Genome-Wide Metatranscriptomics Crosswalk of Diseased Common Beans (Phaseolus vulgaris L.) Unravels Critical Metabolic Pathways Involved in Plant Defense Mechanisms
Abstract
1. Introduction
2. Research Methods
3. RNA Extraction
4. Library Construction, Quality Control & Sequencing
5. Data Quality Control
6. Transcriptome Assembly and Analysis
7. Data Availability and Accession
8. Results
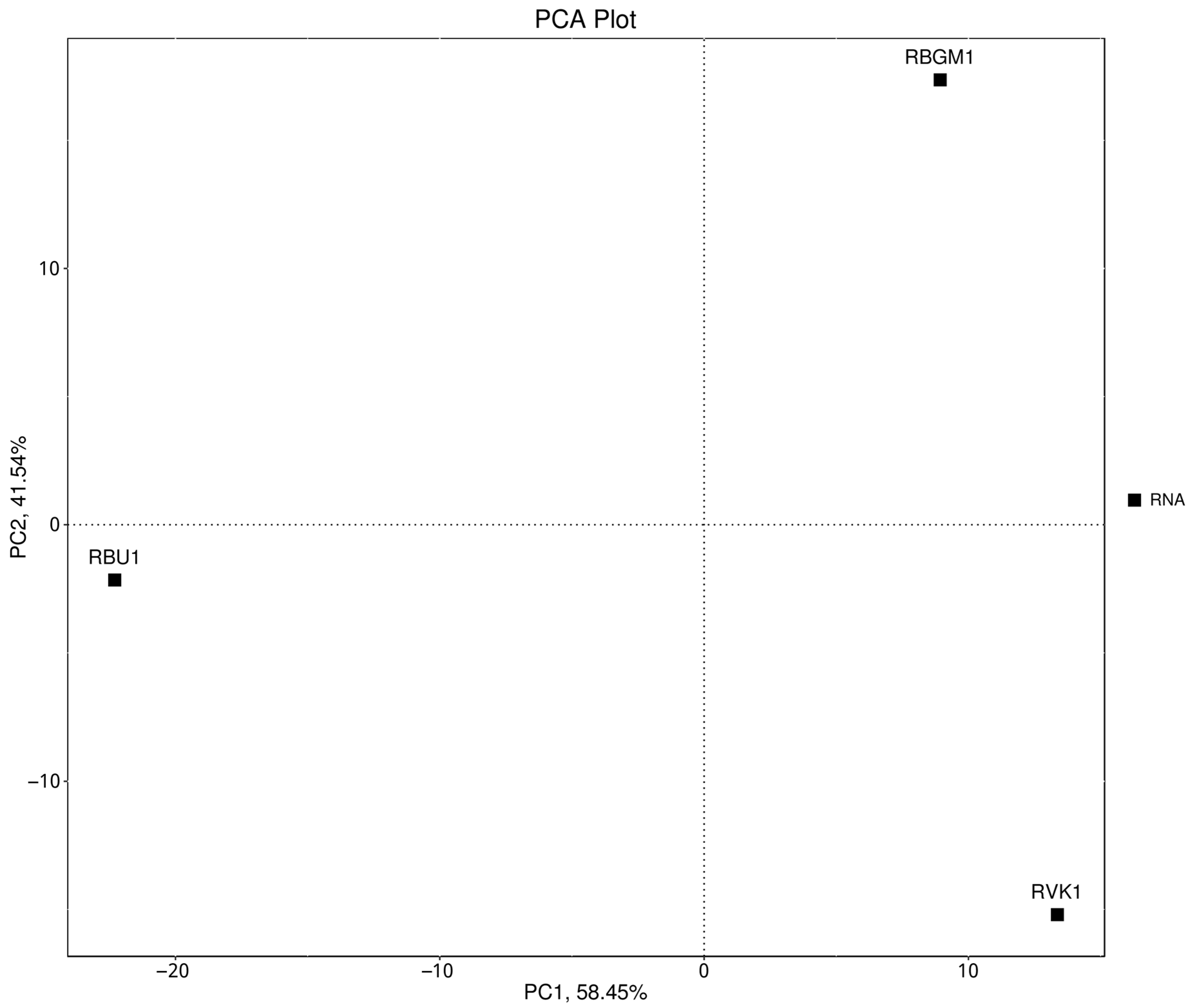
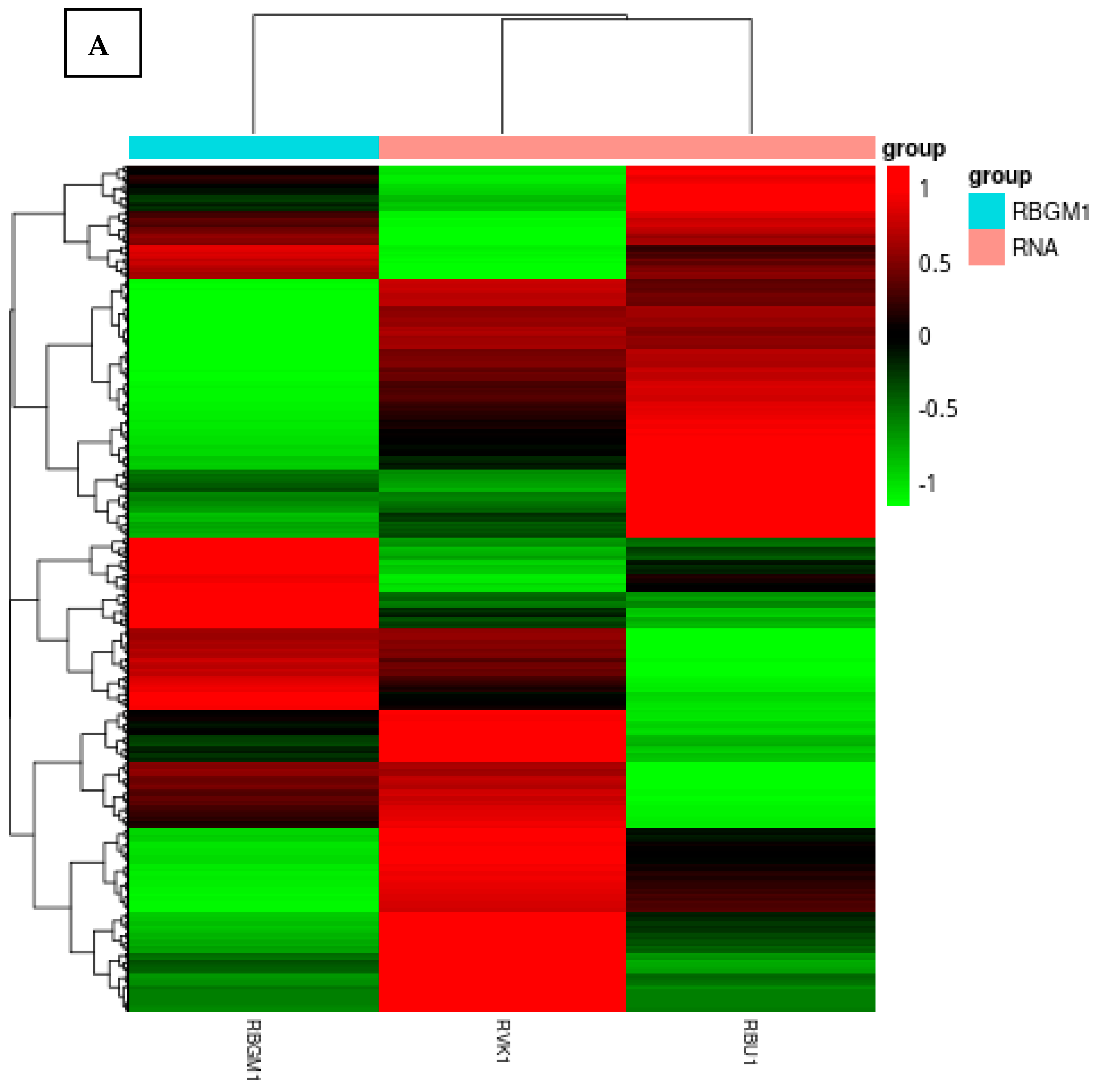
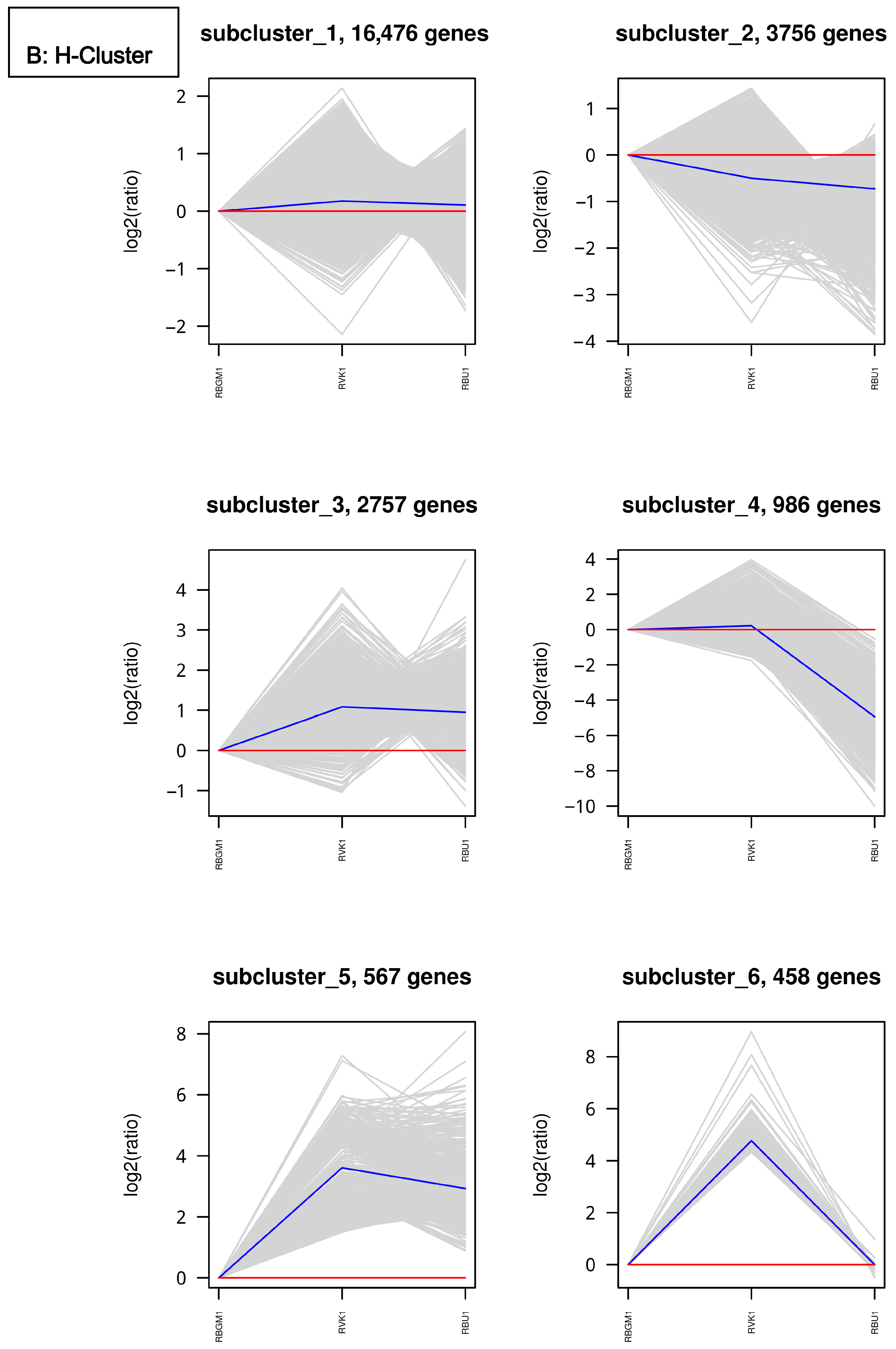
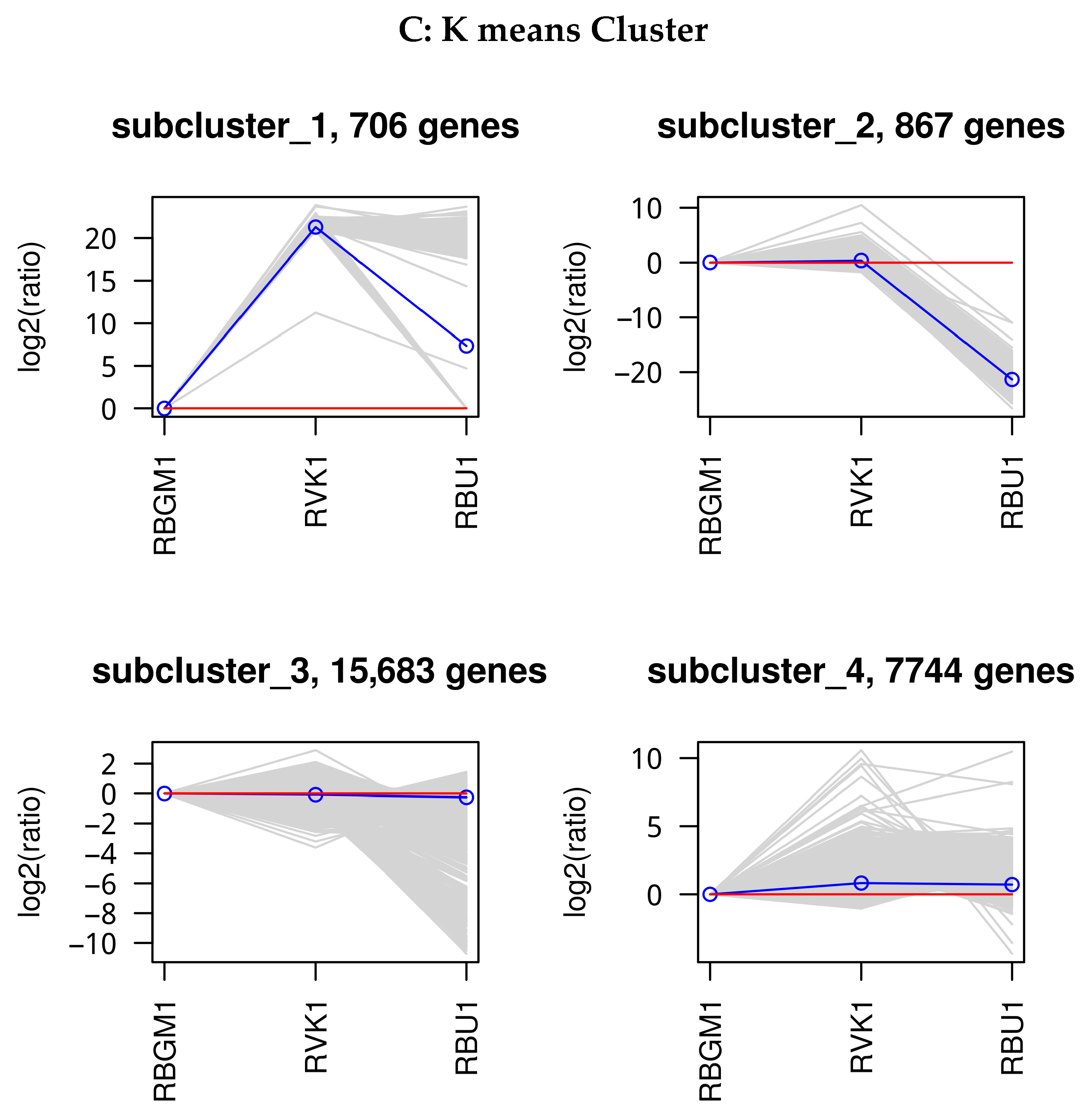
8.1. Quality Check (QC) of the Transcriptome
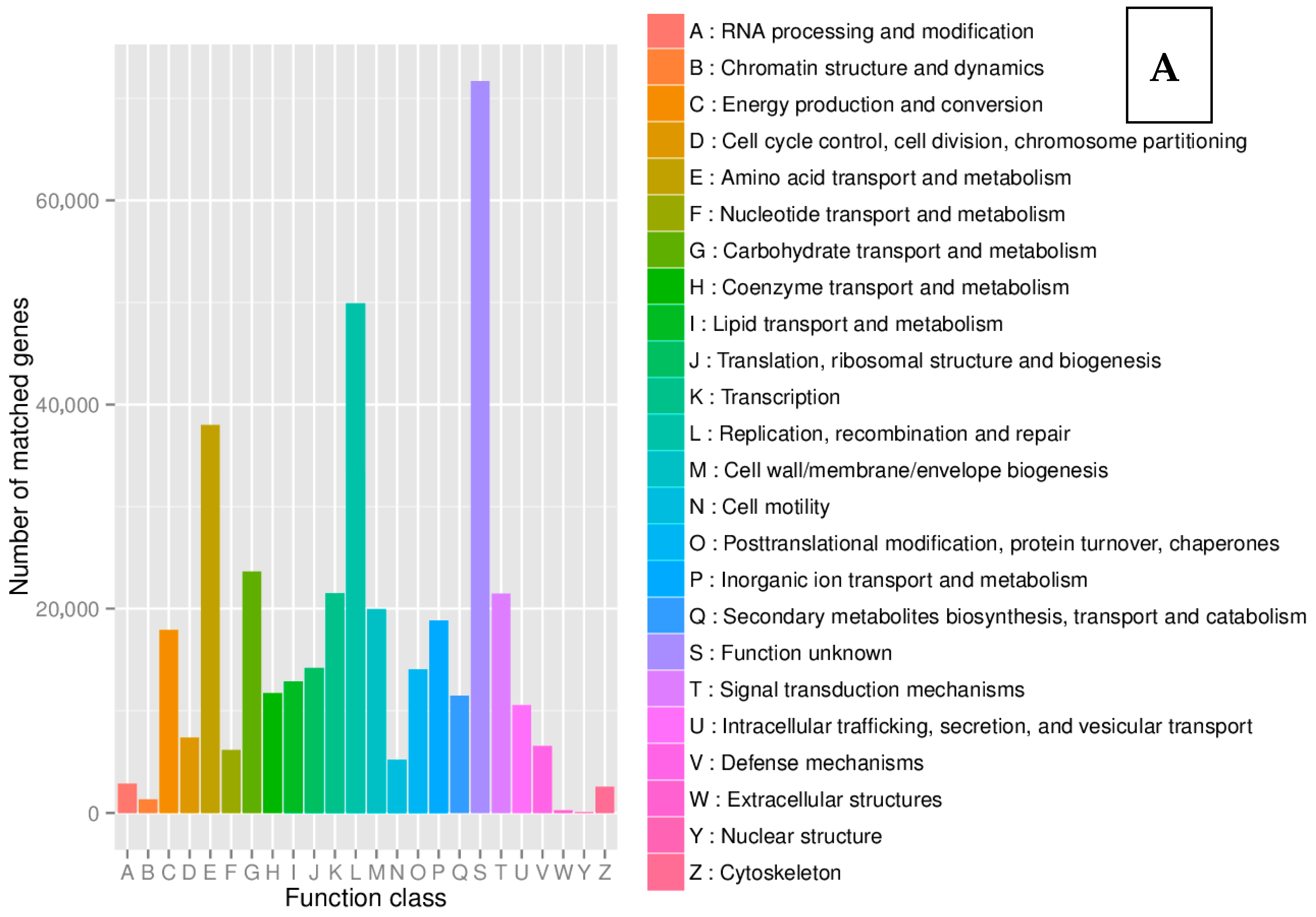
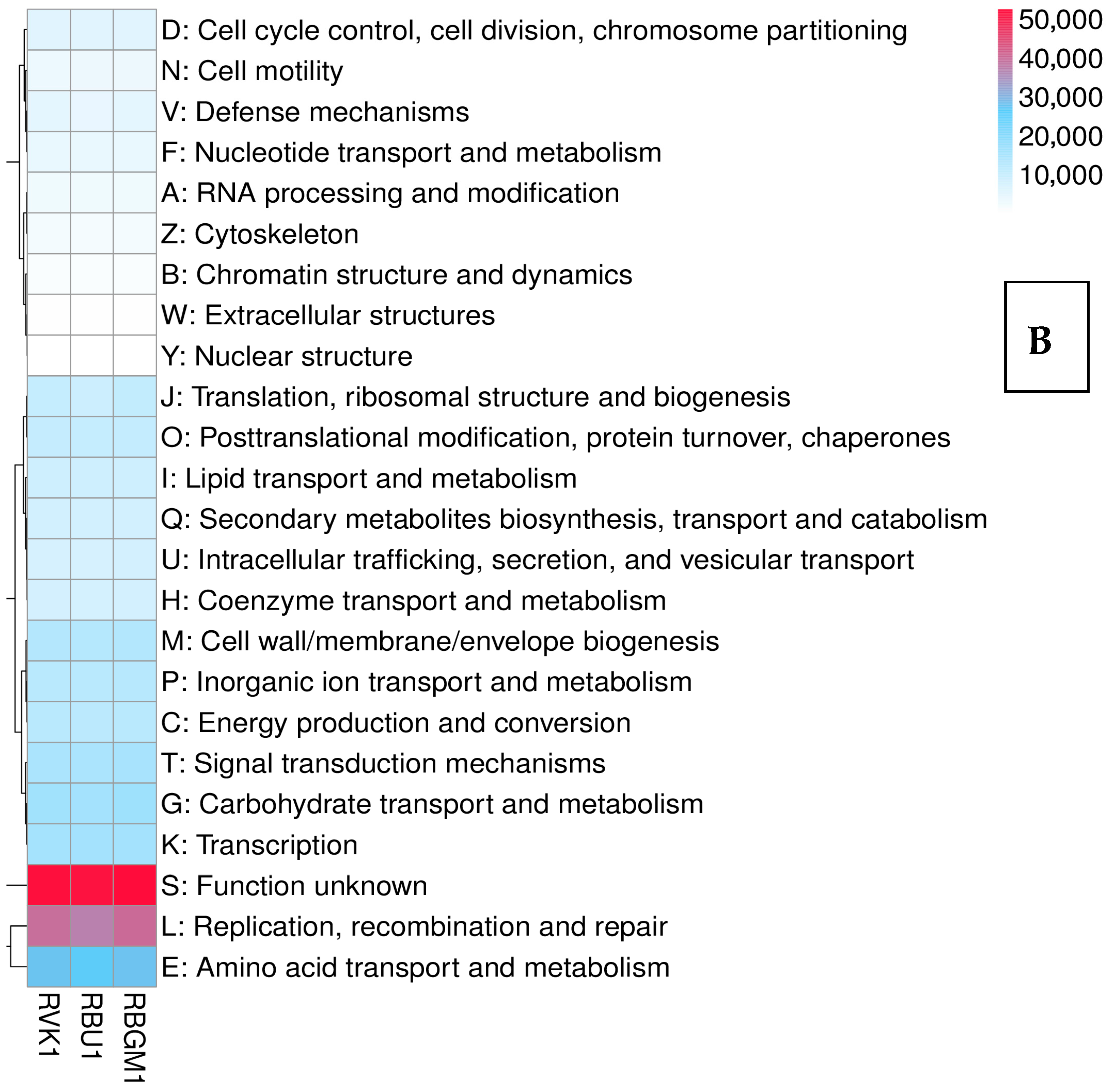
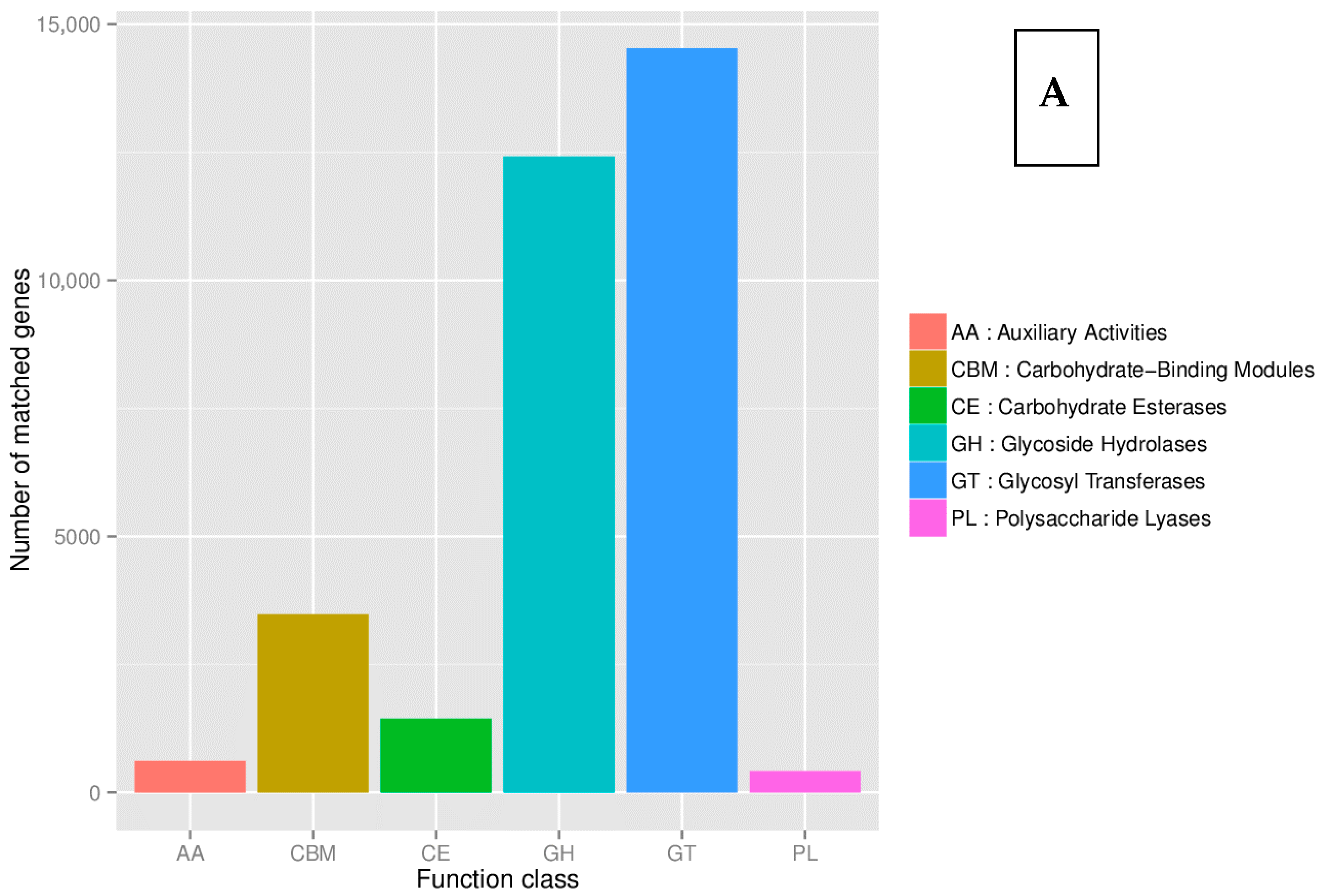
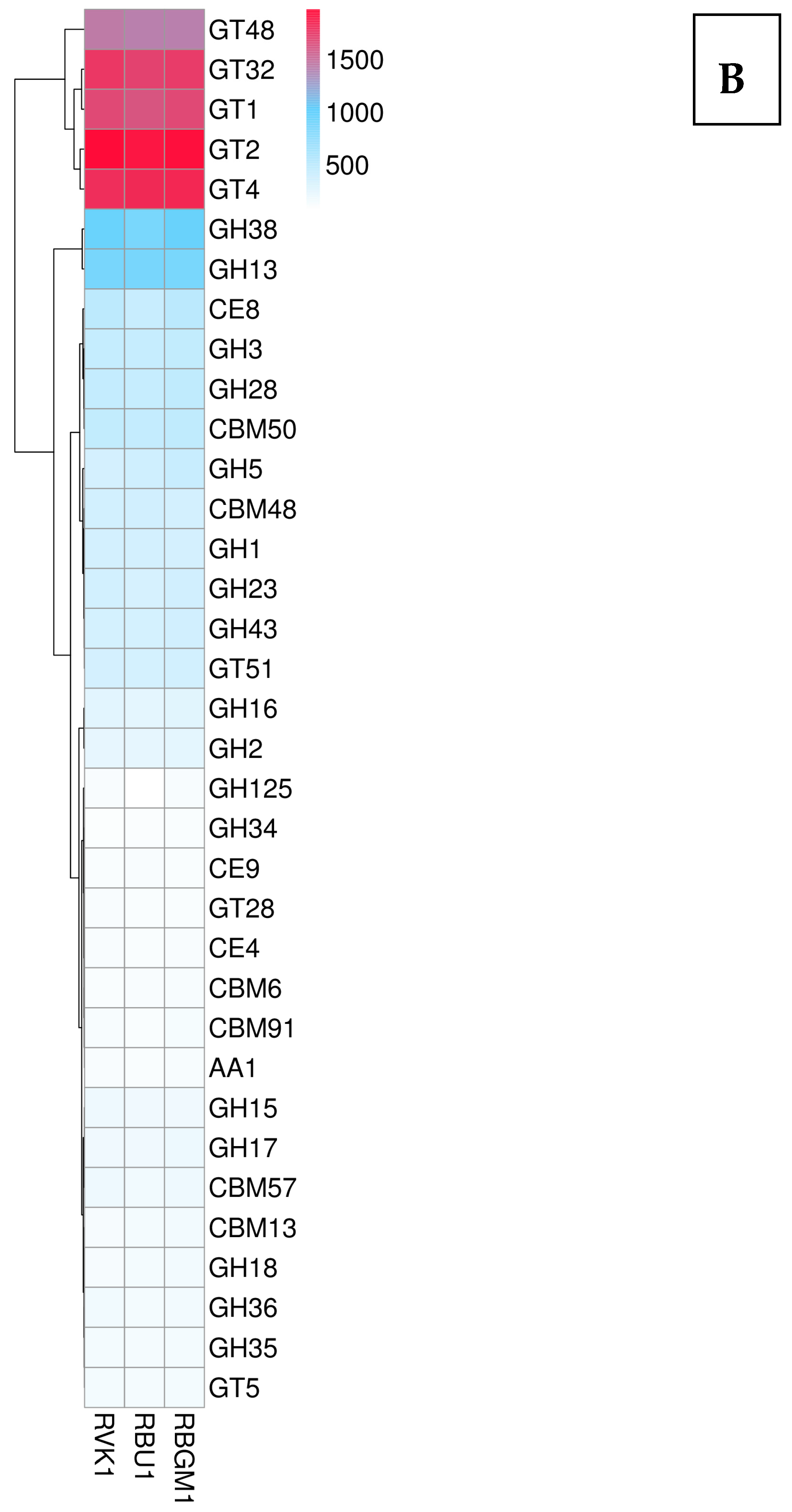
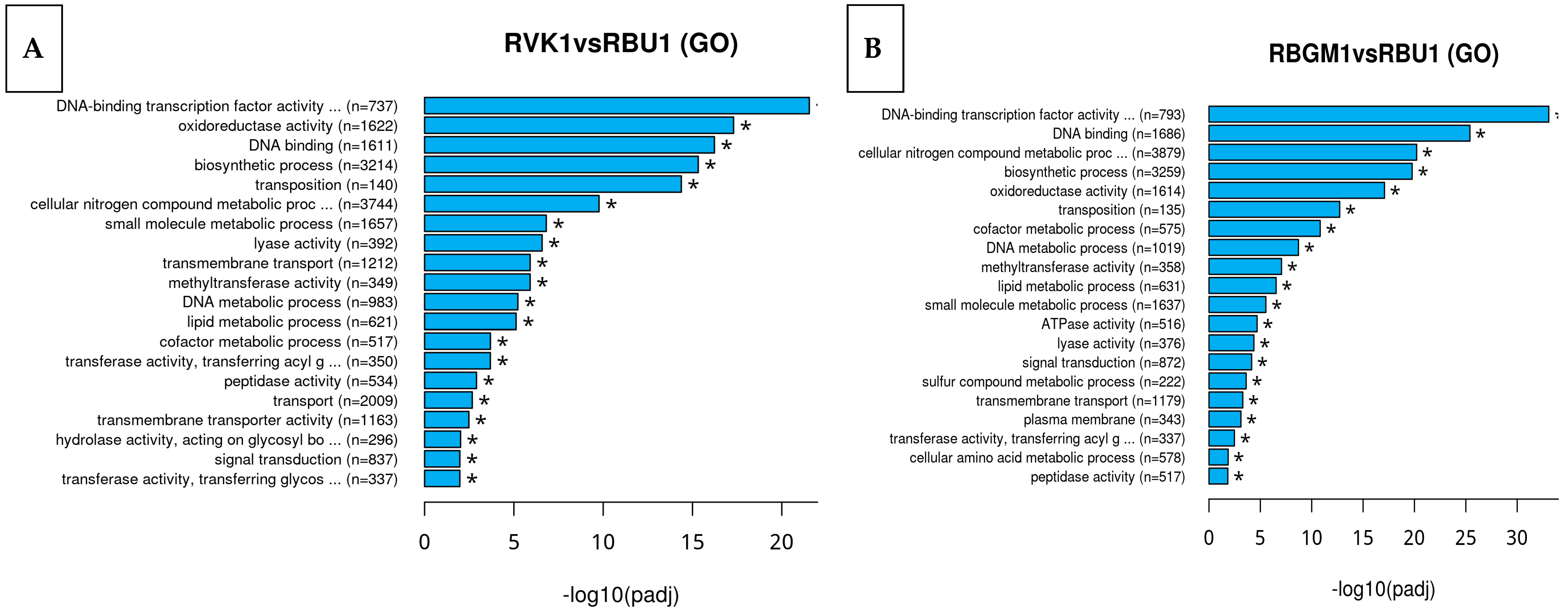
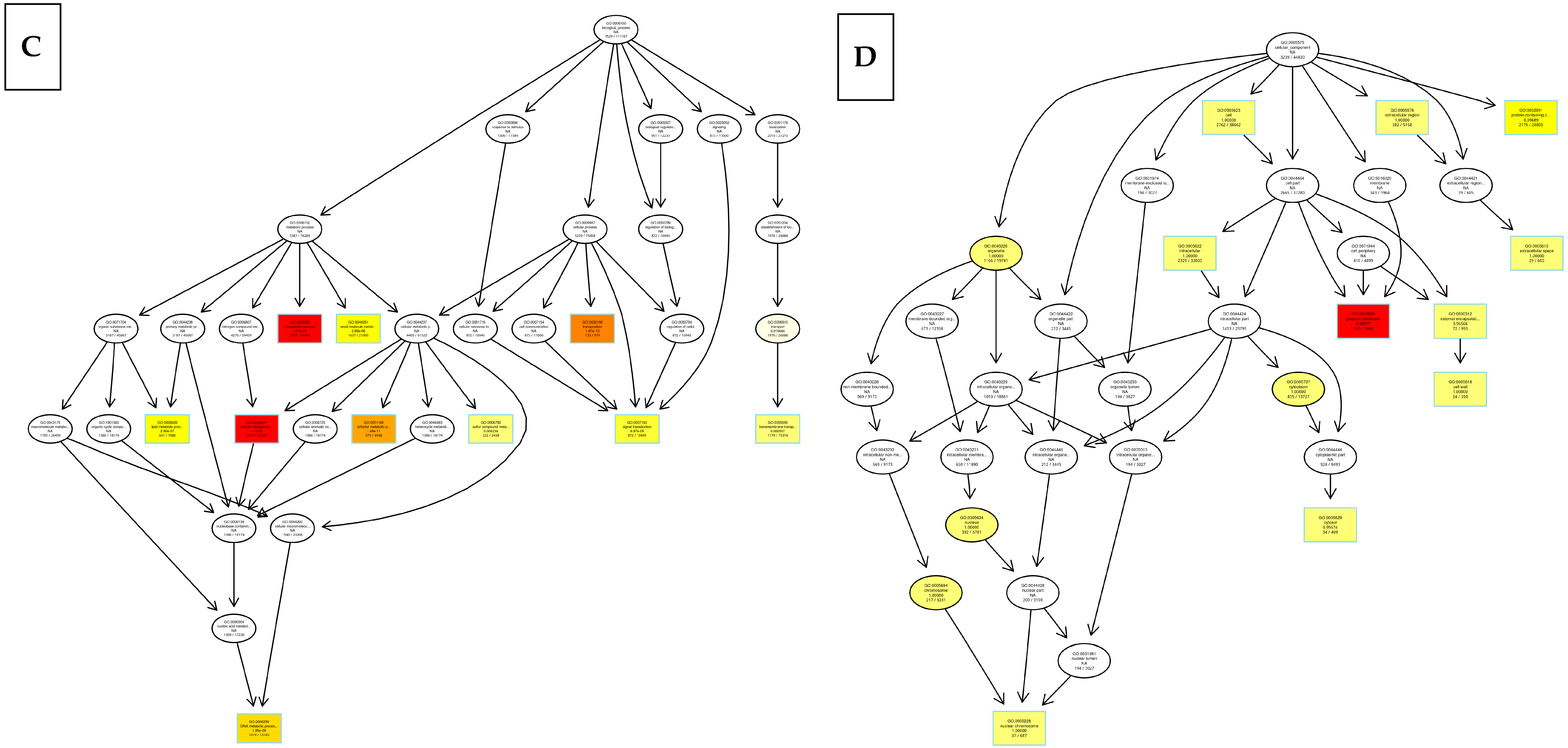
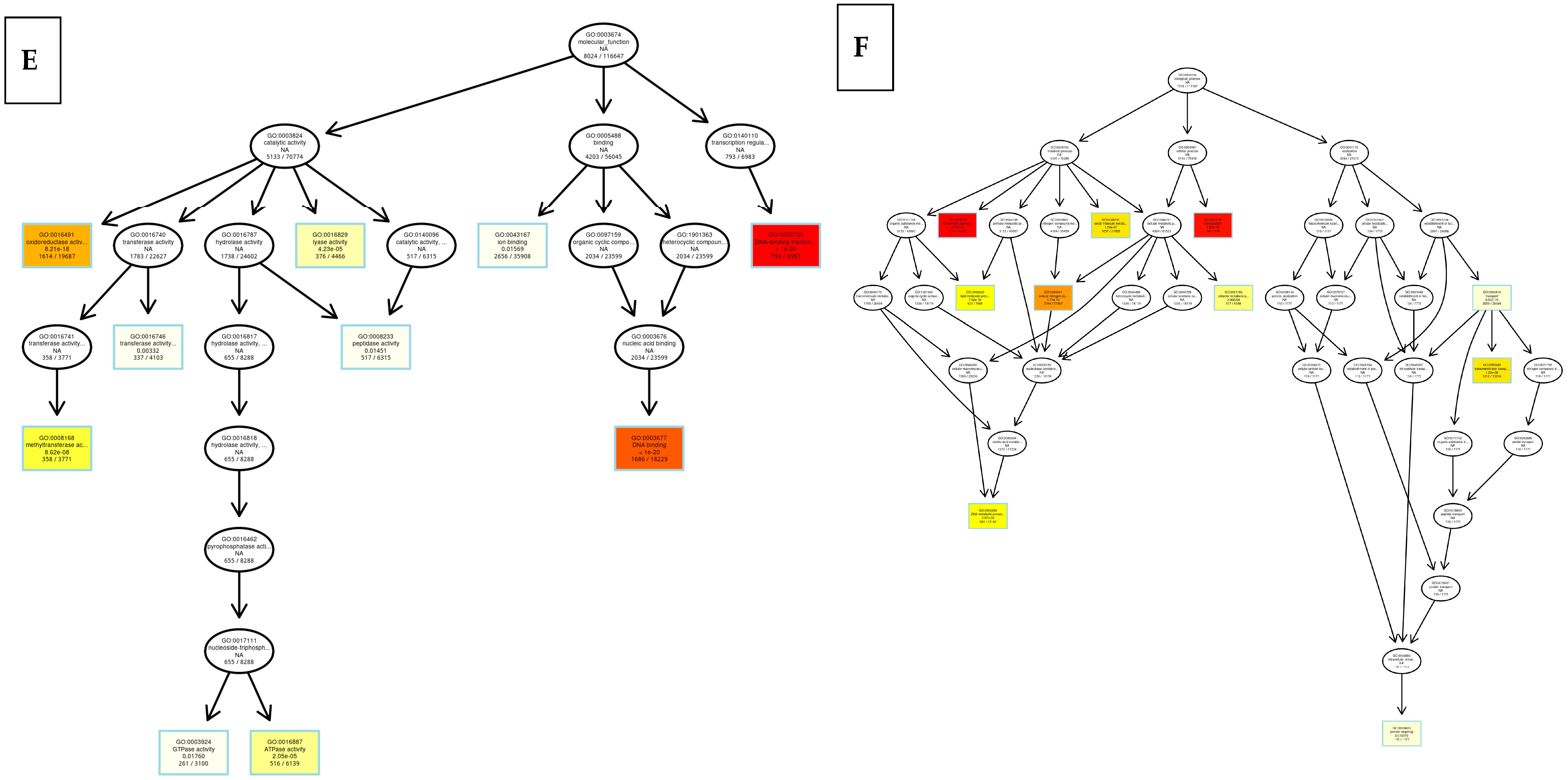
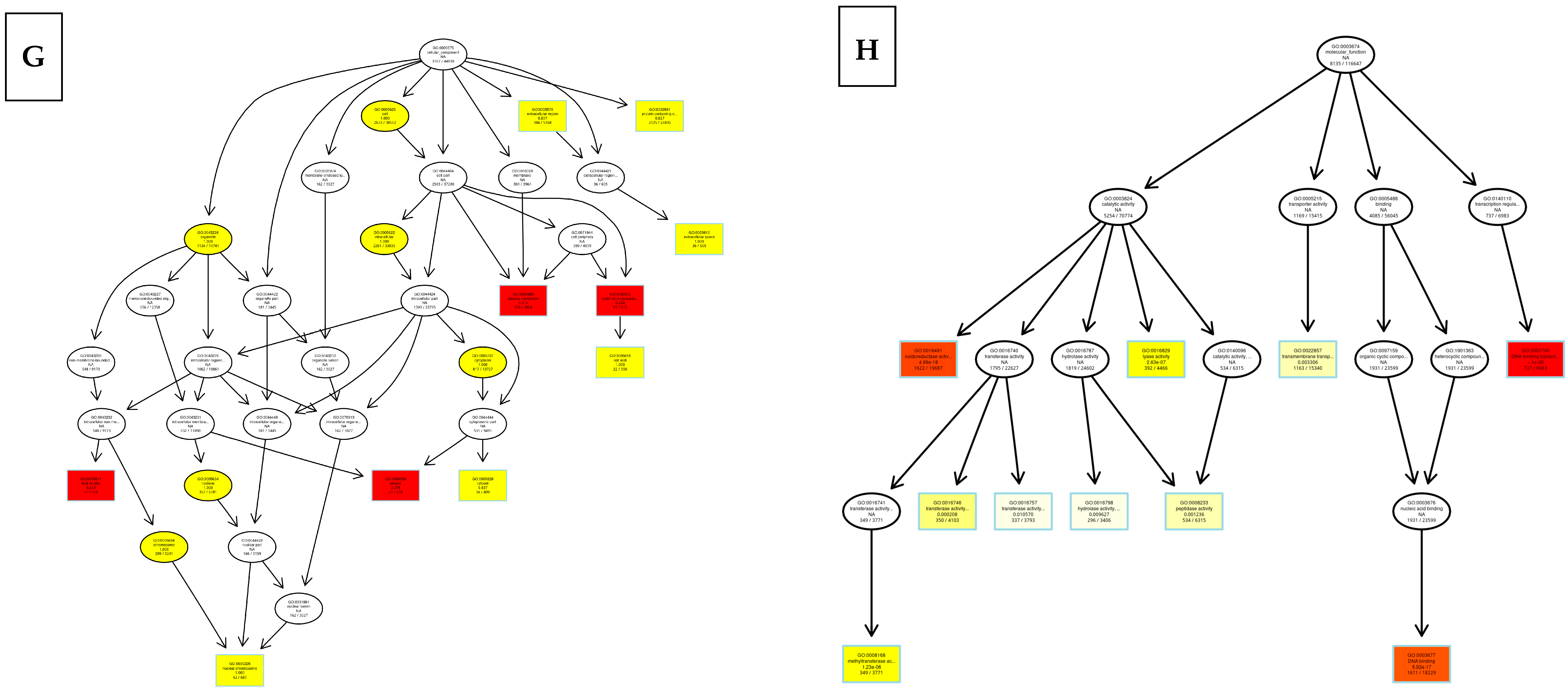
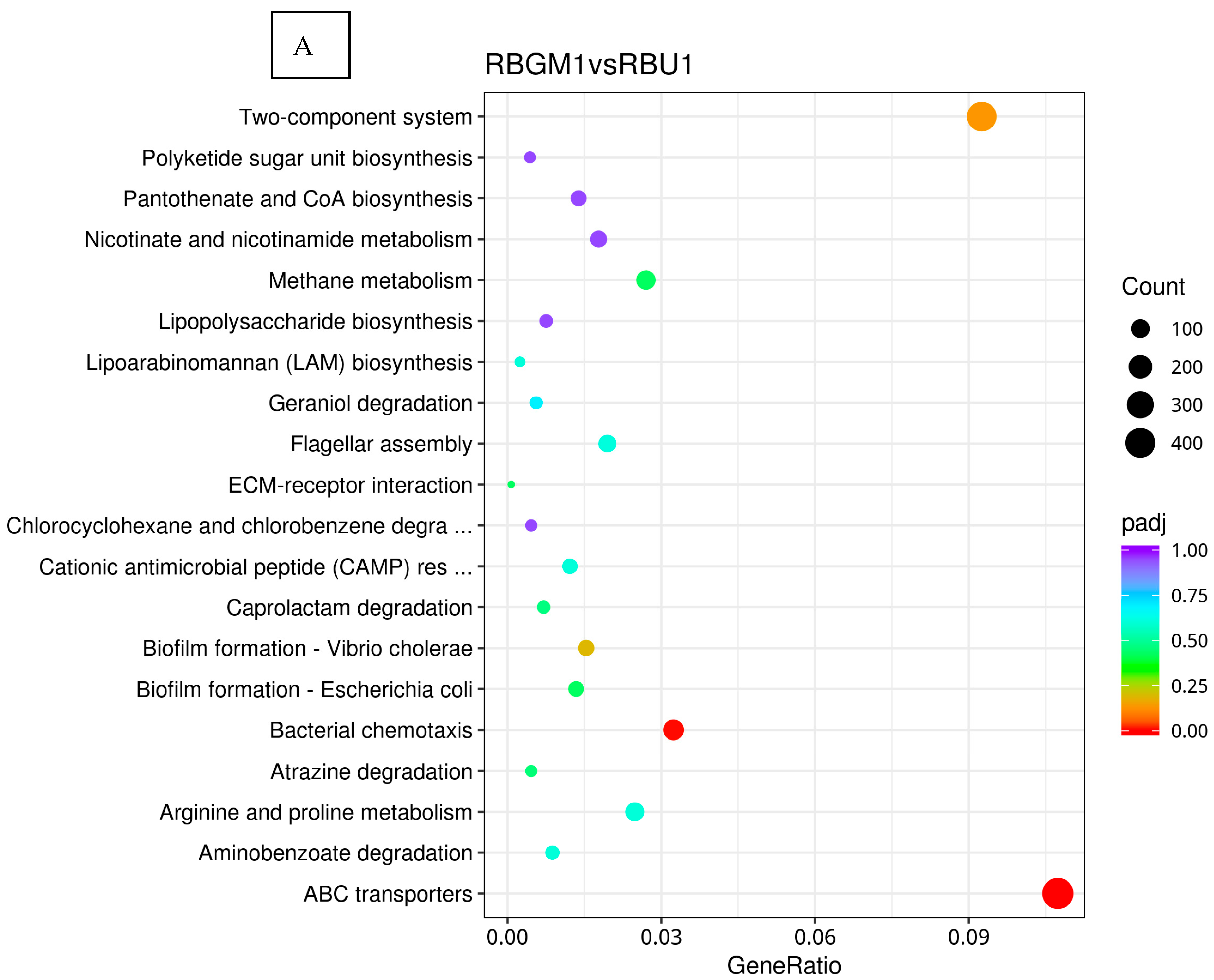
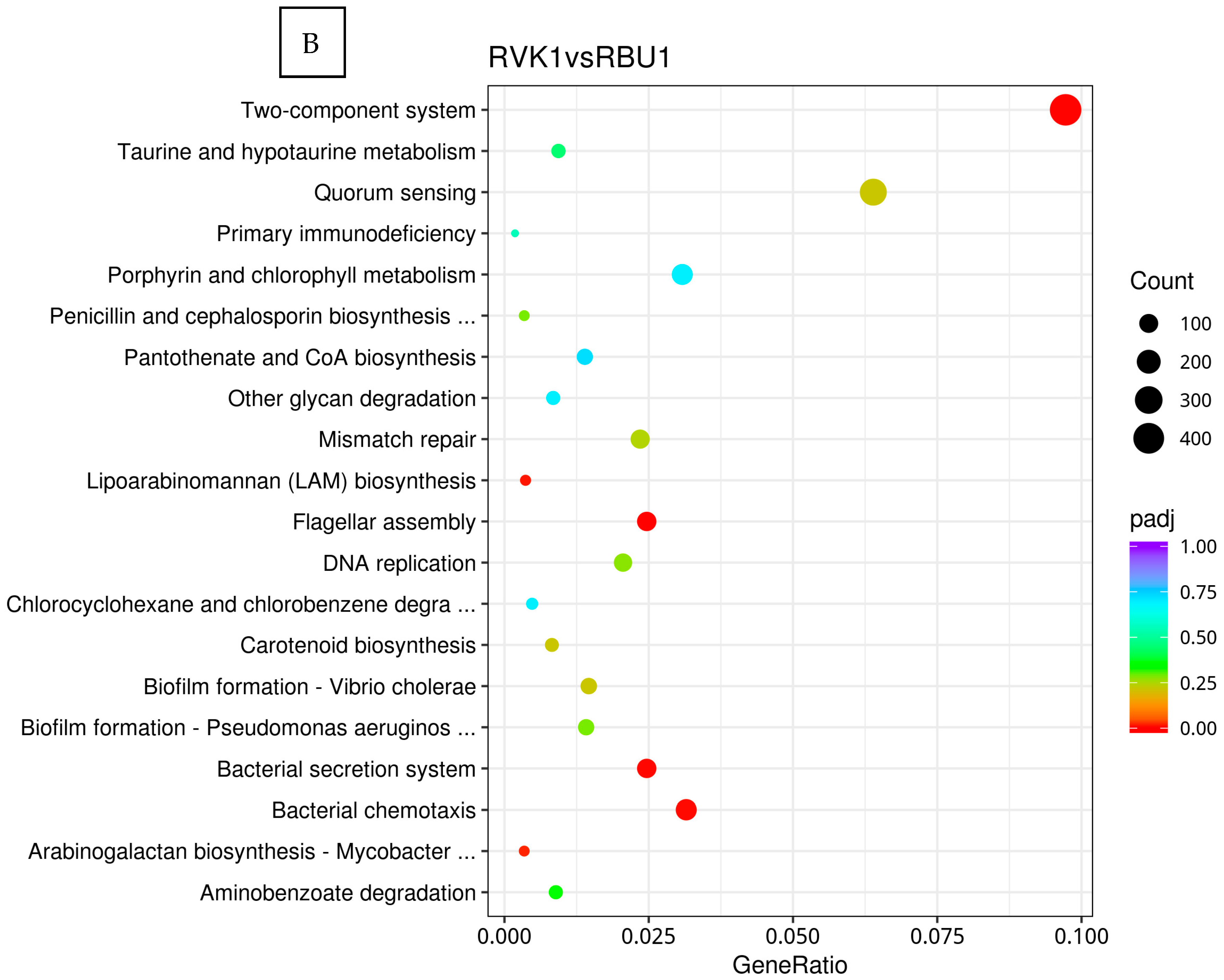
8.2. Kyoto Encyclopedia of Genes and Genomes (KEGG)
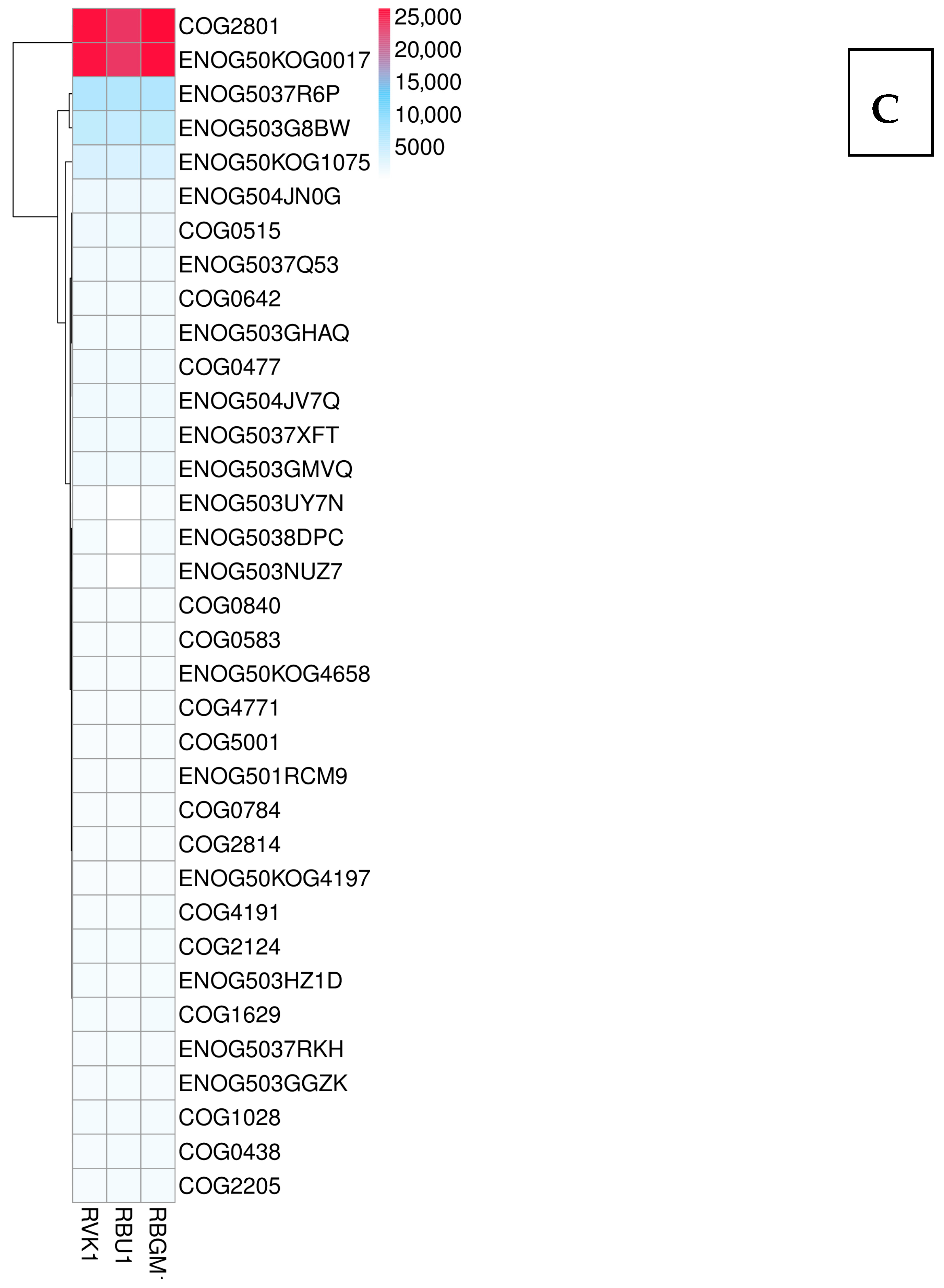
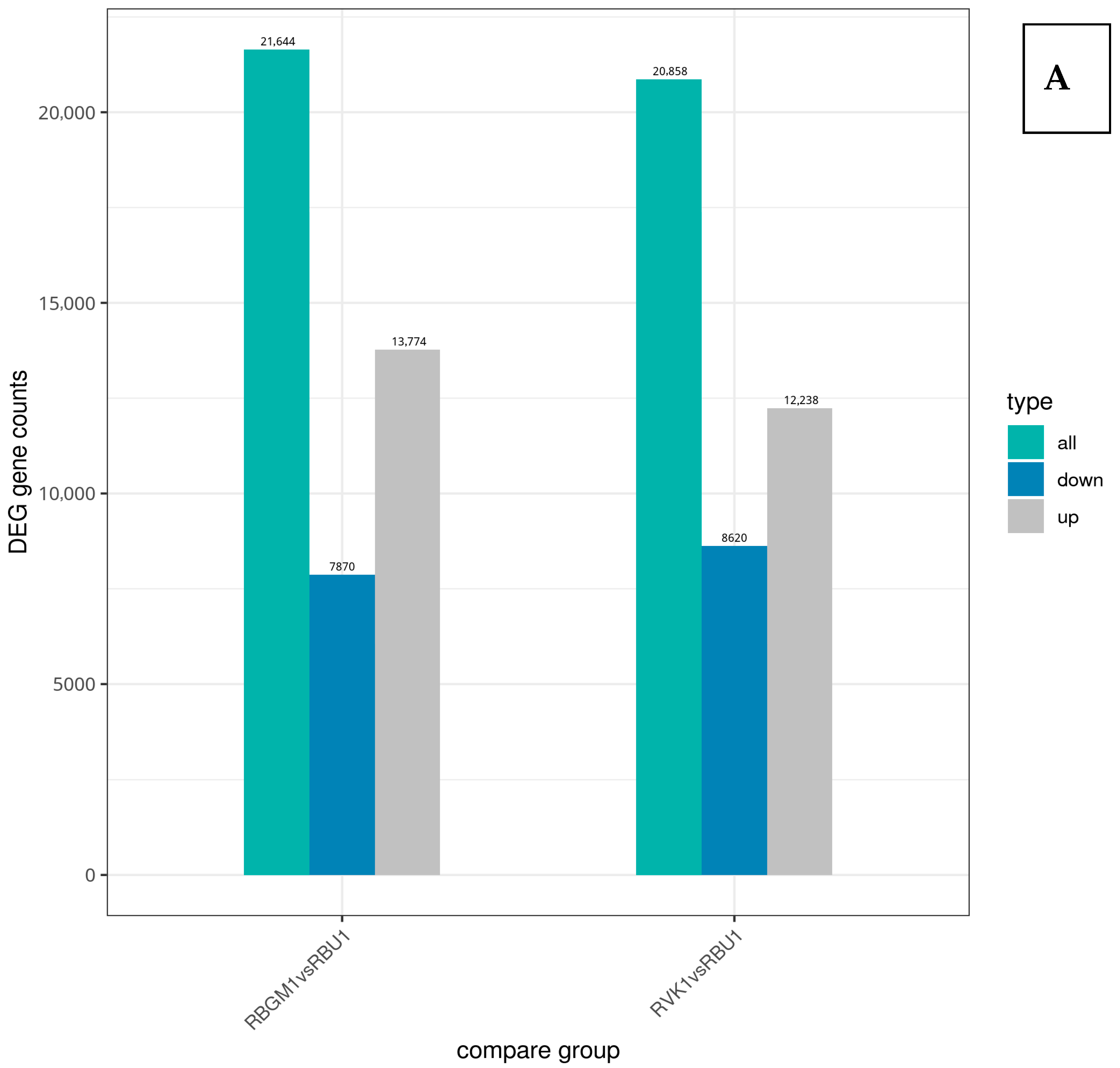
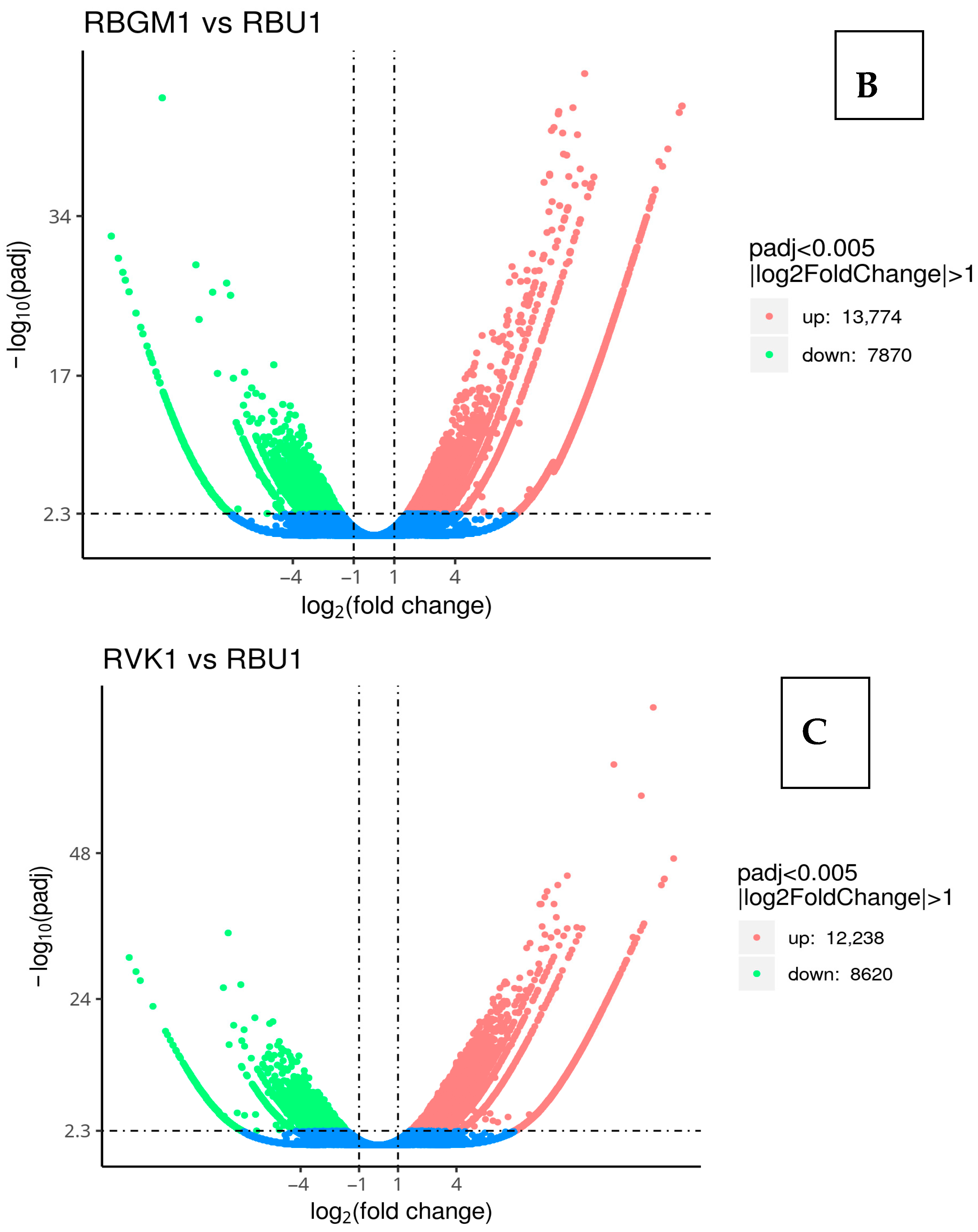
8.3. Differentially Expressed Genes
9. Discussion
10. Conclusions
11. Recommendations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blair, M.W.; Li, H.; Nekkalapudi, L.; Becerra, V.; Paredes, M. Nutritional Traits of Beans (Phaseolus vulgaris): Nutraceutical Characterization and Genomics. In Compendium of Crop Genome Designing for Nutraceuticals; Kole, C., Ed.; Springer Nature Singapore: Singapore, 2023; pp. 611–638. ISBN 978-981-19416-8-9. [Google Scholar][Green Version]
- Islam, S.S.; Adhikary, S.; Mostafa, M.; Hossain, M.M. Vegetable beans: Comprehensive insights into diversity, production, nutritional benefits, sustainable cultivation and future prospects. OnLine J. Biol. Sci. 2024, 24, 477–494. [Google Scholar] [CrossRef]
- Mtonga, A.; Maruthi, M.N. Diseases of common bean. In Handbook of Vegetable and Herb Diseases; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–52. [Google Scholar]
- Savary, S.; Ficke, A.; Aubertot, J.-N.; Hollier, C. Crop losses due to diseases and their implications for global food production losses and food security. Food Sec. 2012, 4, 519–537. [Google Scholar] [CrossRef]
- Wani, S.; Nisa, Q.; Fayaz, T.; Nabi, N.; Nabi, A.; Lateef, I.; Bashir, A.; Rashid, R.J.; Rashid, Z.; Gulzar, G.; et al. An Overview of Major Bean Diseases and Current Scenario of Common Bean Resistance. In Diseases in Legume Crops; Jha, U.C., Nayyar, H., Sharma, K.D., Von Wettberg, E.J.B., Singh, P., Siddique, K.H.M., Eds.; Springer Nature Singapore: Singapore, 2023; pp. 99–123. ISBN 978-981-9933-57-0. [Google Scholar]
- Zhu, Y.; Gao, F. Involvement of Pathogenesis-Related Proteins and Their Roles in Abiotic Stress Responses in Plants. Biomolecules 2025, 15, 1103. [Google Scholar] [CrossRef]
- Yang, W.; Feng, H.; Zhang, X.; Zhang, J.; Doonan, J.H.; Batchelor, W.D.; Xiong, L.; Yan, J. Crop phenomics and high-throughput phenotyping: Past decades, current challenges, and future perspectives. Mol. Plant 2020, 13, 187–214. [Google Scholar] [CrossRef] [PubMed]
- Bharti, M.K.; Chandra, D.; Siddique, R.A.; Ranjan, K.; Kumar, P. Recent advancement in high-throughput “omics” technologies. In Current Omics Advancement in Plant Abiotic Stress Biology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 343–355. [Google Scholar]
- Tamchek, N.; Lee, P.-C. Comparative Metatranscriptomics of Rhizosphere Microbiomes in Survived and Dead Cocoa Plants Under Drought Condition. Agric. Res. 2024, 14, 339–350. [Google Scholar] [CrossRef]
- Aranda, P.S.; LaJoie, D.M.; Jorcyk, C.L. Bleach gel: A simple agarose gel for analyzing RNA quality. Electrophoresis 2012, 33, 366–369. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Fåk, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Bäckhed, F.; Nielsen, J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012, 3, 1245. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2013, 2, e01202. [Google Scholar] [CrossRef]
- Li, J.; Jia, H.; Cai, X.; Zhong, H.; Feng, Q.; Sunagawa, S.; Arumugam, M.; Kultima, J.R.; Prifti, E.; Nielsen, T. An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 2014, 32, 834–841. [Google Scholar] [CrossRef]
- Feng, Q.; Liang, S.; Jia, H.; Stadlmayr, A.; Tang, L.; Lan, Z.; Zhang, D.; Xia, H.; Xu, X.; Jie, Z. Gut microbiome development along the colorectal adenoma–carcinoma sequence. Nat. Commun. 2015, 6, 6528. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Hattori, M.; Aoki-Kinoshita, K.F.; Itoh, M.; Kawashima, S.; Katayama, T.; Araki, M.; Hirakawa, M. From genomics to chemical genomics: New developments in KEGG. Nucleic Acids Res. 2006, 34, D354–D357. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Cepas, J.; Szklarczyk, D.; Forslund, K.; Cook, H.; Heller, D.; Walter, M.C.; Rattei, T.; Mende, D.R.; Sunagawa, S.; Kuhn, M. eggNOG 4.5: A hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Nucleic Acids Res. 2016, 44, D286–D293. [Google Scholar] [CrossRef] [PubMed]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; Le Chatelier, E.; Yao, J.; Wu, L. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014, 513, 59–64. [Google Scholar] [CrossRef]
- Backhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 852. [Google Scholar] [CrossRef]
- Koner, S.; De Sarkar, N.; Laha, N. False discovery rate control: Moving beyond the Benjamini–Hochberg method. bioRxiv 2024. [Google Scholar] [CrossRef]
- Saif, R.; Mahmood, T.; Ejaz, A.; Zia, S. Pathway enrichment and network analysis of differentially expressed genes in pashmina goat. Gene Rep. 2022, 27, 101606. [Google Scholar] [CrossRef]
- Xu, S.; Hu, E.; Cai, Y.; Xie, Z.; Luo, X.; Zhan, L.; Tang, W.; Wang, Q.; Liu, B.; Wang, R. Using clusterProfiler to characterize multiomics data. Nat. Protoc. 2024, 19, 3292–3320. [Google Scholar] [CrossRef]
- ul Qamar, M.T.; Noor, F.; Guo, Y.-X.; Zhu, X.-T.; Chen, L.-L. Deep-HPI-pred: An R-Shiny applet for network-based classification and prediction of Host-Pathogen protein-protein interactions. Comput. Struct. Biotechnol. J. 2024, 23, 316–329. [Google Scholar] [CrossRef]
- Uebersax, M.A.; Cichy, K.A.; Gomez, F.E.; Porch, T.G.; Heitholt, J.; Osorno, J.M.; Kamfwa, K.; Snapp, S.S.; Bales, S. Dry beans (Phaseolus vulgaris L.) as a vital component of sustainable agriculture and food security—A review. Legume Sci. 2023, 5, e155. [Google Scholar] [CrossRef]
- McCartney, N.; Kondakath, G.; Tai, A.; Trimmer, B.A. Functional annotation of insecta transcriptomes: A cautionary tale from Lepidoptera. Insect Biochem. Mol. Biol. 2024, 165, 104038. [Google Scholar] [CrossRef]
- Marmion, M.; Macori, G.; Ferone, M.; Whyte, P.; Scannell, A.G.M. Survive and thrive: Control mechanisms that facilitate bacterial adaptation to survive manufacturing-related stress. Int. J. Food Microbiol. 2022, 368, 109612. [Google Scholar] [CrossRef]
- Wang, Y.; Pruitt, R.N.; Nuernberger, T.; Wang, Y. Evasion of plant immunity by microbial pathogens. Nat. Rev. Microbiol. 2022, 20, 449–464. [Google Scholar] [CrossRef]
- Kumar, S.; Korra, T.; Thakur, R.; Arutselvan, R.; Kashyap, A.S.; Nehela, Y.; Chaplygin, V.; Minkina, T.; Keswani, C. Role of plant secondary metabolites in defence and transcriptional regulation in response to biotic stress. Plant Stress 2023, 8, 100154. [Google Scholar] [CrossRef]
- Saini, N.; Anmol, A.; Kumar, S.; Bakshi, M.; Dhiman, Z. Exploring phenolic compounds as natural stress alleviators in plants—A comprehensive review. Physiol. Mol. Plant Pathol. 2024, 133, 102383. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y. Current understanding of the interplays between host hormones and plant viral infections. PLoS Pathog. 2021, 17, e1009242. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, K.K. Defensive Strategies of ROS in Plant–Pathogen Interactions. In Plant Pathogen Interaction; Springer: Berlin/Heidelberg, Germany, 2024; pp. 163–183. [Google Scholar]
- Sahu, P.K.; Jayalakshmi, K.; Tilgam, J.; Gupta, A.; Nagaraju, Y.; Kumar, A.; Hamid, S.; Singh, H.V.; Minkina, T.; Rajput, V.D. ROS generated from biotic stress: Effects on plants and alleviation by endophytic microbes. Front. Plant Sci. 2022, 13, 1042936. [Google Scholar] [CrossRef]
- Jha, Y.; Mohamed, H.I. Plant secondary metabolites as a tool to investigate biotic stress tolerance in plants: A review. Gesunde Pflanz. 2022, 74, 771–790. [Google Scholar] [CrossRef]
- Singh, R.; Choudhary, P.; Kumar, S.; Daima, H.K. Mechanistic approaches for crosstalk between nanomaterials and plants: Plant immunomodulation, defense mechanisms, stress resilience, toxicity, and perspectives. Environ. Sci. Nano 2024, 11, 2324–2351. [Google Scholar] [CrossRef]
- Alghsham, R.; Rasheed, Z.; Shariq, A.; Alkhamiss, A.S.; Alhumaydhi, F.A.; Aljohani, A.S.; Althwab, S.A.; Alshomar, A.; Alhomaidan, H.T.; Hamad, E.M. Recognition of pathogens and their inflammatory signaling events. Open Access Maced. J. Med. Sci. 2022, 10, 462–467. [Google Scholar] [CrossRef]
- Kaur, S.; Samota, M.K.; Choudhary, M.; Choudhary, M.; Pandey, A.K.; Sharma, A.; Thakur, J. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants 2022, 28, 485–504. [Google Scholar] [CrossRef]
- Appu, M.; Ramalingam, P.; Sathiyanarayanan, A.; Huang, J. An overview of plant defense-related enzymes responses to biotic stresses. Plant Gene 2021, 27, 100302. [Google Scholar] [CrossRef]
- Dos Santos, C.; Franco, O.L. Pathogenesis-related proteins (PRs) with enzyme activity activating plant defense responses. Plants 2023, 12, 2226. [Google Scholar] [CrossRef]
- Akbar, M.U.; Aqeel, M.; Shah, M.S.; Jeelani, G.; Iqbal, N.; Latif, A.; Elnour, R.O.; Hashem, M.; Alzoubi, O.M.; Habeeb, T. Molecular regulation of antioxidants and secondary metabolites act in conjunction to defend plants against pathogenic infection. S. Afr. J. Bot. 2023, 161, 247–257. [Google Scholar] [CrossRef]
- Lian, N.; Wang, X.; Jing, Y.; Lin, J. Regulation of cytoskeleton-associated protein activities: Linking cellular signals to plant cytoskeletal function. J. Integr. Plant Biol. 2021, 63, 241–250. [Google Scholar] [CrossRef]
- Kumar, S.; Jeevaraj, T.; Yunus, M.H.; Chakraborty, S.; Chakraborty, N. The plant cytoskeleton takes center stage in abiotic stress responses and resilience. Plant Cell Environ. 2023, 46, 5–22. [Google Scholar] [CrossRef]
- Spitzer, J. Physicochemical origins of prokaryotic and eukaryotic organisms. J. Physiol. 2024, 602, 2383–2394. [Google Scholar] [CrossRef]
- Su, J.; Song, Y.; Zhu, Z.; Huang, X.; Fan, J.; Qiao, J.; Mao, F. Cell–cell communication: New insights and clinical implications. Signal Transduct. Target. Ther. 2024, 9, 196. [Google Scholar] [CrossRef]
- Solanki, S.; Das, H.K. Antimicrobial resistance: Molecular drivers and underlying mechanisms. J. Med. Surg. Public Health 2024, 3, 100122. [Google Scholar] [CrossRef]
- Krasavina, M.S.; Burmistrova, N.A.; Raldugina, G.N. The role of carbohydrates in plant resistance to abiotic stresses. In Emerging Technologies and Management of Crop Stress Tolerance; Elsevier: Amsterdam, The Netherlands, 2014; pp. 229–270. [Google Scholar]
- Weng, J.-K.; Lynch, J.H.; Matos, J.O.; Dudareva, N. Adaptive mechanisms of plant specialized metabolism connecting chemistry to function. Nat. Chem. Biol. 2021, 17, 1037–1045. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Rashmi, R.; Toppo, V.; Chole, P.B.; Banadka, A.; Sudheer, W.N.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q.; Alessa, F.M. Plant secondary metabolites: The weapons for biotic stress management. Metabolites 2023, 13, 716. [Google Scholar] [CrossRef]
- Ahmad, N.; Hussain, H.; Naeem, M.; Rahman, S.U.; Khan, K.A.; Iqbal, B.; Umar, A.W. Metabolites-induced co-evolutionary warfare between plants, viruses, and their associated vectors: So close yet so far away. Plant Sci. 2024, 346, 112165. [Google Scholar] [CrossRef]
- EL Sabagh, A.; Islam, M.S.; Hossain, A.; Iqbal, M.A.; Mubeen, M.; Waleed, M.; Reginato, M.; Battaglia, M.; Ahmed, S.; Rehman, A. Phytohormones as growth regulators during abiotic stress tolerance in plants. Front. Agron. 2022, 4, 765068. [Google Scholar] [CrossRef]
- Chang, H.; Ma, M.; Gu, M.; Li, S.; Li, M.; Guo, G.; Xing, G. Acyl-CoA-binding protein (ACBP) genes involvement in response to abiotic stress and exogenous hormone application in barley (Hordeum vulgare L.). BMC Plant Biol. 2024, 24, 236. [Google Scholar] [CrossRef] [PubMed]
- Abulfaraj, A.A.; Shami, A.Y.; Alotaibi, N.M.; Alomran, M.M.; Aloufi, A.S.; Al-Andal, A.; AlHamdan, N.R.; Alshehrei, F.M.; Sefrji, F.O.; Alsaadi, K.H. Exploration of genes encoding KEGG pathway enzymes in rhizospheric microbiome of the wild plant Abutilon fruticosum. AMB Express 2024, 14, 27. [Google Scholar] [CrossRef]
- Yanamadala, V. Carbohydrate Metabolism. In Essential Medical Biochemistry and Metabolic Disease: A Pocket Guide for Medical Students and Residents; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–34. [Google Scholar]
- Zhu, X.; Tian, H.; Li, X.; Yan, H.; Yang, S.; He, G. Transcriptome analysis of cadmium accumulation characteristics and fruit response to cadmium stress in Zunla 1 chili pepper. Cogent Food Agric. 2024, 10, 2437136. [Google Scholar] [CrossRef]
- Lika, J.; Fan, J. Carbohydrate metabolism in supporting and regulating neutrophil effector functions. Curr. Opin. Immunol. 2024, 91, 102497. [Google Scholar] [CrossRef] [PubMed]
- Munzert, K.S.; Engelsdorf, T. Plant cell wall structure and dynamics in plant–pathogen interactions and pathogen defence. J. Exp. Bot. 2025, 76, 228–242. [Google Scholar] [CrossRef] [PubMed]
- King, D.G. Mutation protocols share with sexual reproduction the physiological role of producing genetic variation within ‘constraints that deconstrain’. J. Physiol. 2024, 602, 2615–2626. [Google Scholar] [CrossRef]
- Baduel, P.; Quadrana, L. Jumpstarting evolution: How transposition can facilitate adaptation to rapid environmental changes. Curr. Opin. Plant Biol. 2021, 61, 102043. [Google Scholar] [CrossRef] [PubMed]
- Alshareef, S.A. Metabolic analysis of the CAZy class glycosyltransferases in rhizospheric soil fungiome of the plant species Moringa oleifera. Saudi J. Biol. Sci. 2024, 31, 103956. [Google Scholar] [CrossRef]
- Marcianò, D.; Kappel, L.; Ullah, S.F.; Srivastava, V. From glycans to green biotechnology: Exploring cell wall dynamics and phytobiota impact in plant glycopathology. Crit. Rev. Biotechnol. 2024, 45, 314–332. [Google Scholar] [CrossRef]
- Molina, A.; Sánchez-Vallet, A.; Jordá, L.; Carrasco-López, C.; Rodríguez-Herva, J.J.; López-Solanilla, E. Plant cell walls: Source of carbohydrate-based signals in plant-pathogen interactions. Curr. Opin. Plant Biol. 2024, 82, 102630. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Ren, Y.; Yan, T.; Jia, X.; Xu, H.; Yang, B.; Zhang, X.; He, J. Melatonin improves the postharvest anthracnose resistance of mango fruit by regulating antioxidant activity, the phenylpropane pathway and cell wall metabolism. Eur. J. Plant Pathol. 2024, 171, 17–36. [Google Scholar] [CrossRef]
- Swaminathan, S.; Lionetti, V.; Zabotina, O.A. Plant cell wall integrity perturbations and priming for defense. Plants 2022, 11, 3539. [Google Scholar] [CrossRef]
- Majeed, H.N.; Shaheen, S.; Kashif, M. Glycosyltransferases: Unraveling Molecular Insights and Biotechnological Implications. Sci. Reviews. Biol. 2024, 3, 16. [Google Scholar] [CrossRef]
- Buckeridge, M.S. The diversity of plant carbohydrate hydrolysis in nature and technology. Polysacch. Degrad. Biocatal. 2023, 55–74. [Google Scholar] [CrossRef]
- Gharechahi, J.; Vahidi, M.F.; Sharifi, G.; Ariaeenejad, S.; Ding, X.-Z.; Han, J.-L.; Salekdeh, G.H. Lignocellulose degradation by rumen bacterial communities: New insights from metagenome analyses. Environ. Res. 2023, 229, 115925. [Google Scholar] [CrossRef]
- Forsberg, Z.; Courtade, G. On the impact of carbohydrate-binding modules (CBMs) in lytic polysaccharide monooxygenases (LPMOs). Essays Biochem. 2023, 67, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Annapure, U.S. Trends in “green” and novel methods of pectin modification—A review. Carbohydr. Polym. 2022, 278, 118967. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, G.; Gugole, E.; Montemiglio, L.C.; Turbé-Doan, A.; Chena, D.; Navarro, D.; Lomascolo, A.; Piumi, F.; Exertier, C.; Freda, I. Crystal structure and functional characterization of an oligosaccharide dehydrogenase from Pycnoporus cinnabarinus provides insights into fungal breakdown of lignocellulose. Biotechnol. Biofuels 2021, 14, 161. [Google Scholar] [CrossRef]
- Balducci, E.; Papi, F.; Capialbi, D.E.; Del Bino, L. Polysaccharides’ structures and functions in biofilm architecture of antimicrobial-resistant (AMR) pathogens. Int. J. Mol. Sci. 2023, 24, 4030. [Google Scholar] [CrossRef]
- Kadirvelraj, R.; Yang, J.-Y.; Kim, H.W.; Sanders, J.H.; Moremen, K.W.; Wood, Z.A. Comparison of human poly-N-acetyl-lactosamine synthase structure with GT-A fold glycosyltransferases supports a modular assembly of catalytic subsites. J. Biol. Chem. 2021, 296, 100110. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, K.; Ahmad, R.; Abd-Elsalam, K.A. Emerging frontiers in nanotechnology for precision agriculture: Advancements, hurdles and prospects. Agrochemicals 2023, 2, 220–256. [Google Scholar] [CrossRef]
- Campos, M.D.; Felix, M.D.R.; Patanita, M.; Materatski, P.; Albuquerque, A.; Ribeiro, J.A.; Varanda, C. Defense strategies: The role of transcription factors in tomato–pathogen interaction. Biology 2022, 11, 235. [Google Scholar] [CrossRef]
- Wani, S.H.; Anand, S.; Singh, B.; Bohra, A.; Joshi, R. WRKY transcription factors and plant defense responses: Latest discoveries and future prospects. Plant Cell Rep. 2021, 40, 1071–1085. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, M.; Mur, L.A.J.; Shen, Q.; Guo, S. Unravelling the roles of nitrogen nutrition in plant disease defences. Int. J. Mol. Sci. 2020, 21, 572. [Google Scholar] [CrossRef] [PubMed]
- Tiku, A.R. Antimicrobial compounds (phytoanticipins and phytoalexins) and their role in plant defense. In Co-Evolution of Secondary Metabolites; Springer: Berlin/Heidelberg, Germany, 2020; pp. 845–868. [Google Scholar]
- Shetty, N.P.; Jørgensen, H.J.L.; Jensen, J.D.; Collinge, D.B.; Shetty, H.S. Roles of reactive oxygen species in interactions between plants and pathogens. Eur. J. Plant Pathol. 2008, 121, 267–280. [Google Scholar] [CrossRef]
- Aller, E.S.; Kanstrup, C.; Hunziker, P.; Kliebenstein, D.J.; Burow, M. Altered defense patterns upon retrotransposition highlights the potential for rapid adaptation by transposable elements. bioRxiv 2023. [Google Scholar] [CrossRef]
- Upadhyay, R.; Saini, R.; Shukla, P.K.; Tiwari, K.N. Role of secondary metabolites in plant defense mechanisms: A molecular and biotechnological insights. Phytochem. Rev. 2024, 24, 953–983. [Google Scholar] [CrossRef]
- Abdel-Hameed, A.A.; Liao, W.; Prasad, K.V.; Reddy, A.S. CAMTAs, a family of calmodulin-binding transcription factors, are versatile regulators of biotic and abiotic stress responses in plants. Crit. Rev. Plant Sci. 2024, 43, 171–210. [Google Scholar] [CrossRef]
- Alves, F.; Lane, D.; Nguyen, T.P.M.; Bush, A.I.; Ayton, S. In defence of ferroptosis. Signal Transduct. Target. Ther. 2025, 10, 2. [Google Scholar] [CrossRef]
- Songire, V.M.; Patil, R.H. Microbial Antioxidative Enzymes: Biotechnological Production and Environmental and Biomedical Applications. Appl. Biochem. Microbiol. 2025, 61, 1–26. [Google Scholar] [CrossRef]
- Samsami, H.; Maali-Amiri, R. Global insights into intermediate metabolites: Signaling, metabolic divergence and stress response modulation in plants. Plant Physiol. Biochem. 2024, 213, 108862. [Google Scholar] [CrossRef]
- Thiruvengadam, R.; Venkidasamy, B.; Easwaran, M.; Chi, H.Y.; Thiruvengadam, M.; Kim, S.-H. Dynamic interplay of reactive oxygen and nitrogen species (ROS and RNS) in plant resilience: Unveiling the signaling pathways and metabolic responses to biotic and abiotic stresses. Plant Cell Rep. 2024, 43, 198. [Google Scholar] [CrossRef] [PubMed]
- Olmo-Uceda, M.J.; Ambrós, S.; Corrêa, R.L.; Elena, S.F. Transcriptomic insights into the epigenetic modulation of turnip mosaic virus evolution in Arabidopsis thaliana. BMC Genom. 2024, 25, 897. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Xie, X.; Zhao, Z. Virulence regulation in plant-pathogenic bacteria by host-secreted signals. Microbiol. Res. 2024, 288, 127883. [Google Scholar] [CrossRef]
- Deng, S.; Chen, C.; Wang, Y.; Liu, S.; Zhao, J.; Cao, B.; Jiang, D.; Jiang, Z.; Zhang, Y. Advances in understanding and mitigating Atrazine’s environmental and health impact: A comprehensive review. J. Environ. Manag. 2024, 365, 121530. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.A.; Saleem, M.H.; Afzal, S.; Hussain, I.; Ameen, F.; Fahad, S. Ferulic acid: Therapeutic potential due to its antioxidant properties, role in plant growth, and stress tolerance. Plant Growth Regul. 2024, 104, 1329–1353. [Google Scholar] [CrossRef]
- Liu, B.; Liu, L.; Liu, Y. Targeting cell death mechanisms: The potential of autophagy and ferroptosis in hepatocellular carcinoma therapy. Front. Immunol. 2024, 15, 1450487. [Google Scholar] [CrossRef]
- Belay, W.Y.; Getachew, M.; Tegegne, B.A.; Teffera, Z.H.; Dagne, A.; Zeleke, T.K.; Abebe, R.B.; Gedif, A.A.; Fenta, A.; Yirdaw, G. Mechanism of antibacterial resistance, strategies and next-generation antimicrobials to contain antimicrobial resistance: A review. Front. Pharmacol. 2024, 15, 1444781. [Google Scholar] [CrossRef] [PubMed]
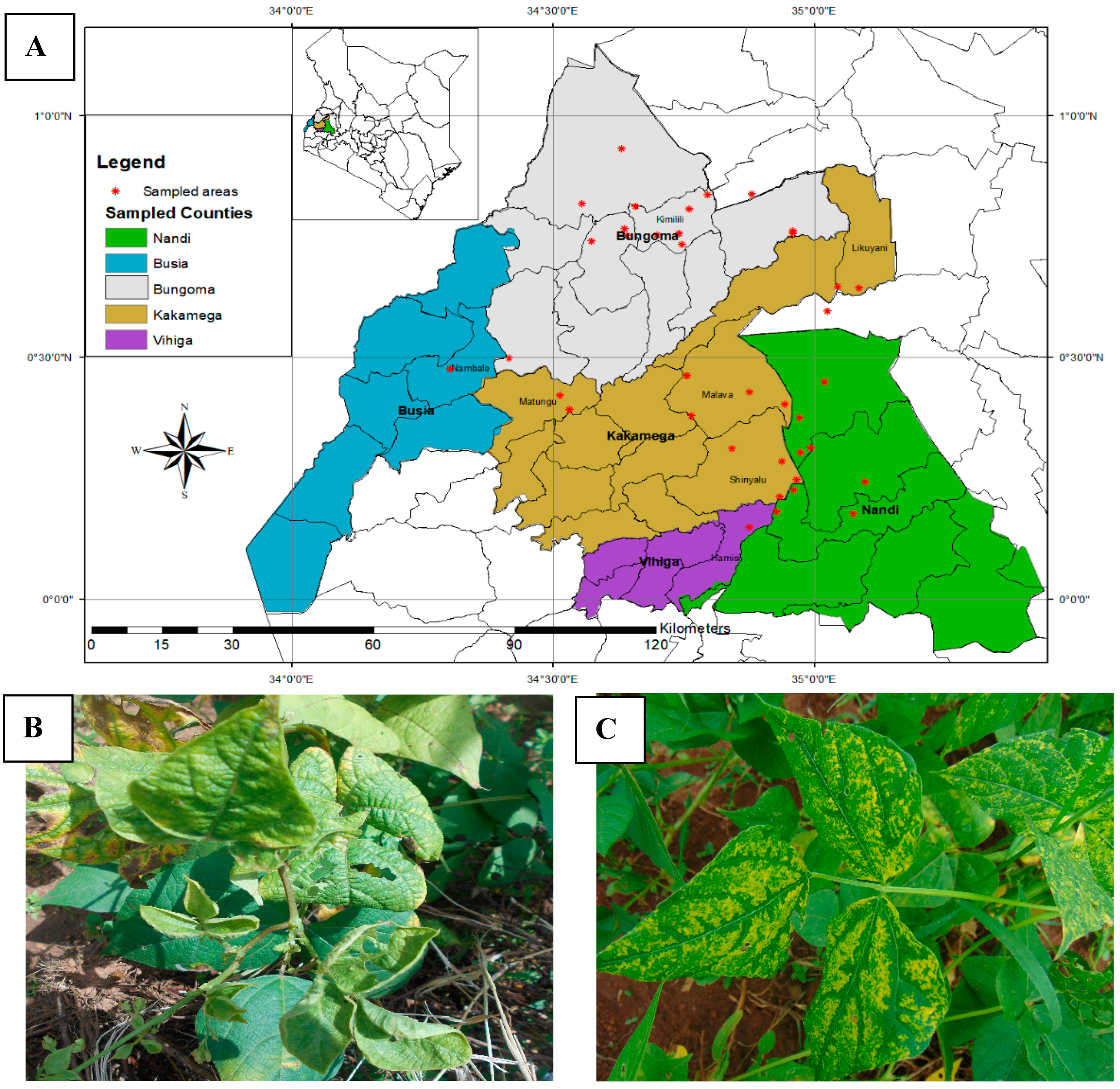

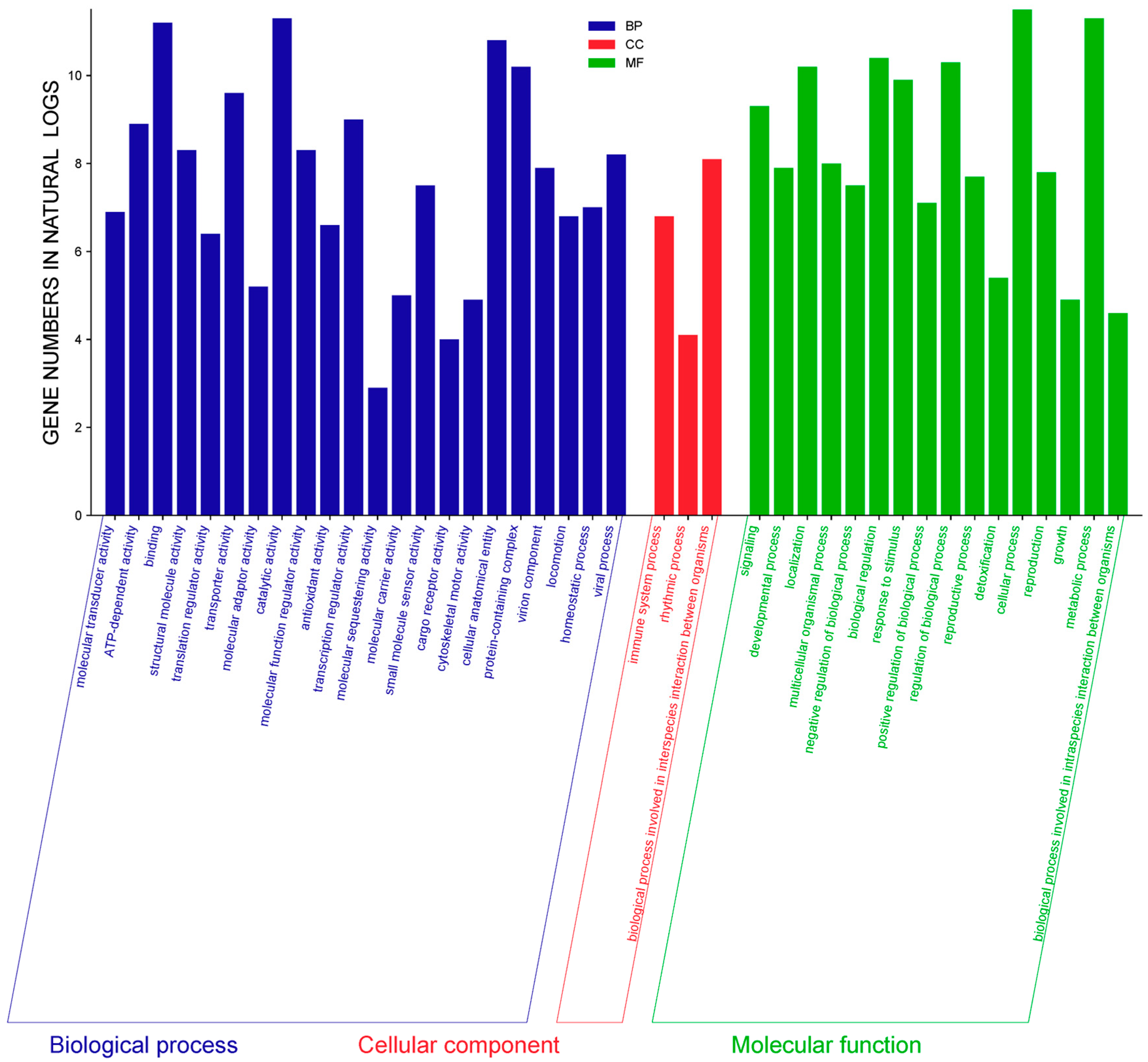

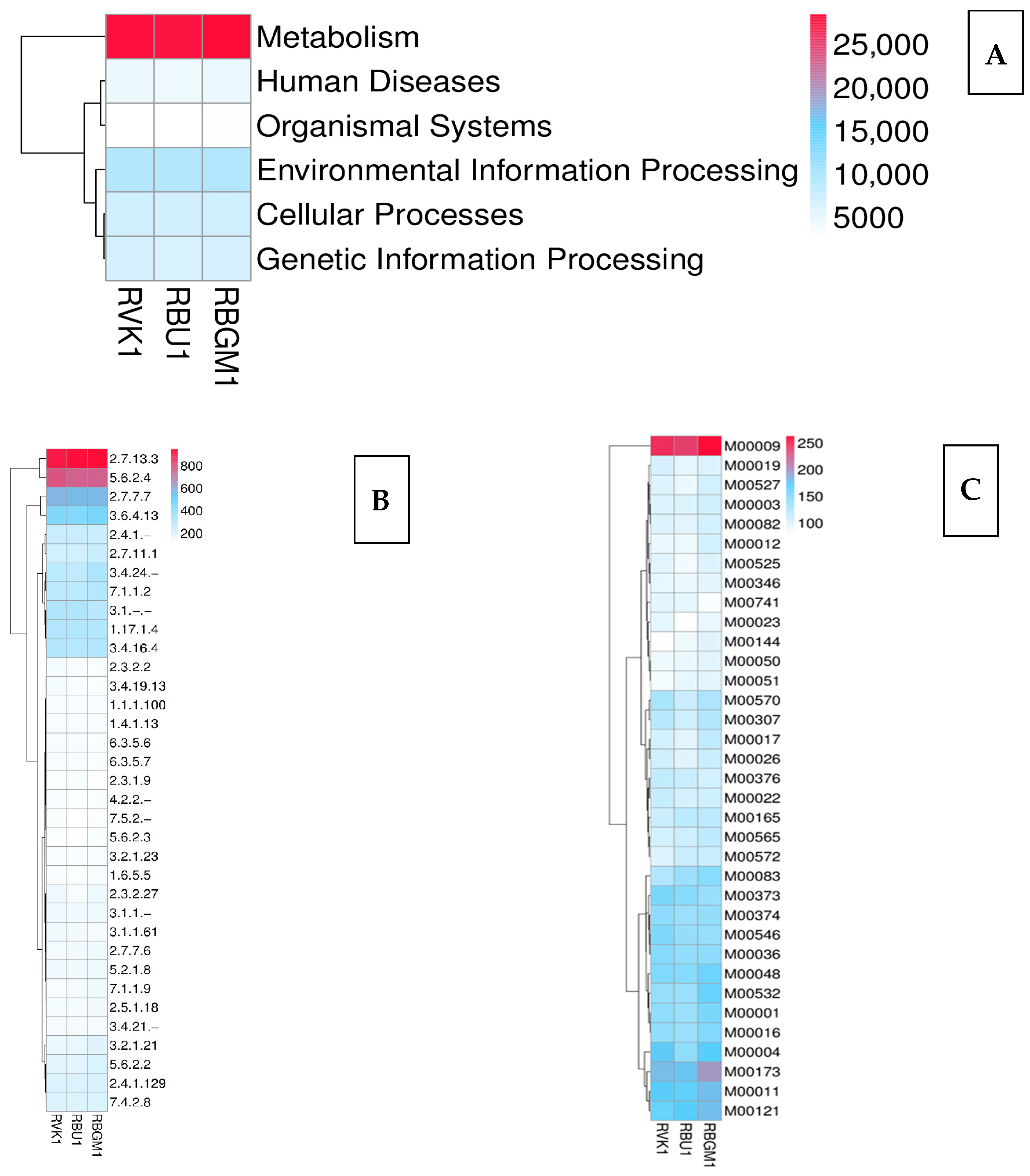

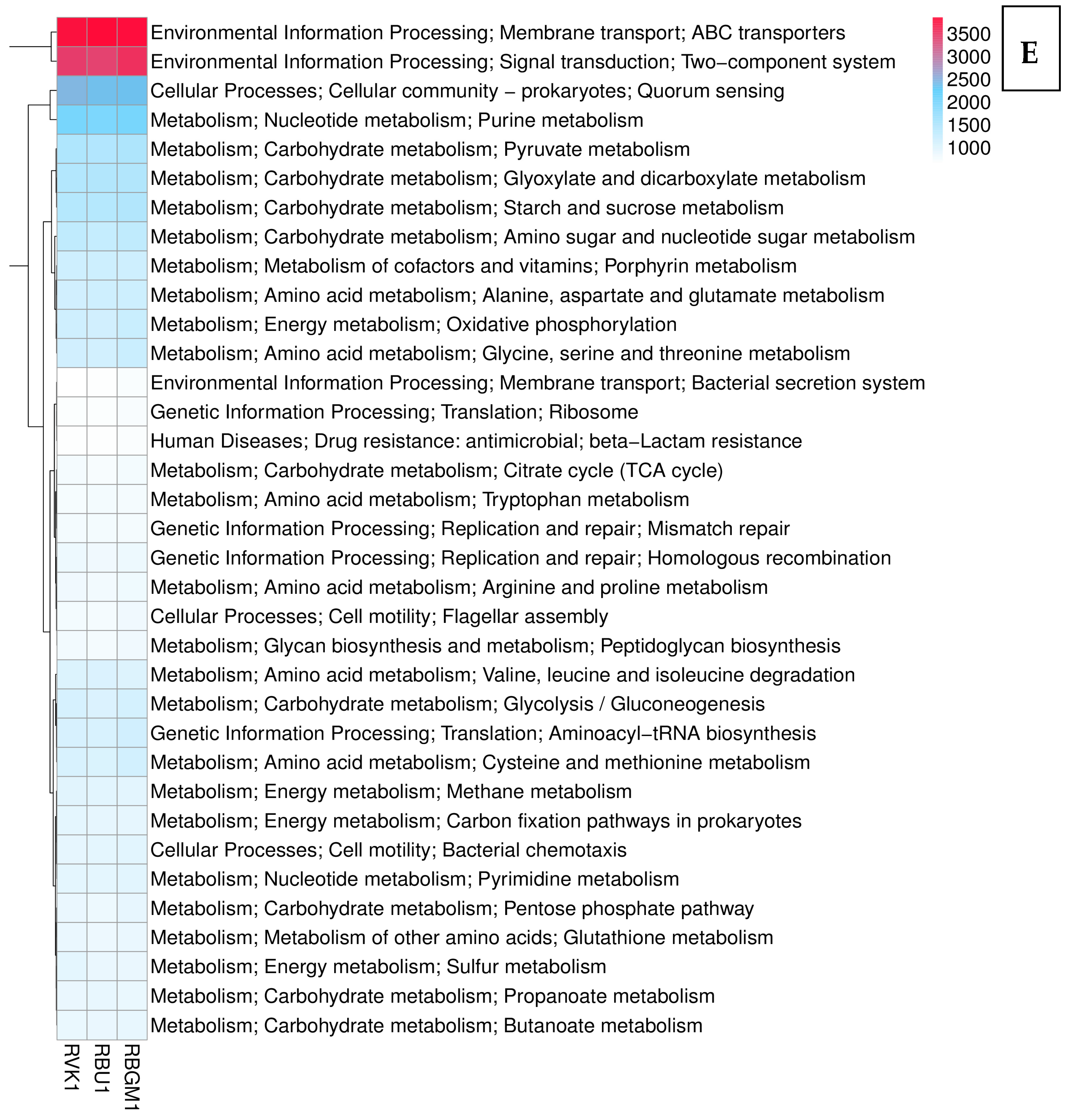


| County | Bean Phenotype | Sub-County | Number of Leaf Samples |
|---|---|---|---|
| Bungoma | Rosecoco | Bungoma town | 2 |
| Rosecoco | Bungoma Central | 2 | |
| Rosecoco | Bungoma N/S | 2 | |
| Rosecoco | Bungoma West | 2 | |
| Rosecoco | Kanduyi-kibuke | 3 | |
| Subtotal | 11 | ||
| Busia | Rosecoco | Butula | 5 |
| Rosecoco | Matayos | 5 | |
| Subtotal | 10 | ||
| Kakamega | Rosecoco | Kakamega East | 3 |
| Rosecoco | Kakamega South | 3 | |
| Rosecoco | Kakamega West | 2 | |
| Rosecoco | Lugari | 2 | |
| Subtotal | 10 | ||
| Nandi | Rosecoco | Nandi South (Aldai) | 10 |
| Rosecoco | Subtotal | 10 | |
| Vihiga | Rosecoco | Hamisi | 5 |
| Rosecoco | Sabatia | 5 | |
| Subtotal | 10 | ||
| Total | 51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osogo, A.K.; Sarkar, S.; Muyekho, F.; Were, H.; Okoth, P. Genome-Wide Metatranscriptomics Crosswalk of Diseased Common Beans (Phaseolus vulgaris L.) Unravels Critical Metabolic Pathways Involved in Plant Defense Mechanisms. Int. J. Plant Biol. 2025, 16, 114. https://doi.org/10.3390/ijpb16040114
Osogo AK, Sarkar S, Muyekho F, Were H, Okoth P. Genome-Wide Metatranscriptomics Crosswalk of Diseased Common Beans (Phaseolus vulgaris L.) Unravels Critical Metabolic Pathways Involved in Plant Defense Mechanisms. International Journal of Plant Biology. 2025; 16(4):114. https://doi.org/10.3390/ijpb16040114
Chicago/Turabian StyleOsogo, Aggrey Keya, Shrabana Sarkar, Francis Muyekho, Hassan Were, and Patrick Okoth. 2025. "Genome-Wide Metatranscriptomics Crosswalk of Diseased Common Beans (Phaseolus vulgaris L.) Unravels Critical Metabolic Pathways Involved in Plant Defense Mechanisms" International Journal of Plant Biology 16, no. 4: 114. https://doi.org/10.3390/ijpb16040114
APA StyleOsogo, A. K., Sarkar, S., Muyekho, F., Were, H., & Okoth, P. (2025). Genome-Wide Metatranscriptomics Crosswalk of Diseased Common Beans (Phaseolus vulgaris L.) Unravels Critical Metabolic Pathways Involved in Plant Defense Mechanisms. International Journal of Plant Biology, 16(4), 114. https://doi.org/10.3390/ijpb16040114








