use and Management of Emicizumab-Kxwh (Hemlibra®) for Hemophilia a with and without Inhibitors. 2022. Available online: https://www.hemophilia.org/sites/default/files/document/files/268_Emicizumab.pdf (accessed on 27 February 2023).
- Mancuso, M.E.; Apte, S.; Hermans, C. Managing invasive procedures in haemophilia patients with limited resources, extended half-life concentrates or non-replacement therapies in 2022. Haemophilia 2022, 28 (Suppl. S4), 93–102. [Google Scholar] [CrossRef]
- Kruse-Jarres, R.; Peyvandi, F.; Oldenburg, J.; Chang, T.; Chebon, S.; Doral, M.Y.; Croteau, S.E.; Lambert, T.; Kempton, C.L.; Pipe, S.W.; et al. Surgical outcomes in people with hemophilia A taking emicizumab prophylaxis: Experience from the HAVEN 1-4 studies. Blood Adv. 2022, 6, 6140–6150. [Google Scholar] [CrossRef]
- Castaman, G.; Windyga, J.; Alzahrani, H.; Robson, S.; Sanabria, F.; Howard, M.; Jiménez-Yuste, V. Surgical Experience from the Phase Iiib Stasey Trial of Emicizumab Prophylaxis in Persons with Hemophilia a (Pwha) with Fviii Inhibitors: Final Analysis. In Proceedings of the 63rd ASH Annual Meeting and Exposition, Atlanta, GA, USA, 11–14 December 2021. Oral presentation #344. [Google Scholar]
- McCary, I.; Guelcher, C.; Kuhn, J.; Butler, R.; Massey, G.; Guerrera, M.F.; Ballester, L.; Raffini, L. Real-world use of emicizumab in patients with haemophilia A: Bleeding outcomes and surgical procedures. Haemophilia 2020, 26, 631–636. [Google Scholar] [CrossRef]
- Lewandowska, M.; Randall, N.; Bakeer, N.; Maahs, J.; Sagar, J.; Greist, A.; Shapiro, A.D. Management of people with haemophilia A undergoing surgery while receiving emicizumab prophylaxis: Real-world experience from a large comprehensive treatment centre in the US. Haemophilia 2021, 27, 90–99. [Google Scholar] [CrossRef]
- Barg, A.A.; Budnik, I.; Avishai, E.; Brutman-Barazani, T.; Bashari, D.; Misgav, M.; Lubetsky, A.; Kuperman, A.A.; Livnat, T.; Kenet, G. Emicizumab prophylaxis: Prospective longitudinal real-world follow-up and monitoring. Haemophilia 2021, 27, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Ebbert, P.T.; Xavier, F.; Seaman, C.D.; Ragni, M.V. Emicizumab prophylaxis in patients with haemophilia A with and without inhibitors. Haemophilia 2020, 26, 41–46. [Google Scholar] [CrossRef]
- Santagostino, E.; Lentz, S.R.; Misgav, M.; Brand, B.; Chowdary, P.; Savic, A.; Kilinc, Y.; Amit, Y.; Amendola, A.; Solimeno, L.P.; et al. Safety and efficacy of turoctocog alfa (NovoEight®) during surgery in patients with haemophilia A: Results from the multinational guardian™ clinical trials. Haemophilia 2015, 21, 34–40. [Google Scholar] [CrossRef]
- Mauser-Bunschoten, E.P.; Bijlsma, W.; Roosendaal, G.; Schutgens, R.E.G. Cataract surgery in haemophilia. Haemophilia 2013, 19, e371–e372. [Google Scholar] [CrossRef]
- Klamroth, R.; Ay, C.; De Moerloose, P.; Fontana, P.; Windyga, J.; Astermark, J.; Berntorp, E.; Carvalho, M.; Dolan, G.; Hermans, C.; et al. Applicability of the European Society of Cardiology Guidelines on the management of acute coronary syndromes to older people with haemophilia A—A modified Delphi consensus by the ADVANCE Working Group. Haemophilia 2023, 29, 21–32. [Google Scholar] [CrossRef]
- Nagao, A.; Koganei, H.; Yamaguchi, T.; Fukutake, K. Successful emicizumab prophylaxis during dual antiplatelet therapy for insertion of drug-eluting stents after acute coronary syndrome: A case report. Haemophilia 2021, 27, E549–E550. [Google Scholar] [CrossRef]
- Schmitt, C.; Mancuso, M.E.; Chang, T.; Podolak-Dawidziak, M.; Petry, C.; Sidonio, R.S., Jr.; Yoneyama, K.; Key, N.S.; Niggli, M.; Lehle, M.; et al. Emicizumab dose up-titration in case of suboptimal bleeding control in people with haemophilia A. Haemophilia 2023, 29, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.S.; Davis, C.; Eyster, M.E. Total knee replacement with and without emicizumab: A unique comparison of perioperative management. Blood Adv. 2020, 4, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Lenting, P.J. Laboratory monitoring of hemophilia A treatments: New challenges. Blood Adv. 2020, 4, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, L.; van Dievoet, M.-A.; Lambert, C.; Hermans, C. Challenges of biological monitoring in a hemophilia A patient without inhibitors on emicizumab undergoing major orthopedic surgery: A case report. Ther. Adv. Hematol. 2021, 12, 20406207211040345. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | N = 12 † |
|---|---|
| Age at the time of surgery, years | |
| Median (range) | 53.5 (32–66) |
| History of inhibitors, n (%) ‡ | 2 (16.7) |
| Surgical classification, n (%) | |
| Major | 9 (75.0) |
| Minor | 3 (25.0) |
| Type of procedure, n (%) | |
| Orthopedic | 8 (66.7) |
| Other | 4 (33.3) |
| Bleeding episode, n (%) | |
| Yes | 3 (25.0) |
| No | 9 (75.0) |
| Duration of emicizumab exposure, days | |
| Median (range) | 405 (26–608) |
| PwHA Case # | Age at Time of Surgery, Years | Inhibitor Status (Yes/No) | Procedure/Surgery Type (Elective/Urgent) | Time on EMI, Days † | Preoperative Factor Dose rFVIIa (µg/kg) rFVIII (IU/kg) | Factor Consumption (Days) | Hospitalization (Days) | Bleeding Episode * (Yes/No) | Overall Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Minor surgical procedures | |||||||||
| 1 | 55 | No | Cataract removal (elective) | 308 | 70 IU/kg | 70 IU/kg (1 day) | 0 | No | Visual acuity improved. |
| 2 | 66 | No | Cystoscopic lithotripsy (elective) | 230 | 50 IU/kg | 285 IU/kg (10 days) | 3 | Yes | Bladder stones were successfully removed. |
| 3 | 32 | No | Percutaneous coronary intervention with stent placement (urgent) ‡ | 362 | 27 IU/kg (1st PCI); 17 IU/kg (2nd PCI) | 130 IU/kg (4 days) | 6 | No | Switching to FVIII prophylaxis while on DAPT. |
| Major surgical procedures | |||||||||
| 4 | 50 | Yes | Osteosynthesis after spiral femur fracture (urgent) | 26 (end of induction) | 94 µg/kg | 5.08 mg/kg (14 days) | 18 | Yes | Successful osteosynthesis. |
| 51 | Yes | Osteosynthesis of pseudoarthrosis (elective) | 279 | 90 µg/kg | 6.54 mg/kg (14 days) | 13 | No | ||
| 5 | 56 | No | Necrectomy of chronic osteomyelitis with re-arthrodesis (elective) | 274 | 40 IU/kg | 386 IU/kg (14 days) | 22 | No | Walks with the help of a leg prosthesis. |
| 56 | No | Below-knee amputation (elective) | 608 | 37 IU/kg | 381 IU/kg (14 days) | 15 | No | ||
| 6 | 56 | No | Bilateral osteosynthesis of the tibia and fibula (urgent) | 596 | 53 IU/kg | 506 IU/kg (14 days) | 36 (one hospitalization) | Yes | Survived, remained disabled. |
| 56 | No | Below-knee amputation (urgent) | 599 | 26 IU/kg | No | ||||
| 7 | 51 | No | External ventricular drainage after intraventricular hemorrhage (urgent) | 448 | 53 IU/kg | 320 IU/kg (14 days) | 58 (one hospitalization) | No | Physical and cognitive improvement. Successful osteosynthesis. Switch to FVIII prophylaxis. |
| 51 | No | Osteosynthesis after hip fracture (urgent) | 478 | 27 IU/kg | 253 IU/kg (14 days) | No | |||
| 8 | 52 | No | Total right knee arthroplasty (elective) | 567 | 50 IU/kg | 442 IU/kg (14 days) | 16 | No | Better mobility, less pain. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rener, K.; Anžej Doma, S.; Fink, M.; Podgornik, H.; Preložnik Zupan, I. Management and Outcomes of Invasive Procedures in Individuals with Hemophilia A on Emicizumab Prophylaxis: A Single Center Experience. Hematol. Rep. 2023, 15, 597-607. https://doi.org/10.3390/hematolrep15040062
Rener K, Anžej Doma S, Fink M, Podgornik H, Preložnik Zupan I. Management and Outcomes of Invasive Procedures in Individuals with Hemophilia A on Emicizumab Prophylaxis: A Single Center Experience. Hematology Reports. 2023; 15(4):597-607. https://doi.org/10.3390/hematolrep15040062
Chicago/Turabian StyleRener, Karla, Saša Anžej Doma, Martina Fink, Helena Podgornik, and Irena Preložnik Zupan. 2023. "Management and Outcomes of Invasive Procedures in Individuals with Hemophilia A on Emicizumab Prophylaxis: A Single Center Experience" Hematology Reports 15, no. 4: 597-607. https://doi.org/10.3390/hematolrep15040062
APA StyleRener, K., Anžej Doma, S., Fink, M., Podgornik, H., & Preložnik Zupan, I. (2023). Management and Outcomes of Invasive Procedures in Individuals with Hemophilia A on Emicizumab Prophylaxis: A Single Center Experience. Hematology Reports, 15(4), 597-607. https://doi.org/10.3390/hematolrep15040062