Mitigating Salinity Stress in Sugar Beet Seedlings Through Exogenous Application of Putrescine and Salicylic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Salt Stress Treatment
2.2. Determination of Plant Height, Fresh Weight, and Dry Weight
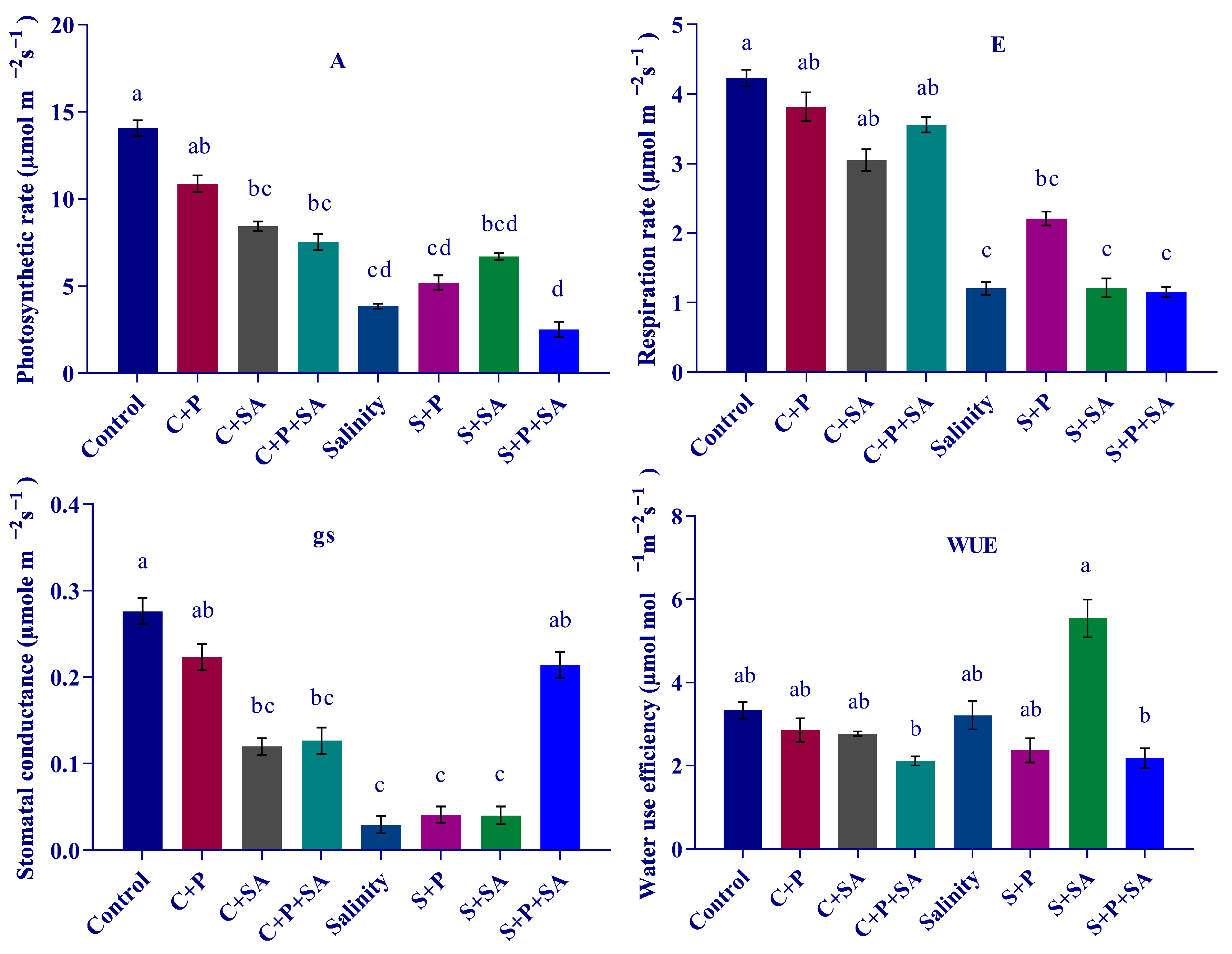
2.3. Leaf Gas Exchange Measurement
2.4. Determination of SPAD Value and Photosystem II Quantum Yield
2.5. Leaf Relative Water Content
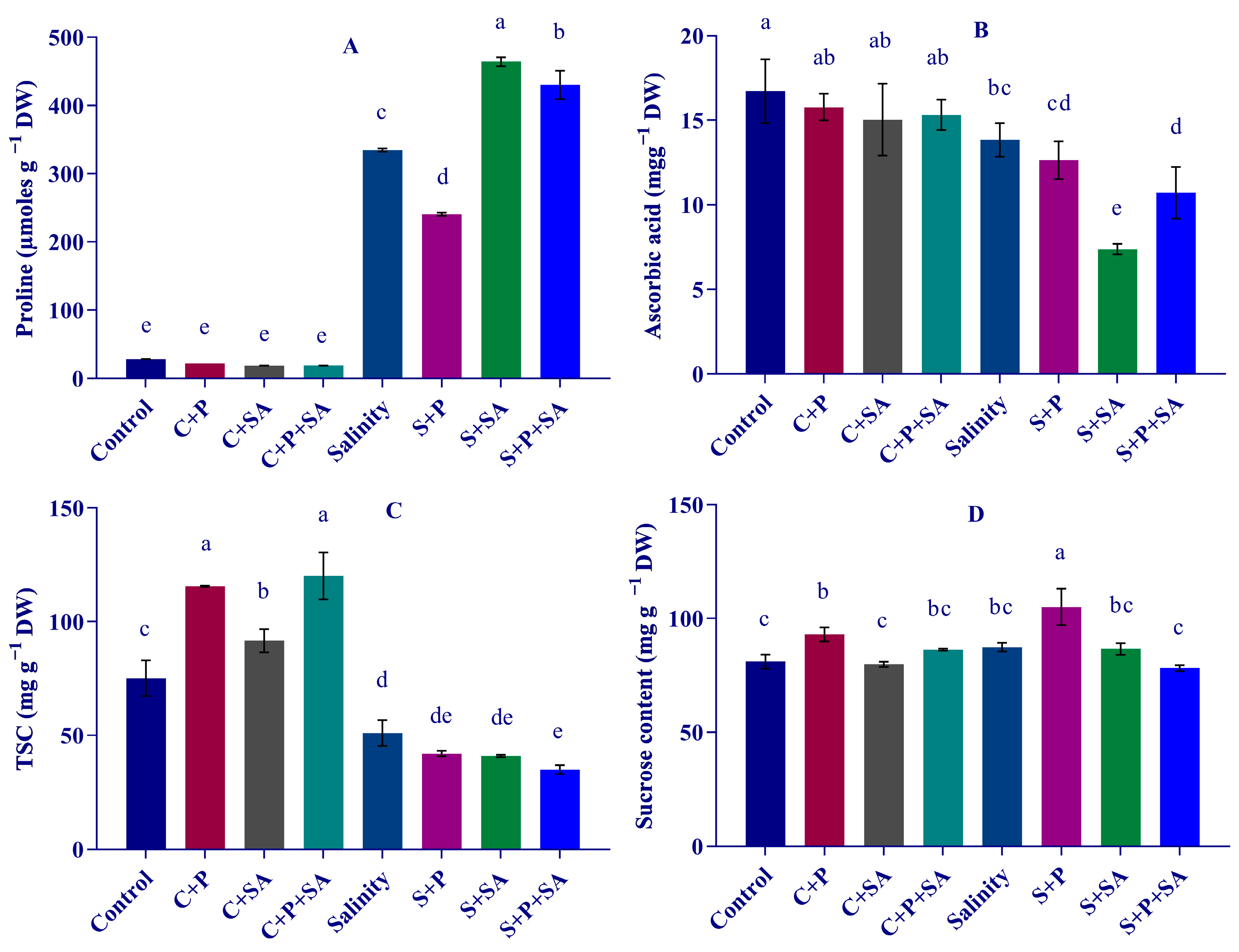
2.6. Estimation of Osmotic Adjustment Molecules
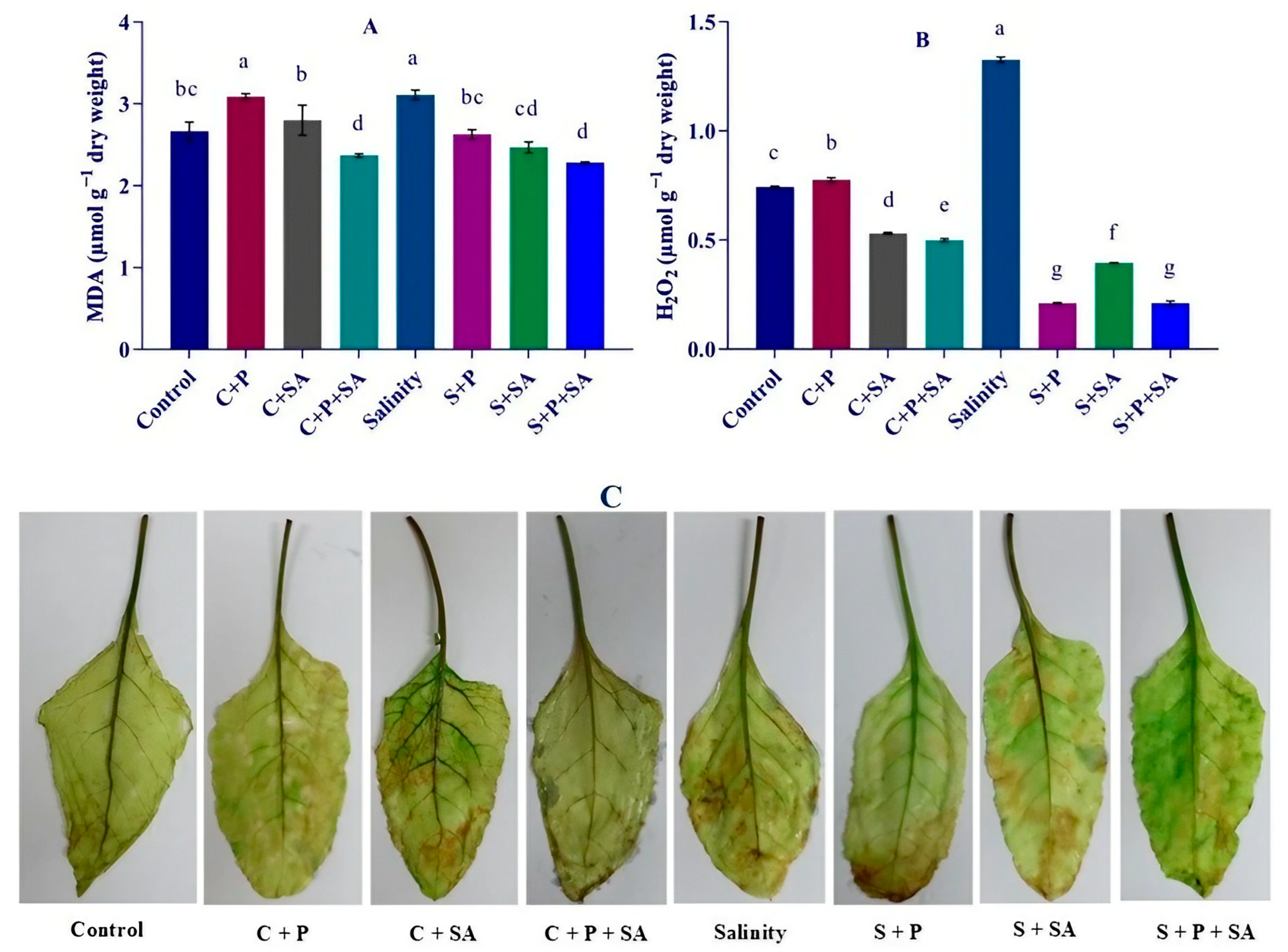
2.7. Determination of Malondialdehyde and H2O2 Content
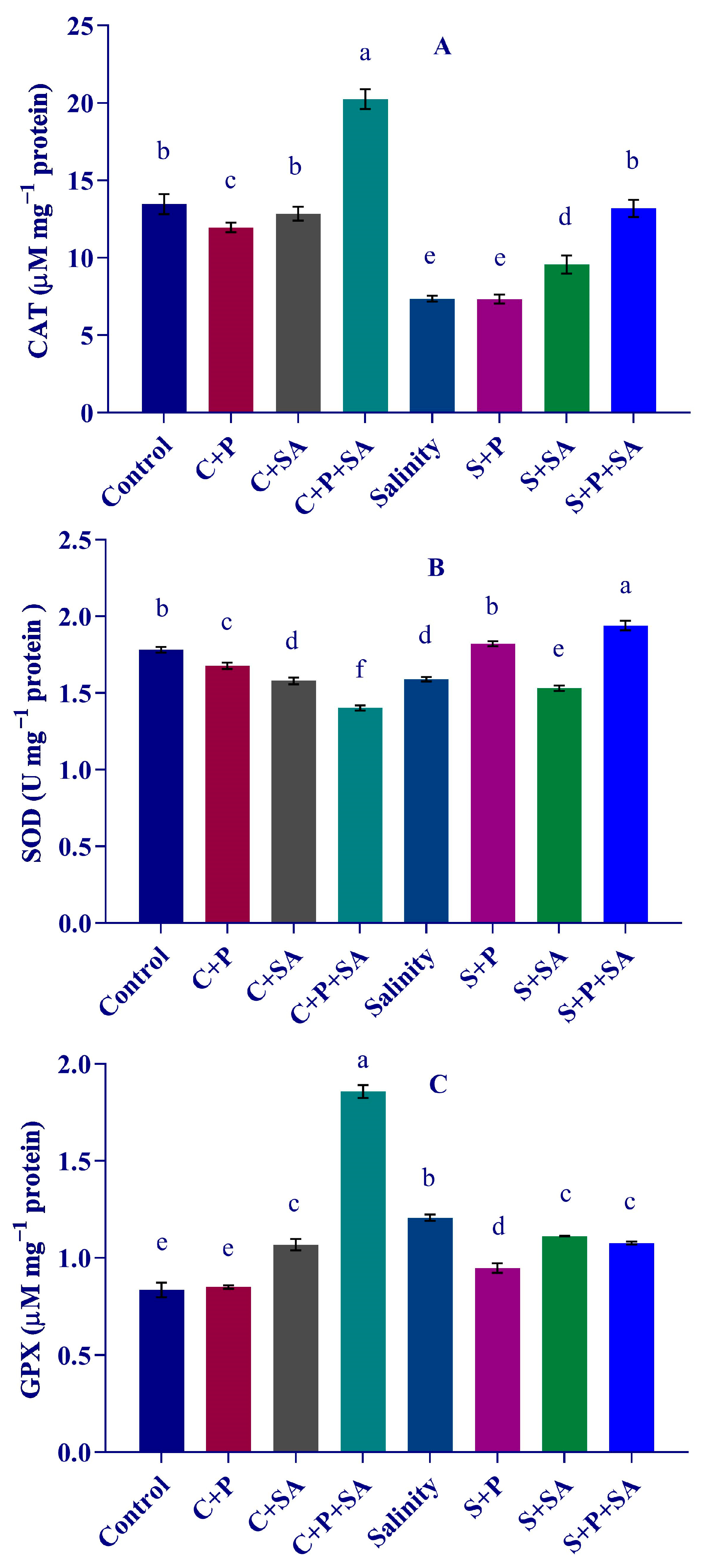
2.8. Determination of Antioxidant Enzyme Activities
2.9. Determination of Protein Content
2.10. Histochemical Confirmation of Reactive Oxygen Species
2.11. Statistical Analysis
3. Results and Discussion
3.1. Growth Parameters
3.2. Changes in Photosynthesis
3.3. Changes in SPAD Value and Fluorescence Parameters
3.4. MDA and H2O2 Concentration
3.5. Changes in Osmotic Adjustment Molecules
3.6. Changes in Antioxidant Enzyme Activity
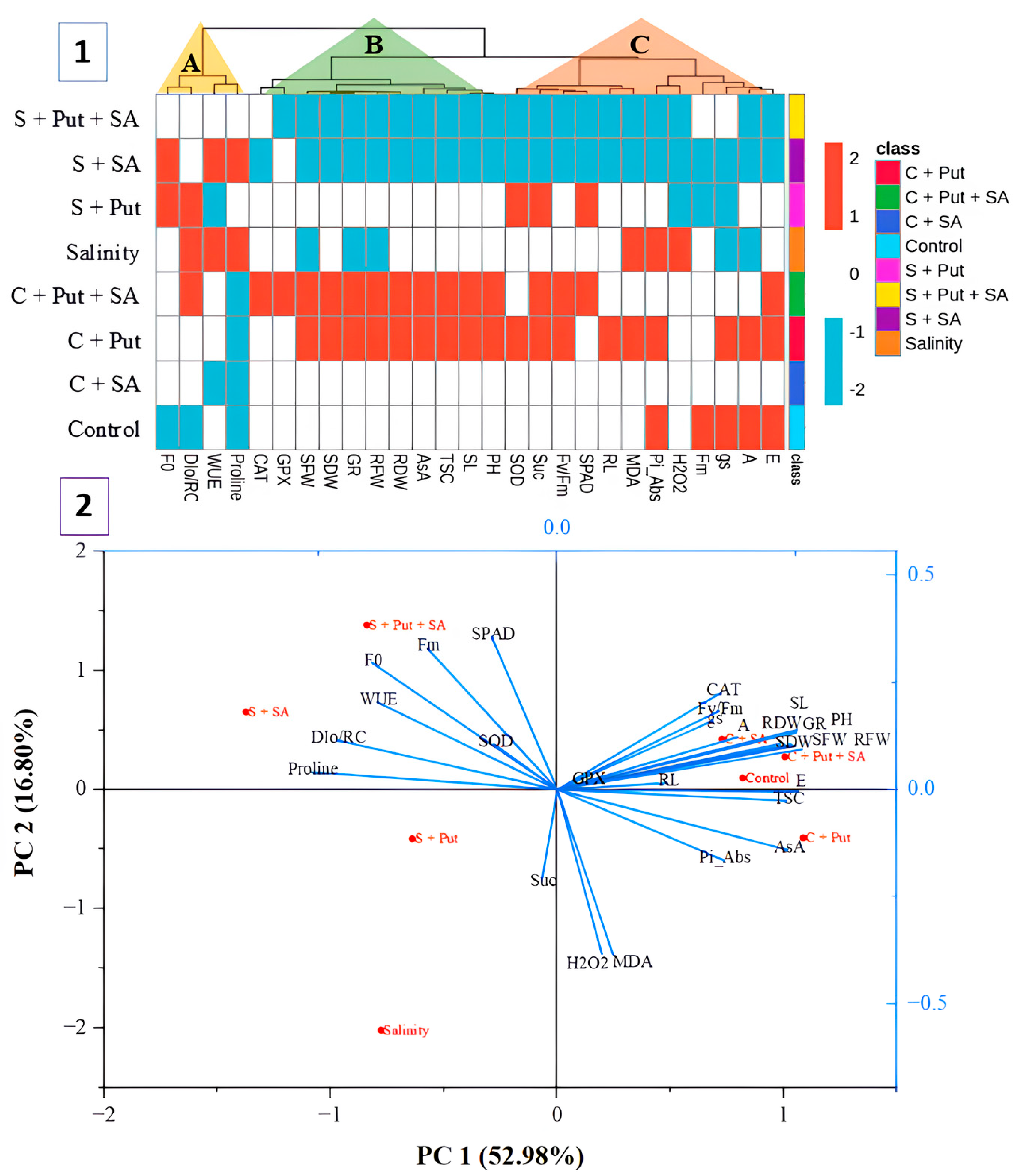
3.7. Hierarchical Clustering and PCA
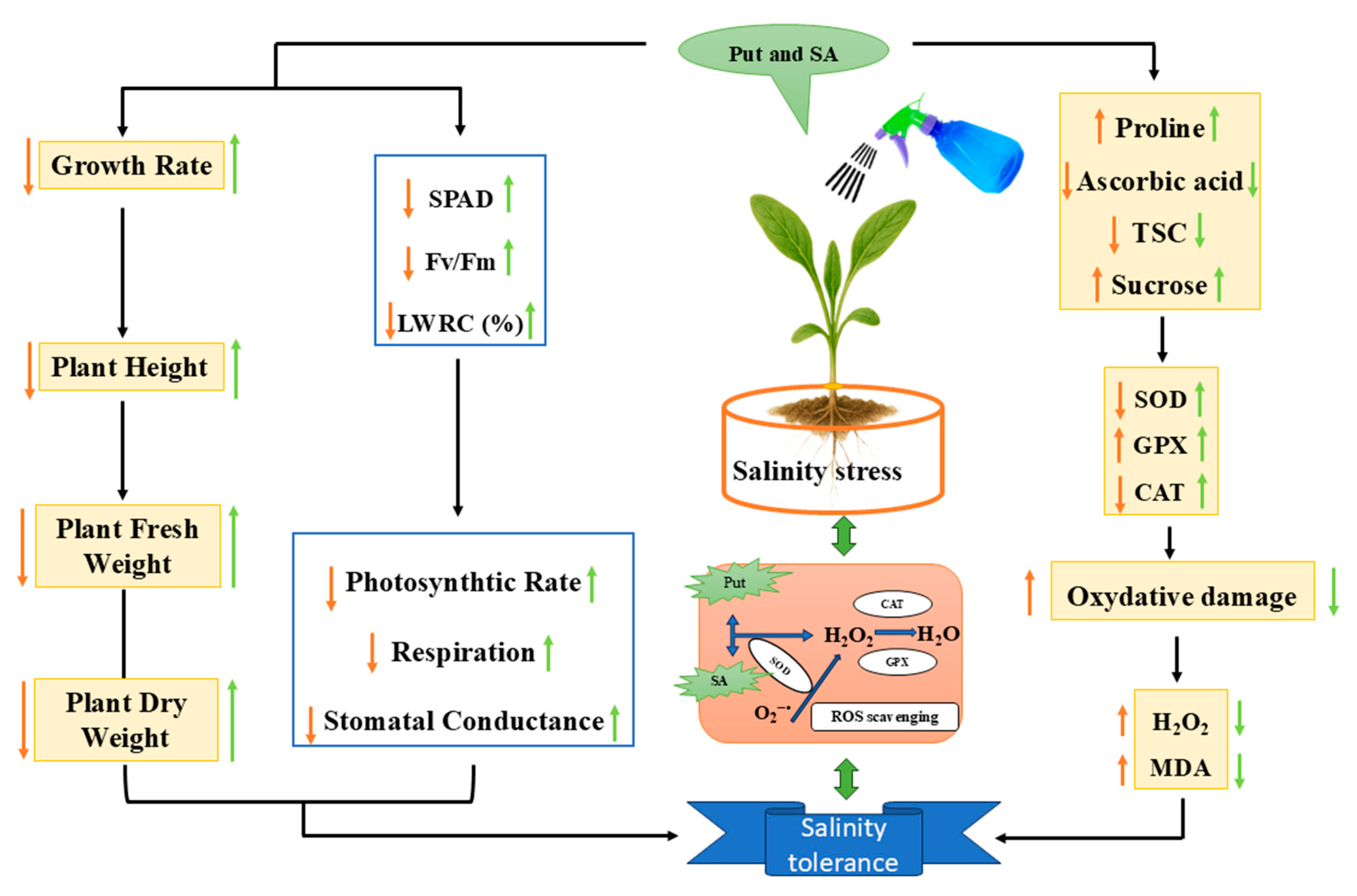
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carillo, P.; Grazia, M.; Pontecorvo, G.; Fuggi, A.; Woodrow, P. Salinity Stress and Salt Tolerance. In Abiotic Stress in Plants—Mechanisms and Adaptations; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Nachshon, U. Cropland Soil Salinization and Associated Hydrology: Trends, Processes and Examples. Water 2018, 10, 1030. [Google Scholar] [CrossRef]
- LI, R.; SHI, F.; FUKUDA, K.; YANG, Y. Effects of Salt and Alkali Stresses on Germination, Growth, Photosynthesis and Ion Accumulation in Alfalfa (Medicago sativa L.). Soil Sci. Plant Nutr. 2010, 56, 725–733. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Mir, B.A.; Yusuf, M.; Ahmad, A. 24-Epibrassinolide and/or Putrescine Trigger Physiological and Biochemical Responses for the Salt Stress Mitigation in Cucumis sativus L. Photosynthetica 2014, 52, 464–474. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Farhangi-Abriz, S. Foliar Sprays of Salicylic Acid and Jasmonic Acid Stimulate H+-ATPase Activity of Tonoplast, Nutrient Uptake and Salt Tolerance of Soybean. Ecotoxicol. Environ. Saf. 2018, 166, 18–25. [Google Scholar] [CrossRef]
- Abdoli, S.; Ghassemi-Golezani, K. Salicylic Acid: An Effective Growth Regulator for Mitigating Salt Toxicity in Plants. J. Plant Physiol. Breed. 2021, 11, 1–15. [Google Scholar]
- Abdoli, S.; Ghassemi-Golezani, K.; Alizadeh-Salteh, S. Responses of Ajowan (Trachyspermum ammi L.) to Exogenous Salicylic Acid and Iron Oxide Nanoparticles under Salt Stress. Environ. Sci. Pollut. Res. 2020, 27, 36939–36953. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Nikpour-Rashidabad, N. Seed Pretreatment and Salt Tolerance of Dill: Osmolyte Accumulation, Antioxidant Enzymes Activities and Essence Production. Biocatal. Agric. Biotechnol. 2017, 12, 30–35. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Tavasolee, A.; Ghassemi-Golezani, K.; Torabian, S.; Monirifar, H.; Rahmani, H.A. Growth-Promoting Bacteria and Natural Regulators Mitigate Salt Toxicity and Improve Rapeseed Plant Performance. Protoplasma 2020, 257, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine Function in Plants: Metabolism, Regulation on Development, and Roles in Abiotic Stress Responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef]
- Islam, M.J.; Ryu, B.R.; Azad, M.O.K.; Rahman, M.H.; Rana, M.S.; Lim, J.-D.; Lim, Y.-S. Exogenous Putrescine Enhances Salt Tolerance and Ginsenosides Content in Korean Ginseng (Panax ginseng Meyer) Sprouts. Plants 2021, 10, 1313. [Google Scholar] [CrossRef]
- Nazar, R.; Umar, S.; Khan, N.A. Exogenous Salicylic Acid Improves Photosynthesis and Growth through Increase in Ascorbate-Glutathione Metabolism and S Assimilation in Mustard under Salt Stress. Plant Signal Behav. 2015, 10, e1003751. [Google Scholar] [CrossRef]
- Kıemde, O.; Kibar, B. Effects of Different Putrescine and Salicylic Acid Applications on Germination, Plant Growth, Quality Properties and Nutrient Content of Lettuce (Lactuca sativa L.) under Saline Conditions. Akad. Ziraat Derg. 2023, 12, 1–14. [Google Scholar] [CrossRef]
- Jayakannan, M.; Bose, J.; Babourina, O.; Rengel, Z.; Shabala, S. Salicylic Acid in Plant Salinity Stress Signalling and Tolerance. Plant Growth Regul. 2015, 76, 25–40. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; An, J.; Yin, M.; Jia, X.; Guan, Y.; He, F.; Hu, J. Cold Plasma Treatment and Exogenous Salicylic Acid Priming Enhances Salinity Tolerance of Oryza Sativa Seedlings. Protoplasma 2019, 256, 79–99. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Abdoli, S. Improving ATPase and PPase Activities, Nutrient Uptake and Growth of Salt Stressed Ajowan Plants by Salicylic Acid and Iron-Oxide Nanoparticles. Plant Cell Rep. 2021, 40, 559–573. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Ghassemi-Golezani, K. How Can Salicylic Acid and Jasmonic Acid Mitigate Salt Toxicity in Soybean Plants? Ecotoxicol. Environ. Saf. 2018, 147, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Pirasteh-Anosheh1, H.; Emam, Y. Role of Chlormequat Chloride and Salicylic Acid in Improving Cereal Crops Production under Saline. In Improving Cereal Productivity Through Climate Smart Practices; Woodhead Publishing: Cambridge, UK, 2020; pp. 145–158. [Google Scholar]
- Abo-Elwafa, S.F.; Abdel-Rahim, H.M.; Abou-Salama, A.M.; Teama, E.A. Sugar Beet Floral Induction and Fertility: Effect of Vernalization and Day-Length Extension. Sugar Tech 2006, 8, 281–287. [Google Scholar] [CrossRef]
- Brar, N.S.; Dhillon, B.S.; Saini, K.S.; Sharma, P.K. Agronomy of Sugarbeet Cultivation-A Review. Agric. Rev. 2015, 36, 184–197. [Google Scholar] [CrossRef]
- Islam, M.J.; Kim, J.W.; Begum, M.K.; Sohel, M.A.T.; Lim, Y.-S. Physiological and Biochemical Changes in Sugar Beet Seedlings to Confer Stress Adaptability under Drought Condition. Plants 2020, 9, 1511. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Seo, B.-S.; Jeong, Y.-J.; Yang, H.I.; Park, S.; Baek, N.; Kwak, J.-H.; Choi, W.-J. Soil Salinity, Fertility and Carbon Content, and Rice Yield of Salt-Affected Paddy with Different Cultivation Period in Southwestern Coastal Area of South Korea. Soil Sci. Plant Nutr. 2022, 68, 53–63. [Google Scholar] [CrossRef]
- He, Y.; Zhu, Z.J. Exogenous Salicylic Acid Alleviates NaCl Toxicity and Increases Antioxidative Enzyme Activity in Lycopersicon Esculentum. Biol. Plant 2008, 52, 792–795. [Google Scholar] [CrossRef]
- Ghahremani, Z.; Alizadeh, B.; Barzegar, T.; Nikbakht, J.; Ranjbar, M.E.; Nezamdoost, D. The Mechanism of Enhancing Drought Tolerance Threshold of Pepper Plant Treated with Putrescine and Salicylic Acid. Plant Stress 2023, 9, 100199. [Google Scholar] [CrossRef]
- Nazar, R.; Umar, S.; Khan, N.A.; Sareer, O. Salicylic Acid Supplementation Improves Photosynthesis and Growth in Mustard through Changes in Proline Accumulation and Ethylene Formation under Drought Stress. S. Afr. J. Bot. 2015, 98, 84–94. [Google Scholar] [CrossRef]
- Abd Elbar, O.H.; Farag, R.E.; Shehata, S.A. Effect of Putrescine Application on Some Growth, Biochemical and Anatomical Characteristics of Thymus vulgaris L. under Drought Stress. Ann. Agric. Sci. 2019, 64, 129–137. [Google Scholar] [CrossRef]
- Islam, M.J.; Uddin, M.J.; Hossain, M.A.; Henry, R.; Begum, M.K.; Sohel, M.A.T.; Mou, M.A.; Ahn, J.; Cheong, E.J.; Lim, Y.-S. Exogenous Putrescine Attenuates the Negative Impact of Drought Stress by Modulating Physio-Biochemical Traits and Gene Expression in Sugar Beet (Beta vulgaris L.). PLoS ONE 2022, 17, e0262099. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.A.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Alam, T.; Islam, M.J.; Habib, M.A.; Begum, M.K.; Arefin, M.S.; Hossain, M.S.; Hossain, M.A. Effects of Various Chemical Treatments on Ripening Acceleration and Quality Attributes of Sukkari Dates. Sugar Tech 2024, 26, 1690–1703. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of Water Stress-induced Changes in the Levels of Endogenous Ascorbic Acid and Hydrogen Peroxide in Vigna Seedlings. Physiol. Plant 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Islam, M.J.; Ryu, B.R.; Rahman, M.H.; Rana, M.S.; Cheong, E.J.; Wang, M.-H.; Lim, J.-D.; Hossain, M.A.; Lim, Y.-S. Cannabinoid Accumulation in Hemp Depends on ROS Generation and Interlinked with Morpho-Physiological Acclimation and Plasticity under Indoor LED Environment. Front. Plant Sci. 2022, 13, 984410. [Google Scholar] [CrossRef]
- Zhang, X.Z. The Measurement and Mechanism of Lipid Peroxidation and SOD, POD and CAT Activities in Biological System. In Research Methodology of Crop Physiology; Agriculture Press: Beijing, China, 1992; pp. 208–211. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kumar, D.; Yusuf, M.A.; Singh, P.; Sardar, M.; Sarin, N.B. Histochemical Detection of Superoxide and H2O2 Accumulation in Brassica Juncea Seedlings. Bio Protoc. 2014, 4, e1108. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil Salinity: A Serious Environmental Issue and Plant Growth Promoting Bacteria as One of the Tools for Its Alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Gilliham, M. Salinity Tolerance of Crops–What Is the Cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.K.; Das, A.B. Salt Tolerance and Salinity Effects on Plants: A Review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Zeid, I.M. Response of Bean (Phaseolus vulgaris) to Exogenous Putrescine Treatment under Salinity Stress. Pak. J. Biol. Sci. 2004, 7, 219–225. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Khan, N.A. Salicylic Acid and Jasmonates: Approaches in Abiotic Stress Tolerance. J. Plant Biochem. Physiol. 2013, 1, e113. [Google Scholar] [CrossRef]
- Islam, M.J.; Mou, M.A.; Razzak, M.A.; Lim, Y.-S. Exogenous Putrescine-Mediated Modulation of Drought Stress Tolerance in Sugar Beet: Possible Mechanisms. In Sugar Beet Cultivation, Management and Processing; Springer Nature: Singapore, 2022; pp. 441–457. [Google Scholar]
- Gill, S.S.; Tuteja, N. Polyamines and Abiotic Stress Tolerance in Plants. Plant Signal Behav. 2010, 5, 26–33. [Google Scholar] [CrossRef]
- Napieraj, N.; Janicka, M.; Reda, M. Interactions of Polyamines and Phytohormones in Plant Response to Abiotic Stress. Plants 2023, 12, 1159. [Google Scholar] [CrossRef]
- Zahra, N.; Al Hinai, M.S.; Hafeez, M.B.; Rehman, A.; Wahid, A.; Siddique, K.H.M.; Farooq, M. Regulation of Photosynthesis under Salt Stress and Associated Tolerance Mechanisms. Plant Physiol. Biochem. 2022, 178, 55–69. [Google Scholar] [CrossRef]
- Khalvandi, M.; Siosemardeh, A.; Roohi, E.; Keramati, S. Salicylic Acid Alleviated the Effect of Drought Stress on Photosynthetic Characteristics and Leaf Protein Pattern in Winter Wheat. Heliyon 2021, 7, e05908. [Google Scholar] [CrossRef]
- Liu, A.; Wang, M.; Dong, J.; Yan, Z.; Wang, X.; Li, J.; Song, H. Foliar Application of Exogenous Salicylic Acid Mitigates the Detrimental Effects Caused by Salt Stress in Sunflower Seedlings. Ind. Crops Prod. 2024, 222, 119854. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with Regulatory Functions in Plant Abiotic Stress Tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Janda, K.; Hideg, É.; Szalai, G.; Kovács, L.; Janda, T. Salicylic Acid May Indirectly Influence the Photosynthetic Electron Transport. J. Plant Physiol. 2012, 169, 971–978. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, H.; Yang, Y.; Feng, X.; Chen, X.; Xiao, F.; Lin, H.; Guo, Y. Insights into Plant Salt Stress Signaling and Tolerance. J. Genet. Genom. 2024, 51, 16–34. [Google Scholar] [CrossRef]
- Zafar, Z.; Rasheed, F.; Mushtaq, N.; Khan, M.U.; Mohsin, M.; Irshad, M.A.; Summer, M.; Raza, Z.; Gailing, O. Exogenous Application of Salicylic Acid Improves Physiological and Biochemical Attributes of Morus Alba Saplings under Soil Water Deficit. Forests 2023, 14, 236. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Hosseinzadeh-Mahootchi, A.; Farhangi-Abriz, S. Chlorophyll a Fluorescence of Safflower Affected by Salt Stress and Hormonal Treatments. SN Appl. Sci. 2020, 2, 1306. [Google Scholar] [CrossRef]
- Khoshbakht, D.; Asgharei, M.R. Influence of Foliar-Applied Salicylic Acid on Growth, Gas-Exchange Characteristics, and Chlorophyll Fluorescence in Citrus under Saline Conditions. Photosynthetica 2015, 53, 410–418. [Google Scholar] [CrossRef]
- Chaparzadeh, N.; Hosseinzad-Behboud, E. Evidence for Enhancement of Salinity Induced Oxidative Damages by Salicylic Acid in Radish (Raphanus sativus L.). J. Plant Physiol. Breed. 2015, 5, 23–33. [Google Scholar]
- Ghassemi-Golezani, K.; Hassanzadeh, N.; Shakiba, M.-R.; Esmaeilpour, B. Exogenous Salicylic Acid and 24-Epi-Brassinolide Improve Antioxidant Capacity and Secondary Metabolites of Brassica Nigra. Biocatal. Agric. Biotechnol. 2020, 26, 101636. [Google Scholar] [CrossRef]
- Foyer, C.H.; Lelandais, M.; Kunert, K.J. Photooxidative Stress in Plants. Physiol. Plant. 1994, 92, 696–717. [Google Scholar] [CrossRef]
- Zeid, F.A.; Omer, E.A.; Amin, A.Y.; Hanafy, S.A.H. Effect of Putrescine and Salicylic Acid on Ajwain Plant (Trachyspermum ammi) at Vegetative Stage Grown under Drought Stress. Int. J. Agric. Sci. Res. 2014, 4, 61–80. [Google Scholar]
- Farooq, A.; Bukhari, S.A.; Akram, N.A.; Ashraf, M.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Exogenously Applied Ascorbic Acid-Mediated Changes in Osmoprotection and Oxidative Defense System Enhanced Water Stress Tolerance in Different Cultivars of Safflower (Carthamus tinctorious L.). Plants 2020, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Besford, R.T.; Richardson, C.M.; Campos, J.L.; Tiburcio, A.F. Effect of Polyamines on Stabilization of Molecular Complexes in Thylakoid Membranes of Osmotically Stressed Oat Leaves. Planta 1993, 189, 201–206. [Google Scholar] [CrossRef]
- Aroca, R.; Ruiz-Lozano, J.M.; Zamarreño, Á.M.; Paz, J.A.; García-Mina, J.M.; Pozo, M.J.; López-Ráez, J.A. Arbuscular Mycorrhizal Symbiosis Influences Strigolactone Production under Salinity and Alleviates Salt Stress in Lettuce Plants. J. Plant Physiol. 2013, 170, 47–55. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, M.; Aroca, R.; Muñoz, Y.; Polón, R.; Ruiz-Lozano, J.M. The Arbuscular Mycorrhizal Symbiosis Enhances the Photosynthetic Efficiency and the Antioxidative Response of Rice Plants Subjected to Drought Stress. J. Plant Physiol. 2010, 167, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Khoshbakht, D.; Asghari, M.R.; Haghighi, M. Influence of Foliar Application of Polyamines on Growth, Gas-Exchange Characteristics, and Chlorophyll Fluorescence in Bakraii Citrus under Saline Conditions. Photosynthetica 2018, 56, 731–742. [Google Scholar] [CrossRef]
- TSIMILLI-MICHAEL, M. Special Issue in Honour of Prof. Reto J. Strasser—Revisiting JIP-Test: An Educative Review on Concepts, Assumptions, Approximations, Definitions and Terminology. Photosynthetica 2020, 58, 275–292. [Google Scholar] [CrossRef]
- Zuo, G.; Mei, W.; Feng, N.; Zheng, D. Photosynthetic Performance Index (PIabs) and Malondialdehyde (MDA) Content Determine Rice Biomass under Combined Salt Stress and Prohexadione-Calcium Treatment. BMC Plant Biol. 2025, 25, 823. [Google Scholar] [CrossRef]
- Warzecha, T.; Skrzypek, E.; Bocianowski, J.; Sutkowska, A. Impact of Selected PSII Parameters on Barley DH Lines Biomass and Yield Elements. Agronomy 2021, 11, 1705. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Gao, H.; Xue, Z.; Yang, C.; Meng, X.; Meng, Q. Mitochondrial Alternative Oxidase Pathway Protects Plants against Photoinhibition by Alleviating Inhibition of the Repair of Photodamaged PSII through Preventing Formation of Reactive Oxygen Species in Rumex K-1 Leaves. Physiol. Plant 2011, 143, 396–407. [Google Scholar] [CrossRef]
- Carillo, P.; Cirillo, C.; De Micco, V.; Arena, C.; De Pascale, S.; Rouphael, Y. Morpho-Anatomical, Physiological and Biochemical Adaptive Responses to Saline Water of Bougainvillea Spectabilis Willd. Trained to Different Canopy Shapes. Agric. Water Manag. 2019, 212, 12–22. [Google Scholar] [CrossRef]
- Munir, N.; Hasnain, M.; Roessner, U.; Abideen, Z. Strategies in Improving Plant Salinity Resistance and Use of Salinity Resistant Plants for Economic Sustainability. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2150–2196. [Google Scholar] [CrossRef]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity Stress Affects Photosynthesis, Malondialdehyde Formation, and Proline Content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Bin, X.U.; Luming, A.N.; Xintao, M. Effect of Exogenous Putrescine (Put) Treatments on Postharvest Chilling Injury and Reactive Oxygen Metabolism of Yellow Melon Fruits. Shipin Gongye Ke-Ji 2022, 43, 360–367. [Google Scholar]
- Mohammadi, M.; Nezamdoost, D.; Khosravi Far, F.; Zulfiqar, F.; Eghlima, G.; Aghamir, F. Exogenous Putrescine Application Imparts Salt Stress-Induced Oxidative Stress Tolerance via Regulating Antioxidant Activity, Potassium Uptake, and Abscisic Acid to Gibberellin Ratio in Zinnia Flowers. BMC Plant Biol. 2024, 24, 865. [Google Scholar] [CrossRef]
- Akter, S.; Rasul, M.G.; Zakaria, M.; Sarker, M.M.; Nila, I.S.; Dutta, S.; Haque, M.M.; Rohman, M.M. Effect of Polyamine on Pigmentation, Reactive Oxidative Species and Antioxidant under Drought in Maize (Zea mays L.). Turk. J. Agric.-Food Sci. Technol. 2018, 6, 799–811. [Google Scholar] [CrossRef]
- Alhasnawi, A.N. Role of Proline in Plant Stress Tolerance: A Mini Review. Res. Crops 2019, 20, 223–229. [Google Scholar]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of Proline under Changing Environments: A Review. Plant Signal Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Verslues, P.E.; Sharma, S. Proline Metabolism and Its Implications for Plant-Environment Interaction. Arab. Book/Am. Soc. Plant Biol. 2010, 8, e0140. [Google Scholar] [CrossRef]
- Liu, L.; Huang, L.; Lin, X.; Sun, C. Hydrogen Peroxide Alleviates Salinity-Induced Damage through Enhancing Proline Accumulation in Wheat Seedlings. Plant Cell Rep. 2020, 39, 567–575. [Google Scholar] [CrossRef]
- Gulen, H.; Turhan, E.; Eris, A. Changes in Peroxidase Activities and Soluble Proteins in Strawberry Varieties under Salt-Stress. Acta Physiol. Plant 2006, 28, 109–116. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-Induced Responses of Photosynthesis and Antioxidant Metabolism in Higher Plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and Biochemical Changes during Drought and Recovery Periods at Tillering and Jointing Stages in Wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef]
- Ebeed, H.T.; Hassan, N.M.; Aljarani, A.M. Exogenous Applications of Polyamines Modulate Drought Responses in Wheat through Osmolytes Accumulation, Increasing Free Polyamine Levels and Regulation of Polyamine Biosynthetic Genes. Plant Physiol. Biochem. 2017, 118, 438–448. [Google Scholar] [CrossRef]
- Farhadi, N.; Ghassemi-Golezani, K. Physiological Changes of Mentha Pulegium in Response to Exogenous Salicylic Acid under Salinity. Sci. Hortic. 2020, 267, 109325. [Google Scholar] [CrossRef]
- Sekmen, A.H.; Turkan, I.; Tanyolac, Z.O.; Ozfidan, C.; Dinc, A. Different Antioxidant Defense Responses to Salt Stress during Germination and Vegetative Stages of Endemic Halophyte Gypsophila Oblanceolata Bark. Environ. Exp. Bot. 2012, 77, 63–76. [Google Scholar] [CrossRef]
- Khan, M.H.; Panda, S.K. Alterations in Root Lipid Peroxidation and Antioxidative Responses in Two Rice Cultivars under NaCl-Salinity Stress. Acta Physiol. Plant 2007, 30, 81–89. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Sheokand, S.; Kumari, A.; Sawhney, V. Effect of Nitric Oxide and Putrescine on Antioxidative Responses under NaCl Stress in Chickpea Plants. Physiol. Mol. Biol. Plants 2008, 14, 355–362. [Google Scholar] [CrossRef]
- Mohammadi, M.; Aelaei, M.; Saidi, M. Pre-Harvest and Pulse Treatments of Spermine, γ-and β-Aminobutyric Acid Increased Antioxidant Activities and Extended the Vase Life of Gerbera Cut Flowers ‘Stanza’. Ornam. Hortic. 2020, 26, 306–316. [Google Scholar] [CrossRef]
- Choudhary, S.P.; Kanwar, M.; Bhardwaj, R.; Gupta, B.D.; Gupta, R.K. Epibrassinolide Ameliorates Cr (VI) Stress via Influencing the Levels of Indole-3-Acetic Acid, Abscisic Acid, Polyamines and Antioxidant System of Radish Seedlings. Chemosphere 2011, 84, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Moschou, P.N.; Paschalidis, K.A.; Delis, I.D.; Andriopoulou, A.H.; Lagiotis, G.D.; Yakoumakis, D.I.; Roubelakis-Angelakis, K.A. Spermidine Exodus and Oxidation in the Apoplast Induced by Abiotic Stress Is Responsible for H2O2 Signatures That Direct Tolerance Responses in Tobacco. Plant Cell 2008, 20, 1708–1724. [Google Scholar] [CrossRef]
- Pottosin, I.; Velarde-Buendía, A.M.; Bose, J.; Fuglsang, A.T.; Shabala, S. Polyamines Cause Plasma Membrane Depolarization, Activate Ca2+-, and Modulate H+-ATPase Pump Activity in Pea Roots. J. Exp. Bot. 2014, 65, 2463–2472. [Google Scholar] [CrossRef]
- Horváth, E.; Szalai, G.; Janda, T. Induction of Abiotic Stress Tolerance by Salicylic Acid Signaling. J. Plant Growth Regul. 2007, 26, 290–300. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic Acid-Induced Abiotic Stress Tolerance and Underlying Mechanisms in Plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef]
- Ma, X.; Zheng, J.; Zhang, X.; Hu, Q.; Qian, R. Salicylic Acid Alleviates the Adverse Effects of Salt Stress on Dianthus superbus (Caryophyllaceae) by Activating Photosynthesis, Protecting Morphological Structure, and Enhancing the Antioxidant System. Front. Plant Sci. 2017, 8, 600. [Google Scholar] [CrossRef]
- Janda, T.; Pál, M.; Darkó, É.; Szalai, G. Use of Salicylic Acid and Related Compounds to Improve the Abiotic Stress Tolerance of Plants: Practical Aspects. In Salicylic Acid: A Multifaceted Hormone; Springer: Singapore, 2017; pp. 35–46. [Google Scholar]
| Chemical Name | A Tank (50 L) * | B Tank (50 L) |
|---|---|---|
| Ca(NO3) | 1.5 kg | |
| KNO3 | 3.79 kg | 3.79 kg |
| (NH4)2HPO4 | 1.6 kg | |
| MgSO4 | 4.3 kg | |
| Fe-EDTA | 460 g | |
| MnSO4 | 30.8 g | |
| H3BO3 | 57.2 g | |
| ZnSO4 | 3.6 g | |
| CuSO4 | 1.3 g | |
| (NH4)6Mo7O24.4H2O | 0.4 g |
| Treatment | Plant GR on DW Basis (g day−1) | SL (cm) | RL (cm) | PH (cm) | SFW (g) | RFW (g) | PFW (g) | SDW (g) | RDW (g) | PDW (g) |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.118 abc | 26.33 ab | 4.17 bc | 30.50 abc | 14.46 a | 3.24 ab | 17.70 a | 1.16 ab | 0.30 bcd | 1.46 ab |
| C+P | 0.158 abc | 28.33 a | 5.50 a | 33.83 a | 14.19 a | 3.48 ab | 17.67 a | 1.41 ab | 0.45 ab | 1.86 ab |
| C+SA | 0.166 ab | 25.83 ab | 5.17 ab | 31.00 ab | 15.58 a | 4.10 a | 19.67 a | 1.45 ab | 0.49 a | 1.94 ab |
| C+P+SA | 0.171 a | 28.97 a | 3.60 c | 32.57 ab | 15.50 a | 3.45 ab | 18.95 a | 1.55 a | 0.44 abc | 1.99 a |
| Salinity | 0.052 bc | 16.33 c | 3.73 c | 20.07 d | 5.42 b | 1.25 c | 6.67 b | 0.62 b | 0.17 d | 0.80 b |
| S+P | 0.077 abc | 20.90 abc | 3.73 c | 24.63 bcd | 7.18 b | 2.32 bc | 9.50 b | 0.78 ab | 0.27 cd | 1.05 ab |
| S+SA | 0.049 c | 18.30 bc | 4.27 bc | 22.57 cd | 5.69 b | 1.17 c | 6.86 b | 0.60 b | 0.17 d | 0.77 b |
| S+P+SA | 0.083 abc | 21.13 abc | 3.73 c | 24.87 bcd | 7.29 b | 1.92 bc | 9.21 b | 0.84 ab | 0.27 d | 1.11 ab |
| Treatment | SPAD | F0 | Fm | Fv/Fm | PI_abs | DIo/RC | LRWC (%) |
|---|---|---|---|---|---|---|---|
| Control | 32.433 ab | 7466 b | 38,037 abc | 0.8027 a | 3.2050 a | 0.3687 d | 82.88 ab |
| C+P | 32.550 ab | 6838 b | 29,964 cd | 0.7633 ab | 2.1810 ab | 0.4407 cd | 89.77 a |
| C+SA | 35.183 a | 8258 b | 331,94 bcd | 0.7507 b | 1.7863 ab | 0.5443 bcd | 87.18 a |
| C+P+SA | 32.583 ab | 6692 b | 24,698 d | 0.7257 bc | 1.4430 ab | 0.5667 bc | 86.51 a |
| Salinity | 28.383 b | 6903 b | 27,461 cd | 0.6960 c | 2.2693 ab | 0.6017 bc | 58.23 c |
| S+P | 34.783 a | 6351 b | 21,663 d | 0.7320 bc | 1.2407 ab | 0.6410 b | 61.73 bc |
| S+SA | 34.483 a | 13,676 a | 46,024 ab | 0.7030 c | 0.7393 b | 0.8817 a | 58.22 c |
| S+P+SA | 35.133 a | 12,332 a | 48,994 a | 0.7487 b | 1.2487 ab | 0.7023 ab | 61.94 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.J.; Ryu, B.R.; Alam, T.; Mou, M.A.; Rahman, M.H.; Salam, M.A.; Lim, Y.-S.; Hossain, M.A. Mitigating Salinity Stress in Sugar Beet Seedlings Through Exogenous Application of Putrescine and Salicylic Acid. Int. J. Plant Biol. 2025, 16, 131. https://doi.org/10.3390/ijpb16040131
Islam MJ, Ryu BR, Alam T, Mou MA, Rahman MH, Salam MA, Lim Y-S, Hossain MA. Mitigating Salinity Stress in Sugar Beet Seedlings Through Exogenous Application of Putrescine and Salicylic Acid. International Journal of Plant Biology. 2025; 16(4):131. https://doi.org/10.3390/ijpb16040131
Chicago/Turabian StyleIslam, Md. Jahirul, Byung Ryeol Ryu, Tanjina Alam, Masuma Akter Mou, Md. Hafizur Rahman, Md. Abdus Salam, Young-Seok Lim, and Mohammad Anwar Hossain. 2025. "Mitigating Salinity Stress in Sugar Beet Seedlings Through Exogenous Application of Putrescine and Salicylic Acid" International Journal of Plant Biology 16, no. 4: 131. https://doi.org/10.3390/ijpb16040131
APA StyleIslam, M. J., Ryu, B. R., Alam, T., Mou, M. A., Rahman, M. H., Salam, M. A., Lim, Y.-S., & Hossain, M. A. (2025). Mitigating Salinity Stress in Sugar Beet Seedlings Through Exogenous Application of Putrescine and Salicylic Acid. International Journal of Plant Biology, 16(4), 131. https://doi.org/10.3390/ijpb16040131









