Interactive Effect of Copper and Herbivory on the Whole-Plant Growth of Leucaena leucocephala
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Species
2.2. Experimental Design
2.3. RGRB Estimation: Morphological and Physiological Components
2.4. Data Analysis
3. Results
3.1. Effects of Cu and Herbivory on the Growth and Survival of L. leucocephala Seedlings
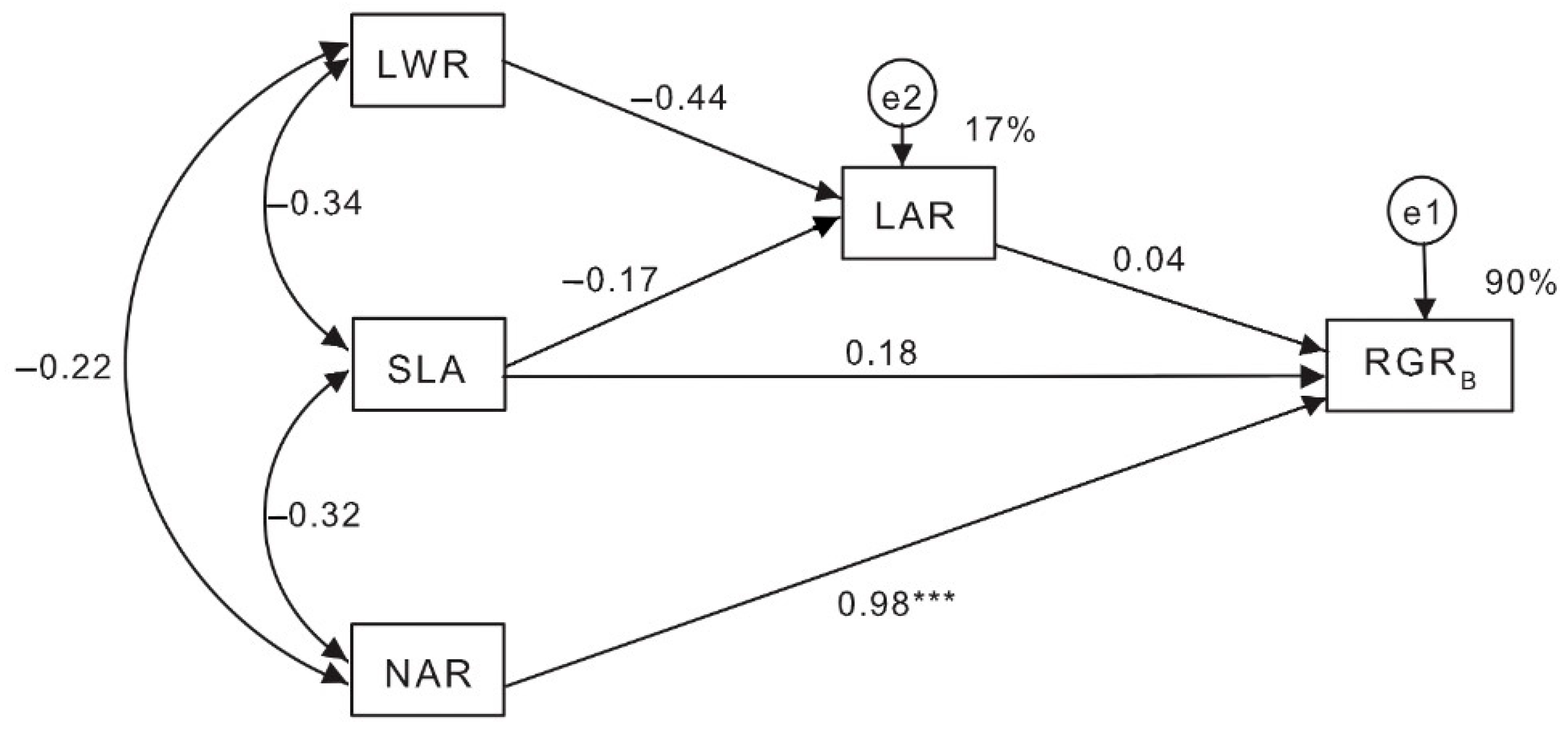
3.2. Effects of Cu and Herbivory on Morphological and Physiological Components of RGRB
3.3. Effect of Cu and Herbivory on Mass Allocation in Seedlings of L. leucocephala
4. Discussion
4.1. Compensatory Growth and Survival of L. leucocephala in Response to Cu Effects
4.2. Contribution of Morphological and Physiological Components to the RGRB of L. leucocephala
4.3. Mass Allocation in L. leucocephala
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RGR | Growth Rate |
| RGRB | Relative Growth Rate in Biomass |
| RGRLA | Relative Growth Rate in Leaf Area |
| LAR | Leaf Area Ratio |
| SLA | Specific Leaf Area |
| NAR | Net Assimilation Rate |
| LWR | Leaf Weight Ratio |
| SWR | Stem Weight Ratio |
| RWR | Root Weight Ratio |
| R:S | Root:Shoot Ratio |
| GRM | Growth Rate Model |
| CCH | Compensatory Continuum Hypothesis |
| LRM | Limiting Resource Model |
| DBH | Diameter at Breast Height |
| ITC | Instituto Tecnológico de Conkal. |
| CESVY | Comité Estatal de Sanidad Vegetal de Yucatán. |
| SE | Standard Error |
| GLM | Generalized Linear Models |
| ANOVA | Analysis of Variance |
| AIC | Akaike Information Criterion |
| RH | Relative Humidity |
| PPFD | Photosynthetic Photon Flux Density |
References
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ejaz, U.; Vithanage, M.; Bolan, N.; Siddique, K.H. Synthesis, characterization, and advanced sustainable applications of copper oxide nanoparticles: A review. Clean Technol. Environ. 2024, 1–26. [Google Scholar] [CrossRef]
- Ali, Z.; Malik, R.N.; Shinwari, Z.K.; Qadir, A. Enrichment, risk assessment, and statistical apportionment of heavy metals in tannery-affected areas. Int. J. Environ. Sci. Technol. 2015, 12, 537–550. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Hussain, S.; Kamran, M.; Chattha, M.S.; Ahmad, S.; Aqeel, M.; Rizwan, M.; Aljarba, N.H.; Alkahtani, S.; et al. Flax (Linum usitatissimum L.): A potential candidate for phytoremediation? Biological and economical points of view. Plants 2020, 9, 496. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Ali, S.; Kamran, M.; Iqbal, N.; Azeem, M.; Javed, M.T.; Ali, Q.; Haider, M.Z.; Irshad, S.; Rizwan, M.; et al. Ethylenediaminetetraacetic acid (EDTA) mitigates the toxic effect of excessive copper concentrations on growth, gaseous exchange and chloroplast ultrastructure of Corchorus capsularis L. and improves copper accumulation capabilities. Plants 2020, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Miñón-Huesca, E.; González-Alcorta, M.J.; Huerta-Bravo, M.; Crespo-López, G.; Carrillo-Domínguez, S.; Casti-llo-Domínguez, R.M.; Cuca-García, J.M.; Morales-Barrera, J.E. Niveles óptimos biológico y económico de cobre dietético en pollos de engorda. Agrociencia 2006, 40, 163–170. [Google Scholar]
- Saleem, M.H.; Fahad, S.; Khan, S.U.; Din, M.; Ullah, A.; Sabagh, A.E.; Hossain, A.; Llanes, A.; Liu, L. Copper-induced oxidative stress, initiation of antioxidants and phytoremediation potential of flax (Linum usitatissimum L.) seedlings grown under the mixing of two different soils of China. Environ. Sci. Pollut. Res. 2020, 27, 5211–5221. [Google Scholar] [CrossRef]
- Ryser, P.; Emerson, P. Growth, root and leaf structure, and biomass allocation in Leucanthemum vulgare Lam. (Asteraceae) as influenced by heavy-metal-containing slag. Plant Soil 2007, 301, 315–324. [Google Scholar] [CrossRef]
- Northfield, T.D.; Ives, A.R. Coevolution and the effects of climate change on interacting species. PLoS Biol. 2013, 11, e1001685. [Google Scholar] [CrossRef]
- Lequeux, H.; Hermans, C.; Lutts, S.; Verbruggen, N. Response to copper excess in Arabidopsis thaliana: Impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiol. Biochem. 2010, 48, 673–682. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, D.; Liu, X. Effects of copper on root growth, cell division, and nucleolus of Zea mays. Biol. Plant. 2001, 44, 105–109. [Google Scholar] [CrossRef]
- Liu, D.; Jiang, W.; Meng, Q.; Zou, J.; Gu, J.; Zeng, M. Cytogenetical and ultrastructural effects of copper on root meristem cells of Allium sativum L. Biocell 2009, 33, 25–32. [Google Scholar] [CrossRef]
- Dirzo, R.; Thompson, J.N. La coevolución y las enseñanzas de Darwin. Cienc. Hoy 2009, 19, 43–48. [Google Scholar]
- Marquis, R.J. Leaf herbivores decrease fitness of a tropical plant. Science 1984, 226, 537–539. [Google Scholar] [CrossRef]
- Boege, K. Influence of plant ontogeny on compensation to leaf damage. Am. J. Bot. 2005, 92, 1632–1640. [Google Scholar] [CrossRef]
- Ballina-Gómez, H.S.; Iriarte, V.S.; Orellana, R.; Santiago, L.S. Crecimiento, supervivencia y herbivoría de plántulas de Brosimum alicastrum (Moraceae), una especie del sotobosque neotropical. Rev. Biol. Trop. 2008, 56, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- Ballina-Gómez, H.S.; Iriarte, V.S.; Orellana, R.; Santiago, L.S. Compensatory growth responses to defoliation and light availability in two native Mexican woody plant species. J. Trop. Ecol. 2010, 26, 163–171. [Google Scholar] [CrossRef]
- Martínez, C.L.; Riquelme Virgala, M.B.; Santadino, M.V.; de Haro, A.M.; Barañao, J.J. Estudios sobre el comportamiento de forrajeo de Acromyrmex lundi Guering (HYMENOPTERA, FORMICIDAE) y su efecto sobre el crecimiento de procedencias de Eucalyptus globulus Labill. (Myrtaceae). Rev. Árvore 2015, 39, 189–198. [Google Scholar] [CrossRef]
- Hicks, S.; Turkington, R. Compensatory growth of three herbaceous perennial species: The effects of clipping and nutrient availability. Can. J. Bot. 2000, 78, 759–767. [Google Scholar] [CrossRef]
- Hilbert, D.W.; Swift, D.M.; Detling, J.K.; Dyer, M.I. Relative growth rates and the grazing optimization hypothesis. Oecologia 1981, 51, 14–18. [Google Scholar] [CrossRef]
- Maschinski, J.; Whitham, T.G. The continuum of plant responses to herbivory: The influence of plant association, nutrient availability, and timing. Am. Nat. 1989, 134, 1–19. [Google Scholar] [CrossRef]
- Wise, M.J.; Abrahamson, W.G. Beyond the compensatory continuum: Environmental resource levels and plant tolerance of herbivory. Oikos 2005, 109, 417–428. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Sullivan, J.J. The Impact of Herbivory on Plants in Different Resource Conditions: A Meta-Analysis. Ecology 2001, 82, 2045–2058. [Google Scholar] [CrossRef]
- Camargo, I.D.; Tapia-López, R.; Núñez-Farfán, J. Ecotypic variation in growth responses to simulated herbivory: Trade-off between maximum relative growth rate and tolerance to defoliation in an annual plant. AoB Plants 2015, 7, plv015. [Google Scholar] [CrossRef] [PubMed]
- Dobarro, I. Respuestas de la Vegetación al Pastoreo: Mecanismos Relacionados Con la Defoliación en Especies Mediterrá-Neas. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2009. [Google Scholar]
- Poorter, H. Interspecific variation in relative growth rate: On ecological causes and physiological consequences. Acta Bot. Neerl. 1989, 38, 237–250. [Google Scholar]
- Poorter, H. Plant growth at elevated CO2. In Encyclopedia of Global Environmental Change; Mooney, H.A., Canadell, J.G., Eds.; Wiley: Chichester, UK, 2002; pp. 489–496. [Google Scholar]
- Khalil Gardezi, A.; Barcelo-Quintal, I.D.; Cetina-Alcalá, V.M.; Bussy, A.L.; Pérez-Nieto, J.; Borja-Salin, M.A. Absorción de cobre y características de Leucaena leucocephala asociada con Glomus spp. y Rhizobium en suelo contaminado del Río Lerma, México. TERRA Latinoam. 2006, 24, 347–354. [Google Scholar]
- Lima Lins, C.E.; Cavalcante, U.M.T.; Sampaio, E.V.S.B.; Messias, A.S.; Maia, L.C. Growth of mycorrhized seedlings of Leucaena leucocephala (Lam.) de Wit. in a copper contaminated soil. Appl. Soil Ecol. 2006, 31, 181–185. [Google Scholar] [CrossRef]
- Grijalva, J.; Ramos, R.; Vera, A. Pasturas Para Sistemas Silvopastoriles: Alternativa Para el Desarrollo Sostenible de la Ganadería en la Amazonía Baja de Ecuador; Boletín Técnico Nº 156; Programa Nacional de Forestería del INIAP: Quito, Ecuador, 2011. [Google Scholar]
- Villar, R.; Marañón, T.; Quero, J.L.; Panadero, P.; Arenas, F.; Lambers, H. Variation in relative growth rate of 20 Aegilops species (Poaceae) in the field: The importance of net assimilation rate or specific leaf area depends on the time scale. Plant Soil 2005, 272, 11–27. [Google Scholar] [CrossRef]
- Arenas, A.D.; Marcó, L.M.; Torres, G. Evaluación de la planta Lemna minor como biorremediadora de aguas contaminadas con mercurio. Av. Cienc. Ing. 2011, 2, 1–11. [Google Scholar]
- Varnagiryte-Kabašinskiene, I.; Araminiene, V.; Stakenas, V. Effects of artificial defoliation and simulated insect damage on the growth of Betula pendula saplings. iForest 2015, 9, 95–100. [Google Scholar] [CrossRef]
- Casanova, L. Caracterización de la Vegetación Secundaria del Área Experimental Que se Ubica al Noreste del Campus Uni-versitario de la Facultad de Medicina Veterinaria y Zootecnia. Bachelor’s Thesis, Universidad Autónoma de Yucatán, Mérida, Mexico, 2000. [Google Scholar]
- Flores, J.S.; Durán, R.; Ortiz, D.J. Comunidades vegetales terrestres. In Biodiversidad y Desarrollo Humano en Yucatán; Durán, R., Méndez, M., Eds.; CICY-PPD-FMAM: Mérida, México, 2010; pp. 125–129. [Google Scholar]
- Flores, J.S.; Espejel, I. Tipos de vegetación de la península de Yucatán. Etnoflora Yucatanense 1994, 3, 1–35. [Google Scholar]
- White, D.A.; Hood, C.S. Vegetation patterns and environmental gradients in tropical dry forest of the northern Yucatan Peninsula. J. Veg. Sci. 2004, 15, 151–160. [Google Scholar] [CrossRef]
- Pennington, T.D.; Sarukhán, J. Árboles tropicales de México. In Manual Para la Identificación de las Principales Especies, 3rd ed.; Universidad Nacional Autónoma de México-Fondo de Cultura Económica: Mexico City, Mexico, 2005. [Google Scholar]
- Gómez-Castro, H.; Pinto-Ruiz, R.; Guevara-Hernández, F.; González-Reyna, A. Estimaciones de biomasa aérea y carbono almacenado en Gliricidia sepium (Lam.) y Leucaena leucocephala (Jacq.) y su aplicación en sistemas silvopastoriles. ITEA 2010, 106, 256–270. [Google Scholar]
- Borges-Gómez, L.; Moo-Kauil, C.; Ruíz-Novelo, J.; Osalde-Balam, M.; González-Valencia, C.; Yam-Chimal, C.; Can-Puc, F. Suelos destinados a la producción de chile habanero en Yucatán: Características físicas y químicas predominantes. Agrociencia 2014, 48, 347–359. [Google Scholar]
- Poorter, H.; Garnier, E. Plant growth analysis: An evaluation of experimental design and computational methods. J. Exp. Bot. 1996, 47, 1343–1351. [Google Scholar] [CrossRef]
- Johnson, M.T.; Bertrand, J.A.; Turcotte, M.M. Precision and accuracy in quantifying herbivory. Ecol. Entomol. 2016, 41, 112–121. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ; U.S. National Institutes of Health: Bethesda, MD, USA, 2012; Available online: https://imagej.net/ (accessed on 25 December 2024).
- McCullagh, P.; Nelder, J.A. Generalized Linear Models, 2nd ed.; Chapman & Hall: London, UK, 1989. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, Version 22.0; IBM Corp.: Armonk, NY, USA, 2013. [Google Scholar]
- Arbuckle, J.L. IBM SPSS Amos 19 User’s Guide; Amos Development Corporation: Crawfordsville, FL, USA, 2012. [Google Scholar]
- Morales, J.M.L.; Sepúlveda-Jiménez, G. El daño por oxidación causado por cobre y la respuesta antioxidante de las plantas. Interciencia 2012, 37, 805–811. [Google Scholar]
- Barceló-Quintal, I.D.; García-Alamilla, P.; Gómez-González, M.A. Fitorremediación de suelos contaminados con metales pesados utilizando Leucaena leucocephala. Rev. Mex. Ing. Quím. 2016, 15, 431–440. [Google Scholar]
- Pires-Bomfim, P.M.C.; Demarco, D.; De Marco, P., Jr. Heavy metal accumulation in Leucaena leucocephala: Implications for phytoremediation in tropical soils. Environ. Sci. Pollut. Res. 2023, 30, 43210–43221. [Google Scholar] [CrossRef]
- Calle, Z.; Murgueitio, E.; Chará, J. Integración de árboles en sistemas ganaderos para la rehabilitación ecológica. Agrofor. Am. 2011, 48, 12–19. [Google Scholar]
- Amaya-Martin, S.M.; Ballina-Gomez, H.S.; Alvarado-López, C.; Ruiz-Sánchez, E.; Gil-Cardeza, M.; Azcorra-Perera, G. Cadmium tolerance and resistance to damage by Bemisia tabaci induced by a consortium of arbuscular mycorrhizal fungi in Solanum lycopersicum L. Trop. Subtrop. Agroecosyst. 2025, 28, 1–12. [Google Scholar] [CrossRef]
- Nanthavong, K.; Sampanpanish, P. Effect of NTA and EDTA on arsenic uptake from contaminated soil by Mimosa pudica. Mod. Appl. Sci. 2015, 9, 280–291. [Google Scholar] [CrossRef]
- Marozzi, M.; Villalba, A.; Polla, W.; Hadad, H.; Devercelli, M. Cambios en la morfología y en la tasa de crecimiento de Pistia stratiotes (Araceae) asociados a la presencia de plomo. Cienc. Agrar. 2003, 2, 1–2. [Google Scholar] [CrossRef][Green Version]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar] [CrossRef]
- Freeman, J.L.; Lindblom, S.D.; Quinn, C.F.; Fakra, S.; Marcus, M.A.; Pilon-Smits, E.A.H. Selenium accumulation protects plants from herbivory by Orthoptera via toxicity and deterrence. New Phytol. 2007, 175, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Wise, M.J.; Mudrak, E.L. Revisiting the Limiting Resource Model: Context-dependent plant tolerance to herbivory. Ecol. Lett. 2023, 26, 512–523. [Google Scholar]
- Wise, M.J.; Abrahamson, W.G. Effects of resource availability on tolerance of herbivory: A review and assessment of three opposing models. Am. Nat. 2007, 169, 443–454. [Google Scholar] [CrossRef]
- Banta, J.A.; Stevens, M.H.H.; Pigliucci, M. A comprehensive test of the limiting resource framework applied to plant tolerance to apical meristem damage. Oikos 2010, 119, 359–369. [Google Scholar] [CrossRef]
- March, P.J.; Wise, M.J.; Abrahamson, W.G. The effect of resource stress on goldenrod’s tolerance of folivory depends more on the identity of the stress than on the severity of the stress. Int. J. Mod. Bot. 2013, 3, 15–25. [Google Scholar]
- Wise, M.J.; Abrahamson, W.G. Applying the limiting resource model to plant tolerance of apical meristem damage. Am. Nat. 2008, 172, 635–647. [Google Scholar] [CrossRef]
- Houghton, J.; Thompson, K.; Rees, M. Does seed mass drive the differences in relative growth rate between growth forms? Proc. R. Soc. B 2013, 280, 20130921. [Google Scholar] [CrossRef]
- Shibuya, T.; Shimizu, K.; Nishioka, N. Growth responses of Arabidopsis thaliana to salinity stress: The role of ion homeostasis. Plant Cell Physiol. 2024, 65, 387–398. [Google Scholar]
- Matsuda, R.; Suzuki, K.; Nakano, A. Light availability and photosynthetic acclimation in understory plants. Front. Plant Sci. 2023, 14, 1123456. [Google Scholar]
- van Staalduinen, M.A.; Anten, N.P.R. Differences in the compensatory growth of two co-occurring grass species in relation to water availability. Oecologia 2005, 146, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Herrera, E.F.; Dzib-Ek, S.A.; Ballina-Gómez, H.S. Influence of herbivory and gap-openness on whole-plant growth of Brosimum alicastrum (Moraceae). Acta Biol. Colomb. 2021, 26, 186–195. [Google Scholar] [CrossRef]
- Eichholtzer, J.; Ballina-Gómez, H.S.; Gómez-Tec, K.; Medina-Dzul, K. Arbuscular mycorrhizal fungi influence whitefly abundance by modifying habanero pepper tolerance to herbivory. Arthropod Plant Interact. 2021, 15, 861–874. [Google Scholar] [CrossRef]
- Baraloto, C.; Bonal, D.; Goldberg, D.E. Differential seedling growth responses to soil resource availability among nine neotropical tree species. J. Trop. Ecol. 2006, 22, 487–497. [Google Scholar] [CrossRef]
- Gandhi, S.; Thangavel, M.; Kumar, R.S. Morpho-physiological responses of Brassica juncea to heavy metal stress: A comparative study. Environ. Exp. Bot. 2020, 178, 104123. [Google Scholar]
- Ali, A.; Khan, B.; Sajid, M. Heavy metal accumulation and tolerance mechanisms in hyperaccumulator plants: A review. Environ. Res. 2024, 215, 114312. [Google Scholar]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol. Monogr. 1992, 62, 365–392. [Google Scholar] [CrossRef]
- Huante, P.; Rincón, E.; Acosta, I. Nutrient availability and growth rate of 34 woody species from a tropical deciduous forest in Mexico. Funct. Ecol. 1995, 9, 849–858. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Castro Diez, P.; Hunt, R. Seedling growth, allocation and leaf attributes in a wide range of woody plant species and types. J. Ecol. 1996, 84, 755–765. [Google Scholar] [CrossRef]
- Antúnez, I.; Retamosa, E.C.; Villar, R. Relative growth rate in phylogenetically related deciduous and evergreen woody species. Oecologia 2001, 128, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Villar, R.; López-Iglesias, B.; Ruiz-Benito, P.; Zavala, M.A.; De la Riva, E.G. Crecimiento de plántulas y árboles de seis especies de Quercus. Ecosistemas 2014, 23, 64–72. [Google Scholar]
- Pérez-Garrido, C.; Fernández-Díaz, L.; Pina, C.M.; Prieto, M. In situ AFM observations of the interaction between calcite (1014) surfaces and Cd-bearing aqueous solutions. Surf. Sci. 2007, 601, 5499–5509. [Google Scholar] [CrossRef]
- Luna, C.M.; González, C.A.; Trippi, V.S. Oxidative damage caused by an excess of copper in oat leaves. Plant Cell Physiol. 1994, 35, 11–15. [Google Scholar]
- Burzyński, M.; Klobus, G. Changes of photosynthetic parameters in cucumber leaves under Cu, Cd and Pb stress. Photosynthetica 2004, 42, 505–510. [Google Scholar] [CrossRef]
- Khatun, S.; Ali, M.B.; Hahn, E.J.; Paek, K.Y. Copper toxicity in Withania somnifera: Growth and antioxidant enzymes responses of in vitro grown plants. Environ. Exp. Bot. 2008, 64, 279–285. [Google Scholar] [CrossRef]
- Russo, M.; Sgherri, C.; Izzo, R.; Navari-Izzo, F. Brassica napus subjected to copper excess: Phospholipases C and D and glutathione system in signaling. Environ. Exp. Bot. 2008, 62, 238–246. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Huu Thanh, N.; Le Ha, T.T.; Viet Ha, C.; Duc Hung, N.; Quoc Hung, P.; Kurosawa, K.; Egashira, K. Uptake of Pb, Zn and Cu by roots and shoots of fast growing plants grown in contaminated soil in Vietnam. J. Soil Sci. Environ. Manag. 2013, 4, 108–115. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Wang, Z. Biomass reallocation in plants under heavy metal stress: A meta-analysis. Environ. Exp. Bot. 2024, 218, 105612. [Google Scholar]
- Hackett, S.C.; Karley, A.J.; Bennett, A.E. Unpredicted impacts of insect endosymbionts on interactions between soil organisms, plants and aphids. Proc. R. Soc. B 2013, 280, 20131275. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; van der Werf, A. Is inherent variation in RGR determined by LAR at low irradiance and by NAR at high irradiance? A review of herbaceous species. In Inherent Variation in Plant Growth; Lambers, H., Poorter, H., Van Vuuren, M.M.I., Eds.; Backhuys: Leiden, The Netherlands, 1998; pp. 309–336. [Google Scholar]
- Barchuk, A.; Iglesias, M.R.; Oviedo, C. Rebrote basal de Aspidosperma quebracho-blanco en estado de plántula: Mecanismo de persistencia en el Chaco Árido. Ecol. Austral 2006, 16, 197–205. [Google Scholar]

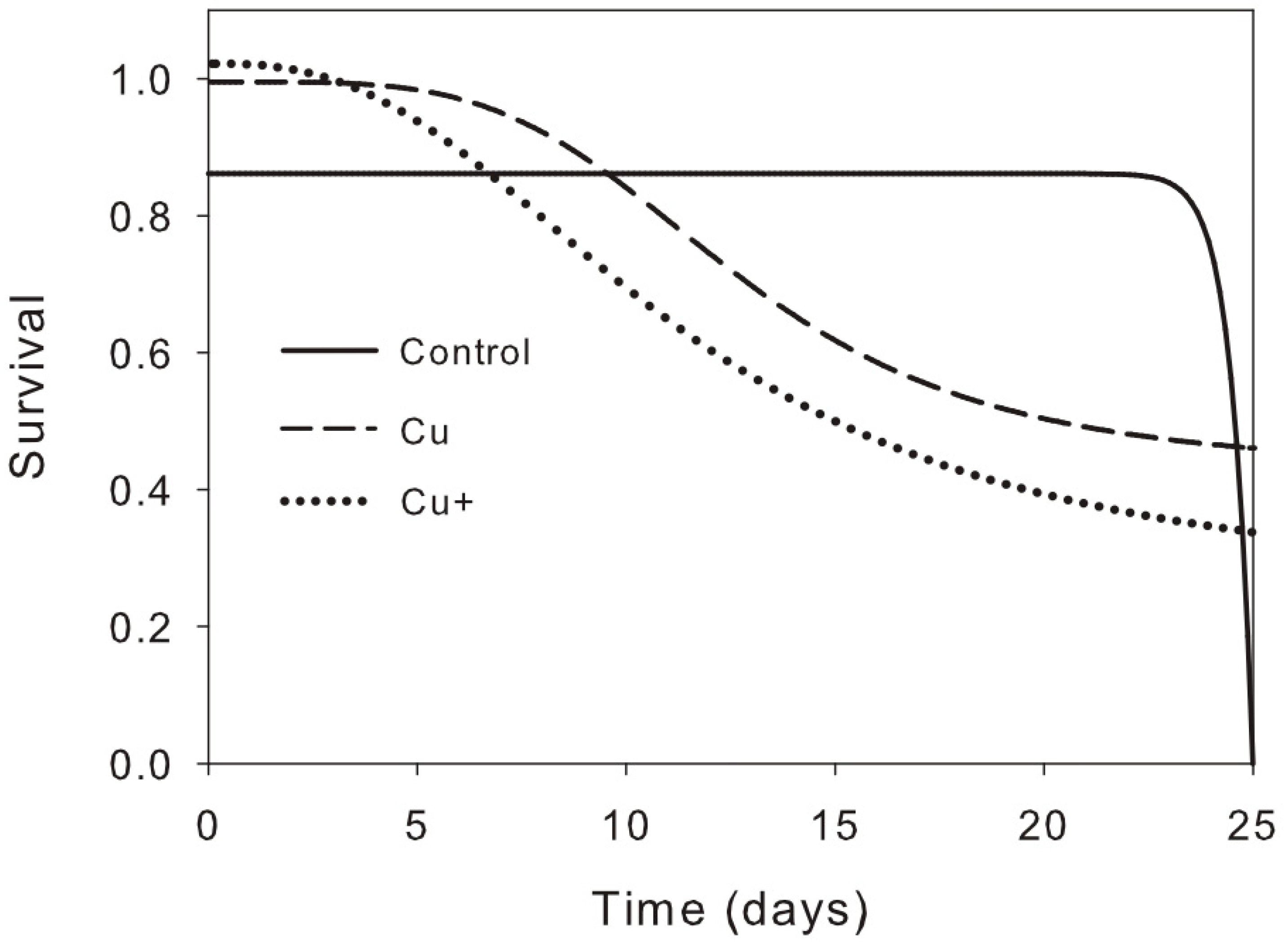

| Effect | RGRB (g g−1 d−1) | χ2 | RGRLA (cm2 cm−2 d−1) | χ2 | LPR (no.) | χ2 | LAR (cm2 g−1) | χ2 | SLA (cm2 g−1) | χ2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Inter-subjects | ||||||||||
| Copper treatment | 1.67 ns | 0.44 ns | 1.35 ns | 0.27 ns | 7.37 * | |||||
| Control | 0.0157 ± 0.0079 a | −0.0534 ± 0.0097 a | −0.1585 ± 0.1184 a | 241.48 ± 15.15 a | 848.96 ± 74.01 a | |||||
| Low copper | 0.0042 ± 0.0090 a | −0.0623 ± 0.0107 a | −0.3063 ± 0.1803 a | 227.05 ± 18.67 a | 772.51 ± 93.94 a | |||||
| High copper | 0.0048 ± 0.0069 a | −0.0518 ± 0.0133 a | −0.2243 ± 0.1979 a | 248.48 ± 24.92 a | 1859.2 ± 605.3 b | |||||
| Intra-subjects | ||||||||||
| Herbivory treatment | 1.03 ns | 0.015 ns | 8.49 ** | 0.36 ns | 7.4 * | |||||
| Control | 0.0150 ± 0.0077 a | −0.0503 ± 0.0085 a | 0.0081 ± 0.1610 a | 231.90 ± 14.89 a | 794.97 ± 81.11 a | |||||
| Herbivory | 0.0035 ± 0.0055 a | −0.0609 ± 0.0091 a | −0.4357 ± 0.0703 b | 244.88 ± 15.68 a | 1379.6 ± 331.2 b | |||||
| Copper × Herbivory | 1.77 ns | 20.24 *** | 0.68 ns | 0.52 ns | 9.48 * |
| Factor | Exp (β) | β | Standard Error | Confidence Intervals 95% Lower Upper | χ2 Wald | df | p Value | |
|---|---|---|---|---|---|---|---|---|
| Copper treatment | 86.3 | 2 | <0.0001 | |||||
| Cu | 0.543 | −0.610 | 0.132 | 0.420 | 0.703 | 21.5 | 1 | <0.0001 |
| Cu+ | 0.288 | −1.244 | 0.181 | 0.222 | 0.375 | 86.1 | 1 | <0.0001 |
| Time (Days) | 0.849 | −0.163 | 0.009 | 0.835 | 0.864 | 349.6 | 1 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaya-Martín, S.M.; Ballina-Gómez, H.S.; Ruíz-Sánchez, E.; Azcorra-Perera, G.J.; Ruiz-Santiago, R.R.; Pierre, J.F. Interactive Effect of Copper and Herbivory on the Whole-Plant Growth of Leucaena leucocephala. Int. J. Plant Biol. 2025, 16, 76. https://doi.org/10.3390/ijpb16030076
Amaya-Martín SM, Ballina-Gómez HS, Ruíz-Sánchez E, Azcorra-Perera GJ, Ruiz-Santiago RR, Pierre JF. Interactive Effect of Copper and Herbivory on the Whole-Plant Growth of Leucaena leucocephala. International Journal of Plant Biology. 2025; 16(3):76. https://doi.org/10.3390/ijpb16030076
Chicago/Turabian StyleAmaya-Martín, Shirley Margarita, Horacio Salomón Ballina-Gómez, Esaú Ruíz-Sánchez, Gabriel Jesús Azcorra-Perera, Roberto Rafael Ruiz-Santiago, and Jacques Fils Pierre. 2025. "Interactive Effect of Copper and Herbivory on the Whole-Plant Growth of Leucaena leucocephala" International Journal of Plant Biology 16, no. 3: 76. https://doi.org/10.3390/ijpb16030076
APA StyleAmaya-Martín, S. M., Ballina-Gómez, H. S., Ruíz-Sánchez, E., Azcorra-Perera, G. J., Ruiz-Santiago, R. R., & Pierre, J. F. (2025). Interactive Effect of Copper and Herbivory on the Whole-Plant Growth of Leucaena leucocephala. International Journal of Plant Biology, 16(3), 76. https://doi.org/10.3390/ijpb16030076






