Synergistic Interactions between the Hypomethylating Agent Thio-Deoxycytidine and Venetoclax in Myelodysplastic Syndrome Cells
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Lines and Reagents
2.2. Analysis of Cell Death
2.3. Measurement of ROS
2.4. Western Blot
2.5. Immunofluorescence
2.6. Isolation of Primary MDS Cells and Normal CD34+ Cells
2.7. Statistical Analysis
3. Results
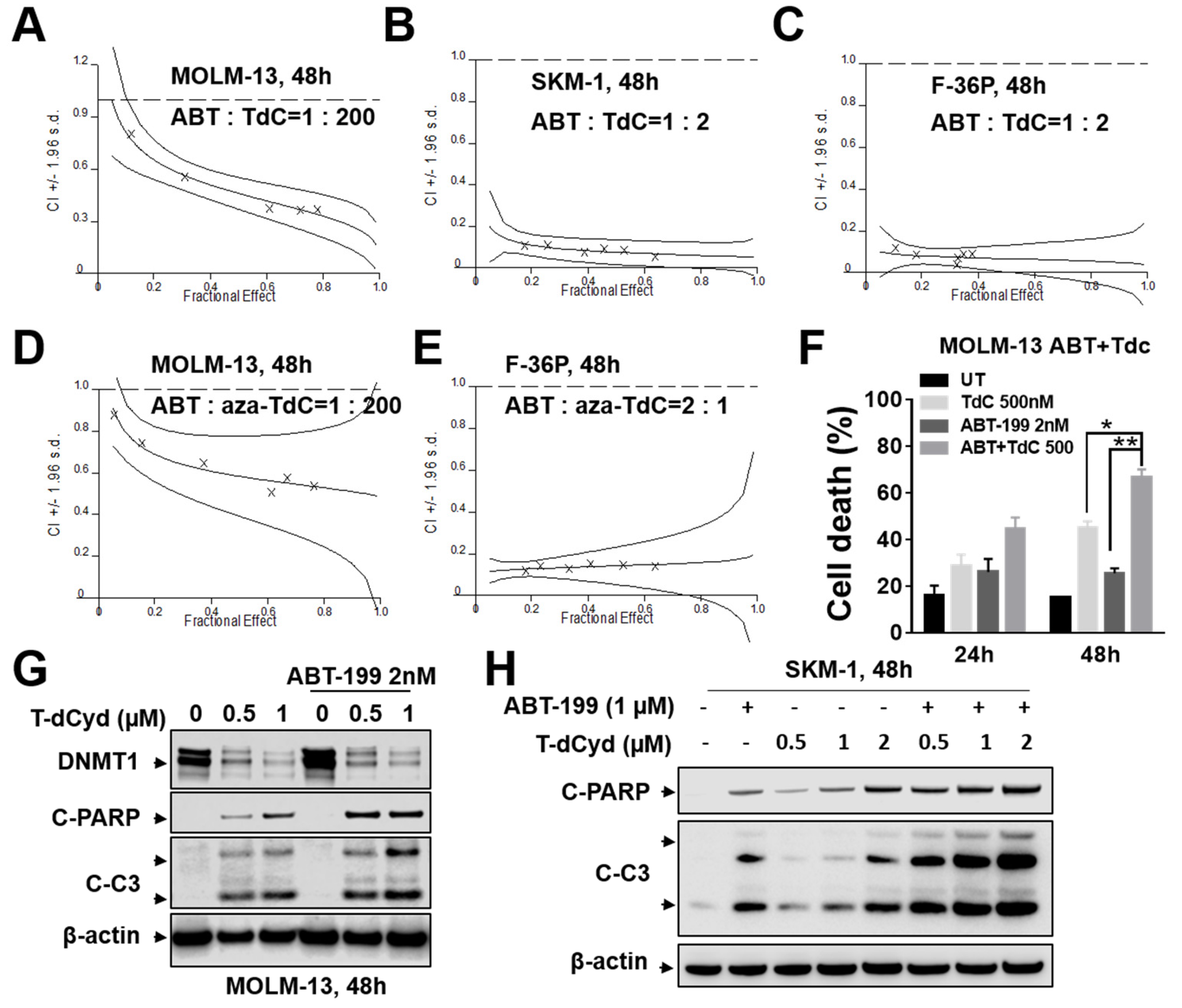
3.1. ABT-199/T-dCyd or aza-T-dCyd Synergism in MDS Cell Lines
3.2. ABT-199/T-dCyd or aza-T-dCyd Synergism in AML Cell Lines
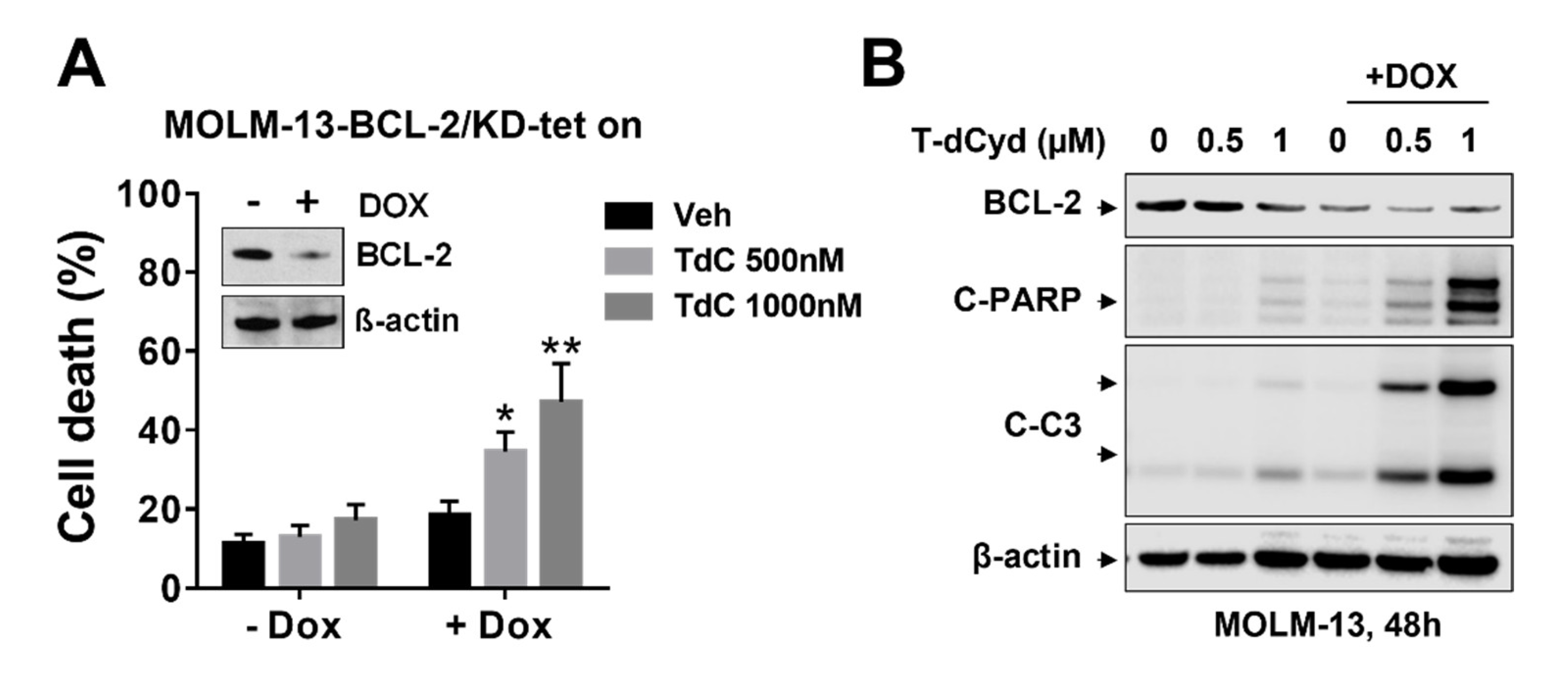
3.3. BCL-2 Interruption Contributes to Enhanced T-dCyd Activity in MDS-Derived Cells
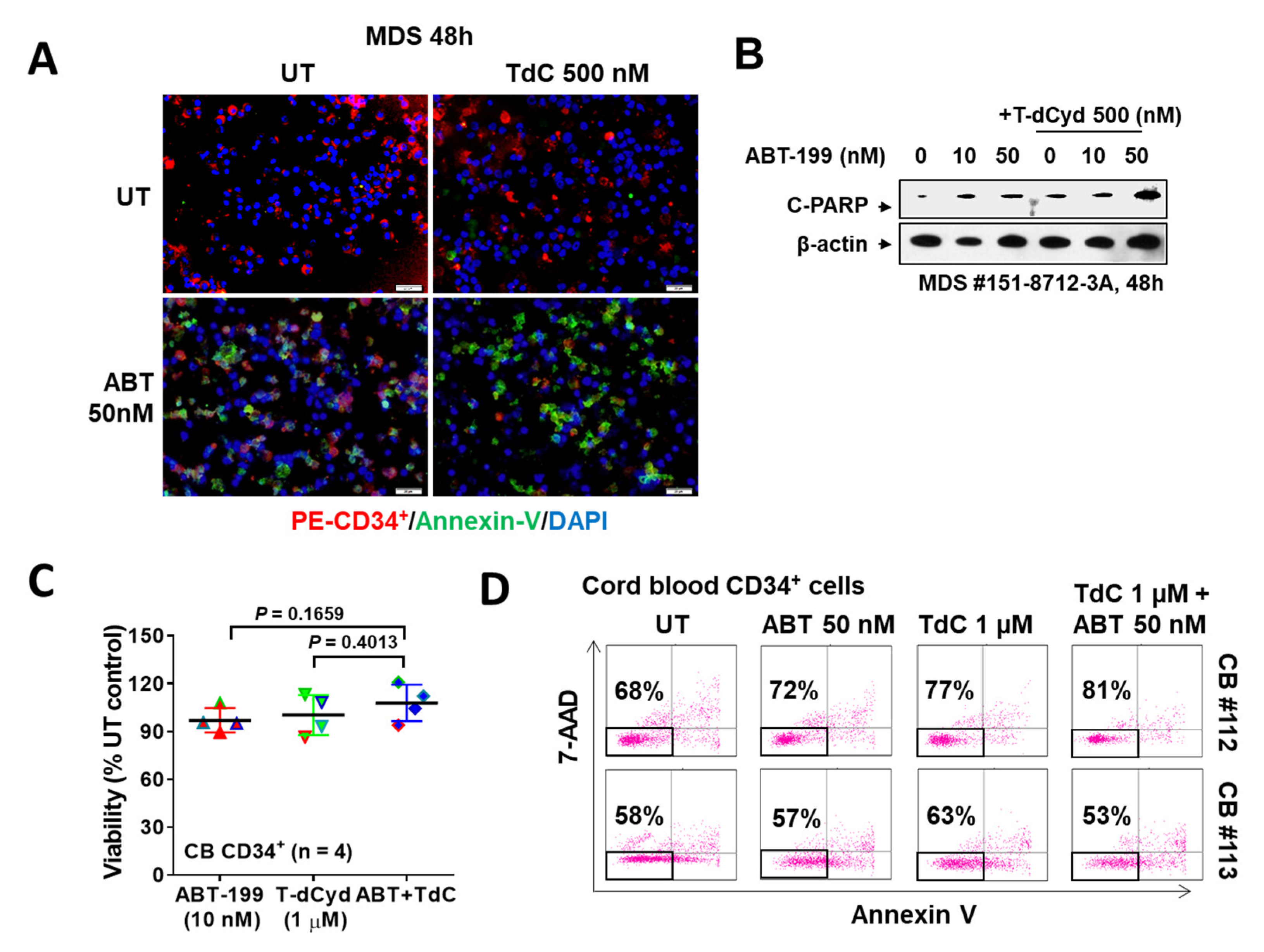
3.4. The T-dCyd/ABT-199 Regimen Is Active against Primary MDS, but Not Normal CD34+ Cells
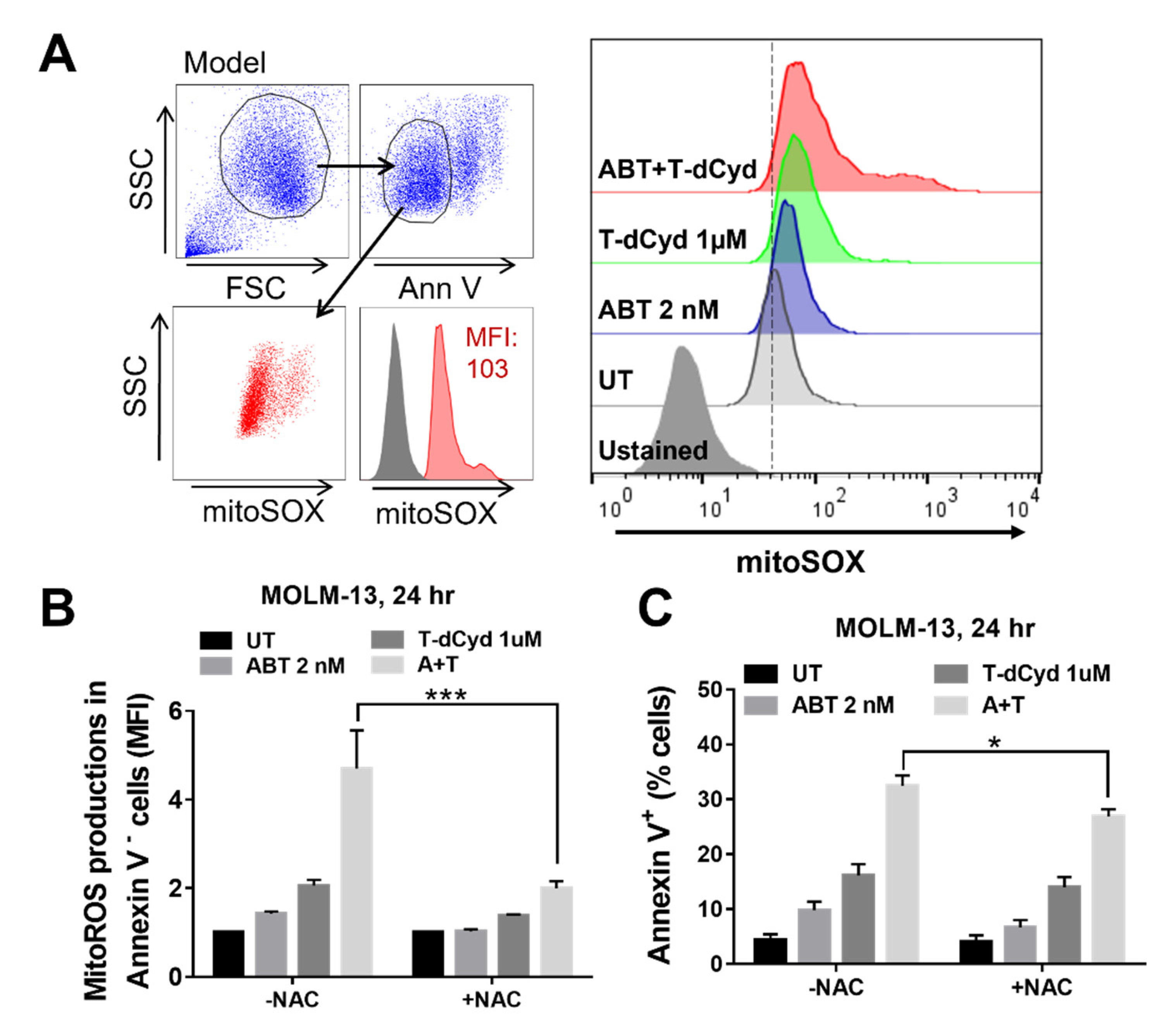
3.5. The T-dCyd/ABT-199 Regimen Increases Mitochondrial ROS Production in MDS-Derived Cell Lines
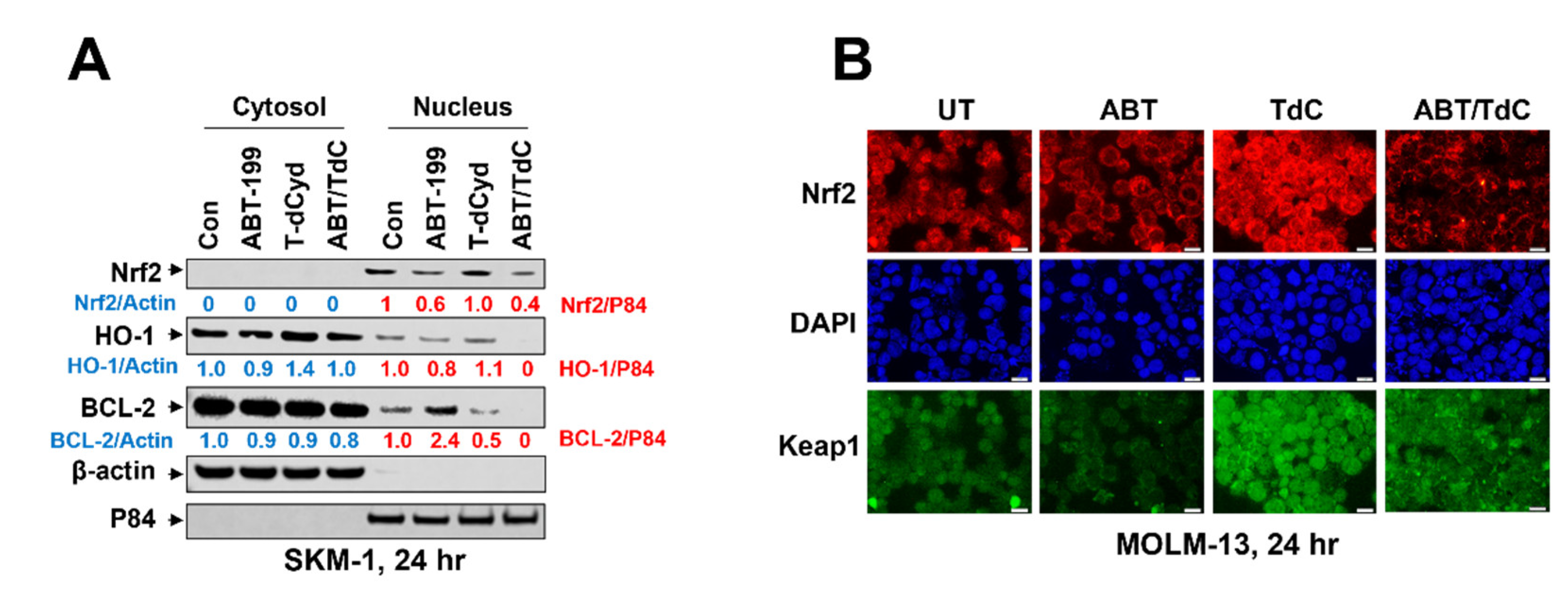
3.6. Combined Exposure of SKM-1 Cells to T-dCyd and ABT-199 Results in Diminished Expression of Nuclear Anti-Oxidant Proteins and BCL-2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia-Manero, G.; Chien, K.S.; Montalban-Bravo, G. Myelodysplastic syndromes: 2021 update on diagnosis, risk stratification and management. Am. J. Hematol. 2020, 95, 1399–1420. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Tohme, R.; Tomlinson, B.; Sakre, N.; Hasipek, M.; Durkin, L.; Schuerger, C.; Grabowski, D.; Zidan, A.; Radivoyevitch, T.; et al. Decitabine- and 5-azacytidine resistance emerges from adaptive responses of the pyrimidine metabolism network. Leukemia 2021, 35, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Lachowiez, C.; DiNardo, C.D.; Konopleva, M. Venetoclax in acute myeloid leukemia—Current and future directions. Leuk. Lymphoma 2020, 61, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Pollyea, D.A.; Stevens, B.M.; Jones, C.L.; Winters, A.; Pei, S.; Minhajuddin, M.; D’Alessandro, A.; Culp-Hill, R.; Riemondy, K.A.; Gillen, A.E.; et al. Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia. Nat. Med. 2018, 24, 1859–1866. [Google Scholar] [CrossRef]
- Nguyen, L.X.T.; Troadec, E.; Kalvala, A.; Kumar, B.; Hoang, D.H.; Viola, D.; Zhang, B.; Nguyen, D.Q.; Aldoss, I.; Ghoda, L.; et al. The Bcl-2 inhibitor venetoclax inhibits Nrf2 antioxidant pathway activation induced by hypomethylating agents in AML. J. Cell. Physiol. 2019, 234, 14040–14049. [Google Scholar] [CrossRef]
- Ball, B.J.; Famulare, C.A.; Stein, E.M.; Tallman, M.S.; Derkach, A.; Roshal, M.; Gill, S.I.; Manning, B.M.; Koprivnikar, J.; McCloskey, J.; et al. Venetoclax and hypomethylating agents (HMAs) induce high response rates in MDS, including patients after HMA therapy failure. Blood Adv. 2020, 4, 2866–2870. [Google Scholar] [CrossRef]
- Thottassery, J.V.; Sambandam, V.; Allan, P.W.; Maddry, J.A.; Maxuitenko, Y.Y.; Tiwari, K.; Hollingshead, M.; Parker, W.B. Novel DNA methyltransferase-1 (DNMT1) depleting anticancer nucleosides, 4′-thio-2′-deoxycytidine and 5-aza-4′-thio-2′-deoxycytidine. Cancer Chemother. Pharmacol. 2014, 74, 291–302. [Google Scholar] [CrossRef]
- Parker, W.B.; Thottassery, J.V. 5-Aza-4′-thio-2′-deoxycytidine, a New Orally Bioavailable Nontoxic “Best-in-Class”: DNA Methyltransferase 1-Depleting Agent in Clinical Development. J. Pharmacol. Exp. Ther. 2021, 379, 211–222. [Google Scholar] [CrossRef]
- Rahmani, M.; Aust, M.M.; Attkisson, E.; Williams, D.C., Jr.; Ferreira-Gonzalez, A.; Grant, S. Dual inhibition of Bcl-2 and Bcl-xL strikingly enhances PI3K inhibition-induced apoptosis in human myeloid leukemia cells through a GSK3- and Bim-dependent mechanism. Cancer Res. 2013, 73, 1340–1351. [Google Scholar] [CrossRef]
- Chen, S.; Dai, Y.; Harada, H.; Dent, P.; Grant, S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007, 67, 782–791. [Google Scholar] [CrossRef]
- Rahmani, M.; Davis, E.M.; Bauer, C.; Dent, P.; Grant, S. Apoptosis induced by the kinase inhibitor BAY 43-9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation. J. Biol. Chem. 2005, 280, 35217–35227. [Google Scholar] [CrossRef]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Palau, A.; Mallo, M.; Palomo, L.; Rodriguez-Hernandez, I.; Diesch, J.; Campos, D.; Granada, I.; Junca, J.; Drexler, H.G.; Sole, F.; et al. Immunophenotypic, cytogenetic, and mutational characterization of cell lines derived from myelodysplastic syndrome patients after progression to acute myeloid leukemia. Genes Chromosomes Cancer 2017, 56, 243–252. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Bewersdorf, J.P.; Carraway, H.; Prebet, T. Emerging treatment options for patients with high-risk myelodysplastic syndrome. Ther Adv. Hematol. 2020, 11, 1–22. [Google Scholar] [CrossRef]
- Jilg, S.; Hauch, R.T.; Kauschinger, J.; Buschhorn, L.; Odinius, T.O.; Dill, V.; Muller-Thomas, C.; Herold, T.; Prodinger, P.M.; Schmidt, B.; et al. Venetoclax with azacitidine targets refractory MDS but spares healthy hematopoiesis at tailored dose. Exp. Hematol. Oncol. 2019, 8, 9. [Google Scholar] [CrossRef]
- Bogenberger, J.M.; Kornblau, S.M.; Pierceall, W.E.; Lena, R.; Chow, D.; Shi, C.X.; Mantei, J.; Ahmann, G.; Gonzales, I.M.; Choudhary, A.; et al. BCL-2 family proteins as 5-Azacytidine-sensitizing targets and determinants of response in myeloid malignancies. Leukemia 2014, 28, 1657–1665. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019, 133, 7–17. [Google Scholar] [CrossRef]
- Liu, B.; Guo, Y.; Deng, L.; Qiao, Y.; Jian, J. The efficacy and adverse events of venetoclax in combination with hypomethylating agents treatment for patients with acute myeloid leukemia and myelodysplastic syndrome: A systematic review and meta-analysis. Hematology 2020, 25, 414–423. [Google Scholar]
- Azizi, A.; Ediriwickrema, A.; Dutta, R.; Patel, S.A.; Shomali, W.; Medeiros, B.; Iberri, D.; Gotlib, J.; Mannis, G.; Greenberg, P.; et al. Venetoclax and hypomethylating agent therapy in high risk myelodysplastic syndromes: A retrospective evaluation of a real-world experience. Leuk. Lymphoma 2020, 61, 2700–2707. [Google Scholar] [CrossRef]
- Zavras, P.D.; Shastri, A.; Goldfinger, M.; Verma, A.K.; Saunthararajah, Y. Clinical Trials Assessing Hypomethylating Agents Combined with Other Therapies: Causes for Failure and Potential Solutions. Clin. Cancer Res. 2021, 27, 6653–6661. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Y.; Sampath, D.; Leverson, J.; Dai, Y.; Kmieciak, M.; Nguyen, M.; Orlowski, R.Z.; Grant, S. Flavopiridol enhances ABT-199 sensitivity in unfavourable-risk multiple myeloma cells in vitro and in vivo. Br. J. Cancer 2018, 118, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Inguva, A.; Pollyea, D.A. SOHO State of the Art Updates and Next Questions: The Past, Present and Future of Venetoclax-Based Therapies in AML. Clin. Lymphoma Myeloma Leuk. 2021, 21, 805–811. [Google Scholar] [CrossRef]
- Ruefli, A.A.; Ausserlechner, M.J.; Bernhard, D.; Sutton, V.R.; Tainton, K.M.; Kofler, R.; Smyth, M.J.; Johnstone, R.W. The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc. Natl. Acad. Sci. USA 2001, 98, 10833–10838. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Li, L.; Nkwocha, J.; Sharma, K.; Zhou, L.; Grant, S. Synergistic Interactions between the Hypomethylating Agent Thio-Deoxycytidine and Venetoclax in Myelodysplastic Syndrome Cells. Hematol. Rep. 2023, 15, 91-100. https://doi.org/10.3390/hematolrep15010010
Hu X, Li L, Nkwocha J, Sharma K, Zhou L, Grant S. Synergistic Interactions between the Hypomethylating Agent Thio-Deoxycytidine and Venetoclax in Myelodysplastic Syndrome Cells. Hematology Reports. 2023; 15(1):91-100. https://doi.org/10.3390/hematolrep15010010
Chicago/Turabian StyleHu, Xiaoyan, Lin Li, Jewel Nkwocha, Kanika Sharma, Liang Zhou, and Steven Grant. 2023. "Synergistic Interactions between the Hypomethylating Agent Thio-Deoxycytidine and Venetoclax in Myelodysplastic Syndrome Cells" Hematology Reports 15, no. 1: 91-100. https://doi.org/10.3390/hematolrep15010010
APA StyleHu, X., Li, L., Nkwocha, J., Sharma, K., Zhou, L., & Grant, S. (2023). Synergistic Interactions between the Hypomethylating Agent Thio-Deoxycytidine and Venetoclax in Myelodysplastic Syndrome Cells. Hematology Reports, 15(1), 91-100. https://doi.org/10.3390/hematolrep15010010





