CRISPR-Editing AsDREBL Improved Creeping Bentgrass Abiotic Stress Tolerance
Abstract
1. Introduction
2. Materials and Methods
2.1. Cloning of Agrostis stolonifera (As) DREB1C-like (AsDREBL) Genomic DNA (gDNA) by PCR
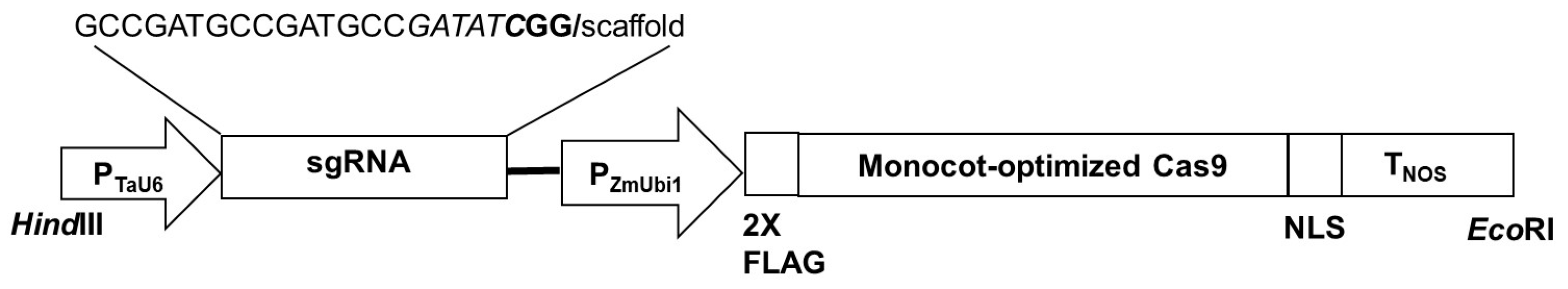
2.2. Construction of AsDREBL CRISPR-Editing Vector
2.3. Transformation of Creeping Bentgrass cv. Crenshaw Embryogenic Calli with pRD303
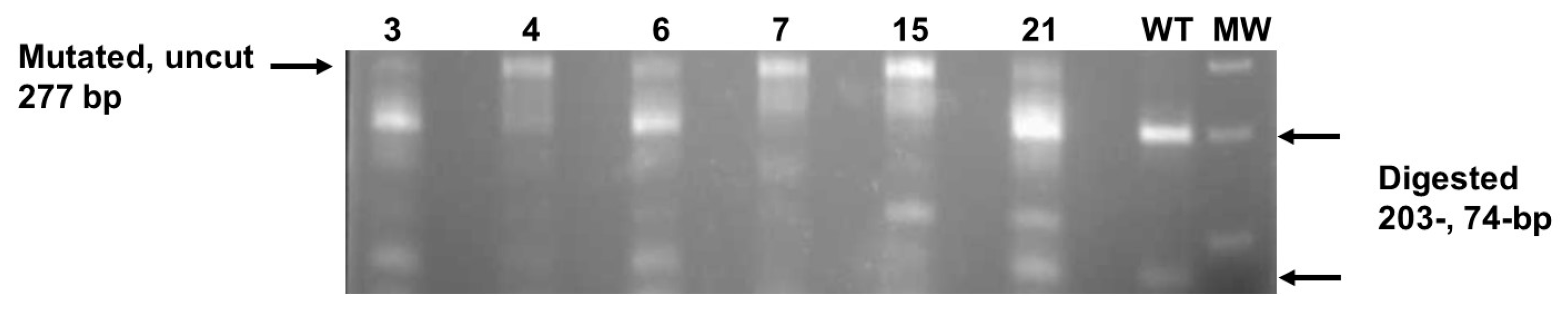
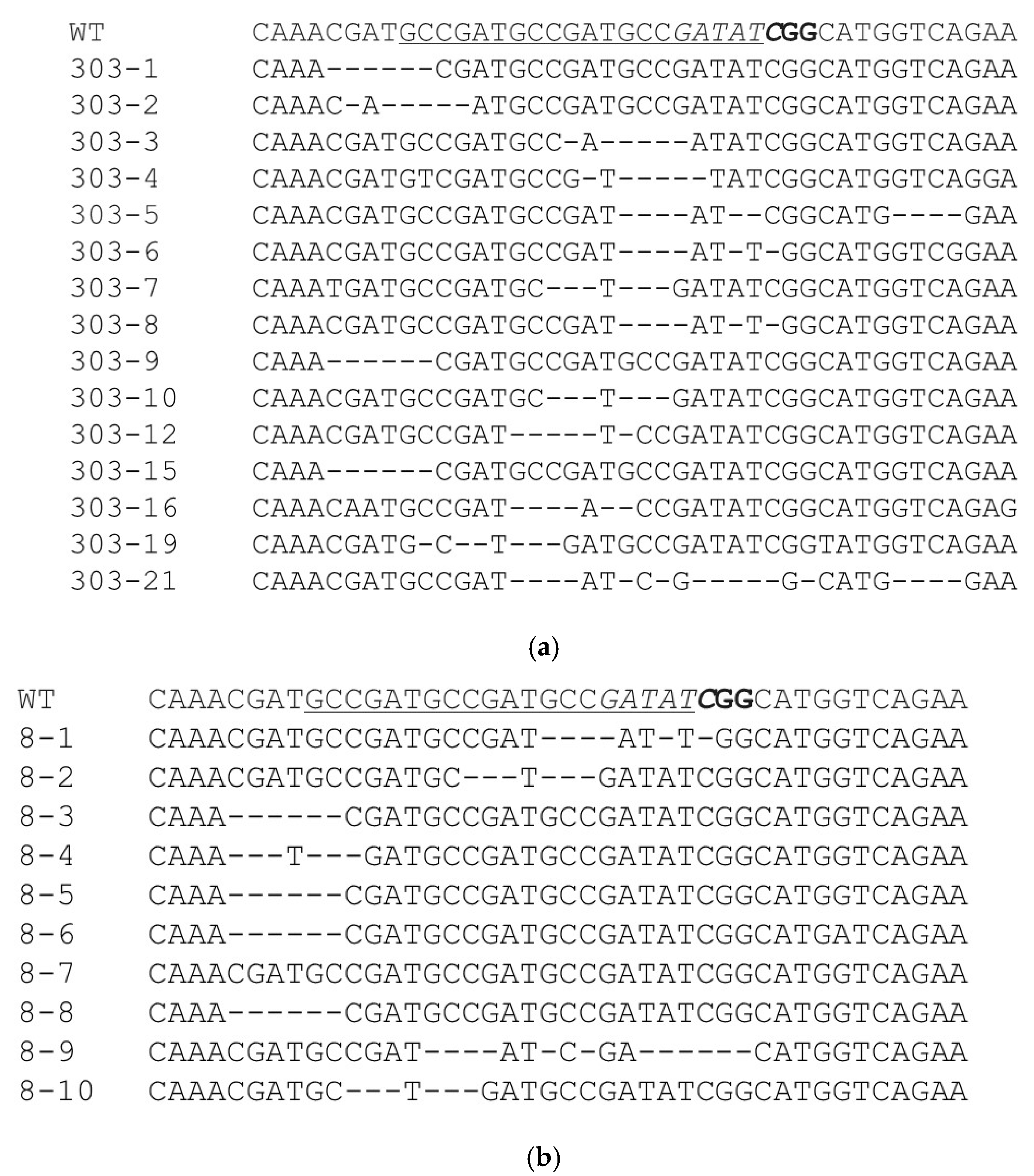
2.4. Identification of AsDREBL-Edited Crenshaw Mutants
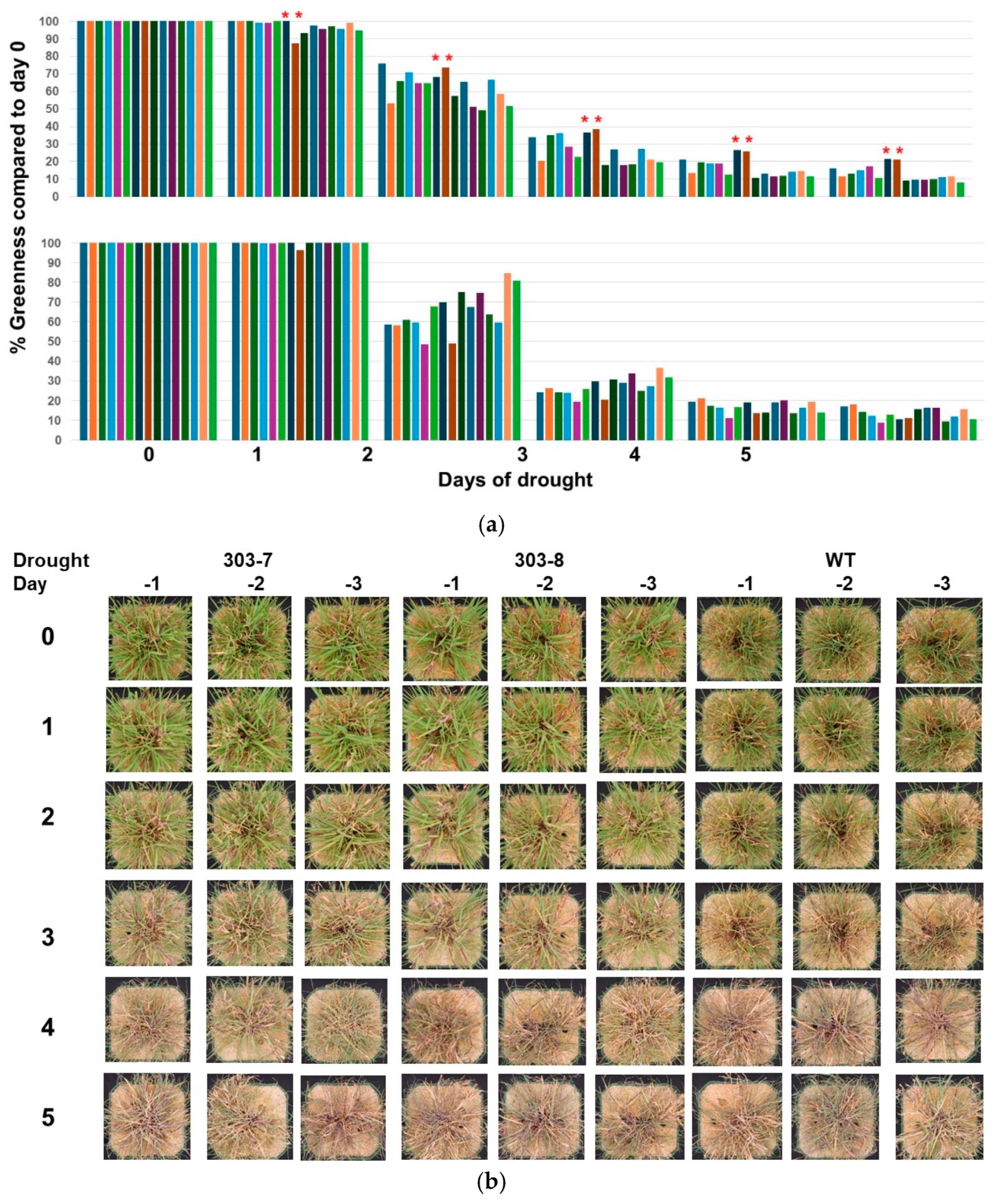
2.5. Drought Tolerance Testing of AsDREBL Mutant Crenshaw Plants
2.6. Salinity Tolerance Testing of AsDREBL Mutant Crenshaw Plants
3. Results
3.1. Identification of the AsDREBL Gene in Creeping Bentgrass Crenshaw and the Construction of AsDREBL CRISPR-Editing Vector
3.2. Production of AsDREBL-Edited Mutant Crenshaw Plants
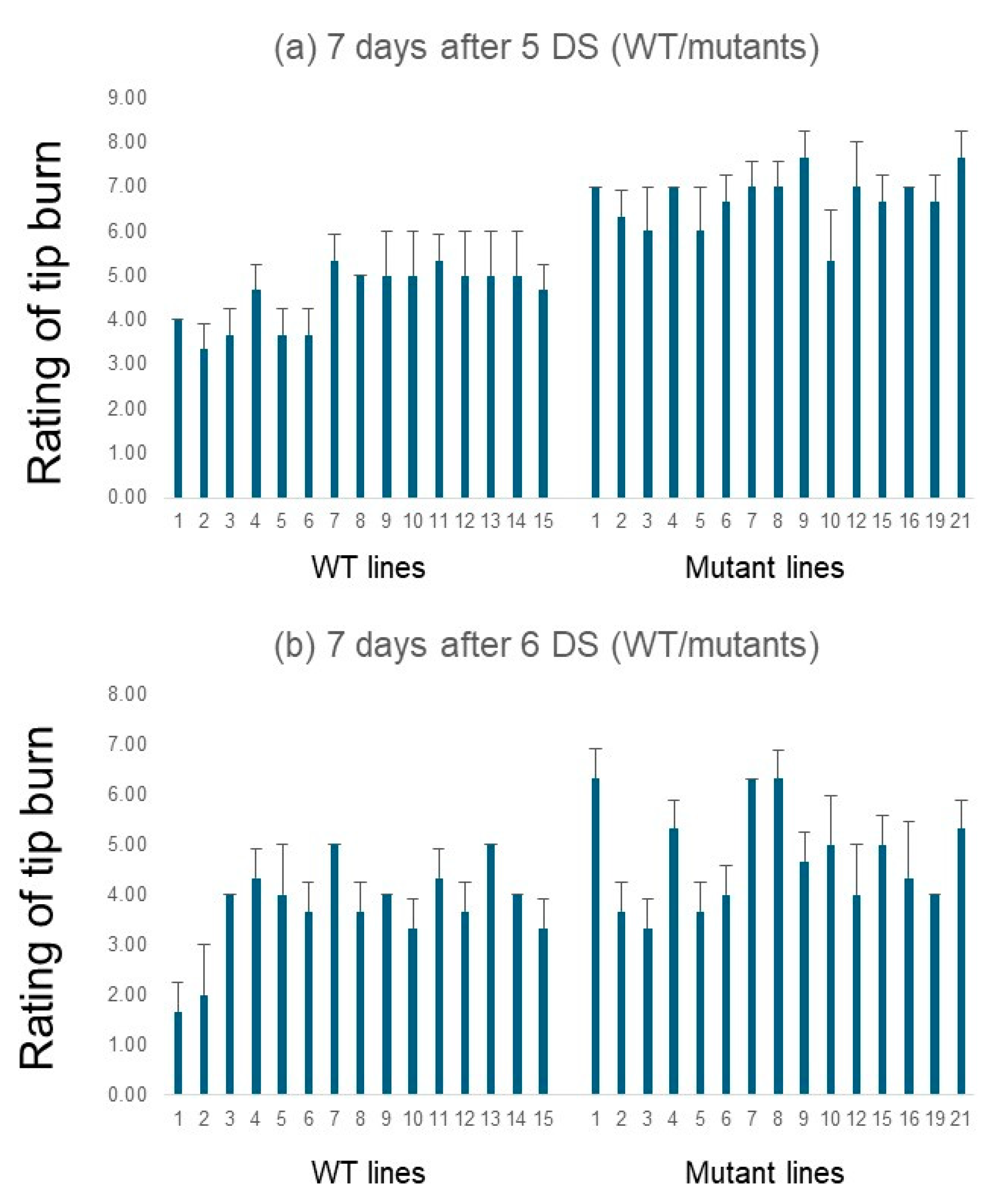
3.3. AsDREBL-Edited Mutant Crenshaw Plants Were More Tolerant to Drought
3.4. AsDREBL-Edited Mutant Crenshaw Plants Were More Tolerant to Salinity
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Bonos, S.A.; Clarke, B.B.; Meyer, W.A. Breeding for disease resistance in the major cool-season turfgrasses. Annu. Rev. Phytopathol. 2006, 44, 213–234. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, P.; Zhang, Z.; Han, B.; Wang, W.; Wang, Y.; Cao, Y.; Hu, T. Isolation and characterization of a buffalograss (Buchloe dactyloides) dehydration responsive element binding transcription factor, BdDREB2. Gene 2014, 536, 123–128. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, Y. Identification of differentially expressed genes under drought stress in perennial ryegrass. Physiol. Plant. 2010, 139, 375–387. [Google Scholar] [CrossRef]
- Hu, L.; Wang, Z.; Du, H.; Huang, B. Differential accumulation of dehydrins in response to water stress for hybrid and common bermudagrass genotypes differing in drought tolerance. J. Plant Physiol. 2010, 167, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Du, H.; Wang, Z.; Huang, B. Identification of proteins associated with water-deficit tolerance in C4 perennial grass species, Cynodon dactylon x Cynodon transvaalensis and Cynodon dactylon. Physiol. Plant. 2011, 141, 40–55. [Google Scholar] [CrossRef]
- Zhou, M.; Luo, H. Role of microRNA319 in creeping bentgrass salinity and drought stress response. Plant Signal. Behav. 2014, 9, e28700. [Google Scholar] [CrossRef]
- Tang, N.; Zhang, H.; Li, X.; Xiao, J.; Xiong, L. Constitutive activation of transcription factor OsbZIP46 improves drought tolerance in rice. Plant Physiol. 2012, 158, 1755–1768. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Park, M.J.; Seo, P.J.; Song, J.S.; Kim, H.J.; Park, C.M. Controlled nuclear import of the transcription factor NTL6 reveals a cytoplasmic role of SnRK2.8 in the drought-stress response. Biochem. J. 2012, 448, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; De Vleesschauwer, D.; Sharma, M.K.; Ronald, P.C. Recent advances in dissecting stress-regulatory crosstalk in rice. Mol. Plant 2013, 6, 250–260. [Google Scholar] [CrossRef]
- Nakashima, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front. Plant Sci. 2014, 5, 170. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Low, Y.C.; Lawton, M.A.; Di, R. Validation of barley 2OGO gene as a functional orthologue of Arabidopsis DMR6 gene in Fusarium head blight susceptibility. Sci. Rep. 2020, 10, 9935. [Google Scholar] [CrossRef]
- Low, Y.C.; Lawton, M.A.; Di, R. Ethylene insensitive 2 (EIN2) as a potential target gene to enhance Fusarium head blight disease resistance. Plant Sci. 2022, 322, 111361. [Google Scholar] [CrossRef]
- Zhang, X.; Low, Y.C.; Lawton, M.A.; Simon, J.E.; Di, R. CRISPR-editing of sweet basil (Ocimum basilicum L.) homoserine kinase gene for improved downy mildew disease resistance. Front. Genome Ed. 2021, 3, 629769. [Google Scholar] [CrossRef]
- Novillo, F.; Alonso, J.M.; Ecker, J.R.; Salinas, J. CBF2/DREB1C is a negative regulator of CBF1/DREB1B and CBF3/DREB1A expression and plays a central role in stress tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 3985–3990. [Google Scholar] [CrossRef] [PubMed]
- Rotter, D.; Bharti, A.K.; Li, H.K.; Luo, C.; Bonos, S.A.; Bughrara, S.; Jung, G.; Messing, J.; Meyer, W.A.; Rudd, S.; et al. Analysis of EST sequences suggests recent origin of allotetraploid colonial and creeping bentgrasses. Mol. Genet. Genom. 2007, 278, 197–209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Shan, Q.; Wang, Y.; Li, J.; Zhang, Y.; Chen, K.; Liang, Z.; Zhang, K.; Liu, L.; Xi, J.J.; Qiu, J.L.; et al. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 686–688. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, H.; Bi, H.; Fromm, M.; Yang, B.; Weeks, D.P. Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res. 2013, 41, e188. [Google Scholar] [CrossRef]
- Dai, W.D.; Bonos, S.A.; Guo, Z.; Meyer, W.A.; Day, P.R.; Belanger, F.C. Expression of pokeweed antiviral proteins in creeping bentgrass. Plant Cell Rep. 2003, 21, 497–502. [Google Scholar] [CrossRef]
- Kumar, R.; Kamda, T.; Budhathoki, R.; Tang, D.; Yer, H.; Zhao, Y.; Li, Y. Agrobacterium- and a single Cas9-sgRNA transcript system-mediated high efficiency gene editing in perennial ryegrass. Front. Genome Ed. 2022, 4, 960414. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.M.; Gondo, T.; Tanaka, H.; Akashi, R. CRISPR/Cas9-mediated knockout of NYC1 gene enhances chlorophyll retention and reduces tillering in Zoysia matrella (L.) Merrill. Plant Cell Rep. 2024, 43, 50. [Google Scholar] [CrossRef] [PubMed]
- Donde, R.; Gupta, M.K.; Gouda, G.; Kumar, J.; Vadde, R.; Sahoo, K.K.; Dash, S.K.; Behera, L. Computational characterization of structural and functional roles of DREB1A, DREB1B and DREB1C in enhancing cold tolerance in rice plant. Amino Acids 2019, 51, 839–853. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di, R.; Ravikumar, S.; Daddio, R.; Bonos, S. CRISPR-Editing AsDREBL Improved Creeping Bentgrass Abiotic Stress Tolerance. Int. J. Plant Biol. 2025, 16, 89. https://doi.org/10.3390/ijpb16030089
Di R, Ravikumar S, Daddio R, Bonos S. CRISPR-Editing AsDREBL Improved Creeping Bentgrass Abiotic Stress Tolerance. International Journal of Plant Biology. 2025; 16(3):89. https://doi.org/10.3390/ijpb16030089
Chicago/Turabian StyleDi, Rong, Sreshta Ravikumar, Ryan Daddio, and Stacy Bonos. 2025. "CRISPR-Editing AsDREBL Improved Creeping Bentgrass Abiotic Stress Tolerance" International Journal of Plant Biology 16, no. 3: 89. https://doi.org/10.3390/ijpb16030089
APA StyleDi, R., Ravikumar, S., Daddio, R., & Bonos, S. (2025). CRISPR-Editing AsDREBL Improved Creeping Bentgrass Abiotic Stress Tolerance. International Journal of Plant Biology, 16(3), 89. https://doi.org/10.3390/ijpb16030089






