1. Introduction
Ascorbic acid (ascorbate—ASC) is an important water-soluble vitamin essential for the biosynthesis of collagen, carnitine and neurotransmitters [
1,
2]. Unlike most animals and plants, humans cannot synthesize vitamin C, making plants the primary source of this vitamin for human health. Beyond its role as a vital nutrient for humans, ascorbic acid also plays a significant role in plant development. The first known report on the effects of ascorbic acid in plants was published in 1937, five years after the discovery of ASC [
3].
Ascorbic acid functions as a major antioxidant and redox buffer in mature plant tissues [
4]. In plants, ascorbate predominantly exists in complexes with flavonoids and other substrates. Beyond its antioxidant properties, ASC serves as a critical reducing substrate for at least eight enzymatic reactions. The highest ascorbate levels are typically associated with differentiated plant tissues. However, the mechanisms by which ASC influences plant development remain poorly understood.
The interaction between ascorbate and plant hormones—particularly auxin—has been known since 1948 [
5]. Since then, numerous studies have investigated the effect of ascorbate feeding on the development of isolated organs and ascorbate interaction with the hormonal system [
6,
7,
8,
9,
10].
One of the first comprehensive reviews on the effects of ascorbate supplementation on plants and plant cells was published in 1994 [
7].
For the most recent comprehensive summary on the topic, I can mention two reviews [
11,
12].
However, ascorbate feeding needs to be considered with some precautions: it is a unique compound with very specific behavior, and in many cases, the observed effects are not due to ascorbic acid itself but rather to its degradation or conversion products [
13].
A common application of ASC in plant tissue culture is for preventing tissue browning by using high concentrations of ascorbate (1–5 mM) [
14,
15]. Surprisingly, in these papers there is little information about the “fate” of ASC in the culture medium or its uptake kinetics. It has been shown that ascorbic acid is unstable in aqueous solutions with a pH range of 5 to 6, the typical pH of plant tissue culture media [
16]. The primary oxidation product of ASC, dehydroascorbate, is also highly unstable and is further oxidized to oxalic acid [
17].
The identification of ascorbate-deficient mutants has allowed researchers to bypass the issue of exogenous ascorbic acid conversion and focus on intracellular ASC. Studies on the effects of low ASC levels on root architecture and its interaction with auxin signaling have been conducted using the
vtc1 and
vtc2 mutants of Arabidopsis thaliana. These mutants exhibit ASC levels of only 25% (
vtc1) [
18] and 10% (
vtc2) [
19] of the wild-type across all tested organs. Interestingly, significant differences in primary root growth, root apical meristem (RAM) organization and lateral root patterning were observed in the
vtc1 mutant, but not in the
vtc2 mutant, when grown on standard agar medium. These differences were linked to stem cell function, auxin maxima formation and the protein level of auxin transporters (PIN genes).
However, the defects observed in the
vtc1 mutant did not appear to be directly caused by low ASC content, as these phenotypes were not replicated when the mutants were grown in liquid medium or in a medium with low ammonium [
20]. In contrast, the
vtc2 mutant showed no significant differences in root architecture under the control conditions.
Pharmacological studies using ASC feeding experiments have revealed strong interactions between ASC and the various auxin transport/metabolism mutants [
10]. At the organ level, ASC feeding significantly downregulated auxin-mediated cell cycle activation [
13].
Moreover, ASC were widely used in plant tissue culture as a browning (secondary metabolites) inhibitor [
15]. In a majority of cases researchers simply added ascorbic acid to the culture medium, ignoring the chemical instability of the compound [
13]. This topic requires more detailed investigations.
In this study, I conducted detailed investigations of the exogenous ascorbate degradation kinetics in the culture medium, and its uptake and recovery within plant cells. Using ASC-deficient mutants and ascorbic acid feeding experiments, I demonstrated a potential link between ascorbate and the plant hormone auxin. Based on the data presented, I propose that ASC plays a regulatory role in plant development, primarily through its interaction with auxin signaling pathways.
2. Materials and Methods
2.1. Plant Material and Growth Conditions
The plant varieties used in these experiments were wild-type
Arabidopsis thaliana (L.) Heyhn. with different Arabidopsis mutant alleles for the Ascorbate biosynthesis genes:
vtc1 NASC ID: N8326 [
18] and
vtc2 NASC ID: N888262 [
19], early auxin response markers DR5::GUS and DR5::GFP [
21], auxin transport mutant
pin3 [
22], auxin transporter markers PIN3::GUS/PIN4::GUS [
22] and auxin response marker HS::AXR3NT–GUS [
23].
Seeds were surface-sterilized and planted in square Petri dishes in ½ Murashige and Skoog medium [
24] or TK1 [
25] containing 1% sucrose, 5 mM MES and 1.1% agar (Roth)set at a pH of 5.6. Seeds sown on these plates were kept at room temperature for 4 h to allow for water uptake, then transferred to a cold room at 4 °C for 12–14 h and finally grown in plates placed vertically in an incubator kept at 22 °C in a 16 h/8 h day night cycle under white light with an intensity of 80 µmol/s/m
2. Dishes were scanned with an Adobe 950 Scanner and root lengths were measured using Image J software version 1.54f (
http://imagej.nih.gov/ij) accessed on 7 April 2024.
For the pharmacological treatments, ASC/DHA were prepared as stocks, where the pH of ASC was adjusted, filter-sterilized through a 0.22 µm filter (Roth) and added to the medium after it had cooled to 40 °C. Plants/seedlings were loaded to the medium immediately after its solidification to avoid ascorbate degradation. All experiments were performed in at least 3 independent biological replications with at least 10 plants in each.
2.2. Isolated Root Experiments
For experiments regarding isolated roots, 6–7-day-old seedlings of all the required marker lines were used. Primary roots were excised under a stereomicroscope and placed in a 40 mm Petri plate containing liquid medium. Subsequently they received treatment with exogenous auxin and ascorbate (as indicated for the different experiments), after which they were used for a GUS assay and morphological analysis.
2.3. Whole-Mount In Situ Immunolocalization
Immunolocalization in Arabidopsis plants was performed according to a whole mounting in situ protocol [
26]. Affinity purified primary anti-PIN1 (mouse, clone 10A7), anti-PIN2 (guinea pig, clone A192) and anti-PIN4 (rabbit, clone 9105) antibodies were diluted 1:40, 1:400 and 1:400 respectively [
27]. The secondary Alexa-488/Alexa 555 conjugated anti-guinea pig, anti-rabbit or anti-mouse antibodies were diluted 1:400.
2.4. GUS Assay
HS::AXR3NT-GUS seedlings were treated with DHA for 6 h. NAA was subsequently added for the next 90 min, and the seedlings were subjected to heat shock at 37 °C for 90 min. Thereafter, the seedlings were soaked in the GUS staining solutions.
DR5::GUS reporter activity was determined using a standard GUS histochemical staining procedure. The seedlings were soaked in the GUS staining solution (1 mg mL−1 X-GLUC (Calbiochem® N203783) in NaP buffer (pH7.0), containing 1 mM EDTA and 2 mM ferric cyanide) at pH 7.0. Incubation for 1–8 h was performed at 37 °C, and explants were immersed in methanol thereafter. After 15 min of incubation, water was added gradually until a final methanol concentration of 15% was obtained. The samples were washed several times with water and cleared in 8:3:1 (w:v:v) chloral hydrate/distilled water/glycerol for 10 min. Samples were visualized using Nomarski optics on an Axioplan 2 (Zeiss, Oberkochen, Germany) microscope, using the Axiovision 3.1 (Zeiss, Jena, Germany) software for image processing.
2.5. HPLC Determination of ASC and GSH
Samples for the determination of the low molecular weight antioxidants were prepared by weighing tissue and grinding samples in 3% (
w/v) m-phosphoric acid (MPA) in Eppendorf tubes. The tubes were subsequently centrifuged at 50,000×
g for 15 min at 4 °C and stored at −80 °C before analysis. For the determination of antioxidant in the medium, an equal amount of the medium was mixed with 6% MPA. Total ASC was determined by reducing 20 µL of each sample with 10 µL of a solution made up of 200 mM dithiothreitol and 400 mM Tris (at a final pH of 6). After 20 min incubation at room temperature, samples were acidified again by addition of 10 µL 3% m-phosphoric acid and kept at 4 °C before analysis. Antioxidant determination was carried out by reverse phase HPLC, as previously described [
28]. In short, separation occurred over a reverse phase type C-18 column (3 µm particle diameter, Polaris 3 C-18, Chromsep SS 100 × 4.6 mm, Varian Europe, Middelburg, The Netherlands), which was kept at a constant temperature of 40 °C in a column oven (CTO-10AVP, Shimadzu, The Netherlands). The mobile phase consisted of 2 mM KCl, set at pH 2.5 by dropwise addition of concentrated o-phosphoric acid. Oxygen was removed from the mobile phase by passing the flow through a degasser (DGU-14A, Shimadzu, The Netherlands). Detection was via a diode array (SPD-M10AVP, Shimadzu, The Netherlands), measuring between 196 nm and 250 nm, and set in tandem with a home-made amperometric detection system (glassy carbon working electrode, calomel reference electrode, reference potential 1000 mV). Chromatogram analysis was performed with the ClassVP software package (Shimadzu HPLC Class VP 612 SP 5, Shimadzu, The Netherlands) [
25].
2.6. Statistical Analysis
Each experiment was independently performed at least three times. Data were statistically analyzed using Student’s t-test for comparisons between two treatment groups.
3. Results
3.1. Ascorbate Behavior in the Culture Medium
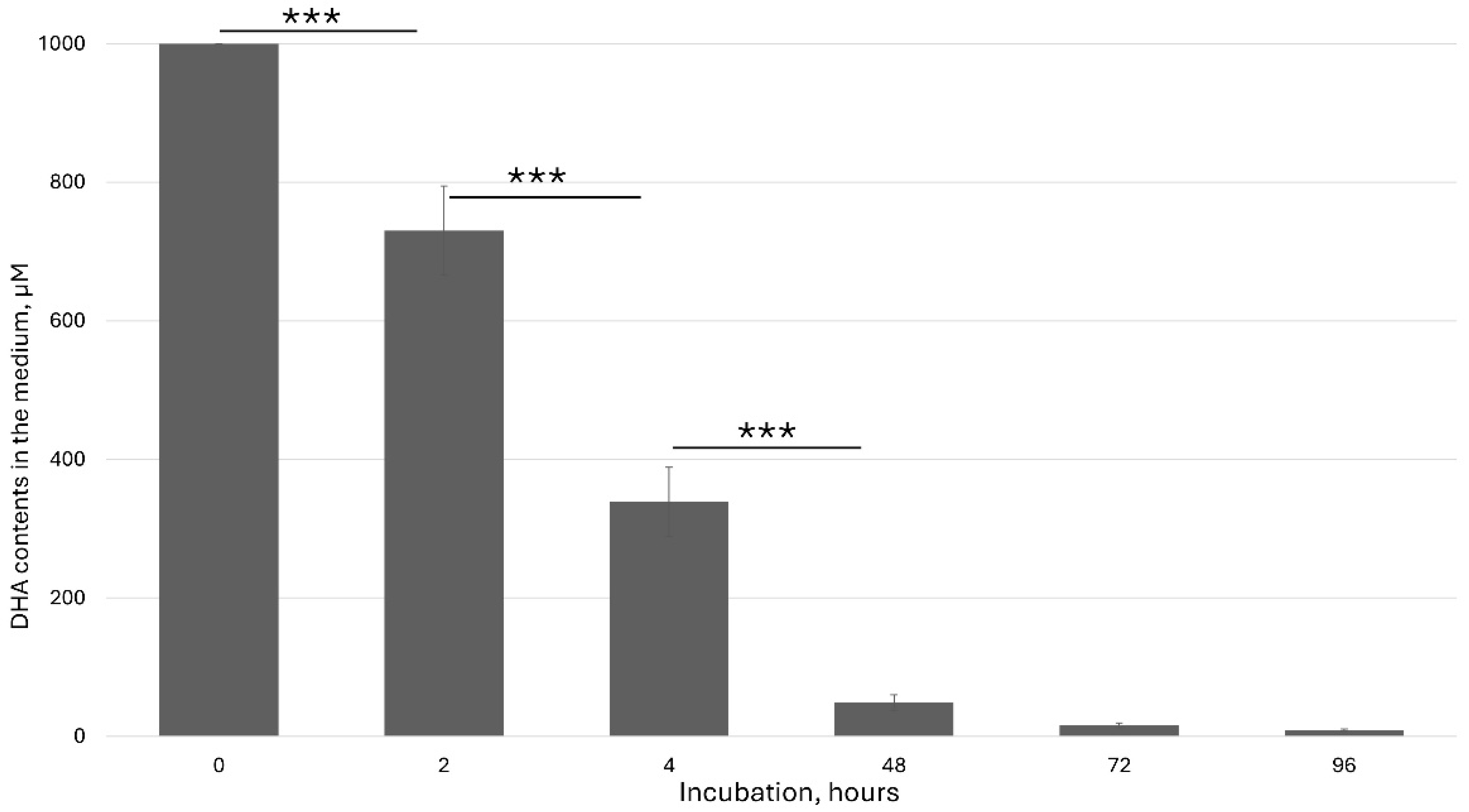
Many experiments with the plants in vitro were performed through ascorbate feeding. In many cases, authors added a high ascorbate concentration to the culture medium and studied effect of this addition. However, the chemical properties of ascorbate in the medium have been ignored. So, before feeding experiments, it is necessary to precisely measure and characterize the ascorbic acid content in the medium. I used HPLC to detect the kinetics of the ascorbate behavior in the culture medium. The degradation of ascorbic acid in the presence of ions in the culture medium has been considered one of the major factors in data interpretation.
Ascorbic acid is a furan-based lactone of 2-ketogluconic acid. However, being a good electron donor, excess ascorbate in the presence of free metal ions, mainly iron, can not only promote but also initiate free radical reactions, thus making it a potentially dangerous pro-oxidative compound in certain metabolic contexts. Ascorbate solution stability is pH dependent. It is the most unstable at pH 5–6, which has a significant effect on ASC feeding in vitro. Under this pH range, the ascorbate rapidly oxidized to dehydroascorbic acid (DHA). Aqueous solutions of DHA, in turn, are also very unstable, undergoing hydrolysis with a half-life of a few hours at room temperature. Decomposition products include diketogulonic acid, xylonic acid, threonic acid and oxalic acid [
29].
In our experiments, freshly added dehydroascorbic acid (DHA) was rapidly taken up by plant cells and also underwent oxidation.
Figure 1 shows the kinetics of the DHA concentration in the culture medium.
3.2. ASC Content in Different Plant Organs and in the Feeding of Arabidopsis Seedlings
In order to better understand ASC’s biological function, I investigated in detail the compound’s distribution between different organs. For this aim, I measured ASC and GSH content in different species and in different organs. It was shown that in Arabidopsis, lime tree, maize, Dactylis, etc., photosynthetic organs are the main ascorbate sources (
Figures S1 and S2). Interestingly, generative organs, while having a higher ASC content per FW, also exhibit a significantly higher GSH/ASC ratio. This suggests that ascorbate and glutathione play a different role in plant development.
Next, I checked the possibility of ascorbate feeding by incubating the plant material in freshly prepared solutions. Since ascorbate is a negatively charged molecule that cannot be taken up directly, and considering its rapid degradation and pH modification, I used DHA and monitored its behavior in plants. Several systems were tested, including
Medicago sativa leaves/protoplasts [
30],
Nicotiana tabacum seeds/protoplasts [
13] and
Arabidopsis thaliana seedlings (
Figure S2).
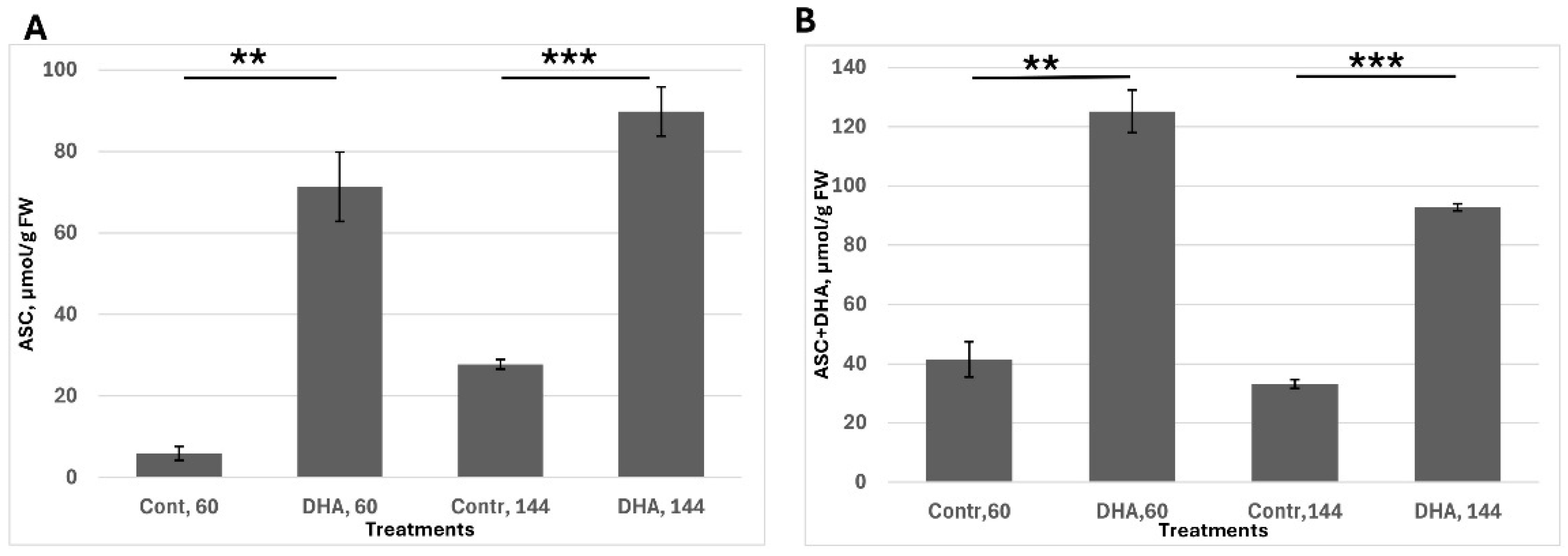
The second task was to assess ascorbate uptake, stability, recovery capacity and the ability of plant tissue to maintain a high ascorbate pool; seeds were pre-germinated for 30 h, corresponding to the stage of endosperm rupture. Following this, seeds were transferred to a medium containing 1 mM dehydroascorbic acid (DHA). Ascorbate levels were measured at 60 h—when radicle emergence is visible but the cotyledons remain inactive—and at 144 h, when seedlings are fully developed and metabolically active. This experimental setup enabled evaluation of the tissue’s capacity to uptake DHA, reduce it back to ascorbate and sustain redox balance over time (
Figure 2).
I found that the ASC content was significantly upregulated after 30 h of DHA exposure (i.e., 60 h from soaking). However, a large proportion of ascorbate remained in its oxidized form, likely due to relatively low internal cellular activity. By 144 h (in 6-day-old seedlings), the redox balance had shifted, with nearly 90% of the ascorbate present in its reduced form (ASC). In general, ASC content after treatment remained significantly higher than in the control.
Considering the previous figure (
Figure 1) about DHA degradation and based on
Figure 2, I could conclude that DHA feeding can serve as an effective tool to study the ascorbate effects on plant development.
3.3. Ascorbate Has a Concentration-Dependent Biphasic Effect on Root Development
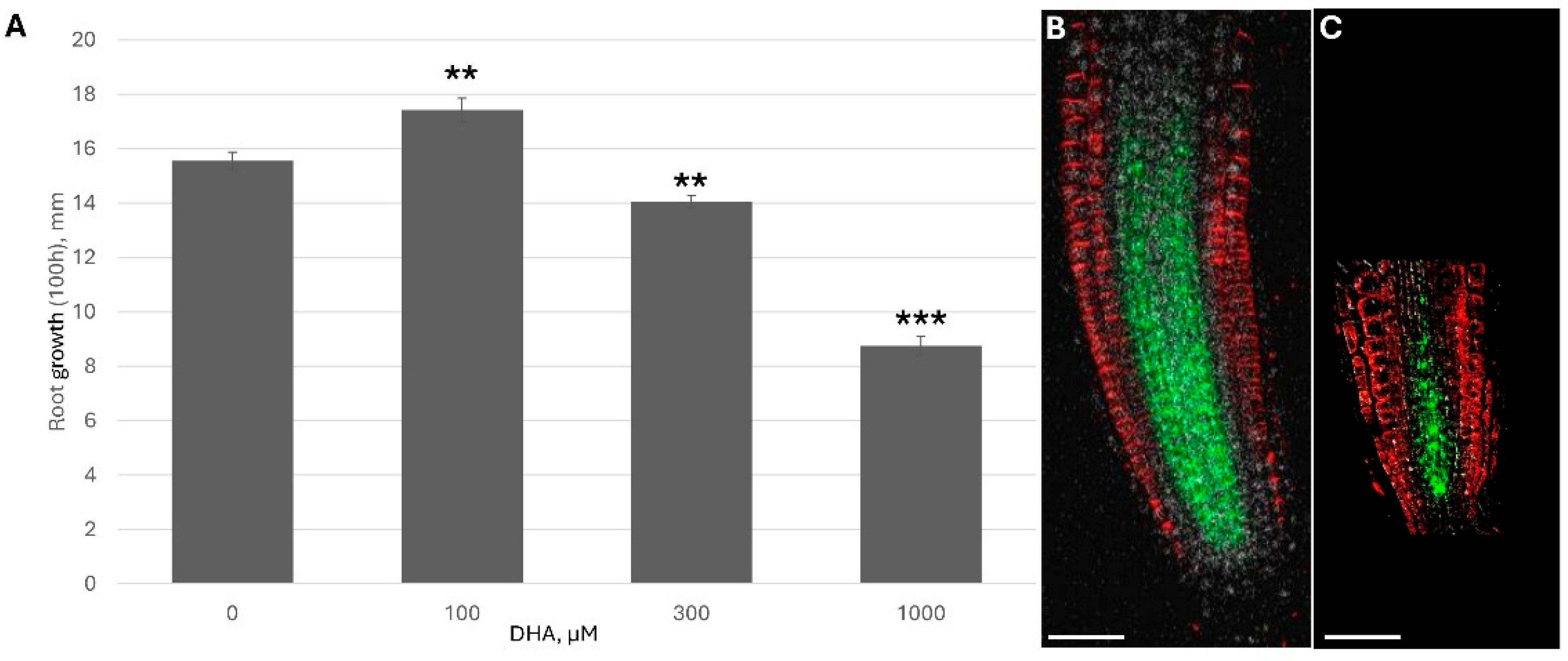
Because DHA/ASC is rapidly uptaken by plants and significantly increases the reduced ascorbate content inside the plant (
Figure 2), I monitored how ascorbate addition affected plant growth under different ASC concentrations.
The dose-response curve showed two clear opposite trends: low ascorbate feeding accelerated root growth while higher feeding showed significant inhibition and shortening of the root apical meristem (RAM) (
Figure 3). A higher concentration of DHA led to the significant downregulation of PINs (
Figure 3B and
Figure S5). For example, under 1 mM DHA, PIN1 fluorescence per RAM was only 38.1 ± 3.5% of the control.
According to the published literature, root growth depends on both the transport of shoot-derived auxin and the auxin response within root cells [
31,
32].
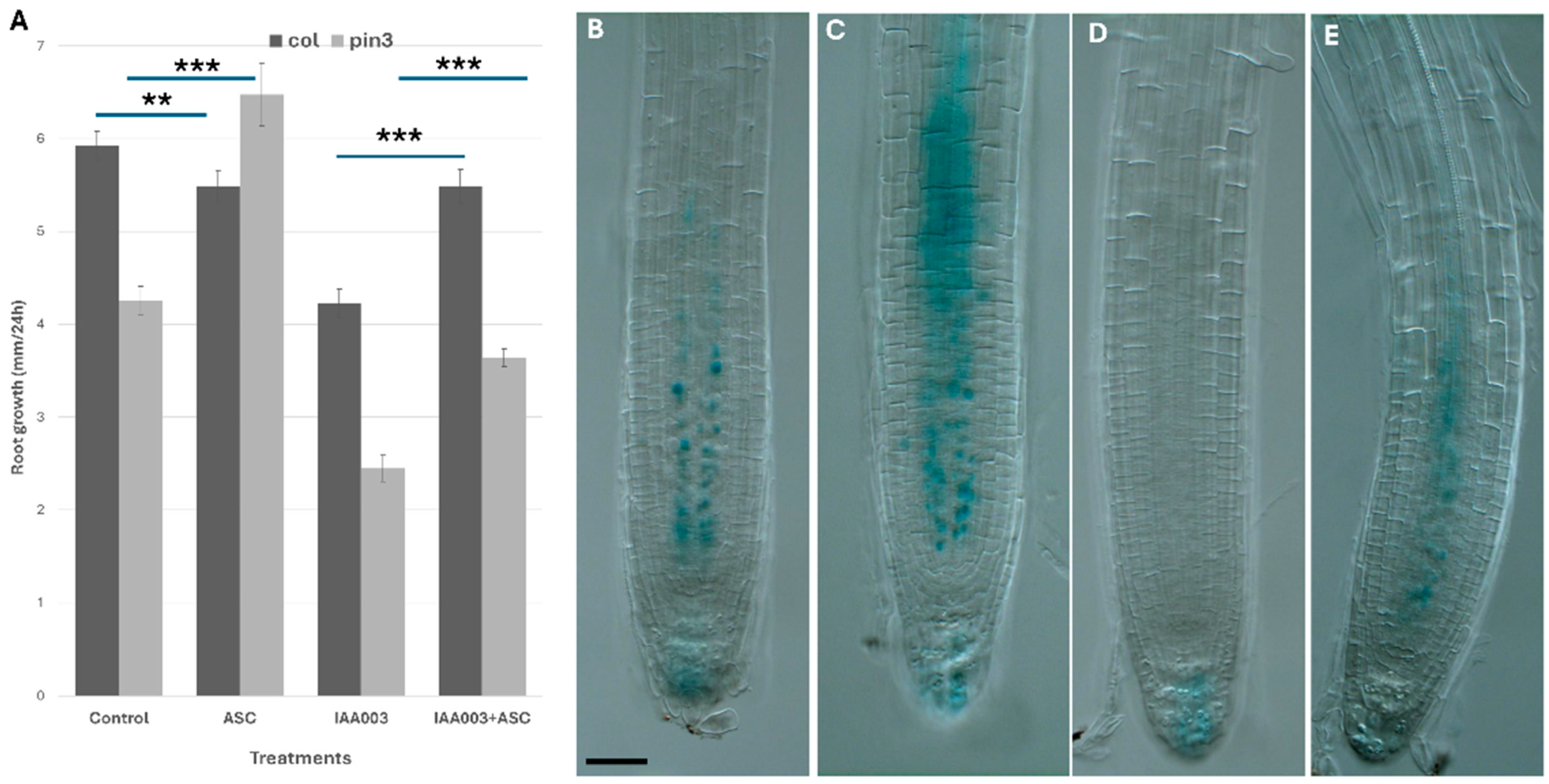
To distinguish between these effects, I studied the auxin transport mutant pin3 and examined the impact of exogenous ascorbate (ASC) and auxin treatments on its growth (
Figure 4A). PIN3 is localized in the stele and facilitates the transport of shoot-derived auxin from the elongation zone to the quiescent center (QC) [
33].
Ascorbate feeding (300 µM) restored the pin3 mutant defect. Moreover, the application of exogenous IAA (150 nM) severely inhibited root growth by suppressing pericycle elongation via an increased auxin response. Co-application of ascorbate partially reversed the inhibitory effects of IAA and restored root growth along the longitudinal axis.
Next, I checked AXR3 degradation after ascorbate feeding and exogenous auxin application. It was shown that a high dose of ascorbate feeding reduced auxin response both in control and after auxin treatments.
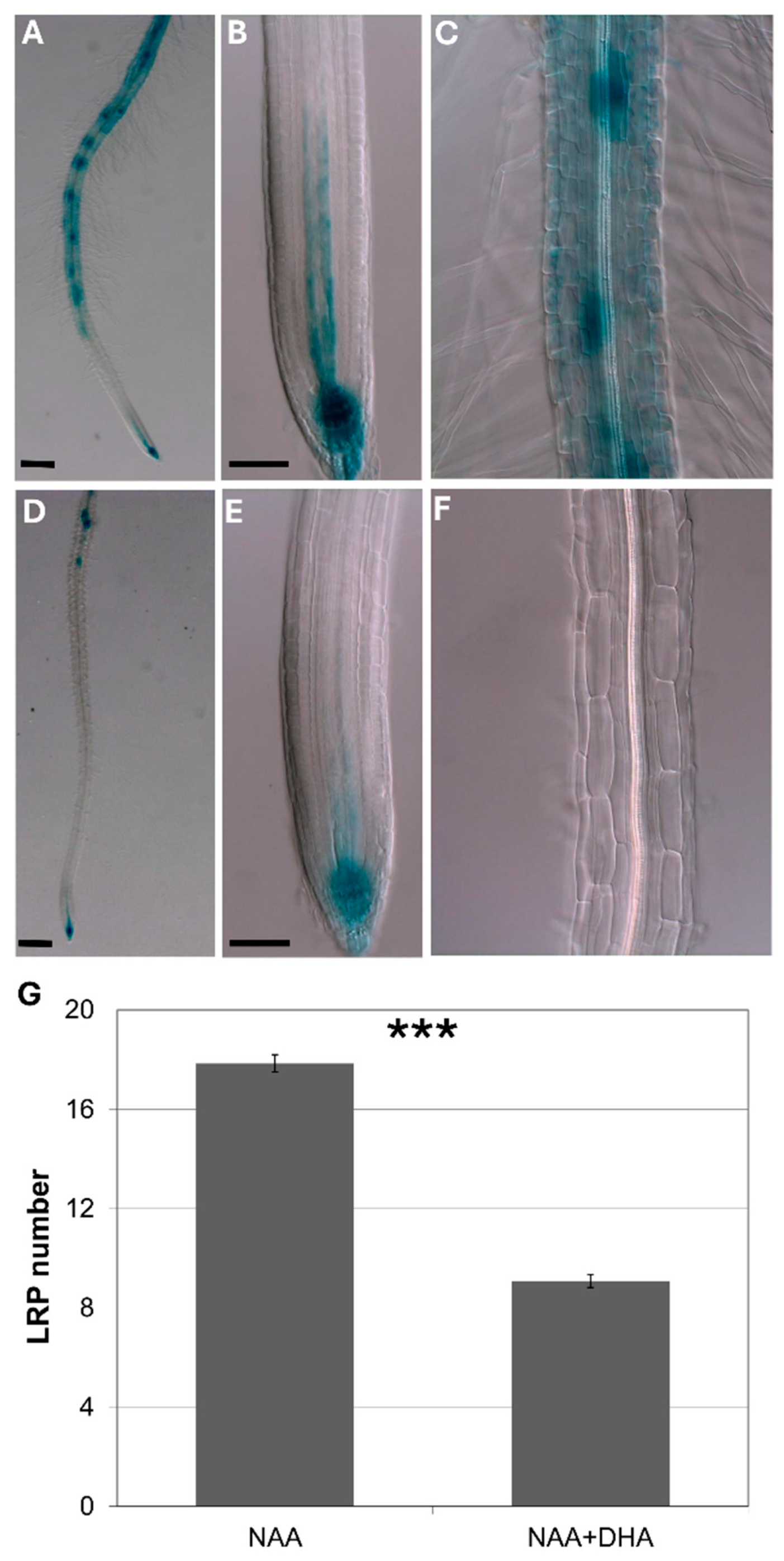
In order to further elucidate the effect of ascorbate on auxin response, I studied how pre-feeding with ascorbate affected early auxin response as the DR5 signal. I found that ascorbate feeding inhibited both early auxin response and later auxin effects on lateral root induction.
To avoid the influence of auxin transport from the shoots, I used isolated roots pre-fed with ascorbate for 8 h, followed by incubation with exogenous auxin.
Figure 5 shows that, in isolated roots, ascorbate feeding significantly inhibited both the DR5 signal and de novo lateral root induction.
3.4. Ascorbate-Deficient Mutants Have Little Effect on Root Development
To further elucidate the role of ascorbate in plant development, I investigated the ascorbate-deficient mutants vtc1 and vtc2.
3.4.1. The vtc1 Mutant as a Tool to Study ASC Deficiency
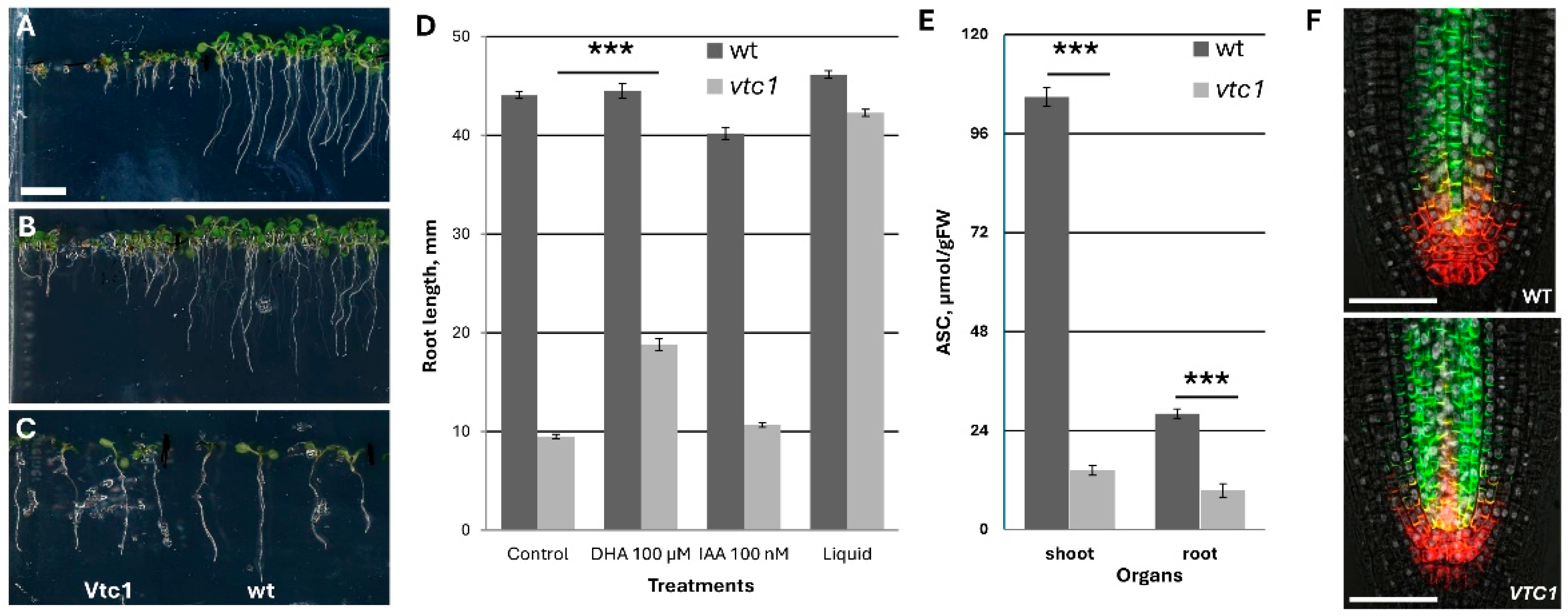
Historically,
vtc1 is the first mutant with ascorbate deficiency and contains only 25% of ascorbate at four and six days in the culture. However, later with leaf maturation, the differences is increased to 12–15% in the shoot part, while it remains at 30% in the aboveground part (
Figure 6E). This mutant is very popular in the investigation of ascorbate deficiency. During growth in ½ MS medium, the root growth demonstrated kinetics similar to wt up to day 5 with similar RAM structure and auxin transporter localization. However, thereafter, the root almost stopped growth, possessing rapid differentiation and formation of a defective lateral root (
Figure 6A). This defect could be partially rescued by the application of 100 µM DHA, and much less by the application of 100 nM IAA. Surprisingly, the transfer of 4-day-old seedlings to the liquid medium with the same composition (½ MS salt) led to the almost full recovery of root and shoot growth (
Figure 6D). Interestingly, in the liquid medium, the ascorbate content remained similar as the content in the seedlings grown on agar medium (only 25% from the control).
Altogether, I concluded that the vtc1 mutant cannot be used in the study of ascorbate metabolism/deficiency in Arabidopsis on a solid medium.
3.4.2. The vtc2 Mutant as a Tool to Study Ascorbate Metabolism
In contrast to
vtc1, the
vtc2 mutant has even less ascorbate content (10%), demonstrated with significant similarity to the
vtc1 mutant compared with the wt (
Figure 7) and was strongly dependent on the conditions. Namely, at a lower temperature (21 °C) and ½ MS salt, the
vtc2 mutant was slightly shorter than the wt and kept similar differences after cultivation in the optimal medium. However, at a higher temperature, the differences between the wt and
vtc2 plants remained only when grown in the optimal medium, which meant a higher sensitivity of
vtc2 to temperature rise. Interestingly,
vtc2 also showed less lateral root primordia under all conditions tested.
4. Discussion
Ascorbate (ASC) is a multifunctional metabolite essential for numerous cellular processes in both plants and animals. One of its key roles in plants is the scavenging of reactive oxygen species (ROS), particularly in mature tissues. This role aligns with its distribution pattern: ASC accumulates most abundantly in mature, photosynthetically active tissues such as chloroplast-rich leaves where ROS production is high. This high ASC content, along with active chloroplast development and elevated photosynthetic activity, underscores the importance of stromal ascorbate peroxidase (sAPX) and thylakoid-bound ascorbate peroxidase (tAPX) in regulating photosynthesis and detoxifying ROS in chloroplasts. This pattern has been consistently observed across multiple plant species [
30,
34].
ROS play a dual role in plant physiology. At low levels, they are essential signaling molecules involved in processes such as cell cycle progression. However, excessive ROS accumulation is toxic. In roots, ROS are linked to polar auxin transport and auxin signaling responses [
35]. ASC helps maintain ROS homeostasis in various tissues by acting as both a redox buffer and a cofactor for ascorbate peroxidases. In addition, ASC interacts with hormonal pathways that regulate growth and stress responses (for a review, see [
36]).
In this study, I demonstrated that ascorbic acid degrades rapidly in the plant culture medium, with a half-life of approximately 5–6 h [
13]. This emphasizes the necessity of using freshly prepared ASC solutions in feeding experiments. Once taken up—primarily in the oxidized form as dehydroascorbic acid (DHA)—ASC is regenerated intracellularly, maintaining optimal redox potential (
Figure 2).
Given ASC’s central role in ROS detoxification, and the influence of ROS on both auxin transport and response [
37], it is logical that exogenous ASC may have a dual effect on root development. Indeed, I observed a two-phase response to ASC feeding. At low concentrations, ASC promoted root growth, likely through the enhancement of auxin transport (
Figure 3). This was supported by the partial rescue of auxin transport mutants and the upregulation of auxin transporters in the root stele (
Figure S6).
Conversely, higher ASC concentrations suppressed root growth, likely by interfering with auxin signaling—possibly through the effects on AUX/IAA protein degradation (
Figure 4 and
Figure S5).
Another key focus was ASC’s role in cell cycle regulation. While high ASC concentrations are known to inhibit proliferation in animal cells [
38,
39], its role in plant cell division is less understood. De Pinto et al. [
40] demonstrated that the ASC:DHA ratio affects the cell cycle in BY-2 cells. Other studies have shown ASC influences cell division in various plant tissues, including the quiescent center [
41] and tapetum cells [
42], though often without accounting for local hormone signaling.
In previous work, I showed that high ASC levels inhibited cell cycle activation in protoplasts [
13]. In the current study, I extended this observation to intact Arabidopsis seedlings, showing that high ASC levels inhibited both root growth and cell cycle activation in the pericycle. Notably, high ASC feeding significantly suppressed the formation of new lateral root primordia, in parallel with a reduced auxin response as shown by DR5::GUS expression (
Figure 5).
I also investigated ASC effects in ascorbate-deficient mutants. A crucial consideration was the degree of ASC deficiency in roots versus shoots. Measurements showed that the ASC deficiency in roots was much less severe, likely explaining the limited mutant phenotypes in root tissues. Notably, the
vtc1 mutant displayed a conditionally strong phenotype that was fully rescued in liquid culture (
Figure 6), suggesting that the
vtc1 mutant should be used with caution when studying ASC deficiency.
In contrast, the
vtc2 mutant presented a more intriguing case. Despite having only 10% of the wild-type ASC content,
vtc2 roots and seedlings developed relatively normally under standard conditions. However, under optimal growth conditions, root growth was inhibited (
Figure 7). Both
vtc1 and
vtc2 mutants produced viable seeds in vitro, though this was only in the presence of exogenous sucrose [
25].
ASC feeding is commonly used in tissue culture, primarily to prevent tissue browning. However, this requires an understanding of ASC chemistry. In culture medium, ASC is rapidly oxidized to DHA [
16], and at pH 5–6, DHA is further degraded to diketogulonic acid [
43]. Thus, only freshly prepared ASC solutions should be used to ensure effective uptake.
Importantly, ASC itself—being a charged molecule—is not taken up directly by plant cells. Rather, DHA is the main form transported into cells, likely via glucose transporters [
44,
45]. Once inside, DHA is efficiently reduced back to ASC, allowing intracellular ASC levels to remain high for at least seven days after treatment.
6. Conclusions
In plants, ascorbate (ASC) predominantly accumulates in mature tissues, with limited involvement in early developmental processes. This study demonstrated that exogenous ASC feeding has concentration-dependent effects on root development: low ASC levels enhanced root growth, likely by promoting auxin transport to the root tip, whereas higher concentrations suppressed auxin responses and inhibited root development.
Despite their low ASC content, the vtc1 and vtc2 mutants exhibited minimal developmental and reproductive defects under standard in vitro conditions, suggesting a restricted role for ASC during early seedling development. Notably, the vtc1 mutant displayed strong condition-dependent phenotypes, highlighting the need for careful experimental design when using ASC-deficient lines in physiological studies.