Abstract
In the coming decades, the agricultural system will predictably rely on organic material to produce crops and maintain food security. Currently, the use of inorganic fertilizers to grow crops and vegetables, such as Swiss chard, spinach, and lettuce, is on the rise and has been proven to be detrimental to the soil in the long run. Hence, there is a growing need to use organic waste material, such as spent coffee grounds (SCGs), to grow crops. Spent coffee grounds are made of depleted coffee beans that contain important soluble compounds. This study aimed to determine the influence of different levels (0.32 g, 0.63 g, 0.92 g, and 1.20 g) of spent coffee grounds on the metabolomic profile of Swiss chard. The 1H-nuclear magnetic resonance (NMR) results showed that Swiss chard grown with different levels of SCGs contains a total of 10 metabolites, which included growth-promoting metabolites (trehalose; betaine), defense mechanism metabolites (alanine; cartinine), energy-reserve metabolites (sucrose; 1,6 Anhydro-β-D-glucose), root metabolites (thymine), stress-related metabolites (2-deoxyadenosine), caffeine metabolites (1,3 Dimethylurate), and body-odor metabolites (trimethylamine). Interestingly, caprate, with the abovementioned metabolites, was detected in Swiss chard grown without the application of SCGs. The findings of the current study suggest that SCGs are an ideal organic material for growing Swiss chard for its healthy metabolites.
1. Introduction
Falcon et al. [1] predicted that by 2050, the world population will increase to 9.7 billion, resulting in 70% more demand for food than is currently consumed. Additionally, the agricultural system will rely heavily on adopting farming methods that minimize environmental impacts and adapt to climate change to ensure stable crop production, food security, and safety [2]. To meet the increased demand and ensure a sustain-able supply of nutritious food, farmers will have to optimize inputs and maximize the use of natural resources, such as organic material [2] like coffee remains known as spent coffee grounds [3], which are made of depleted coffee beans that contains important soluble compounds. A recent study by Cervera-Mata et al. [4] revealed that organic remains of coffee are the solid remains obtained after coffee preparation and are found in homes and/or commercial establishments, such as restaurants [5]. The rapid increase in the consumption of coffee throughout the world increases the quantity of organic waste from the coffee processing industry [6]. As a result, high amounts of dry SCGs are produced every year [7]. The composition of SCGs makes it suitable for it to be used as soil organic amendment [8] due to its positive effects on the soil’s chemistry, physico-chemistry, biological, and physical properties [8], such as increased nitrogen, potassium, phosphorus, and organic carbon content in soils [9,10], hence the need to use it as an organic waste material.
Swiss chard (Beta vulgaries var. cicla), also known as ‘stem chard’, belongs to the Chenopodiaceae family. It was first discovered and cultivated in early 350 before Christ (B.C) [11] and is closely related to garden beets (Beta vulgaris) and spinach (Spinaccia oleraccea) [12]. It is classified as a dicotyledonous biennial crop, generally treated as an annual crop, and can be harvested for five months consecutively [13]. Swiss chard is a dark green leafy vegetable (GLV) that can be planted in mid-spring and late summer [14]. A recent study by Sindesi [15] reported that chard is a desired and highly cultivated crop due to its adaptability to saline soil, ability to grow in water-scarce regions, and tolerance to extremely high and low temperatures.
According to Ninfali and Angelino [16], cultivation of Swiss chard should be encouraged as it is a great source of essential micro and macronutrients and phytochemicals, and is an affordable crop. Due to its high nutritional value, including carbohydrates, proteins, lipids, lignins, and minerals [17], and its delicious taste, Swiss chard is consumed as food and added to salads throughout the world, but mostly in Africa [18]. Nair [19] recommended the consumption of green leafy vegetables (GVLs), such as Swiss chard, due to their free radical activity, which is reported to protect against chronic diseases, such as Alzheimer’s disease and atherosclerosis. Medicinally, Swiss chard is reported to cure kidney and liver diseases and cancers [16]. In addition, Swiss chard contributes to the reinforcement of the gastric mucosa, and possesses antiseptic, mineralizing, and choleretic activities [20].
In South Africa, Swiss chard is a nutrient-rich leafy green vegetable that has gained popularity due to its adaptability to local climate conditions and high nutritional value. According to a study by Ndololwana [21], it is widely cultivated in the Western Cape and KwaZulu-Natal provinces for local markets and is consumed primarily by low-income households, who value its affordability and nutritional benefits. Matlala [22] found that Swiss chard is among the top 10 most consumed vegetables, including tomatoes and onions, in South Africa. Furthermore, a study by Oelofse et al. [23] revealed the potential of Swiss chard as a valuable source of essential micronutrients, such as iron, and macronutrients such as calcium, as well as vitamin A, which are often lacking in the diets of South African households. Currently, there are few scientific studies conducted and literature available that report the effect of SCGs on the metabolomic profile of Swiss chard. Therefore, the current study aimed to determine the impact of different levels of SCGs on the metabolites released by Swiss chard.
Metabolomics, which is the comprehensive study of small molecules or metabolites within biological systems, has emerged as a pivotal tool in understanding complex biological processes and disease mechanisms [24], and, as reported by Nemadodzi et al. [25], metabolomics has been widely applied in several human and plant studies. Recent advancements in mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy have enabled high-throughput metabolomics analyses, facilitating the identification and quantification of thousands of metabolites in various biological samples [26]. Applications of metabolomics include biomarker discovery [27], disease diagnosis [28], and understanding plant–microbe interactions [29].
A study by Thovhogi et al. [30] reported that Swiss chard contains five metabolites, such as Apiin, Apigein 7–6-malonyl, Crotonoside, Isorhamnetin-3,4′-diglucoside, and uridine. Furthermore, a previous study by Liu et al. [31] discovered that Swiss chard grown under controlled conditions accumulated a relatively high level of glycine betaine (GB), which is responsible for regulating various biochemical processes via systemic signaling pathways. To date, there is no scientific literature available on the metabolites released by Swiss chard grown in South Africa using SCGs. Therefore, the current study aimed to determine the effect of different concentrations of SCGs on the metabolomic profile of Swiss chard.
2. Materials and Methods
The pot experiment was conducted in a greenhouse at the University of South Africa’s Science Campus, situated in Florida, Roodepoort (Latitude: −26°9′29.274′′; Longitude: 27°55′17.663′′), South Africa, with air temperatures ranging from 7.4 to 44.9 °C, and 68% average relative humidity [32] during the planting period. Planting pots were laid out in a randomized complete block design (RCBD) with five treatments: T1 (0%), T2 (2.25%), T3 (4.5%), and T4 (6.5%), and T5 (8.75%) in weight of SCGs from Arabica coffee beans, as recommended by Abubbaker et al. [33]. This was replicated four times and arranged in 3 separate blocks with a total of 60 plants [34]. Plastic pots (18 cm diameter, 14.5 cm height, and 18 cm width) were filled with Agromix growth medium (see Table 1), which was used for growing seeds.

Table 1.
Descriptive characteristics of potting soil.
Transplanting was done when four leaves were present. Three seedlings were transplanted in each pot and later thinned to one plant per pot. At the beginning of the experiment, the plants were irrigated daily; however, they were later irrigated as and when the need arose, as daily irrigation retarded the plants’ growth, as described by Seidel et al. [35]. Furthermore, weeding was done manually when a need arose. The growth cycle started in late winter and terminated in early spring, which lasted for a period of 3 months (July–September 2023).
2.1. Leaf Collection and Preparation
The leaves were manually collected, with the first and second harvest done a month apart to allow regrowth of the leaves. Post-harvest, the leaves were dried at room temperature (22 °C), as prescribed by Nemadodzi and Managa [36], and taken to the Council for Scientific Industrial Research (CSIR) for metabolomic analysis.
2.2. NMR Metabolomics Analysis
For NMR analysis, deuterated methanol (CD3OD), KH2PO4, sodium deuterium oxide (NaOD), trimethylsilyl propionic acid sodium salt (TSP), and deuterium oxide (D2O) were supplied by Sigma-Aldrich (Darmstadt, Germany). Buffer was prepared by adding 1.232 g KH2PO4 to 100 mL of D2O with 10 mg TSP (0.1%) added as a reference standard. The pH of the solution was adjusted to pH = 6. The protocol reported by Kim et al. [37] was implemented for the extraction procedures, with a few adjustments. A dried leaf sample of 50 mg was transferred to 2 mL Eppendorf tubes and was extracted with 750 µL of deuterated methanol and 750 µL of KH2PO4 buffer in D2O (pH 6.0) containing 0.1% TSP. The Eppendorf tubes were vortexed for 1 min at room temperature and ultra-sonicated for 20 min without heating. The solutions were centrifuged for another 15 min at 10,000 rpm to separate the supernatant from the precipitate. The supernatant was transferred to standard 5 mm NMR tubes and subjected to 1H NMR analysis. The 1H NMR measurements were performed on a Varian 600 MHz spectrometer (Varian Inc., Palo Alto, CA, USA) with a frequency of 599.74 MHz. The acquisition time of each 1H NMR spectrum was 7 min, which consisted of 32 scans with a width of 20 ppm. Gradient shimming was used to improve homogeneity in the magnetic field, and each sample was replicated five times. All spectra were phase-corrected and binned from 0.04 ppm to 10.00 ppm using MestReNova [38] before being statistically analyzed with SIMCA 17.0.2 (Umetrics, Umea, Sweden), which is multivariate data analysis (MDVA) program. Data scaling was done using the Pareto method, and an unsupervised principal component analysis (PCA) model was adapted to illustrate the distinctive separation between the leaf samples collected from different levels of SCGs, as shown in Figure 1.
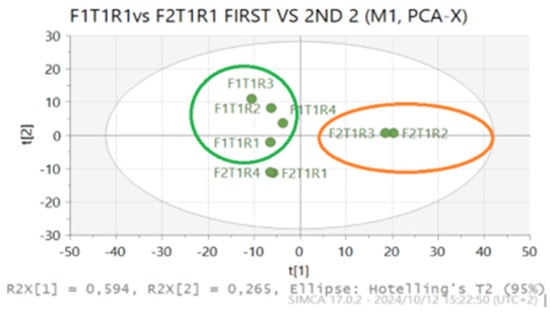
Figure 1.
PCA (unsupervised) model scatter plot showing clusters of untreated Swiss chard leaves (T1), with first harvest treatment herein referred to as F1T1R1, and second harvest herein referred to as F2T1R1.
In addition, the PCA model was put to a 95% probability test and showed a good fit (R2 = 0.968) and predictive ability (Q2 = 0.904) as shown in the Table 2 below.

Table 2.
The goodness of fit and predictability values of the PCA.
Furthermore, the raw NMR data were pre-processed with Mestrenova version 15.0.1 to detect the NMR peaks in parts per million (ppm). Annotation, detection, and identification of metabolites were carried out using Chenomx NMR suite 10.1 (Edmonton, AB, Canada), which has specific characteristics and was used to annotate non-targeted metabolites that were profiled [39]. Chenomx library manager was used to obtain the compounds database, whilst Chenomx compound builder version 11 was used to create 1D NMR chemical structures in relation to the cluster/number of peaks, shapes, locations, and/or NMR signal regions. External reference databases, such as Chenomx and Human Metabolome Data Base (HMDB), were used to compare and confirm NMR-specific characteristics [39].
3. Results
The Impact of Varying Levels of SCGs on the Metabolomic Profile of Swiss Chard at Initial and Second Harvest
The findings of the current study showed that there was no distinct separation on the samples analyzed from the first and second harvest, except for two samples that were separated in the second harvest, as shown in Figure 1.
Additionally, the results showed that there were common metabolites released between the treatments, such as thymine, carnitine, trimethylamine, betaine, trehalose, 2-deoxyadenosine, and sucrose, at the first harvest (see Figure 2). Interestingly, caprate was only detected at the first harvest where no SCGs were applied, as shown in Figure 2.
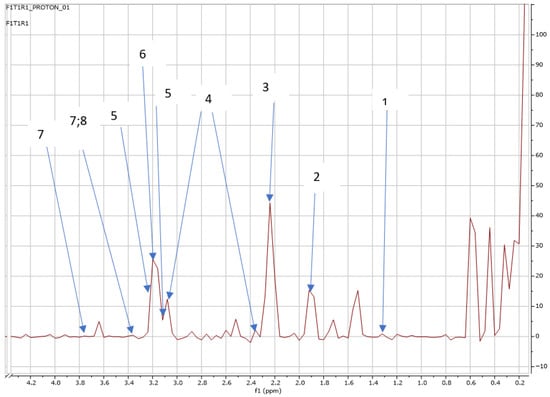
Figure 2.
NMR spectra showing peaks detected in Swiss chard when no SCGs were applied (T1: First harvest). 1. Caprate (1.3 ppm), 2. Thymine (ppm 1.9), 3. Alanine (2.2; 3.1 ppm), 4. Carnitine (3.1; 2.3 ppm), 5. Trimethylamine (3.2; 3.3 ppm), 6. Betaine (3.2 ppm), 7. Trehalose (3.7; 3.4 ppm), and 8. 2-Deoxyadenosine (3.4 ppm).
Interestingly, at the second harvest, which occurred a month after the first harvest, Swiss chard grown without application of SCGs (T1) also released other metabolites, such as sucrose, 1,3 Dimenthylurate, and 1,6 Anhydro-β-D-glucose, as shown in Figure 3. in addition to the few that were detected at the early growth stage. A similar number of metabolites released by Swiss chard after the second harvest were detected in T2, T3, T4, and T5.
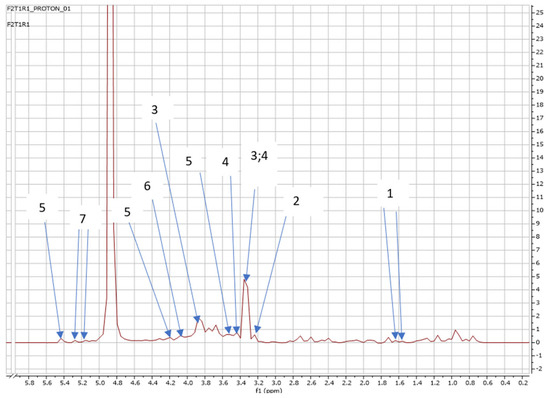
Figure 3.
NMR spectra showing peaks detected in Swiss chard when T1 (no application: second harvest) was used: 1. Alanine (1.5 ppm), 2. Carnitine (3.2 ppm), 3. Betaine (3.3; 3.9 ppm), 4. 1,3 Dimethylurate (3.3; 3.4 ppm), 5. Sucrose (3.5; 4.2; 5.4 ppm), 6. 1,6 Anhydro-β-D-glucose (4.1 ppm), and 7. Trehalose (5.2 ppm).
However, T2 showed a reduction in the number of metabolites released by T2 at the first harvest, from seven to four metabolites, as shown in Figure 4.
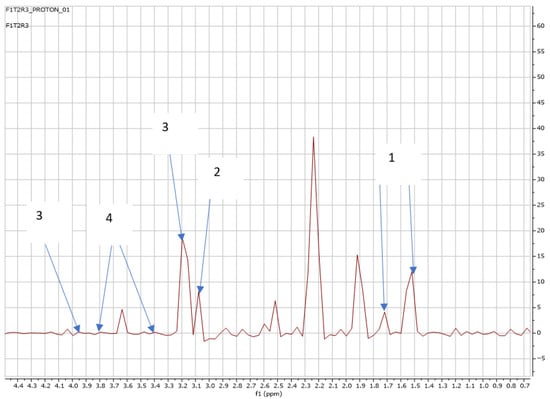
Figure 4.
NMR spectra showing peaks detected in Swiss chard when T2 (0.32 g: first harvest) was applied: 1. Alanine (1.5 ppm), 2. Carnitine (3.2 ppm), 3. Betaine (3.3; 3.8 ppm), and 4. Trehalose (3.4; 3.8 ppm).
However, during the second harvest, T2 showed an increase in the number of metabolites by releasing metabolites such as 1,3 Dimethylurate; 1,6 Anhydro-β-D-glucose; and sucrose as indicated in Figure 5.
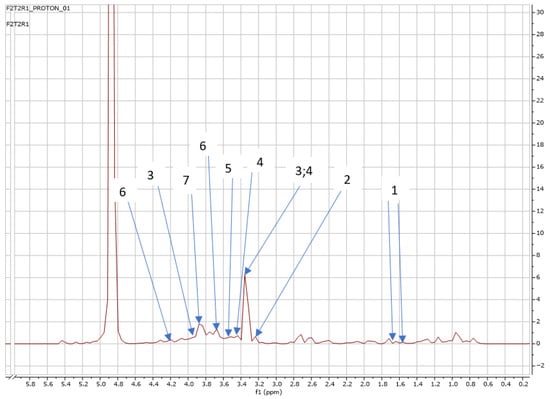
Figure 5.
NMR spectra showing peaks detected in Swiss chard when T2 (0.32 g: second harvest) was used: 1. Alanine (1.5 ppm), 2. Carnitine (3.2 ppm), 3. Betaine (3.3; 3.9 ppm), 4. 1,3 Dimethylurate (3.3; 3.4 ppm), 5. 1,6 Anhydro-β-D-glucose (3.5 ppm), 6. Sucrose (4.2; 3.6 ppm), and 7. Trehalose (3.8 ppm).
Below is Figure 6, which features the NMR spectra showing the effect of T3 on the metabolomic profile of Swiss chard at the first harvest, showing the release of five metabolites.
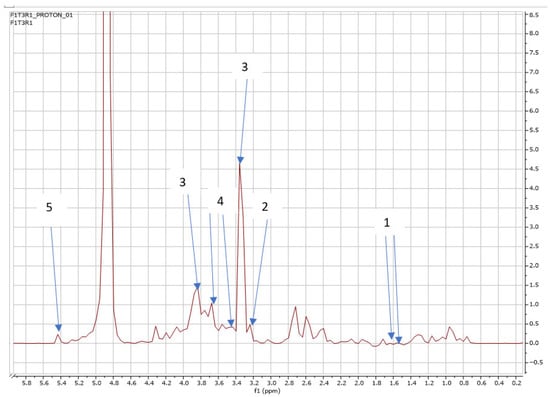
Figure 6.
NMR spectra showing peaks detected in Swiss chard when T3 (0.63 g: first harvest) was used: 1. Alanine (1.5 ppm) 2. Carnitine (3.2 ppm) 3. Betaine (3.3; 3.87 ppm), 4. Trehalose (3.4; 3.6 ppm), and 5. Sucrose (5.4 ppm).
However, at the second harvest, metabolites such as 1,3 Dimethylurate and 1,6 Anhydro-β-D-glucose were released together with alanine, carnitine, trehalose, betaine, and sucrose, which remained constant from the first harvest (see Figure 7).
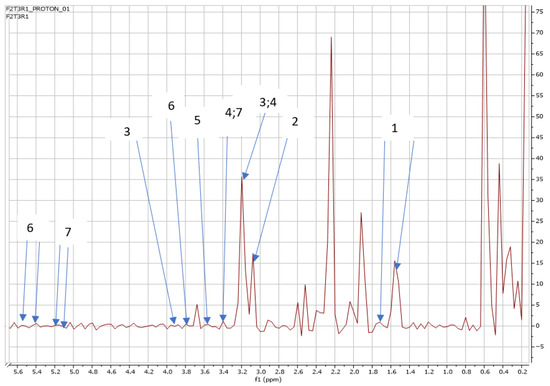
Figure 7.
NMR spectra showing peaks detected in Swiss chard when T3 (0.63 g: second harvest) was used: 1. Alanine (1.5 ppm), 2. Carnitine (3.2 ppm), 3. Betaine (3.3; 3.9 ppm), 4. 1,3 Dimethylurate (3.3; 3.4 ppm), 5. 1,6 Anhydro-β-D-glucose (3.5 ppm), 6. Sucrose (3.8; 5.4 ppm), and 7. Trehalose (3.4; 5.2 ppm).
The NMR spectra showing the effect of T4 on the metabolomic profile of Swiss chard at the first and second harvest are shown in Figure 8 and Figure 9 below, indicating that there were no differences in the metabolites released from T3 at first and second harvest.
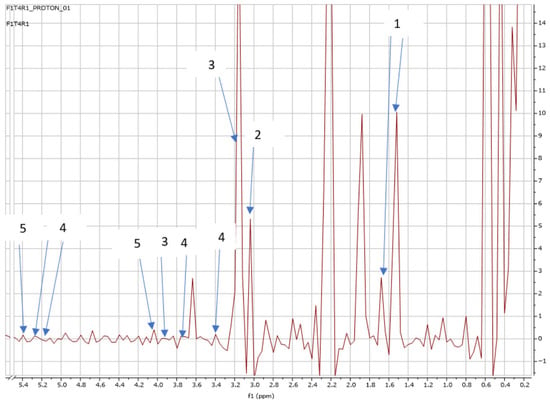
Figure 8.
NMR spectra showing peaks detected in Swiss chard when T4 (0.92 g: first harvest) was used: 1. Alanine (1.5; 1.6 ppm), 2. Carnitine (3.1 ppm), 3. Betaine (3.2; 3.87 ppm), 4. Trehalose (3.4; 3.8; 5.2 ppm), and 5. Sucrose (4.0; 5.4 ppm).
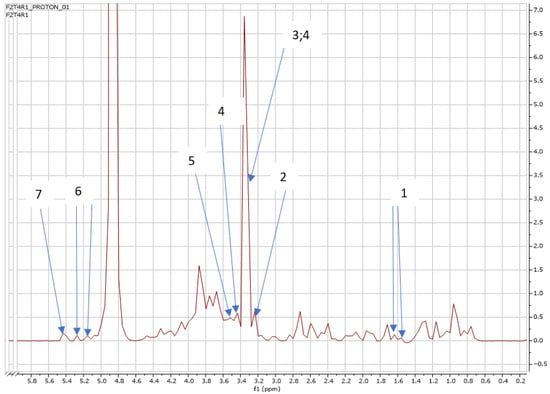
Figure 9.
NMR spectra showing peaks detected in Swiss chard when T4 (0.92 g: second harvest) was used: 1. Alanine (1.5 ppm), 2. Carnitine (3.2 ppm), 3. Betaine (3.3; 3.9 ppm), 4. 1,3 Dimethylurate (3.3; 3.4 ppm), 5. 1,6 Anhydro-β-D-glucose (3.5 ppm), 6. Trehalose (5.2 ppm), and 7. Sucrose (5.4 ppm).
Finally, Figure 10 and Figure 11 below indicate the NMR spectra showing the effect of T5 on the metabolomic profile of Swiss chard at the first and second harvest, showing the same metabolites released from T3 and T4.
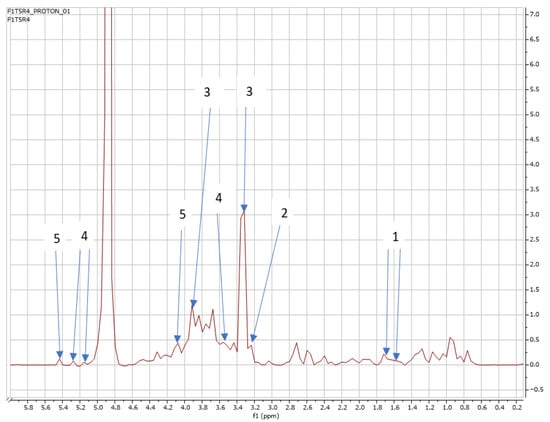
Figure 10.
NMR spectra showing peaks detected in Swiss chard when T5 (1.20 g: first harvest) was used: 1. Alanine (1.5 ppm), 2. Carnitine (3.2 ppm), 3. Betaine (3.3; 3.87 ppm), 4. Trehalose (3.5; 5.2 ppm), and 5. Sucrose (4.0; 5.4 ppm).
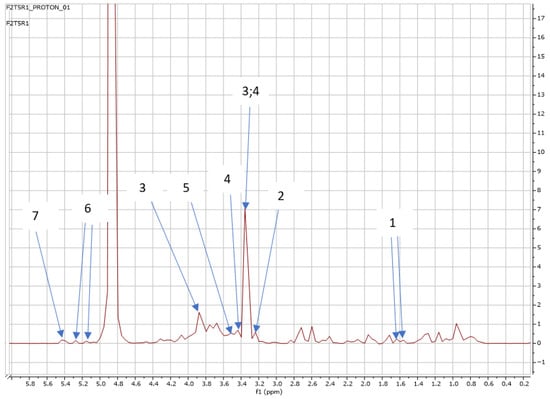
Figure 11.
NMR spectra showing peaks detected in Swiss chard when T5 (1.20 g: second harvest) was used: 1. Alanine (1.5 ppm), 2. Carnitine (3.2 ppm), 3. Betaine (3.3; 3.9 ppm), 4. 1,3 Dimethylurate (3.3; 3.4 ppm), 5. 1,6 Anhydro-β-D-glucose (3.5 ppm), 6. Trehalose (5.2 ppm), and 7. Sucrose (5.4 ppm).
In addition, the NMR peaks in the present study were compared to and conformed with the external database references, such as Chenomx and HMDB, as shown in Table 3 below.

Table 3.
Comparison of NMR chemical shifts (ppm) of metabolites detected in Swiss with two databases.
4. Discussion
The harvesting of green leafy vegetables (GLVs) in farms or rural communities is mainly done at various crop growth stages, at which new leaves continue to grow after each harvest [36]. Furthermore, the stage at which a crop is harvested influences the type and concentration of metabolites present in the crop [36]. Nemadodzi and Managa [36] reported that GLVs are mostly harvested at a stage of development when palatability and flavour are favourable for human consumption. In the present study, harvesting occurred based on the maturity stage; therefore, it is assumed that the metabolites detected could be influenced by the growth stage of the crop. The metabolomic analysis and chemical profile in the current study revealed that a total of 11 metabolites were released by Swiss chard across different SCGs levels.
The current study showed that caprate was detected at first harvest and was released at the early growth/ seedling stage of Swiss chard when no treatment was applied, and is assumed to be crucial for its development. Additionally, Zhao et al. [41] reported that a release of fatty acids such as caprate from the plant membrane is reported to be involved in plant tolerance to biotic and abiotic stresses. Furthermore, Valverde et al. [42], indicated that organic acids and carbohydrates such as caprate played a huge role in the root growth of Phaseolus vulgaries (common bean).
Consuming Swiss chard rich in caprate has been linked to several health benefits [43]. For instance, caprate’s properties can support gut health [43] and it exhibits antioxidant activity, protecting cells from oxidative damage and potentially reducing cancer risk [44]. Furthermore, caprate’s ability to modulate lipid metabolism may contribute to improved weight management and reduced risk of metabolic disorders [45].
The current study showed that thymine was the second metabolite detected at the first harvest when no treatment was applied. A very recent study revealed that thymine was crucial in Swiss chard’s early growth stage [46] and is essential for deoxyribonucleic acid (DNA) synthesis and repair, supporting cell division and growth in young seedlings [46]. Witte and Herde [47] revealed that, at the early stage of any crop development, nucleotides such as thymine serve as building blocks for both DNA and RNA. A study on plant nucleotide metabolism found that thymine is involved in cell differentiation and ensures proper plant development [47]. The DNA in crops is responsible for plant stress responses and helps crops adapt to environmental fluctuations [48]. Thymine is a pyrimidine that plays an important role in cellular regulation and metabolism [49]. It is a substrate for DNA/RNA biosynthesis, a regulator of the biosynthesis of some amino acids, and a cofactor in the biosynthesis of phospholipids, glycolipids, sugars, and polysaccharides [50]. Furthermore, Cansev et al. [51] reported that pyrimidine nucleosides, such as thymine, could enhance stress resistance in crops.
The application of different concentrations of SCGs to determine their effect on the metabolomic profile of Swiss chard showed the presence of alanine in all treatment levels at both harvests. Although the role of alanine is still not clearly described [36], its presence in all the 1H NMR spectra could be due to its functions as a stress regulator and allelochemical in crops [36]. Furthermore, it is detected in Swiss chard because, according to Nemadodzi and Managa [36], alanine is a defence mechanism metabolite (DMM), hence its detection at all levels. Metabolites such as alanine prepared plants to defend themselves from herbivores, insects, and pathogens when grown in a greenhouse [36]. A study by De Sousa and Sodek [52], revealed that the pronounced increase in alanine was observed on the root system of the soybean plant following hypoxia (waterlogging), which is a characteristic response to oxygen deficiency as seen in many plant tissues.
Another study by Guo et al. [53] reported that alanine is released to regulate salt stress. The findings of the current study are in agreement with those of Khan et al. [54], which revealed that alanine accumulates more in the leaves of crops grown in greenhouses. A human study by Holeček [55] reported that alanine is the primary amino acid responsible for gluconeogenesis in the liver and kidneys. Furthermore, alanine is an amino acid that manufactures proteins [56], it metabolizes vitamin B6 and tryptophan [57], and is a source of energy for the central nervous system and muscles [56]. Therefore, the consumption of Swiss chard could help in synthesizing proteins, as a source of energy, and assist in the build-up of muscles.
The 1H-NMR results obtained in the current study showed that the application of various levels of SCGs resulted in carnitine in Swiss chard leaves. Carnitine was detected at both (first and second) harvests across all the treatments. According to Dos Santos et al. [58], carnitine is responsible for the promotion of root growth and elongation, and influences leaf expansion [59] and chlorophyll content [60]. In the current study, the presence of carnitine in all the growth stages of Swiss chard was essential to ensure plant development and establishment. On the contrary, the presence of carnitine is assumed to serve as a defence mechanism for metabolites [61].
Carnitine, an essential nutrient that plays a vital role in energy production and fatty acid metabolism, offers numerous health benefits in humans [62]. It alleviates neuropathic pain, improves nerve conduction in uremic patients [63], enhances immunological function in diabetic individuals [64], and is potentially lifesaving for those with primary carnitine deficiency [65]. Additionally, carnitine has therapeutic potential in treating various neurological disorders, including Alzheimer’s disease, Parkinson’s disease, hepatic encephalopathy, autism spectrum disorder, and painful neuropathies [66]. Moreover, topical carnitine application provides osmoprotection and modulates immune as well as inflammatory responses in dry eye syndrome [67]. However, a study by McCann et al. [68] revealed that high doses of carnitine may cause hypotension and arrhythmias, seizures, as well as kidney and liver damage.
Trimethylamine was detected only at the first harvest under T1. According to Wagh et al. [69], it is responsible for plant growth and development in young crops, and serves as a plant growth regulator and hormone modulator. In the current study, trimethylamine (TMA) was detected at the first harvest because it directly influences seed germination and root development, as reported by Hughes [70]. Additionally, Seth et al. [71] reported that TMA prevents the denaturation of plant proteins from adverse environmental conditions such as varying temperatures and soil salinity. In plant–microbe interactions, TMA facilitates communication between plants and beneficial microbes and promotes symbiotic relationships [72]. Furthermore, it affects plant volatile organic compound (VOC) emissions, influencing plant–plant and plant–insect interactions [73]. From the above-mentioned roles, TMA is assumed to function as a growth-promoting and defense mechanism metabolite in the growth and development of Swiss chard.
Trimethylamine has negative implications on human health and has been linked to various health conditions, such as trimethylaminuria, which is a rare genetic disorder characterized by excessive TMA production, resulting in a strong “fishy” body odor [74]. Additionally, excess levels of TMA have also been associated with cardiovascular disease, kidney disease, and certain cancers [75]. Furthermore, TMA has been identified as a potential biomarker [76,77] for neurological disorders, such as Alzheimer’s and Parkinson’s diseases [78]. Exposure to TMA has been shown to cause respiratory and ocular irritation, headaches, and nausea [79]. Therefore, overconsumption of Swiss chard grown without fertilizer application could cause health risks to humans. Future studies should include determining the minimum threshold of TMA that can be recommended for consumption without causing ailments.
Betaine was detected in Swiss chard at both harvests in all the treatments. Betaine (growth-promoting metabolite) was found in both harvests because it is crucial for crop development from the seedling stage to maturity [25,36]. On the other hand, other findings showed that betaine accumulation enhances crop tolerance to abiotic stresses, such as drought [80] and extreme temperatures [81]. According to Fedotova and Kruchinin [82], betaine protects crops by maintaining cellular hydration, stabilizing membranes, and scavenging reactive oxygen species (ROS).
Betaine has been recognized for its medicinal properties, particularly in mitigating homocysteine-related cardiovascular diseases [83] and enhancing exercise performance [84,85]. Additionally, betaine has shown neuroprotective effects, potentially benefiting individuals with neurodegenerative disorders [86]. Betaine is reported to have a positive impact on obesity, alcohol-induced and metabolic-associated liver disease, cardiovascular diseases, and certain cancers [87].
Trehalose was detected in Swiss chard throughout the two harvests, and it is reported as a growth-promoting metabolite that is responsible for the development and growth of crops [36]; hence, its constant presence in Swiss chard is observed in all stages of its growth and development. In a study by Nemadodzi et al. [25], similar results were reported, indicating that trehalose sustains and promotes the growth of B. africana trees. According to Tian et al. [88], the lack of functional trehalose leads to no transition from vegetative growth, which will eventually retard the leaves. On the contrary, Kosar et al. [89] revealed that trehalose inhibits growth and is toxic to seedlings.
Trehalose is a naturally occurring disaccharide that has demonstrated numerous medicinal benefits. Previous studies have shown that trehalose exhibits neuroprotective effects, mitigating neurodegenerative diseases, such as Alzheimer’s [90] and Parkinson’s [91]. Additionally, trehalose possesses anti-inflammatory properties, reduces oxidative stress and inflammation in various diseases [92]. In drought-sensitive and tolerant cultivars, trehalose accumulation improved photosynthesis, ROS-antioxidant balance, and upregulated stress responses, protecting chloroplasts and proteins [93], while its antioxidant activity scavenges reactive oxygen species, and promotes cellular health [94].
Furthermore, 2-deoxyadenosine was detected when no treatment was applied to Swiss chard at the first harvest and has been shown to impact Swiss chard growth and development. Findings by Becerra and Carmona et al. [95] demonstrated that 2-deoxyadenosine causes spindle dysfunction at the end of mitosis, inhibits the progress of cell division, reduces ATP enzyme activity, suppresses the synthesis of proteins and carbohydrates, inhibits energy metabolism, and blocks cytokinesis, thus limiting root growth.
In humans, it has been reported that 2-deoxyadenosine may contribute to neurodegenerative disorders, such as Parkinson’s disease, due to its ability to inhibit adenosine deaminase (ADA) and disrupt purine metabolism [96]. Furthermore, 2-deoxyadenosine has been linked to cardiotoxicity, as shown by its ability to induce apoptosis in cardiac cells [49].
The current results showed the presence of sucrose in Swiss chard at the first harvest when T3 and T4 were applied, whilst, in the second harvest, sucrose was detected in all treatments, including T1. Our results from the current study are in agreement with those reported by Wang et al. [97], which suggests that the application of organic manure induces the presence of sucrose. According to Mzoughi et al. [20], high levels of sucrose in Swiss chard from the first to second harvest may be attributed to various factors, including environmental conditions, genetic predisposition, and agricultural practices. According to Steindal et al. [98], Swiss chard plants grown under cooler temperatures (15–20 °C) and longer photoperiods tend to accumulate higher sucrose levels. Additionally, soil nutrient availability, particularly potassium and nitrogen, can significantly impact sucrose synthesis and accumulation in Swiss chard [99], which may have been the case in the current study.
Consuming Swiss chard with high concentrations of sucrose might have different effects on human health. For instance, Swiss chard’s nutrient-dense profile, rich in vitamins, minerals, and antioxidants, can offer numerous health benefits, such as supporting eye health and immune function, due to high vitamin K and beta-carotene content [84], and reducing inflammation and oxidative stress due to its antioxidant properties [97]. In addition, the high sucrose content can provide a rapid source of energy; however, excessive consumption may lead to an increased glycemic index, which is potentially problematic for individuals with diabetes or high blood pressure conditions [24]. Furthermore, high sucrose intake has been linked to enhanced calorie consumption, which contributes to weight gain or obesity [100].
The 1,3-Dimethylurate has been reported to modulate ethylene biosynthesis, delay leaf yellowing and senescence, and result in increased leaf expansion and chlorophyll content [77]. Therefore, the presence of 1,3-Dimethylurate in Swiss chard is assumed to enhance quality and delay senescence, thus extending the shelf-life of Swiss chard post-harvest. Scavenging reactive oxygen species reduces oxidative damage and protects cellular components, which leads to improved membrane integrity and photosynthetic rates [101].
Furthermore, 1,3-Dimethylurate is a naturally occurring compound found in coffee [102] and a few medications, such as 1,3-Dimethylurate acid, and is reported to have numerous human health benefits [103]. For instance, 1,3-Dimethylurate may improve respiratory function, enhance cognitive performance, and support weight management [103].
The results of the current study showed that 1,6-Anhydro-β-D-glucose was detected at the second harvest after the application of T2-T5 treatments, as shown in Figure 3, Figure 5, Figure 7, Figure 9 and Figure 11. The increased levels of 1,6-Anhydro-β-D-glucose in the second harvest of Swiss chard can be attributed to several factors. At the second harvest, Swiss chard’s cellular walls change, resulting in increased cellulose degradation [41], which may have increased the 1,6-Anhydro-β-D-glucose level. Additionally, 1,6-Anhydro-β-D-glucose, which is a bioactive compound identified in Swiss chard, exhibits promising medicinal properties and has been reported to possess both antioxidant and anti-inflammatory activities, which may contribute to its protective effects against chronic diseases [104]. Notably, this compound has demonstrated the ability to scavenge free radicals and reduce oxidative stress in plant systems [105], suggesting potential translational benefits for human health. Furthermore, its antimicrobial properties indicate a capacity to help prevent infections and promote wound healing, supporting its therapeutic relevance in both preventive and clinical applications [104]. The consumption of Swiss chard rich in 1,6-Anhydro-β-D-glucose may offer health benefits, including enhanced immune function and cardiovascular health.
5. Conclusions
The presence of the detected metabolites in the Swiss chard positively impacts its nutritional composition and enhances its value as a nutrient-rich green leafy vegetable. Therefore, spent coffee grounds are an ideal organic material that can be recommended to grow Swiss chard for healthy metabolites and nutritional content, which are vital for human health. Therefore, T3, T4, and T5 are recommended due to the consistency in the number of metabolites released at the first and second harvest, particularly to the smallholder and commercial farmers growing Swiss chard for marketing purposes. Future study that include an open-field grown Swiss chard is recommended to compare the metabolomic profile and/or the impact of environmental factors on the release of metabolites.
Author Contributions
Conceptualization, T.M. and L.E.N.; methodology, L.E.N.; software, L.E.N.; validation, T.M. and L.E.N.; formal analysis, L.E.N. and T.M.; investigation, T.M.; resources, L.E.N. and T.M.; data curation, T.M.; writing—original draft preparation, T.M.; writing—review and editing, T.M. and L.E.N.; visualization, L.E.N.; supervision, L.E.N.; project administration, L.E.N.; funding acquisition, T.M. and L.E.N. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the University of South Africa M and D bursary and scholarship, REF: 18026443.
Institutional Review Board Statement
This study was conducted in accordance with and approved by the Ethics Committee of the University of South Africa 2023/CAES_HREC/059) before data collection commenced.
Data Availability Statement
The data will be made available when under review from the corresponding author.
Acknowledgments
The authors would love to thank the University of South Africa for permitting us to use the greenhouse for conducting the experiment.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Falcon, W.P.; Naylor, R.L.; Shankar, N.D. Rethinking global food demand for 2050. Popul. Dev. Rev. 2022, 48, 921–957. [Google Scholar] [CrossRef]
- Rivelli, A.R.; Libutti, A. Effect of biochar and inorganic or organic fertilizer co-application on soil properties, plant growth and nutrient content in Swiss chard. Agronomy 2022, 12, 2089. [Google Scholar] [CrossRef]
- Franca, A.S.; Oliveira, L.S. Potential uses of spent coffee grounds in the food industry. Foods 2022, 11, 2064. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Delgado, G.; Fernández-Arteaga, A.; Fornasier, F.; Mondini, C. Spent coffee grounds by-products and their influence on soil C–N dynamics. J. Environ. Manag. 2022, 302, 114075. [Google Scholar] [CrossRef]
- Vu, D.C.; Vu, Q.T.; Huynh, L.; Lin, C.H.; Alvarez, S.; Vo, X.T.; Nguyen, T.H. Evaluation of fatty acids, phenolics and bioactivities of spent coffee grounds prepared from Vietnamese coffee. Int. J. Food Prop. 2021, 24, 1548–1558. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Pina, G.; Vergara-Castaneda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Tech. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Hardgrove, S.J.; Livesley, S.J. Applying spent coffee grounds directly to urban agriculture soils greatly reduces plant growth. Urban For. Urban Green. 2016, 18, 1–8. [Google Scholar] [CrossRef]
- Comino, F.; Cervera-Mata, A.; Aranda, V.; Martín-García, J.M.; Delgado, G. Short-term impact of spent coffee grounds over soil organic matter composition and stability in two contrasted Mediterranean agricultural soils. J. Soils Sediments 2020, 20, 1182–1198. [Google Scholar] [CrossRef]
- Yamane, K.; Kono, M.; Fukunaga, T.; Iwai, K.; Sekine, R.; Watanabe, Y.; Iijima, M. Field evaluation of coffee grounds application for crop growth enhancement, weed control, and soil improvement. Plant Prod. Sci. 2014, 17, 93–102. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Pastoriza, S.; Rufán-Henares, J.Á.; Párraga, J.; Martín-García, J.M.; Delgado, G. Impact of Spent Coffee Grounds as organic amendment on soil fertility and lettuce growth in two Mediterranean agricultural soils. Arch. Agron. Soil Sci. 2018, 64, 790–804. [Google Scholar] [CrossRef]
- Ustundag, U.V.; Tunali, S.; Alev, B.; Ipekci, H.; Emekli-Alturfan, E.; Akbay, T.T.; Yanardag, R.; Yarat, A. Effects of Chard (Beta vulgaris L. var. Cicla) on Cardiac Damage in Valproic Acid–Induced Toxicity. J. Food Biochem. 2016, 40, 132–139. [Google Scholar] [CrossRef]
- Abu-Ellail, F.F.; Salem, K.F.; Saleh, M.M.; Alnaddaf, L.M.; Al-Khayri, J.M. Molecular Breeding Strategies of Beetroot (Beta vulgaris ssp. vulgaris var. conditiva Alefeld). In Advances in Plant Breeding Strategies, Vegetable Crops, Bulbs, Roots, and Tubers; Springer: Berlin/Heidelberg, Germany, 2021; pp. 157–212. [Google Scholar]
- Libutti, A.; Rivelli, A.R. Quanti-qualitative response of Swiss chard (Beta vulgaris L. var. cycla) to soil amendment with biochar-compost mixtures. Agronomy 2021, 11, 307. [Google Scholar] [CrossRef]
- Simko, I.; Hayes, R.J.; Mou, B.; McCreight, J.D. Lettuce and spinach. Yield Gains Major US Field Crops 2014, 33, 53–85. [Google Scholar]
- Sindesi, O.A. Water and Nutrient Retention Under Swiss Chard (Beta vulgaris var. cicla) and Cabbage (Brassica oleracea var. capitata L.) Cultivated in Soil Amended with Zeolite. Master’s Thesis, Cape Peninsula University of Technology, Cape Town, South Africa, 2020. [Google Scholar]
- Ninfali, P.; Angelino, D. Nutritional and functional potential of Beta vulgaris cicla and rubra. Fitoterapia 2013, 89, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Ivanović, L.; Milašević, I.; Topalović, A.; Ðurović, D.; Mugoša, B.; Knežević, M.; Vrvić, M. Nutritional and phytochemical content of Swiss chard from Montenegro, under different fertilization and irrigation treatments. Br. Food J. 2019, 121, 411–425. [Google Scholar] [CrossRef]
- Maboko, M.M.; Du Plooy, C.P.; Sithole, M.A.; Mbave, A. Swiss chard (Beta vulgaris L.) water use efficiency and yield under organic and inorganic mulch application. J. Agric. Sci. Technol. 2017, 19, 1345–1354. [Google Scholar]
- Nair, L.P.S. Consumption of Fruits and Vegetable Among Adolescent Girls. Bachelor’s Thesis, St Teresa’s College (Autonomous), Ernakulam, India, 2023. [Google Scholar]
- Mzoughi, Z.; Chahdoura, H.; Chakroun, Y.; Cámara, M.; Fernández-Ruiz, V.; Morales, P.; Mosbah, H.; Flamini, G.; Snoussi, M.; Majdoub, H. Wild edible Swiss chard leaves (Beta vulgaris L. var. cicla): Nutritional, phytochemical composition and biological activities. Food Res Int. 2019, 119, 612–621. [Google Scholar]
- Ndololwana, N.G. The Use of Winery Waste Compost to Establish Cabbage (Brassica oleracea var. capitata L.) and Swiss Chard (Beta vulgaris subsp. cycla) on Sandy Soil at Bien Donné Experimental Farm Near Paarl in the Western Cape Region. Master’s Thesis, Cape Peninsula University of Technology, Cape Town, South Africa, 2015. [Google Scholar]
- Matlala, M.N. Estimating the Volumetric Water Footprint of SWISS Chard (Beta vulgaris) and Carrot (Daucus carota) Grown in the Highveld Region, South Africa. Master’s Thesis, University of Pretoria, Pretoria, South Africa, 2017. [Google Scholar]
- Oelofse, A.; Van Averbeke, W.; Van Jaarsveld, P. The Nutritional Value, Water Use and Nutrient Contribution of Traditional African Leafy Vegetables. Ann. Nutr. Metab. 2013, 63, 495. [Google Scholar]
- Johnson, R.K.; Appel, L.J.; Brands, M.; Howard, B.V.; Lefevre, M.; Lustig, R.H. American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism and the Council on Epidemiology and Prevention. Dietary sugars intake and cardiovascular health: A scientific statement from the American Heart Association. Circulation 2009, 120, 1011–1020. [Google Scholar]
- Nemadodzi, L.E.; Vervoort, J.; Prinsloo, G. NMR-Based Metabolomic Analysis and Microbial Composition of Soil Supporting Burkea africana Growth. Metabolites 2020, 10, 402. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Saito, K. Higher dimensional metabolomics using stable isotope labeling for identifying the missing specialized metabolism in plants. Curr. Opin. Plant Biol. 2020, 55, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, W.J.; Koal, T.; Wang, Y.; Kohl, M.; Enot, D.P.; Deigner, H.P. Targeted metabolomics for biomarker discovery. Angew. Chem. Int. Ed. Engl. 2010, 49, 5426–5445. [Google Scholar] [CrossRef] [PubMed]
- Bujak, R.; Struck-Lewicka, W.; Markuszewski, M.J.; Kaliszan, R. Metabolomics for laboratory diagnostics. J. Pharm. Biomed. Anal. 2015, 113, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Schillaci, M.; Roessner, U. Metabolomics as an emerging tool to study plant–microbe interactions. Emerg. Top. Life Sci. 2022, 6, 175–183. [Google Scholar] [CrossRef]
- Thovhogi, F.; Ntushelo, N.; Gwata, E.T. A Comparative Study of the Presence of Minerals, Flavonoids and Total Phenolic Compounds in the Leaves of Common Traditional Vegetables. Appl. Sci. 2023, 13, 8503. [Google Scholar] [CrossRef]
- Liu, L.; Ueda, A.; Saneoka, H. Physiological responses of white Swiss chard (Beta vulgaris L. subsp. cicla) to saline and alkaline stresses. Aust. J. Crop Sci. 2013, 7, 1046. [Google Scholar]
- Mthimunye, L.M.; Managa, G.M.; Nemadodzi, L.E. The influence of Lablab Purpureus growth on nitrogen availability and mineral composition concentration in nutrient-poor savanna soils. Agronomy 2023, 13, 622. [Google Scholar] [CrossRef]
- Abubbaker, H.M.; Alshareef, I.M.; Binyahmed, F. The Effect of Spent Coffee Residues on the Growth and Productivity of Watercress and Swiss Chard Plants. Sebha Univ. J. Pure Appl. Sci. 2022, 21, 42022. [Google Scholar]
- Nemadodzi, L.E. Growth and Development of Baby Spinach (Spinacia oleracea L.) with Reference to Mineral Nutrition. Master’s Thesis, University of South Africa, Pretoria, South Africa, 2015. [Google Scholar]
- Seidel, S.J.; Werisch, S.; Schütze, N.; Laber, H. Impact of irrigation on plant growth and development of white cabbage. Agric. Water Manag. 2017, 187, 99–111. [Google Scholar] [CrossRef]
- Nemadodzi, L.E.; Managa, G.M. 1H NMR-Based Metabolomics Profile of Green and Red Amaranthus Grown in Open Field versus Greenhouse Cultivation System. Metabolites 2023, 14, 21. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based metabolomic analysis of plants. Nature Protoc. 2010, 5, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Maree, J.; Viljoen, A. Phytochemical distinction between Pelargonium sidoides and Pelargonium reniforme—A quality control perspective. S. Afr. J. Bot. 2012, 82, 83–91. [Google Scholar] [CrossRef]
- Mungofa, N.; Sibanyoni, J.J.; Mashau, M.E.; Beswa, D. Prospective role of indigenous leafy vegetables as functional food ingredients. Molecules 2022, 27, 7995. [Google Scholar] [CrossRef]
- Wishart, D.S.; Oler, E.; Peters, H.; Guo, A.; Girod, S.; Han, S.; Saha, S.; Lui, V.W.; LeVatte, M.; Gautam, V.; et al. MiMeDB: The human microbial metabolome database. Nucleic Acids Res. 2023, 51, 611–620. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Hu, J.; Zhou, H.; Adeleye, A.S.; Keller, A.A. 1H NMR and GC-MS based metabolomics reveal defense and detoxification mechanism of cucumber plant under nano-Cu stress. Environ. Sci. Technol. 2016, 50, 2000–2010. [Google Scholar] [CrossRef]
- Valverde, A.; Velazquez, E.; Gutierrez, C.; Cervantes, E.; Ventosa, A.; Igual, J.M. Herbaspirillum lusitanum sp. nov., a novel nitrogen-fixing bacterium associated with root nodules of Phaseolus vulgaris. Int. J. Syst. Evol. Microbiol. 2003, 53, 1979–1983. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, X.; Sun, S.; Yu, C.; Huang, X.; Ness, R.; Dugan, L.L.; Shu, L.; Seidner, D.L.; Murff, H.J.; et al. Ca: Mg ratio, medium-chain fatty acids, and the gut microbiome. Clin. Nutr. 2022, 41, 2490–2499. [Google Scholar] [CrossRef]
- Narayana, A.; Baskaran, S.A.; Amalaradjou, M.A.; Venkitanarayanan, K. Anticarcinogenic properties of medium chain fatty acids on human colorectal, skin and breast cancer cells in vitro. Int. J. Mol. Sci. 2015, 16, 5014–5027. [Google Scholar] [CrossRef]
- Nagao, K.; Yanagita, T. Medium-chain fatty acids: Functional lipids for the prevention and treatment of the metabolic syndrome. Pharmacol. Res. 2010, 61, 208–212. [Google Scholar] [CrossRef]
- Niehaus, M.; Straube, H.; Specht, A.; Baccolini, C.; Witte, C.P.; Herde, M. The nucleotide metabolome of germinating Arabidopsis thaliana seeds reveals a central role for thymidine phosphorylation in chloroplast development. Plant Cell 2022, 34, 3790–3813. [Google Scholar] [CrossRef]
- Witte, C.P.; Herde, M. Nucleotide metabolism in plants. Plant Physiol. 2020, 182, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yang, Z.; Liu, L.; Duan, L. DNA Methylation in Plant Responses and Adaption to Abiotic Stresses. Int. J. Mol. Sci. 2022, 23, 6910. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.; Ceppi, P. A non-proliferative role of pyrimidine metabolism in cancer. Mol. Metab. 2020, 35, 100962. [Google Scholar] [CrossRef]
- Santoso, D.; Thornburg, R. Uridine 5′-monophosphate synthase is transcriptionally regulated by pyrimidine levels in Nicotiana plumbaginifolia. Plant Physiol. 1998, 116, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Cansev, A.; Gülen, H.; Zengin, M.K.; Ergin, S.; Cansev, M. Use of Pyrimidines in Stimulation of Plant Growth and Development and Enhancement of Stress Tolerance; Uludağ Ünïversïtesï Teknolojï Transfer Ofïsï Tïcaret Ve Sanayï Anonïm Şïrketï: Nïlüfer/Bursa, Türkiye, 2014. [Google Scholar]
- De Sousa, C.A.F.; Sodek, L. Alanine metabolism and alanine aminotransferase activity in soybean (Glycine max) during hypoxia of the root system and subsequent return to normoxia. Environ. Exp. Bot. 2003, 50, 1–8. [Google Scholar] [CrossRef]
- Guo, S.H.; Hu, N.; Li, Q.S.; Yang, P.; Wang, L.L.; Xu, Z.M.; Chen, H.J.; He, B.Y.; Zeng, E.Y. Response of edible amaranth cultivar to salt stress led to Cd mobilization in rhizosphere soil: A metabolomic analysis. Environ. Pollut. 2018, 241, 422–431. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Rahman, M.A.; Rathinasabapathi, B.; Babar, M.A. UPLC-HRMS-based untargeted metabolic profiling reveals changes in chickpea (Cicer arietinum) metabolome following long-term drought stress. Plant Cell Environ. 2019, 42, 115–132. [Google Scholar] [CrossRef]
- Holeček, M. Origin and roles of Alanine and glutamine in Gluconeogenesis in the liver, kidneys, and small intestine under physiological and pathological conditions. Int. J. Mol. Sci. 2024, 25, 7037. [Google Scholar] [CrossRef]
- Wang, L.; Mao, Y.; Wang, Z.; Ma, H.; Chen, T. Advances in biotechnological production of β-alanine. World J. Microbiol. Biotechnol. 2021, 37, 79. [Google Scholar] [CrossRef]
- Smolen, A. Vitamin B-6 metabolism and the maternal-fetal relationship. In Vitamin B-6 Metabolism in Pregnancy, Lactation, and Infancy; CRC Press: Boca Raton, FL, USA, 2021; pp. 93–108. [Google Scholar]
- Dos Santos, S.K.; de Azevedo Soares, V.; Dantas, E.F.O.; Dos Santos, L.W.O.; da Silva Gomes, D.; Henschel, J.M.; Batista, D.S. Exogenous carnitine application enhances the growth of culantro (Eryngium foetidum) plants. Vegetables 2023, 36, 393–399. [Google Scholar] [CrossRef]
- Wang, K.; Li, F.; Gao, M.; Huang, Y.; Song, Z. Mechanisms of trehalose-mediated mitigation of Cd toxicity in rice seedlings. J. Clean. Prod. 2020, 267, 121982. [Google Scholar] [CrossRef]
- Turk, H.; Erdal, S.; Dumlupinar, R. Carnitine-induced physio-biochemical and molecular alterations in maize seedlings in response to cold stress. Arch. Agron. Soil Sci. 2020, 66, 925–941. [Google Scholar] [CrossRef]
- Latham, L.E.; Wang, C.; Patterson, T.A.; Slikker, W., Jr.; Liu, F. Neuroprotective effects of carnitine and its potential application to ameliorate neurotoxicity. Chem. Res. Toxicol. 2021, 34, 1208–1222. [Google Scholar] [CrossRef] [PubMed]
- Alhasaniah, A.H. L-carnitine: Nutrition, pathology, and health benefits. Saudi J. Biol. Sci. 2023, 30, 103555. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.C.B.; Arnold, R.; Dhanapalaratnam, R.; Markoulli, M.; Krishnan, A.V. Current and emerging pharmacotherapeutic interventions for the treatment of peripheral nerve disorders. Pharmaceuticals 2022, 15, 607. [Google Scholar] [CrossRef]
- Foster, D.W. The role of the carnitine system in human metabolism. Ann. N. Y. Acad. Sci. 2004, 1033, 1–16. [Google Scholar] [CrossRef]
- Petric, D. Potential clinical relevance of L-Carnitine supplementation in oncology. Food Ther. Health Care 2022, 4, 7. [Google Scholar] [CrossRef]
- Wang, W.; Pan, D.; Liu, Q.; Chen, X.; Wang, S. L-Carnitine in the Treatment of Psychiatric and Neurological Manifestations: A Systematic Review. Nutrients 2024, 16, 1232. [Google Scholar] [CrossRef]
- Flanagan, J.L.; Simmons, P.A.; Vehige, J.; Willcox, M.D.; Garret, Q. Role of carnitine in disease. Nutr. Metab. 2010, 7, 30. [Google Scholar] [CrossRef]
- McCann, M.R.; George De la Rosa, M.V.; Rosania, G.R.; Stringer, K.A. L-carnitine and acylcarnitines: Mitochondrial biomarkers for precision medicine. Metabolites 2021, 11, 51. [Google Scholar] [CrossRef]
- Wagh, M.S.; Osborne, W.J.; Sivarajan, S. Volatile organic compounds for enhancement of plant growth through plant growth promoting rhizobacteria. In Volatiles and Metabolites of Microbes; Academic Press: Cambridge, MA, USA, 2021; pp. 325–347. [Google Scholar]
- Hughes, M.D., Jr. Investigating the Influence of Fresh and Aged Garlic Extracts on the Biosynthesis of Trimethylamine N-Oxide. Ph.D. Thesis, Virgina Tech, Blacksburg, VA, USA, 2021. [Google Scholar]
- Seth, M.; Mondal, P.; Ghosh, D.; Mukhopadhyay, S.K. The foul play of two dietary metabolites trimethylamine (TMA) and trimethylamine N-oxide (TMAO) on human health and the role of microbes in mitigating their effects. Nutrire 2023, 48, 52. [Google Scholar] [CrossRef]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced systemic resistance for improving plant immunity by beneficial microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef]
- Jorge, N.C.; Souza-Silva, É.A.; Alvarenga, D.R.; Saboia, G.; Soares, G.L.G.; Zini, C.A.; Cavalleri, A.; Isaias, R.M.S. Structural and chemical profiles of Myrcia splendens (Myrtaceae) leaves under the influence of the galling Nexothrips sp. (Thysanoptera). Front. Plant Sci. 2018, 9, 1521. [Google Scholar] [CrossRef]
- Fraser-Andrews, E.A.; Manning, N.J.; Ashton, G.H.; Eldridge, P.; McGrath, J.; Menage Hdu, P. Fish odour syndrome with features of both primary and secondary trimethylaminuria. Clin. Exp. Dermatol. 2003, 28, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.S.; Fernandez, M.L. Trimethylamine N-oxide (TMAO), diet and cardiovascular disease. Curr. Atheroscler. Rep. 2021, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of trimethylamine N-oxide (TMAO) in disease: Potential biomarker or new therapeutic target. Nutrients 2018, 10, 398. [Google Scholar] [CrossRef]
- Chhibber-Goel, J.; Singhal, V.; Parakh, N.; Bhargava, B.; Sharma, A. The metabolite trimethylamine-N-oxide is an emergent biomarker of human health. Curr. Med. Chem. 2017, 24, 3942–3953. [Google Scholar] [CrossRef]
- Caradonna, E.; Nemni, R.; Bifone, A.; Gandolfo, P.; Costantino, L.; Giordano, L.; Mormone, E.; Macula, A.; Cuomo, M.; Difruscolo, R.; et al. The brain–gut axis, an important player in alzheimer and parkinson disease: A narrative review. J. Clin. Med. 2024, 13, 4130. [Google Scholar] [CrossRef]
- Ghosh, D.; Bernstein, J.A. Health effects of trimellitic anhydride occupational exposure: Insights from animal models and immunosurveillance programs. Clin Rev Allergy Immunol. 2020, 59, 61–77. [Google Scholar] [CrossRef]
- Wang, G.P.; Li, F.; Zhang, J.; Zhao, M.R.; Hui, Z.; Wang, W. Overaccumulation of glycine betaine enhances tolerance of the photosynthetic apparatus to drought and heat stress in wheat. Photosynthetica 2010, 48, 30–41. [Google Scholar] [CrossRef]
- Jain, P.; Pandey, B.; Singh, P.; Singh, R.; Singh, S.P.; Sonkar, S.; Gupta, R.; Rathore, S.S.; Singh, A.K. Plant performance and defensive role of glycine betaine under environmental stress. In Plant Performance Under Environmental Stress: Hormones, Biostimulants and Sustainable Plant Growth Management; Springer: Berlin/Heidelberg, Germany, 2021; pp. 225–248. [Google Scholar]
- Fedotova, M.V.; Kruchinin, S.E. Hydration and ion-binding of glycine betaine: How they may be involved in protection of proteins under abiotic stresses. J. Mol. Liq. 2017, 244, 489–498. [Google Scholar] [CrossRef]
- Alvarenga, L.; Ferreira, M.S.; Kemp, J.A.; Mafra, D. The role of betaine in patients with chronic kidney disease: A narrative review. Curr. Nutr. Rep. 2022, 11, 395–406. [Google Scholar] [CrossRef]
- Apicella, J.M. The Effect of Betaine Supplementation on Performance and Muscle Mechanisms. Master’s Thesis, University of Connecticut, Storrs, CT, USA, 2011. [Google Scholar]
- Volpe, S.L. Choline, Betaine, and Exercise Performance. ACSM’s Health Fit J. 2024, 28, 44–46. [Google Scholar] [CrossRef]
- Bhatt, M.; Di Iacovo, A.; Romanazzi, T.; Roseti, C.; Bossi, E. Betaine—The dark knight of the brain. Bas. Clin. Pharmacol. Toxicol. 2023, 133, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.K.; Paal, M.C.; Donohue, T.M.; Ganesan, M.; Osna, N.A.; Kharbanda, K.K. Beneficial effects of betaine: A comprehensive review. Biology 2021, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Xie, Z.; Lu, C.; Hao, X.; Wu, S.; Huang, Y.; Li, D.; Chen, L. The trehalose-6-phosphate synthase TPS5 negatively regulates ABA signalling in Arabidopsis thaliana. Plant Cell Rep. 2019, 38, 869–882. [Google Scholar] [CrossRef]
- Kosar, F.; Alshallash, K.S.; Akram, N.A.; Sadiq, M.; Ashraf, M.; Alkhalifah, D.H.M.; Abdel Latef, A.A.H.; Elkelish, A. Trehalose-Induced Regulations in Nutrient Status and Secondary Metabolites of Drought-Stressed Sunflower (Helianthus annuus L.) Plants. Plants 2022, 11, 2780. [Google Scholar] [CrossRef]
- Xu, Y.; McRae, D.M.; Leonenko, Z. Quantitative Analysis of the Influence of Trehalose on Amyloid-β Binding to Membranes by Localized Surface Plasmon Resonance Spectroscopy. ACS Omega 2025, 10, 12872–12879. [Google Scholar] [CrossRef]
- Yu, S.; Park, H.; Kim, W. Trehalose inhibits inflammatory responses through mitochondrial reprogramming in raw 264.7 macrophages. Antioxidants 2023, 12, 1166. [Google Scholar] [CrossRef]
- Echigo, R.; Shimohata, N.; Karatsu, K.; Yano, F.; Kayasuga-Kariya, Y.; Fujisawa, A.; Ohto, T.; Kita, Y.; Nakamura, M.; Suzuki, S.; et al. Trehalose treatment suppresses inflammation, oxidative stress, and vasospasm induced by experimental subarachnoid hemorrhage. J. Transl. Med. 2022, 10, 80. [Google Scholar] [CrossRef]
- Mohanan, A.; Kodigudla, A.; Raman, D.R.; Bakka, K.; Challabathula, D. Trehalose accumulation enhances drought tolerance by modulating photosynthesis and ROS-antioxidant balance in drought sensitive and tolerant rice cultivars. Physiol. Mol. Biol. Plants 2023, 29, 2035–2049. [Google Scholar] [CrossRef]
- Nery, D.D.C.M.; da Silva, C.G.; Mariani, D.; Fernandes, P.N.; Pereira, M.D.; Panek, A.D.; Eleutherio, E.C.A. The role of trehalose and its transporter in protection against reactive oxygen species. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2008, 1780, 1408–1411. [Google Scholar] [CrossRef] [PubMed]
- Becerra, J.; Carmona, M.C. Inhibition of plant cytokinesis by deoxyguanosine and caffeine. Plant Cell Rep. 1983, 2, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gil, M.; Tozzi, M.G.; Varani, S.; Della Verde, L.; Petrotto, E.; Balestri, F.; Colombaioni, L.; Camici, M. The combination of adenosine deaminase inhibition and deoxyadenosine induces apoptosis in a human astrocytoma cell line. Neurochem Int. 2015, 80, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Shi, Q.; Wang, X.; Wei, M.; Hu, J.; Liu, J.; Yang, F. Influence of cow manure vermicompost on the growth, metabolite contents, and antioxidant activities of Chinese cabbage (Brassica campestris ssp. chinensis). Biol. Fertil. Soils 2010, 46, 689–696. [Google Scholar] [CrossRef]
- Steindal, A.L.H.; Rødven, R.; Hansen, E.; Mølmann, J. Effects of photoperiod, growth temperature and cold acclimatisation on glucosinolates, sugars and fatty acids in kale. Food Chem. 2015, 174, 44–51. [Google Scholar] [CrossRef]
- Slavin, J.L. Carbohydrates, Dietary Fiber, and Resistant Starch in White Vegetables: Links to Health Outcomes. Adv. Nutr. 2013, 4, 351–355. [Google Scholar] [CrossRef]
- Stanhope, K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, L.; Jeon, S.J.; Yan, J.; Giraldo, J.P.; Matyjaszewski, K.; Tilton, R.D.; Lowry, G.V. Star polymers with designed reactive oxygen species scavenging and agent delivery functionality promote plant stress tolerance. ACS Nano 2022, 16, 4467–4478. [Google Scholar] [CrossRef]
- Playdon, M.; Sampson, J.N.; Cross, A.J.; Sinha, R.; Guertin, K.A.; Moy, K.A.; Rothman, N.; Irwin, M.L.; Mayne, S.T.; Stolzenberg-Solomon, R. Comparing metabolite profiles of habitual diet in serum and urine. Am. J. Clin. Nutr. 2016, 104, 776–789. [Google Scholar] [CrossRef]
- Guertin, K.A.; Loftfield, E.; Boca, S.M.; Sampson, J.N.; Moore, S.C.; Xiao, Q.; Huang, W.Y.; Xiong, X.; Freedman, N.D.; Cross, A.J.; et al. Serum biomarkers of habitual coffee consumption may provide insight into the mechanism underlying the association between coffee consumption and colorectal cancer. Am. J. Clin. Nutr. 2015, 101, 1000–1011. [Google Scholar] [CrossRef]
- Gamba, M.; Raguindin, P.F.; Asllanaj, E.; Merlo, F.; Glisic, M.; Minder, B.; Bussler, W.; Metzger, B.; Kern, H.; Muka, T. Bioactive compounds and nutritional composition of Swiss chard (Beta vulgaris L. var. cicla and flavescens): A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3465–3480. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Dziki, L.; Anisiewicz, J.; Habza-Kowalska, E.; Sikora, M.; Dziki, D. Leaves of White Beetroot As a New Source of Antioxidant and Anti-Inflammatory Compounds. Plants 2020, 9, 944. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).