Understanding Salt Stress in Watermelon: Impacts on Plant Performance, Adaptive Solutions, and Future Prospects
Abstract
1. Introduction
2. Search Methodology
3. Soil Salinity and Plant Development
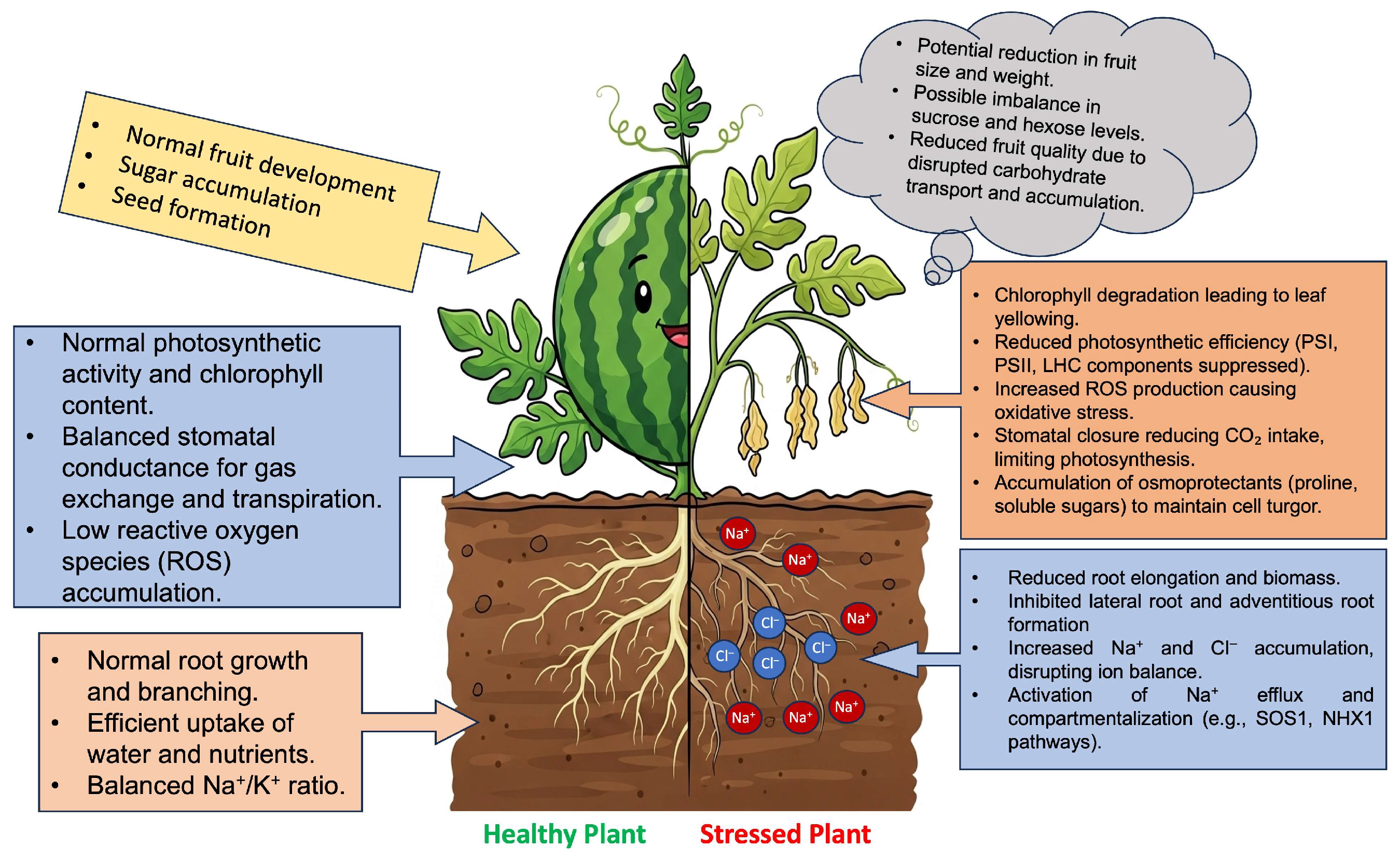
4. Effects of Soil Salinity on Watermelon Growth and Development
4.1. Seed Germination, Early Growth, and Plant Establishment
4.2. Vegetative Growth
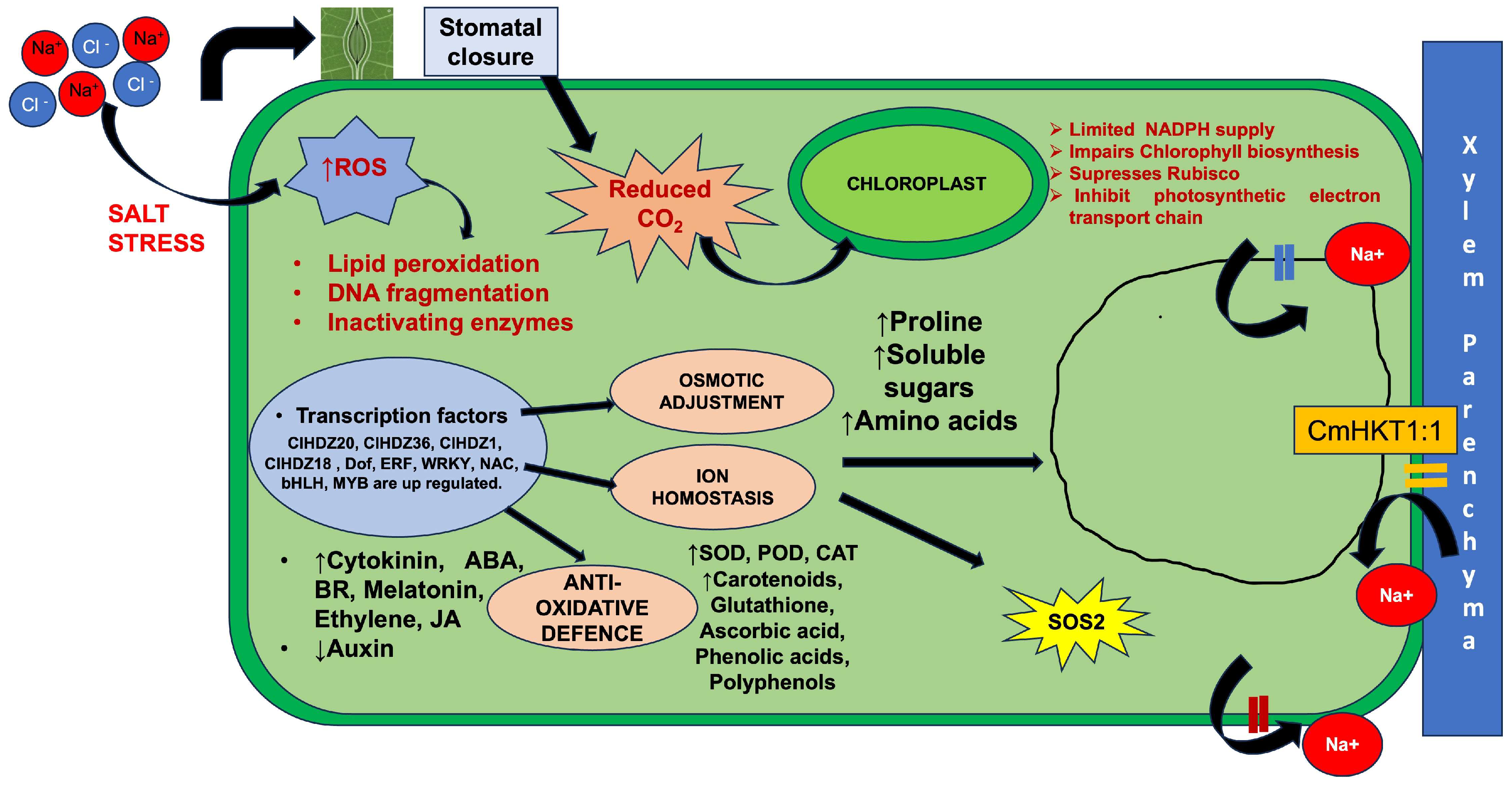
4.3. Physiological, Biochemical, and Molecular Responses
4.3.1. Physiological Responses
4.3.2. Biochemical Responses
4.3.3. Molecular Response
4.4. Yield and Quality
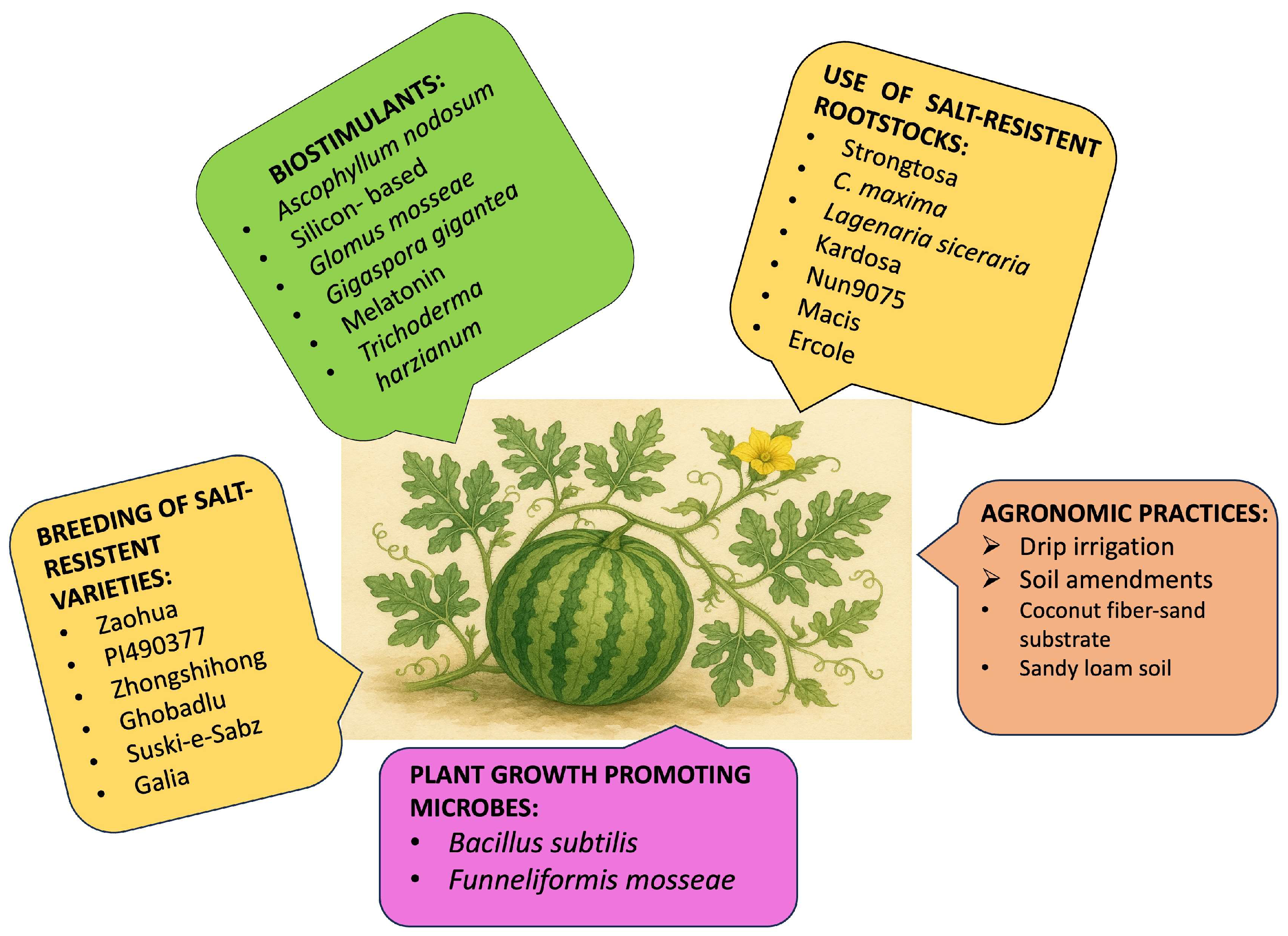
5. Strategies to Mitigate Salt Stress in Watermelon
5.1. Use of Salt-Tolerant Rootstocks
5.2. Breeding Salt-Resistant Varieties
5.3. Agronomic Practices Such as Drip Irrigation and Soil Amendment
5.4. Nanobiochar and Nanoparticles
5.5. Application of Biostimulants and Plant Growth Regulators
5.5.1. Biostimulants
5.5.2. Plant Growth-Promoting Microbes
6. Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abumhadi, N.; Todorovska, E.; Assenov, B.; Tsonev, S.; Vulcheva, D.; Atanasova, A.; Savoav, S.; Atanassov, A.; Keith, W. Agricultural research in 21st century: Challenges facing the food security under the impacts of climate change. Bulg. J. Agri. Sci. 2012, 18, 801–818. [Google Scholar]
- Hasanuzzaman, M.; Oku, H.; Nahar, K.; Bhuyan, M.H.M.B.; Mahmud, J.A.; Baluska, F.; Fujita, M. Nitric oxide-induced salt stress tolerance in plants: ROS metabolism, signaling, and molecular interactions. Plant Biotechnol. Rep. 2018, 12, 77–92. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Furtak, K.; Wolińska, A. The impact of extreme weather events as a consequence of climate change on the soil moisture and on the quality of the soil environment and agriculture—A review. CATENA 2023, 231, 107378. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global Warming, Climate Change, and Environmental Pollution: Recipe for a Multifactorial Stress Combination Disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef]
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Increase in crop losses to insect pests in a warming climate. Science 2018, 361, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2008, 103, 551–560. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather. Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting Mechanisms of Defense Against Biotrophic and Necrotrophic Pathogens. Ann. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, S.; Pellegrini, E.; Contin, M.; Bravo, C.; Nobili, M. Impacts of salinization caused by sea level rise on the biological processes of coastal soils—A review. Front. Environ. Sci. 2022, 10, 909415. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Sandhu, D.; Kaundal, A. Dynamics of Salt Tolerance: Molecular Perspectives. In Biotechnologies of Crop Improvement, Volume 3: Genomic Approaches; Gosal, S.S., Wani, S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 25–40. [Google Scholar]
- Butcher, K.; Wick, A.F.; DeSutter, T.; Chatterjee, A.; Harmon, J. Soil Salinity: A Threat to Global Food Security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How Plant Hormones Mediate Salt Stress Responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Hayat, K.; Jochen, B.; Farooq, J.; Saiqa, M.; Sikandar, H.; Fazal, H.; Ali, S.M.; Javed, C.H.; Abid, U.; Dan, Z. Combating soil salinity with combining saline agriculture and phytomanagement with salt-accumulating plants. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1085–1115. [Google Scholar] [CrossRef]
- Munns, R.; Millar, A.H. Seven plant capacities to adapt to abiotic stress. J. Exp. Bot. 2023, 74, 4308–4323. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS Metabolism in Plants under Environmental Stress: A Review of Recent Experimental Evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Soares, C.; Carvalho, M.E.A.; Azevedo, R.A.; Fidalgo, F. Plants facing oxidative challenges—A little help from the antioxidant networks. Environ. Exp. Bot. 2019, 161, 4–25. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef]
- Rai, V. Role of amino acids in plant responses to stresses. Biol. Plant. 2002, 45, 481–487. [Google Scholar] [CrossRef]
- Sytar, O.; Mbarki, S.; Zivcak, M.; Brestic, M. The Involvement of Different Secondary Metabolites in Salinity Tolerance of Crops. In Salinity Responses and Tolerance in Plants, Volume 2: Exploring RNAi, Genome Editing and Systems Biology; Kumar, V., Wani, S.H., Suprasanna, P., Tran, L.-S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 21–48. [Google Scholar]
- Naz, A.; Butt, M.S.; Sultan, M.T.; Qayyum, M.M.; Niaz, R.S. Watermelon lycopene and allied health claims. Excli J. 2014, 13, 650–660. [Google Scholar]
- Asfaw, M.D. Review on watermelon production and nutritional value in Ethiopia. J. Nutr. Sci. Res. 2022, 7, 173. [Google Scholar]
- Frelier, J.; Cummins, D.; Motsenbocker, C. Sustainable Gardening for School and Home Gardens: Cantaloupe and Watermelon. 2021. Available online: https://repository.lsu.edu/susgard/7 (accessed on 16 June 2025).
- FAO. Watermelon Production by Country 2025. 2025. Available online: https://worldostats.com/agriculture-food/watermelon-production-by-country-2025/ (accessed on 16 June 2025).
- da Silva, S.S.; de Lima, G.S.; de Lima, V.L.A.; Gheyi, H.R.; Soares, L.A.d.A.; Oliveira, J.P.M.; Araujo, A.C.; Gomes, J.P. Production and quality of watermelon fruits under salinity management strategies and nitrogen fertilization. 2020. Semina Ciências Agrárias 2020, 41, 2923–2936. [Google Scholar] [CrossRef]
- Cova, A.M.W.; de Azevedo Neto, A.D.; da Sliva, N.D.; Silva, P.C.C.; Gheyi, H.R.; da Silva, L.L. Osmotic adjustment, production, and post-harvest quality of mini watermelon genotypes differing in salt tolerance. Sci. Hortic. 2022, 306, 111463. [Google Scholar] [CrossRef]
- Yanyan, Y.; Shuoshuo, W.; Min, W.; Biao, G.; Qinghua, S. Effect of different rootstocks on the salt stress tolerance in watermelon seedlings. Hortic. Plant J. 2018, 4, 239–249. [Google Scholar] [CrossRef]
- da Silva, S.S.; de Lima, G.S.; de Lima, V.L.A.; Gheyi, H.R.; dos Anjos Soares, L.A.; Moreira, R.C.L.; Fernandes, P.D.; Andrade, E.M.G.; Pinheiro, F.W.A. Salinity management strategies and potassium fertilization in watermelon (‘Citrullus lanatus’) cultivation. Aust. J. Crop Sci. 2020, 14, 1601–1607. [Google Scholar] [CrossRef]
- Shahbaz, M.; Ashraf, M. Improving salinity tolerance in cereals. Crit. Rev. Plant Sci. 2013, 32, 237–249. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Limitation of salt stress to plant growth. In Plant Toxicology; CRC Press: Boca Raton, FL, USA, 2004; pp. 205–238. [Google Scholar]
- Abou-Baker, N.H.A.; El-Dardiry, E.A. Integrated Management of Salt Affected Soils in Agriculture: Incorporation of Soil Salinity Control Methods; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Yetisir, H.; Caliskan, M.E.; Soylu, S.; Sakar, M. Some physiological and growth responses of watermelon [Citrullus lanatus (Thunb.) Matsum. and Nakai] grafted onto Lagenaria siceraria to flooding. Environ. Exp. Bot. 2006, 58, 1–8. [Google Scholar] [CrossRef]
- Lessa, C.I.N.; de Sousa, G.G.; Sousa, H.C.; da Silva, F.D.B.; Gomes, S.P.; de Araujo Viana, T.V. Agricultural ambience and salt stress in production of yellow passion fruit seedlings. Comun. Sci. 2022, 13, e3703. [Google Scholar] [CrossRef]
- Taheri, S.; Barzegar, T.; Zadeh, A.Z. Effect of salicylic acid pre-treatment on cucumber and watermelon seeds germination under salinity stress. Iran. J. Seed Sci. Res. 2016, 3, fa15–fa27. [Google Scholar]
- de Albuquerque Ribeiro, A.; de Lima Sales, M.A.; Eloi, W.M.; Moreira, F.J.C.; de Lima Sales, F.A. Emergence and Initial Growth of Watermelon Under Salt Stress. Rev. Bras. Eng. Biossistemas 2012, 6, 30–38. [Google Scholar]
- Colla, G.; Roupahel, Y.; Cardarelli, M.; Rea, E. Effect of salinity on yield, fruit quality, leaf gas exchange, and mineral composition of grafted watermelon plants. HortScience 2006, 41, 622. [Google Scholar] [CrossRef]
- Naseer, M.N.; Rahman, F.U.; Hussain, Z.; Khan, L.A.; Aslam, M.M.; Aslam, A.; Waheed, H.; Khan, A.U.; Iqbal, S. Effect of salinity stress on germination, seedling growth, mineral uptake and chlorophyll contents of three Cucurbitaceae species. Braz. Arch. Biol. Technol. 2022, 65, e22210213. [Google Scholar] [CrossRef]
- Yetisir, H.; Uygur, V. Plant growth and mineral element content of different gourd species and watermelon under salinity stress. Turk. J. Agric. For. 2009, 33, 65–77. [Google Scholar] [CrossRef]
- Coşkun, Ö.F.; Toprak, S.; Mavi, K. Genetic Diversity and Association Mapping for Salinity Tolerance in Watermelon (Citrullus lanatus L.). J. Crop Health 2025, 77, 73. [Google Scholar] [CrossRef]
- Huang, Y.; Bie, Z.; He, S.; Hua, B.; Zhen, A.; Liu, Z. Improving cucumber tolerance to major nutrients induced salinity by grafting onto Cucurbita ficifolia. Environ. Exp. Bot. 2010, 69, 32–38. [Google Scholar] [CrossRef]
- Ali, M.; Ayyub, C.; Shaheen, M.R.; Qadri, R.W.K.; Khan, I.; Azam, M.; Akhtar, N. Characterization of Water Melon (Citrullus lanatus) Genotypes under High Salinity Regime. Am. J. Plant Sci. 2015, 6, 3260. [Google Scholar] [CrossRef]
- Ekbic, E.; Cagıran, C.; Korkmaz, K.; Kose, M.A.; Aras, V. Assessment of watermelon accessions for salt tolerance using stress tolerance indices. Ciência E Agrotecnologia 2017, 41, 616–625. [Google Scholar] [CrossRef]
- Fang, S.; Hou, X.; Liang, X. Response mechanisms of plants under saline-alkali stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef]
- Santos, G.L.D.; Pereira, F.H.F.; Sousa, V.F.D.O.; Suassuna, C.D.F.; Santos, A.P.D.L.; Barros Junior, A.P. Cytokinin and Auxin Influence on Growth and Quality of Watermelon Irrigated with Saline Water. Rev. Caatinga 2022, 35, 677–685. [Google Scholar] [CrossRef]
- Zhang, G.; Ding, Q.; Wei, B. Genome-wide identification of superoxide dismutase gene families and their expression patterns under low-temperature, salt and osmotic stresses in watermelon and melon. 3 Biotech 2021, 11, 194. [Google Scholar] [CrossRef]
- Sarabi, B.; Bolandnazar, S.; Ghaderi, N.; Ghashghaie, J. Genotypic differences in physiological and biochemical responses to salinity stress in melon (Cucumis melo L.) plants: Prospects for selection of salt tolerant landraces. Plant Physiol. Biochem. 2017, 119, 294–311. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.; Chen, H.; Wang, Z.; Gu, X.; Wei, C.; Zhang, Y.; Ma, J.; Zhang, X. Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci. 2017, 295. [Google Scholar] [CrossRef]
- Zhu, Y.; Yuan, G.; Gao, B.; An, G.; Li, W.; Si, W.; Sun, D.; Liu, J. Comparative transcriptome profiling provides insights into plant salt tolerance in watermelon (Citrullus lanatus). Life 2022, 12, 1033. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Dong, S.; Miao, H.; Liu, X.; Li, C.; Han, J.; Zhang, S.; Gu, X. A large-scale genomic association analysis identifies the candidate genes regulating salt tolerance in cucumber (Cucumis sativus L.) seedlings. Int. J. Mol. Sci. 2022, 23, 8260. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Dong, S.; Bo, K.; Miao, H.; Li, C.; Zhang, Y.; Zhang, S.; Gu, X. Identification of QTLs controlling salt tolerance in cucumber (Cucumis sativus L.) seedlings. Plants 2021, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Wang, L.; Zhang, Y.; Huang, D. Identification of early response genes to salt stress in roots of melon (Cucumis melo L.) seedlings. Mol. Biol. Rep. 2013, 40, 2915–2926. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, F.; Huang, D.; Zhang, L.; Zhang, Y. Transcriptome analysis of transcription factors in two melon (Cucumis melo L.) cultivars under salt stress. Plant Physiol. J. 2014, 50, 150–158. [Google Scholar]
- Ji, H.; Pardo, J.M.; Batelli, G.; Van Oosten, M.J.; Bressan, R.A.; Li, X. The salt overly sensitive (SOS) pathway: Established and emerging roles. Mol. Plant 2013, 6, 275–286. [Google Scholar] [CrossRef]
- Ma, L.; Ye, J.; Yang, Y.; Lin, H.; Yue, L.; Luo, J.; Long, Y.; Fu, H.; Liu, X.; Zhang, Y. The SOS2-SCaBP8 complex generates and fine-tunes an AtANN4-dependent calcium signature under salt stress. Dev. Cell 2019, 48, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yue, Z.; Pan, X.; Si, F.; Li, J.; Chen, X.; Li, X.; Luan, F.; Yang, J.; Zhang, X.; et al. The HD-ZIP Gene Family in Watermelon: Genome-Wide Identification and Expression Analysis under Abiotic Stresses. Genes 2022, 13, 2242. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, Y.; Wan, C.; Li, J.; Yang, Y.; Chen, J. Genome-wide characterization and expression analysis of the Dof gene family related to abiotic stress in watermelon. PeerJ 2020, 8, e8358. [Google Scholar] [CrossRef]
- Song, Q.; Joshi, M.; Joshi, V. Transcriptomic analysis of short-term salt stress response in watermelon seedlings. Int. J. Mol. Sci. 2020, 21, 6036. [Google Scholar] [CrossRef] [PubMed]
- Boonyaves, K.; Ang, M.C.-Y.; Park, M.; Cui, J.; Khong, D.T.; Singh, G.P.; Koman, V.B.; Gong, X.; Porter, T.K.; Choi, S.W. Near-infrared fluorescent carbon nanotube sensors for the plant hormone family gibberellins. Nano Lett. 2023, 23, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Ribba, T.; Garrido-Vargas, F.; O’Brien, J.A. Auxin-mediated responses under salt stress: From developmental regulation to biotechnological applications. J. Exp. Bot. 2020, 71, 3843–3853. [Google Scholar] [CrossRef]
- Chang, J.; Gao, Y.; Yan, J.; Zhang, Z.; Yuan, L.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X.; et al. The role of watermelon caffeic acid O-methyltransferase (ClCOMT1) in melatonin biosynthesis and abiotic stress tolerance. Hortic. Res. 2021, 8, 210. [Google Scholar] [CrossRef]
- Kusvuran, S.; Dasgan, H.Y.; Abak, K. Citrulline is an important biochemical indicator in tolerance to saline and drought stresses in melon. Sci. World J. 2013, 1, 253414. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Gao, L.; Zhang, Y.; Zhang, F.; Yang, X.; Haund, D. Genome-wide investigation of the NAC transcription factor family in melon (Cucumis melo L.) and their expression analysis under salt stress. Plant Cell Rep 2016, 35, 1827–1839. [Google Scholar] [CrossRef]
- Flores-León, A.; García-Martínez, S.; González, V.; Garcés-Claver, A.; Martí, R.; Julián, C.; Sifres, A.; Pérez-de-Castro, A.; Díez, M.J.; López, C.; et al. Grafting Snake Melon [Cucumis melo L. subsp. melo Var. flexuosus (L.) Naudin] in Organic Farming: Effects on Agronomic Performance; Resistance to Pathogens; Sugar, Acid, and VOC Profiles; and Consumer Acceptance. Front. Plant Sci. 2021, 12, 613845. [Google Scholar] [CrossRef]
- Sanoubar, R.; Orsini, F.; Gianquinto, G. Ionic partitioning and stomatal regulation: Dissecting functional elements of the genotypic basis of salt stress adaptation in grafted melon. Plant Signal Behav. 2013, 8, e27334. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Very, A.A.; Wang, L.M.; Deng, Y.W.; Sentenac, H.; Huang, D.F. A K+ channel from salt-tolerant melon inhibited by Na+. New Phytol. 2011, 189, 856–868. [Google Scholar] [CrossRef]
- Gao, L.-W.; Yang, S.-L.; Wei, S.-W.; Huang, D.-F.; Zhang, Y.-D. Supportive role of the Na+ transporter CmHKT1; 1 from Cucumis melo in transgenic Arabidopsis salt tolerance through improved K+/Na+ balance. Plant Mol. Biol. 2020, 103, 561–580. [Google Scholar] [CrossRef]
- Suárez-Hernández, Á.M.; Vázquez-Angulo, J.C.; Grimaldo-Juárez, O.; Duran, C.C.; González-Mendoza, D.; Bazante-González, I.; Mendoza-Gómez, A. Production and quality of grafted watermelon in saline soil. Hortic. Bras. 2019, 37, 215–220. [Google Scholar] [CrossRef]
- Silva, J.S.D.; Sá, F.V.D.S.; Dias, N.D.S.; Ferreira, M.; Jales, G.D.; Fernandes, P.D. Morphophysiology of mini watermelon in hydroponic cultivation using reject brine and substrates. Rev. Bras. De Eng. Agrícola E Ambient. 2021, 25, 402–408. [Google Scholar] [CrossRef]
- Zong, L.; Tedeschi, A.; Xue, X.; Wang, T.; Menenti, M.; Huang, C. Effect of different irrigation water salinities on some yield and quality components of two field-grown Cucurbit species. Turk. J. Agric. For. 2011, 35, 297–307. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Leonardi, C.; Bie, Z. Role of grafting in vegetable crops grown under saline conditions. Sci. Hortic. 2010, 127, 147–155. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Schwarz, D.; Franken, P.; Colla, G. Effects of drought on nutrient uptake and assimilation in vegetable crops. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Springer: Berlin/Heidelberg, Germany, 2012; pp. 171–195. [Google Scholar]
- Gaion, L.A.; Braz, L.; Carvalho, R. Grafting in Vegetable Crops: A Great Technique for Agriculture. Int. J. Veg. Sci. 2017, 24, 1–18. [Google Scholar] [CrossRef]
- Hashem, A.; Bayoumi, Y.A.; El-Shafik, A.; El-Zawily, E.-S.; Tester, M.; Rakha, M.T. Interspecific Hybrid Rootstocks Improve Productivity of Tomato Grown under High-temperature Stress. HortScience 2024, 59, 129–137. [Google Scholar] [CrossRef]
- Goreta, S.; Bucevic-Popovic, V.; Selak, G.V.; Pavela-Vrancic, M.; Perica, S. Vegetative growth, superoxide dismutase activity and ion concentration of salt-stressed watermelon as influenced by rootstock. J. Agric. Sci. 2008, 146, 695–704. [Google Scholar] [CrossRef]
- Yetisir, H.; Uygur, V. Responses of grafted watermelon onto different gourd species to salinity stress. J. Plant Nutr. 2010, 33, 315–327. [Google Scholar] [CrossRef]
- Edelstein, M.; Ben-Hur, M.; Cohen, R.; Burger, Y.; Ravina, I. Boron and salinity effects on grafted and non-grafted melon plants. Plant Soil 2005, 269, 273–284. [Google Scholar] [CrossRef]
- Ulas, A.; Aydin, A.; Ulas, F.; Yetisir, H.; Miano, T.F. Cucurbita rootstocks improve salt tolerance of melon scions by inducing physiological, biochemical and nutritional responses. Horticulturae 2020, 6, 66. [Google Scholar] [CrossRef]
- Napolitano, M.; Terzaroli, N.; Kashyap, S.; Russi, L.; Jones-Evans, E.; Albertini, E. Exploring heterosis in melon (Cucumis melo L.). Plants 2020, 9, 282. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, S.; Lu, X.; He, N.; Gao, L.; Dou, J.; Bie, Z.; Liu, W. Genome duplication improves the resistance of watermelon root to salt stress. Plant Physiol. Biochem. 2018, 133, 11–21. [Google Scholar] [CrossRef]
- Mo, Y.; Wang, Y.; Yang, R.; Zheng, J.; Liu, C.; Li, H.; Ma, J.; Zhang, Y.; Wei, C.; Zhang, X. Regulation of plant growth, photosynthesis, antioxidation and osmosis by an arbuscular mycorrhizal fungus in watermelon seedlings under well-watered and drought conditions. Front. Plant Sci. 2016, 7, 644. [Google Scholar] [CrossRef]
- Gao, B.-W.; Sun, D.-X.; Yuan, G.-P.; An, G.-L.; Li, W.; Liu, J.-P.; Zhu, Y.-C. Identification of salt tolerance of 121 watermelon (Citrullus lanatus L.) germplasm resources. J. Fruit. Sci. 2022, 39, 1597–1606. [Google Scholar]
- Fan, R.; Zhang, B.; Li, J.; Zhang, Z.; Liang, A. Straw-derived biochar mitigates CO2 emission through changes in soil pore structure in a wheat-rice rotation system. Chemosphere 2020, 243, 125329. [Google Scholar] [CrossRef]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Qiang, X.; Sun, Z.; Li, X.; Li, S.; Yu, Z.; He, J.; Li, Q.; Han, L. The impacts of planting patterns combined with irrigation management practices on soil water content, watermelon yield and quality. Agrofor. Syst. 2024, 98, 979–994. [Google Scholar] [CrossRef]
- Alves, A.D.S.; Oliveira, F.D.A.D.; Silva, D.D.D.; Santos, S.T.D.; Oliveira, R.R.; Góis, H.M.D.M. Production and quality of mini watermelon under salt stress and K+/Ca2+ ratios. Rev. Bras. De Eng. Agrícola E Ambient. 2023, 27, 441–446. [Google Scholar] [CrossRef]
- Silva, S.S.D.; Lima, G.S.D.; Lima, V.L.A.D.; Gheyi, H.R.; Soares, L.A.D.A.; Lucena, R.C.M. Gas exchanges and production of watermelon plant under salinity management and nitrogen fertilization. Pesqui. Agropecuária Trop. 2019, 49, e54822. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Wang, H.; Nie, S.; Liang, Z. Effects of soil salinity on the content, composition, and ion binding capacity of glomalin-related soil protein (GRSP). Sci. Total Environ. 2017, 581, 657–665. [Google Scholar] [CrossRef]
- Chang, R.; Sohi, S.P.; Jing, F.; Liu, Y.; Chen, J. A comparative study on biochar properties and Cd adsorption behavior under effects of ageing processes of leaching, acidification and oxidation. Environ. Pollu. 2019, 254, 113123. [Google Scholar] [CrossRef]
- Sharma, G.; Banik, D.; Mehta, C.M.; Eiji, N.; Inubushi, K. Influence of Biochar Application Rates on Watermelon Growth, Yield, and Soil Nutrient Availability. J. Food Chem. Nanotechnol. 2025, 11, S36–S42. [Google Scholar] [CrossRef]
- Cele, T. Preparation of Nanoparticles. In Engineered Nanomaterials—Health and Safety; Avramescu, S.M., Fierascu, I., Akhtar, K., Khan, S.B., Ali, F., Asiri, A.M.A., Eds.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Rai, P.K.; Kumar, V.; Lee, S.; Raza, N.; Kim, K.-H.; Ok, Y.S.; Tsang, D.C.W. Nanoparticle-plant interaction: Implications in energy, environment, and agriculture. Environ. Int. 2018, 119, 1–19. [Google Scholar] [CrossRef]
- Rastogi, A.; Zivcak, M.; Tripathi, D.K.; Yadav, S.; Kalaji, H.M. Phytotoxic effect of silver nanoparticles in Triticum aestivum: Improper regulation of photosystem I activity as the reason for oxidative damage in the chloroplast. Photosynthetica 2019, 57, 209–216. [Google Scholar] [CrossRef]
- Ahmad, I.; Akhtar, M.S. Use of Nanoparticles in Alleviating Salt Stress. In Salt Stress, Microbes, and Plant Interactions: Causes and Solution: Volume 1; Akhtar, M.S., Ed.; Springer Singapore: Singapore, 2019; pp. 199–215. [Google Scholar]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci 2019, 289, 110270. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Raza, M.A.; Awan, S.A.; Shah, G.A.; Rizwan, M.; Ali, B.; Tariq, R.; Hassan, M.J.; Alyemeni, M.N.; Brestic, M. Amelioration of salt induced toxicity in pearl millet by seed priming with silver nanoparticles (AgNPs): The oxidative damage, antioxidant enzymes and ions uptake are major determinants of salt tolerant capacity. Plant Physiol. Biochem. 2020, 156, 221–232. [Google Scholar] [CrossRef]
- Jacuinde-Guzmán, J.K.; Escalona-Buendía, H.B.; Barbosa-Martínez, C.; Rivera-Cabrera, F.; Raddatz-Mota, D.; Soriano-Melgar, L.D.A.A. The potential of calcium nanoparticles in posthaverst conservation of fresh-cut seedless watermelon (Citrullus lanatus). Postharvest Biol. Technol. 2024, 216, 113069. [Google Scholar] [CrossRef]
- Acharya, P.; Jayaprakasha, G.K.; Crosby, K.M.; Jifon, J.L.; Patil, B.S. Nanoparticle-Mediated Seed Priming Improves Germination, Growth, Yield, and Quality of Watermelons (Citrullus lanatus) at multi-locations in Texas. Sci. Rep. 2020, 10, 5037. [Google Scholar] [CrossRef]
- Chen, L.; Peng, Y.; Zhu, L.; Huang, Y.; Bie, Z.; Wu, H. CeO2 nanoparticles improved cucumber salt tolerance is associated with its induced early stimulation on antioxidant system. Chemosphere 2022, 299, 134474. [Google Scholar] [CrossRef]
- Wang, S.; Liu, P.; Chen, D.; Yin, L.; Li, H.; Deng, X. Silicon enhanced salt tolerance by improving the root water uptake and decreasing the ion toxicity in cucumber. Front. Plant Sci. 2015, 6, 759. [Google Scholar] [CrossRef]
- Yin, J.; Jia, J.; Lian, Z.; Hu, Y.; Guo, J.; Huo, H.; Zhu, Y.; Gong, H. Silicon enhances the salt tolerance of cucumber through increasing polyamine accumulation and decreasing oxidative damage. Ecotoxicol. Environ. Saf. 2019, 169, 8–17. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. (2020) Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Singh, M.; Subahan, G.M.; Sharma, S.; Singh, G.; Sharma, N.; Sharma, U.; Kumar, V. Enhancing Horticultural Sustainability in the Face of Climate Change: Harnessing Biostimulants for Environmental Stress Alleviation in Crops. Stresses 2025, 5, 23. [Google Scholar] [CrossRef]
- Bantis, F.; Koukounaras, A. Ascophyllum nodosum and silicon-based biostimulants differentially affect the physiology and growth of watermelon transplants under abiotic stress factors: The case of salinity. Plants 2023, 12, 433. [Google Scholar] [CrossRef] [PubMed]
- Bijalwan, P.; Jeddi, K.; Saini, I.; Sharma, M.; Kaushik, P.; Hessini, K. Mitigation of saline conditions in watermelon with mycorrhiza and silicon application. Saudi J. Biol. Sci. 2021, 28, 3678–3684. [Google Scholar] [CrossRef]
- Ghani, M.I.; Yi, B.; Rehmani, M.S.; Wei, X.; Siddiqui, J.A.; Fan, R.; Liu, Y.; El-Sheikh, M.A.; Chen, X.; Ahmad, P. Potential of melatonin and Trichoderma harzianum inoculation in ameliorating salt toxicity in watermelon: Insights into antioxidant system, leaf ultrastructure, and gene regulation. Plant Physiol. Biochem. 2024, 211, 108639. [Google Scholar] [CrossRef]
- Afrin, S.; Tamanna, T.; Shahajadi, U.F.; Bhowmik, B.; Jui, A.H.; Miah, M.A.S.; Bhuiyan, M.N.I. Characterization of protease-producing bacteria from garden soil and antagonistic activity against pathogenic bacteria. Microbe 2024, 4, 100123. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.; Vishwakarma, K.; Sharma, S.; Kumar, V.; Upadhyay, N.; Kumar, N.; Verma, R.K.; Mishra, R.; Tripathi, D.K.; Upadhyay, R. Emerging significance of rhizospheric probiotics and its impact on plant health: Current perspective towards sustainable agriculture. In Probiotics Plant Health; Springer: Singapore, 2017; pp. 233–251. [Google Scholar] [CrossRef]
- Ganesh, J.; Hewitt, K.; Devkota, A.R.; Wilson, T.; Kaundal, A. IAA-producing plant growth promoting rhizobacteria from Ceanothus velutinus enhance cutting propagation efficiency and Arabidopsis biomass. Front. Plant Sci. 2024, 15, 1374877. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, P.; Singh, P.K.; Chakraborty, D.; Mishra, S.; Pattnaik, R. Insight into the role of PGPR in sustainable agriculture and environment. Front. Sustain. Food Syst. 2021, 5, 667150. [Google Scholar] [CrossRef]
- Coats, V.C.; Rumpho, M.E. The rhizosphere microbiota of plant invaders: An overview of recent advances in the microbiomics of invasive plants. Front. Microbiol. 2014, 5, 368. [Google Scholar] [CrossRef]
- Acharya, B.R.; Gill, S.P.; Kaundal, A.; Sandhu, D. Strategies for combating plant salinity stress: The potential of plant growth-promoting microorganisms. Front. Plant Sci. 2024, 15, 1406913. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, J.; Singh, V.; Hewitt, K.; Kaundal, A. Exploration of the rhizosphere microbiome of native plant Ceanothus velutinus–an excellent resource of plant growth-promoting bacteria. Front. Plant Sci. 2022, 13, 979069. [Google Scholar] [CrossRef]
- Chaudhary, A.; Poudyal, S.; Kaundal, A. Role of Arbuscular Mycorrhizal Fungi in Maintaining Sustainable Agroecosystems. Appl. Microbiol. 2025, 5, 6. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, X.; Bao, E.; Cao, K.; Zou, Z. Effects of Arbuscular Mycorrhizal Fungi on Watermelon Growth, Elemental Uptake, Antioxidant, and Photosystem II Activities and Stress-Response Gene Expressions Under Salinity-Alkalinity Stresses. Front. Plant Sci. 2019, 10, 863. [Google Scholar] [CrossRef] [PubMed]
| Growth stages | References |
|---|---|
| Seed germination and early growth | [39,40] |
| Vegetative growth | [37,44] |
| Physiological, biochemical, and molecular responses | Physiological responses [46,47] |
| Biochemical responses [49,50] | |
| Molecular responses [53,57,60,61,62,63,64,65,66,67,68,69,70,71] | |
| Yield and Quality | [72,73,74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, S.; Oliveira, M.M.T.d.; Kaundal, A. Understanding Salt Stress in Watermelon: Impacts on Plant Performance, Adaptive Solutions, and Future Prospects. Int. J. Plant Biol. 2025, 16, 93. https://doi.org/10.3390/ijpb16030093
Kaur S, Oliveira MMTd, Kaundal A. Understanding Salt Stress in Watermelon: Impacts on Plant Performance, Adaptive Solutions, and Future Prospects. International Journal of Plant Biology. 2025; 16(3):93. https://doi.org/10.3390/ijpb16030093
Chicago/Turabian StyleKaur, Sukhmanjot, Milena Maria Tomaz de Oliveira, and Amita Kaundal. 2025. "Understanding Salt Stress in Watermelon: Impacts on Plant Performance, Adaptive Solutions, and Future Prospects" International Journal of Plant Biology 16, no. 3: 93. https://doi.org/10.3390/ijpb16030093
APA StyleKaur, S., Oliveira, M. M. T. d., & Kaundal, A. (2025). Understanding Salt Stress in Watermelon: Impacts on Plant Performance, Adaptive Solutions, and Future Prospects. International Journal of Plant Biology, 16(3), 93. https://doi.org/10.3390/ijpb16030093







