Abstract
This investigation proposes an effective protocol (cutting) for Paulownia tomentosa production in Tunisia during the 2022–2024 period. The effects of the three interactive parameters: root cutting diameter (L1, 0.5; L2, 0.8; L3, 1.25; and L4, 2 cm), indole-3-butyric acid (IBA) hormone concentrations (C, 0; T1, 0.1%; and T2, 0.3%), and soil type (S1: 50% silt + 50% potting soil, and S2: 43% potting soil + 43% silt + 14% sand), were investigated. The data showed that cutting roots with 0.5 cm dimensions, a cutting treatment with hormone (0.3%), and the S2 soil type corresponded to a growth enhancement in height. These results revealed the broad changes in flowering of P. tomentosa. Also, data revealed that the root cutting diameter had the greatest influence on the biochemical contents of 4-month-old P. tomentosa sprouts. The studied pathway revealed that the auxin precursor IBA contributes toward active auxin [indole-3-acetic acid (IAA)] biosynthesis. Overall, this study found substantial changes in the morphological, biochemical, and floral features of new P. tomentosa sprouts under the interactive factors. To summarize, vegetative propagation of Paulownia, particularly through root cutting, allows for proliferation and plantation development. These findings can be applied to future breeding efforts with Paulownia to improve and protect it as a woody species, forage, and medicinal plant.
1. Introduction
As part of the valorization of Tunisian flora, Paulownia tomentosa (Thunb.) Steud plantations of hardwood trees were studied; this is a majestic tree originating from Asia and which belongs to the Paulowniaceae family [1]. There are different species of Paulownia but the most important of them include P. kawakamii, P. catalpifolia, P. australis, P. albiphloea, P. fargesii, P. fortunei, P. elongata, and P. tomentosa [2]. P. tomentosa is considered a park and ornamental tree, and it is widespread in temperate zones [3]. In fact, this tree is largely planted for its multiple uses in the environment (afforestation, reclamation of degraded soils), economy (wood production), and for medicinal purposes [4].
Moreover, P. tomentosa has various assets for biodiversity: it is considered a soil builder, its leaves are rich in lipids, proteins, and minerals, and its flowers are appreciated by bees thanks to the scent of lavender. In addition, it is a nitrogen-fixing tree very resistant to high salt levels and retains soil well. This pioneer species always seeks light and lives in open habitats. In places where several other young plantations are developing, the native vegetation can be affected by a lack of light due to the rapid growth of P. tomentosa [5].
The bioclimatic boundaries of Paulownia will probably be pushed back to altitude because this species, thanks to its robustness, hardiness, and rapid growth, is one of the trees that will be able to adapt to this climate change [5]. This miraculous tree can absorb up to ten times more CO2 than most other plant species, which allows for purifying the atmospheric air in a more efficient way [6]. In addition, P. tomentosa presents a renowned ability to grow and produces important biomass (60–80 ton/ha) [6]. It is truly a providential-specific source of wood, which provides excellent material for furniture, toys, plywood, musical instruments, and packing boxes [7]. Another specificity of this species is its ability to grow in dense plantations of about 2000 trees per hectare, which makes it quite profitable from an economic point of view [8].
P. tomentosa can withstand different temperatures, and it is also known to grow at an altitude of 2000 m. Thus, under optimal conditions, P. tomentosa can expect a chest height increase of 6 m per year. However, it is a tree that has a good rooting system and adapts to various types of soils except waterlogged soils [9]. The horizontally extended root system of P. tomentosa prevents surface erosion of the soil, and thanks to the regeneration of the roots and its rapid growth, this species can survive forest fires [5]. The P. tomentosa tree adapts to various soil types, even if it resists unfavorable conditions. This species requires hard peat and prefers medium loamy soil as well as deep soil. It can grow in alkaline and acidophilic soils with a pH of 5–8 but preferably a neutral pH. P. tomentosa also grows very well in very salty and nutrient-poor soils thanks to its ability to absorb Ca++ and Mg++ ions [9].
Paulownia has been used for various herbal purposes to relieve bronchitis, especially to reduce coughing, asthma and phlegm, conjunctivitis, dysentery, enteritis, erysipelas, gonorrhea, hemorrhoids, mumps, traumatic hemorrhage, tonsillitis, and lower blood pressure [10,11]. It is previously indicated that P. tomentosa flower extracts are of particular interest, including the presence of flavonoids, and especially apigenin, which has antioxidant, anti-inflammatory, antispasmodic, and antihypertensive effects [1]. Ibrahim et al. [12] reported that the essential oil extracted from P. tomentosa flowers has antibacterial activity (Bacillus subtilis NRRL B-543, Staphylococcus aureus NRRL B-313, and Escherichia coli NRRL B-210), potential efficacy against bronchial asthma in Cavia porcellus, and neuroprotective effects to inhibit glutamate toxicity [1]. According to Bahri [13], the Paulownia cortex is also largely used to cure several diseases (dysentery, gonorrhea, and erysipelas).
P. tomentosa plants can be established using numerous types of planting stock, including bare root seedlings, seeds, containerized seedlings, and cuttings [14]. Several studies showing that Paulownia is effectively propagated by seed and several vegetative means have been limited to plant production without determining the plants’ subsequent field growth. Using traditional methods for propagation of the Paulownia tree requires large areas and large numbers of workers [15]. However, increasing demand on Paulownia in the market has pushed researchers to find appropriate methods of propagation. In another way, the plant tissue culture technique is one of methods used in the propagation of the Paulownia tree. However, there are also some inherent problems with in vitro culturing, such as (a) excessive callus growth at the base of explants and weakened axillary shoot proliferation; (b) development of adventitious shoots on the callus at the explant base; (c) explant pushed out of the medium by emerging leaves; and (d) difficulty in cultures’ observation due to large-sized leaves [16]. The dramatic success of P. tomentosa and other species’ (P. elongata, P. fortunei, and their hybrids’ results) nursery production is the major rapid and low-cost way to meet multiple human needs. The secret of this success in P. tomentosa nursery production is its ability to tolerate temperature fluctuations [17].
To succeed in the propagation of Paulownia through cutting, it is crucial to exogenously apply commercial growth hormone, such as indole-3-butyric acid (IBA) (auxin), to increase rooting. In fact, to improve the acclimatization of the new successful explants, an appropriate auxin concentration resulted in optimal rooting medium [18].
Tryptophan aminotransferase of Arabidopsis1 (TAA1) and the YUCCA enzyme family are used in the primary auxin biosynthesis pathway [19]. Tryptophan is transformed into indole-3-pyruvic acid (IPyA) by the TAA1 family of aminotransferase enzymes. The YUCCA family of flavin monooxygenase-like enzymes then transforms IPyA into IAA. The conversion of IPyA to IAA is the rate-limiting step in this process; overexpression of YUCCA family members causes high auxin levels. Furthermore, tissue-specific expression of distinct YUCCA family members allows for de novo auxin production, which drives specific elements of plant development [20]. In addition to IAA production via the IPyA route, the pool of active auxin can be regulated by inputs from other storage forms and precursors, including as IAA conjugates and indole-3-butyric acid (IBA) [21].
It is true that the Paulownia tree is able to grow on different soil types, even if the soil is degraded. But it is important to note that the different soil properties (pH, permeability, organic compounds content, etc.) determine if it is the most appropriate soil [22]. The essential idea to mention is that regardless of the texture of soil, Paulownia species improve the soil quality, resulting in its growth enhancement over time [22]. The need for the Paulownia tree is not only linked to economic, medicinal, and other needs but also to the major problem of carbon dioxide pollution caused by climatic changes on Earth. Plantations of such fast-growing tree species are required. Accordingly, scientific researchers are focused on the evaluation of the several growth conditions, seeking to produce these species under optimum conditions and not lose time.
To this end, our specific objectives were to identify a suitable method to propagate P. tomentosa trees and obtain large quantities, marking a successful procedure for its cultivation, by testing the growth ability of sprouts derived from different root cuttings, diameters, IBA (indole-3-butyric acid) levels, and types of soil under Tunisian climatic conditions. Also, we performed a phytochemical characterization of the new resulting plants based on the determination of the contents of pigments and phenolic compounds. In addition, the phenology of P. tomentosa flowers was estimated.
2. Materials and Methods
2.1. Origin and Collection of Plant Materials
Paulownia tomentosa (Thunb.) Steud grown in a parcel extending over 0.25 hectares and located at the Kef Higher School of Agriculture (ESAK) (N 36°7′15.398″ and E 8°43′12.813″; semi-arid bioclimate), Tunisia were used as a source of root cuttings. This plot was created by the Forest Ecology Laboratory at the National Institute for Research in Rural Engineering, Water and Forests (INRGREF), Tunisia in June 2021, as shown in Figure 1A.

Figure 1.
(A,B) Photographs of parcel of the original mother P. tomentosa trees at the plantation site (Kef Higher School of Agriculture) (ESAK), Tunisia in October 2021 (A) and 10 cm of root cutting diameters (L1, 0.5; L2, 0.8; L3, 1.25; and L4, 2 cm) (B).
2.2. Experimental Design: Sprout Growth
In March 2022, selected roots were cut at 10 cm length but at four different diameters (0.5, 0.8, 1.25, and 2 cm), as shown in Figure 1B. All cuttings were treated with fungicide (Fosétyl Al 80%, Aliette express) solution (0.1%) for 30 min. After that, cuttings were dried before soaking in IBA (indole-3-butyric acid) solution at three different concentrations (C, 0; T1, 0.1%; and T2, 0.3%). Cuttings were placed horizontally and immediately in plastic bags containing two types of mixed soils (S1: 50% silt + 50% potting soil, and S2: 43% potting soil + 43% silt + 14% sandy soil).
A complete randomized block design was used for field planting. At the beginning of the experiment, cuttings were moistened daily to keep a moderate moisture level. Subsequently, the roots cuttings’ irrigation was performed three times per week.
2.3. Morphological Measures of Growth Parameters of P. tomentosa Sprouts Under the Three Interactive Parameters
2.3.1. Dynamics of First-Year Stem Height and Leaf Number
One month later, the morphological measures (leaf number and stem length) were recorded every week from May to October (2022) under the effect of the three interactive parameters (diameters of root cuttings (L1, 0.5; L2, 0.8; L3, 1.25; and L4, 2 cm), hormone concentrations of indole-3-butyric acid (IBA) (C, 0; T1, 0.1%; and T2, 0.3%), and soil types (S1: 50% silt + 50% potting soil, and S2: 43% potting soil + 43% silt + 14% sandy soil).
2.3.2. Measures of Stem Height and Leaf Number of P. tomentosa Which Were Taken out Every Year in October of Both 2023 and 2024
The measures were taken in October during 2023 and 2024 of the three parameters studied.
2.3.3. Phenology of Flower Buds and Flower Numbers of P. tomentosa Under Different Interactive Treatments Effects Every Year During 2022–2024 Period
In the analysis of flower morphological and growth dynamic traits, 20 individual flowers were studied as replicates. In the fruit morphological analysis, the numbers of flower buds and flowers were recorded every year.
2.4. Biochemical Parameters of P. tomentosa Sprouts Under the Three Interactive Parameters
At the age of 4 months (5 September 2022), collection of leaves took place. Fresh samples were used to determine photosynthetic pigments, whereas other leaf samples were dried at 70 °C to be used for biomolecule content determination.
2.4.1. Photosynthetic Pigments
Fresh leaves were immersed in acetone solution (80%) and stored for 48 h at 4 °C. The plant material was ground in a mortar before centrifugation. Absorbance was detected at different wavelengths (645 and 663 nm) by a UV spectrophotometer (PG instrument, T60 UV-V/United Kingdom, Lutterworth, UK) to determine the total chlorophyll content according to Lichenthaler et al. [23].
2.4.2. Total Polyphenol Compounds
Maceration of dried leaf samples was adapted from the protocol described by Romani et al. [24]. Maceration was conducted at room temperature for 48 h with 20 mL of 2 aqueous solutions of the solvents (70% methanol) and under continuous stirring. After filtration on filter paper, the filtrates were centrifuged for 15 min at 7000 rpm and room temperature, filtered on filter paper No. 1, dried by rotavapor, and stored at 4 °C until use.
2.4.3. Determination of Total Polyphenol Compound Content
The determination of total polyphenols was carried out according to the Folin Ciocalteu (FC) method [25]. Leaf extract (100 µL) was mixed with 500 µL of the FC reagent (50%) and 400 µL of 7.5% (m/v) Na2CO3. Incubation of the mixture was performed in the dark and at room temperature for ten minutes. Absorbance was measured at 760 nm by a UV spectrophotometer. The results are expressed in mg gallic acid equivalent/g of dry plant matter with reference to the gallic acid calibration curve.
2.4.4. Total Flavonoid Content
The determination of total flavonoids was carried out according to the method described by Depheur et al. [26]. Leaf extract (1 mL) was mixed with 1 mL of 10% (m/v) AlCl3. The mixture was shaken and then incubated in the dark at room temperature for 10 min. Absorbance was measured at 415 nm using a UV spectrophotometer (PG instrument, T60 UV-V/United Kingdom). The results are expressed in mg quercetin equivalent/g of dry plant matter by referring to the quercetin calibration curve.
2.4.5. Condensed Tannin Content
Condensed tannins were determined by the acid vanillin method described by Ba et al. [27]. A volume of 200 µL of leaf extract was added to 1000 µL of vanillin reagent. The mixture was stirred and incubated in the dark at 30 °C for 20 min. The absorbance was measured at 500 nm by a UV spectrophotometer against a blank consisting of a mixture of methanol (37%) and HCl (8%) in equal volumes. The results are expressed in mg catechol equivalent/g of dry plant matter by referring to the catechol calibration curve.
2.4.6. Proline Content
Proline was extracted and assayed mainly according to the Bates method [28]. This is a colorimetric assay based on the complexation of proline with ninhydrin, after one hour at 100 °C. The complex is extracted with toluene and assayed at 520 nm compared to a standard range established with proline. Proline was extracted with 3% aqueous sulfosalicylic acid, where the dry matter/volume ratio was 10 mg of DM for 1.5 mL of acid. After centrifugation at 14,000× g for 20 min at 4 °C, the supernatant was recovered, and the pellet was taken up in 1.5 mL of sulfosalicylic acid. The absorbance was measured at 500 nm by a UV spectrophotometer.
2.4.7. Total Antioxidant Activity
Total antioxidant activity was determined as described by Prieto et al. [29]. A volume (100 µL) of the sample was added to sulfuric acid (0.6 M), sodium phosphate (25 mM), and molybdate ammonium (4 mM). The mixture was incubated in bath water at 90 °C for 90 min. Absorbance was measured at 695 nm by a UV spectrophotometer.
2.5. Data Analysis
Results from the experimental field (parameters related to the growth dynamics (stem height and leaf number), phenotypic fruit production (flower buds and flowers), and laboratory experiment (biochemical estimations) were estimated. Data of the morphological and phonological traits were submitted to one-way ANOVA in IBM SPSS (IBM SPSS Statistics for Windows, Version 30.0, IBM Corp, Armonk, NY, USA). A comparison of the means ± standard errors (SE) of 4 replicates (n = 4) was performed using Tukey’s test. Measures of the line of fit with the F test of growth parameters of P. tomentosa, which were recorded every year in October (2023/2024), and the scatter plots of biochemical data were plotted by JMP Pro 18.0.2 software (JMP statistical discovery LLC, Cary, NC, USA, 2024). The phonology of flower buds and flower numbers were plotted by Microsoft® Excel® for Microsoft 365 MSO (Version 2411). Biochemical data were analyzed with a three-way ANOVA test, and the differences between the means of 3 replicates (n = 3) were compared through Tukey’s test (p ≤ 0.05) by using IPM SPSS v. 30.0 software.
3. Results and Discussion
Because of the economic potential of Paulownia, it is propagated to utilize the advantage of its wood in many businesses; thus, certain governments invest in these trees to reduce poverty and combat COVID-19 [30]. Obtaining large quantities of plants in a short period is one of the most important challenges facing scientific researchers. The propagation of these trees faces various issues such as seed-borne diseases and pests, poor seed germination, and altered growth habits. Also, seedling growth is slow compared to root cuttings originating from plants [31]. In the asexual propagation technique, the survival and acclimation ability and costs of production (cost influences new plant price) of the new sprouts must be studied [32]. In Paulownia clonal forestry, effective vegetative propagation is crucial for producing high-quality clones of the plant species [33]. According to Magar et al. [34] and Bochnia [16], Paulownia production is more successful by the traditional technique (cutting root) when compared to an in vitro culture presenting inherent problems. Growth hormones promote plant growth, productivity, and quality. Growth regulators can also be combined with natural materials, followed by conventional breeding, to increase the Paulownia tree output [35]. In this regard, Bhojwani and Dantu [36] noted that cytokinins kinetin and zeatin are frequently utilized to increase cell differentiation and cell division, whilst auxins such as (IAA), (NAA), and (IBA) are commonly employed to boost root formation. For these reasons, in our study, cutting roots were used to test Paulownia propagation depending on the cutting diameter, soil type, and growth hormone concentration.
3.1. Morphological Traits of P. tomentosa Sprouts Under the Three Interactive Parameters
3.1.1. Kinetics of First-Year Growth Parameters
The kinetics and dynamics of growth parameters (stem height and leaf number) were recorded from May to November 2022. During the first period (May–July) of growth, the elongation of stems was more pronounced in the studied seedlings under the three interactive factors. After six months, the growth parameters dropped during September 2022, then restored their increase until the end of the year, as can be observed in Figure 2 and Figure 3. According to Maqbool and Aftab [37] and Preece et al. [38], the growth of woody species shoots is possible in winter, spring, and until fall. Accordingly, we can assess the growth of the new sprouts detected in our study until October (2022).
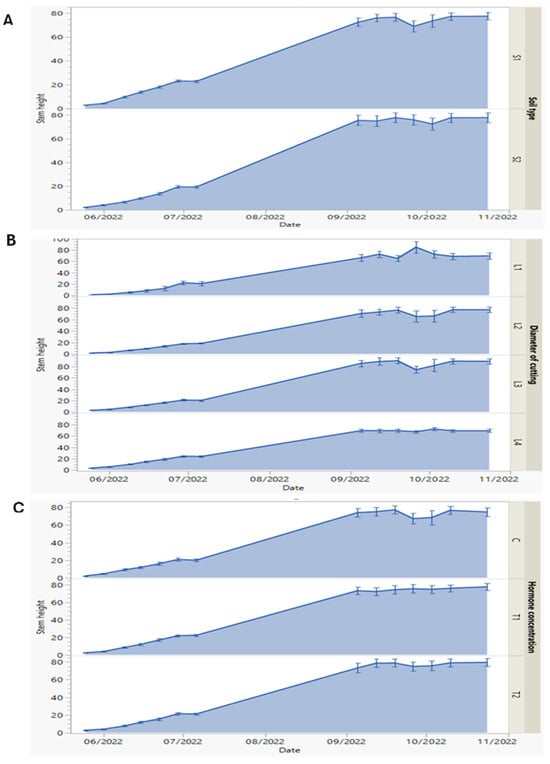
Figure 2.
(A–C) Dynamics of first-year morphological measures of stem height of P. tomentosa sprouts against soil types (S1: 50% silt + 50% potting soil, and S2: 43% potting soil + 43% silt + 14% sandy soil) (A), diameter of root cuttings (L1, 0.5; L2, 0.8; L3, 1.25; and L4, 2 cm) (B), and hormone (indole-3-butyric acid, IBA) concentrations (C, 0; T1, 0.1%; and T2, 0.3%) (C), measured every week from May to October 2022. Data represented as means and error bars.
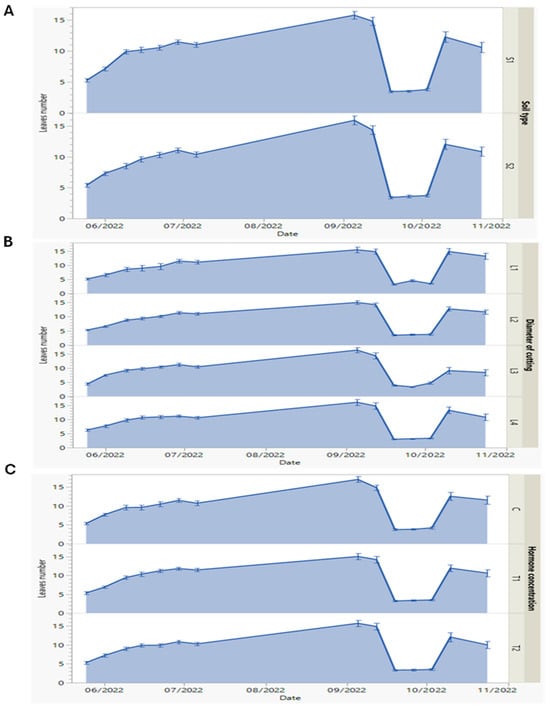
Figure 3.
(A–C) Dynamics of first-year morphological measures of leaf number of P. tomentosa sprouts against soil type (S1: 50% silt + 50% potting soil, and S2: 43% potting soil + 43% silt + 14% sandy soil) (A), diameter of root cuttings (L1, 0.5; L2, 0.8; L3, 1.25; and L4, 2 cm), (B) and hormone (indole-3-butyric acid, IBA) concentrations (C, 0; T1, 0.1%; and T2, 0.3%) (C), measured every week from May to October 2022. Data represented as means and error bars.
Regarding the interaction among the three interactive factors, the results showed mostly the same trend in elevation and decline in stem height records with some slight differences. According to the soil type factor, the stem height records increased gradually over time until reaching 26 September, when there was a slight decline, with the values 68.97 and 75.94 cm for the S1 and S2 soil types, respectively.
While the decline of stem height under the S2 soil type was recorded as 72.40 cm on 3rd October, and then it increased until reaching the final stem height record of 80 cm, as shown in Figure 2A. Moreover, we found that the noted one-week shift in growth of the juvenile Paulownia grown on the S2 soil type was related to the distribution of three types of soil (potty, silt, and sandy) when mixed. We suggest that the water state in the rhizospheres, reflecting the soil water retention capacity, affects roots development. In fact, high water retention results in an oxygen (O2) deficiency, causing a shoot growth reduction [37].
The stem height records showed differences with root cutting diameters (L1, L2, L3, and L4). On 26th September, the highest stem value was recorded as 85.33 cm with the L1 diameter. With the L2 cutting diameter, the stem height decreased to 65.79 cm, while the decline was less (74.85 cm) with the L3 diameter at the same date. In addition, the stem height showed the lowest results with L4 among the root cutting diameter records. When comparing the root cutting diameter effects, the increase in stem height was more greatly correlated when the root cutting diameter was small, as can be observed in Figure 2B. In this investigation, the cutting diameter did not directly affect the shoot growth in height. It seems that the cutting diameter affects the rooting density, causing an enhancement of shoot growth and development. In addition, the results led us to conclude that the smaller diameter (L1) and horizontal positioning correspond to more pronounced growth. Similar suggestions that there is a positive relationship between the cutting root diameter and ability to regenerate have been noted by Ede et al. [39].
Regarding hormone concentrations’ effect on the stem height (Figure 2C), we noted that the T1 and T2 concentrations showed a slight increase in their values compared to C (control). The stem height was more than 20 cm in July (2022). After four months (November 2022), the height reached 80 cm depending on the concentration of the hormone. On 26th September, the stem height decreased to 67.40 cm with the C treatment, while it increased to 75.87 and 75.22 cm under the T1 and T2 concentrations, respectively. Among the three interactive factors, the root cutting diameter factor was the most effective, as with the L1 diameter, the stem height showed the greatest value.
In our study, regardless of the hormone concentration, the root cuttings showed rooting success and shoot development, similarly to the findings of Antwi-Wiredi et al. [40]. Also, the positive effect of growth hormone application was more pronounced when the root cutting diameter was small (L1). The beneficial effect of the growth hormone was mentioned by Mohamad et al. [31], who assayed the micro-propagation of Paulownia and found that the proliferation of roots and shoots was largely induced by the presence of growth hormone (IBA).
The number of leaves is one of the important aspects of plant growth due to its direct relation to the photosynthetic capacity of the plant. Leaf numbers, which make up the second studied vegetative growth parameter, were more dramatically affected by the root cutting diameter than the two other factors (soil type and hormone concentration) (Figure 3). Firstly, we distinguished that the leaf number increased from May to September 2022. In the next month (October), spectacular numbers of leaves fell in all conditions of growth, and then their increase was restored to the end of the year. The leaf number seemed to be unaffected by the S1 or S2 type of soil, as illustrated in Figure 3A. On 26th September, the number of leaves showed the lowest value (3.53) under the S1 soil type and one almost as low (3.60) under the S2 soil type. The maximum values were recorded as 15.77 and 15.92 under the S1 and S2 soil types, respectively.
There was no significant variation (p ≤ 0.05) observed in leaf number, which confirmed that once the rooting process is well established and stem growth is pronounced, the soil type loses its influence on leaf development. This is important, because our principal aim is to research the factors affecting the growth, biomass production, and principal survival ability of the new sprouts produced. These trends were previously discussed and affirmed by Antwi-Wiredu et al. [40].
In contrast, when focusing on the effect of root cutting diameters, we found that the L3 condition produced the maximum number of leaves (16.41) in the new seedlings (Figure 3B). On 26th September, the leaves’ values showed different behavior, as 4.57, 3.68, 3.35, and 3.18 cm for the L1, L2, L3, and L4 diameters, respectively. In contrast to the rooting process, which preferred a smaller root cutting diameter (L1), the development of photosynthetic organs favored a bigger diameter (L3). This can be explained, as mentioned by Stenvall et al. [41], by suggesting that a bigger diameter of root cuttings supports the regeneration of new leaves.
The different hormone concentrations showed the same behavior as the two other factors. On 26th September, the leaf numbers decreased and were recorded as 3.82, 3.35, 3.35, and 3.38 under C, T1, and T2, respectively, as shown in Figure 3C. This is logical because the primordial role of the hormone is assigned in the first step of experiment, when forcing the rooting process and growth in height, as shown in the previous sections. Maqbool and Aftab [37] discussed similar findings in their study. On the other hand, Gerson Riffo et al. [42] found that Paulownia plantlets’ generation based on cuttings’ root propagation technique and their growth performance were principally linked to the cutting material and age of trees.
3.1.2. Measures of Growth Parameters of P. tomentosa Every Year in October of Both 2023 and 2024
To monitor new trees’ growth during 2023 and 2024, we tested their survival ability and acclimation under experimental conditions. We chose October month because after this time, growth starts to slow until it stops. In addition, Dubova et al. [43] confirmed that April–November is the vegetative period of P. tomentosa and June–October is the period of its intense growth. In fact, growth recovers every year, and comfort reflects the trees’ responses to environmental, physiological, genetic, and several other factors [44]. More precisely, factors control the growth recovery of woody species after traversing dormancy [45].
The data in this section, as demonstrated by Figure 4 and Figure 5, measure the line of fit with the F test of stem height and leaf number of P. tomentosa in October of both 2023 and 2024 under the interactive effect of the studied treatments (soil type, cutting diameters, and hormone concentrations). F statistics with two degrees of freedoms (3, 8) and stem height values showed moderate significance (p ≤ 0.01) among the T2 concentration and the other cutting diameters in the S1 soil type in 2023, while the significance was p ≤ 0.05 in 2024, as shown in Figure 4A.
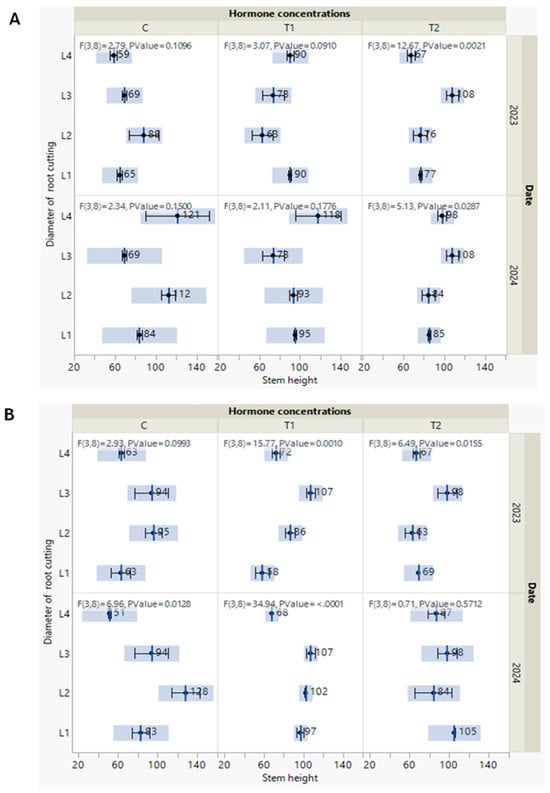
Figure 4.
(A,B) Measures of line of fit with F test of stem height of P. tomentosa, which were taken out every year in October of both 2023 and 2024 under the interactive effect of the studied treatments. Under the S1 soil type (A) and S2 soil type (B). Black point indicates the mean with standard error and F statistics, with two degrees of freedoms and the p-value.
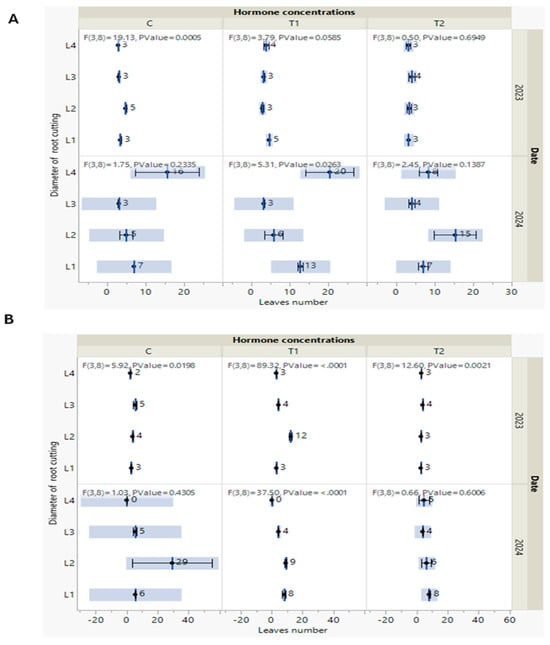
Figure 5.
(A,B) Measures of leaf number of P. tomentosa, as recorded every year in October of both 2023 and 2024, under S1 soil type (A) and under S2 soil type (B). Black point indicates the mean with standard error and F statistics, with two degrees of freedoms and the p-value.
Regarding stem height, we noted that, in October 2023, there were no distinguished effects of hormone concentrations on the stem height of the new P. tomentosa grown on S1-type soil. Except in P. tomentosa seedlings germinated from the L3 cutting diameter, stem height increased (69, 73, and 108 cm) with the hormone concentration (C, T1, and T2), respectively, in both years (Figure 4A). Concerning plants grown on S2-type soil, a dramatic change in stem height was observed in October 2024. P. tomentosa derived from the L4 and L1 cutting diameters showed an increase in stem height in parallel with augmentation of the hormone concentration. Precisely, from the control (C) to T1 and T2 concentrations, stem height increased from 51 to 68 and 87 cm in L4 and from 83 to 97 and 105 cm in L1. According to F statistics, stem height values under the T1 concentration with other root cutting diameters showed high significance (p ≤ 0.001) in both 2023 and 2024, followed by the T2 concentration, with diameters in which it displayed a moderate significance (p ≤ 0.001) in 2023 (Figure 4B).
According to these observations, and regardless of the details, all the new trees continued to grow and had distinct morphological responses. These findings are persuasive as we seek approaches to make experiments effective and grow P. tomentosa capable of producing biomass and meeting covering requirements. In addition, our findings align with the study of Antwi-Wiredi et al. [40], who consider that cutting survival is the first important success factor, followed by the second aim to produce biomass. Regarding the other vegetative growth parameter (leaf number) measured in October 2023, there were no significant effects of the hormone concentration and soil type (S1 and S2) (Figure 5). Meanwhile, in October 2024, a higher stem height was detected in P. tomentosa treated with L4/T1 and grown on the S1 soil type (Figure 5A).
In October 2024, the greater number of leaves was detected in P. tomentosa treated by L2/C (Figure 5B). It is logical that after passing the steps of survival and acclimation, reflected by shoot development in height, growth of leaves follows to secure general growth. Even though the measured parameters during 2023 and their marginal variation did not affect the leaf number, the S2 soil type was found to optimize P. tomentosa macro-propagation.
3.1.3. Generative Flowering Phenology of P. tomentosa
The flowering time is under the influence of endogenous factors (genetic) and several environmental signals (photoperiod, temperature), as well as stress factors (biotic and abiotic) [46,47]. The follow-up of flower phenology and flower buds’ emergence in P. tomentosa under different interactive treatments (root cutting diameter, hormone concentration, and soil type) was performed every year during 2022, 2023, and 2024. The general overview demonstrated different times and seasons of flower buds’ and flowers’ appearance, as illustrated in Figure 6.

Figure 6.
(A–C) Monitoring of flower bud’s emergence and maturing at the flowering stage under different interactive treatment effects over successive years in December 2022 (A), December 2023 (B), and October 2024 (C).
The first appearance of buds, which were not detected in all seedlings, was in December 2022. At this time, only L4S2 (C, T1, and T2), S2L3T2, and S1L4T2 presented flowering buds. The greater number of buds was found in S2L4C (16), followed by S1L4T2 (12)-treated seedlings (Figure 7).
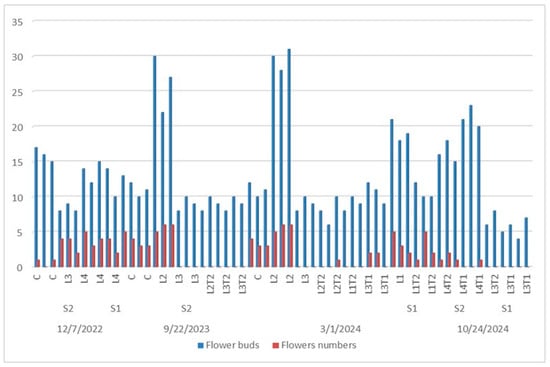
Figure 7.
Phenological (adult individuals) flower buds and flower numbers of P. tomentosa under different interactive treatments effects every year during 2022, 2023, and 2024.
The next year, bud flowers appeared in September (2023) in L4C, L4T2, L1C, L2T2, L2T1, and L3T1 growing on the S2 soil type. The greatest bud flowers (26) were detected in S2L4T2 seedlings. But, in 2024, flower buds’ appearance and flowering process took place two times in several plants. Firstly, in March, L1T1, L1T2, L2C, L2T2, L3T1, and L3T2 grew on the S2 soil type and S1L3 (T2 and C). Secondly, in October 2024, L4T1S2, L4T2S2, L3T2S1, and L3T1S1 grew. Based on these data, during the three cycles (2022–2023–2024), a higher frequency of flower buds’ appearance was found, with the maximum flower buds noted in S2L4T2 (26) and S2L2T2 (29), respectively, in March and October (Figure 7).
In this investigation, these data demonstrated the broad changes in flowering of P. tomentosa issued from a root cutting culture under several experimental conditions (cutting diameters, hormone concentrations, and soil types). The molecular study of Wang and Ding [48] suggests that the seasonal activity, dormancy growth, pleiotropic effects, juvenility, and habitat media of trees are specific factors regulating the flowering time in perennial trees. Also, Dubova et al. [43] suggested that generative organs’ development in Paulownia is largely affected by temperature. More precisely, when spring is warm, flowering starts in April. Alternatively, after a strong winter, this bloom does not take place.
As explained by Wang and Ding [48], in flowering plants, the length of the life cycle, which is considered as vegetative growth, reproductive development, and senescence succession, varies according to many factors. Linked to one factor or all these previously described ones, we can understand the flowering state shown in this study. More than that, this study suggests that there is more than one endogenous difference in new Paulownia trees grown with different parameters of micro-propagation, as fixed in our experiment.
For a greater understanding, we can compare our data to the studies of Wang [49] and Wang et al. [50], who suggested that the induction of the flowering process is related to regulating the transition from the juvenile vegetative phase to the adult one (age pathway in flowering). To that end, we must understand the margin of differences in the flowering process shown in our study.
3.2. Biochemical Contents in P. tomentosa Sprouts Under the Three Interactive Parameters
P. tomentosa is a rich source of biologically active secondary metabolites (such as flavonoids, phenolic glycosides, lignans, quinones, terpenoids, glycerides, and phenolic acids), which are traditionally used in herbal medicine. Because plants’ richness in biochemical compounds is an important parameter to evaluate [51,52], we measured the contents of several compounds.
In this investigation, data showed that compared to the hormone concentration and type of soil, the diameter of root cuttings was the major factor influencing the biochemical contents of the new 4-month-old P. tomentosa sprouts (Table 1). As shown in scatter plots of certain biochemical parameters (Figure 8), the total chlorophyll content was mostly affected by the cuttings’ root diameter. The increase in cuttings’ root diameter was accompanied by an increase in photosynthetic pigments (total chlorophyll). Under the control condition (without hormone treatment), a higher content (1.32 mg/mg FW) was observed in sprouts treated with L4CS2 and L1CS1 (Table 1). Alternatively, when cutting roots were treated with hormone concentration T1, we found that the higher content of total chlorophyll was detected in L1T2S1 and L4T2S2 (1.25 mg/mg FW) followed by L3T2S2 (1.10 mg/mg FW), L2T1S2 (0.95 mg/mg FW), and L1T1S2 (0.09 mg/mg FW).

Table 1.
Mean ± standard deviation of some biochemical parameters under the effect of three estimated variables.
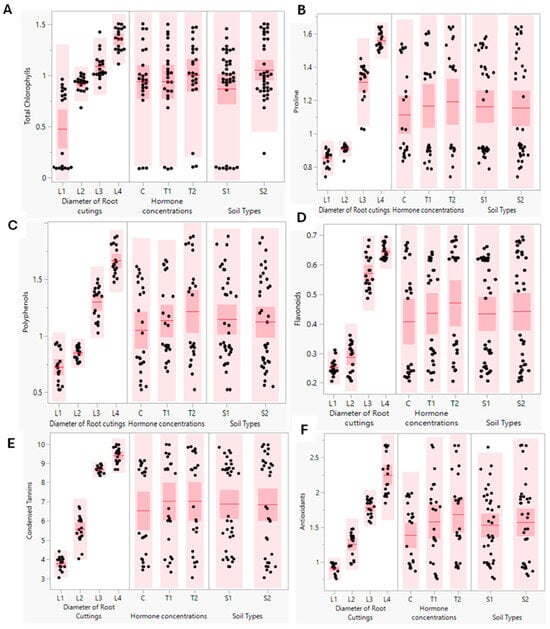
Figure 8.
(A–F) Scatter plots of some of biochemical parameters. Total chlorophyll (mg/g FW) (A), proline (nmol/g DW) (B), polyphenols (mg eq AG/g DW) (C), flavonoids (mg eq QU/g DW) (D), condensed tannins (mg eq catechol/g DW) (E), and antioxidants (mg EAG/g DW) (F) of P. tomentosa grown under three different factors (diameter of root cuttings (L1, 0.5; L2, 0.8; L3, 1.25; and L4, 2 cm), hormone concentrations of indole-3-butyric acid (IBA) (C, 0; T1, 0.1%; and T2, 0.3%), and soil types (S1: 50% silt + 50% potting soil, and S2: 43% potting soil + 43% silt + 14% sandy soil).
For proline (Figure 8), the results showed that the content of this osmoregulator was widely affected by the diameter of the cutting and the concentration of the hormone used before the start of our experiment. When the diameter of the root cutting was larger than 0.8 cm (L3 and L4), proline was highly accumulated. Plus, the increase in hormone dose was accompanied by the accumulation of proline. More precisely, the proline content varied, with 1.631, 1.41, and 0.80 (nmol/g DW) reported for L4T2S2, L3T2S2, and L1T2S2 sprouts, successively (Table 1).
According to results presented in Figure 8, there was a dramatic impact of roots’ cutting diameter on secondary metabolite contents in new sprouts of P. tomentosa. Flavonoids represented the largest groups of secondary metabolites isolated from P. tomentosa. The results revealed that total polyphenols, total flavonoids, and condensed tannin contents were augmented by increasing the root cutting diameters used to propagate P. tomentosa. Also, the increase in cutting roots’ diameter resulted in an elevation of all these measured compounds. In contrast, the soil type had no effect on the previously described contents. Based on the values presented in Table 1, we found that the total polyphenol compounds contents were detected as follows: 1.82, 1.44, and 0.57 (mg eq AG/g DW), respectively, in sprout treated with L4T1S2, L3T2S2, and L1CS2 (Table 1). These same sprouts’ total flavonoid contents were 0.68, 0.65, and 0.24 (mg eq QU/g DW), respectively. Moreover, Table 1 shows that the condensed tannin contents reached 9.81, 8.78, and 3.50 (mg eq catechol/g DW) in L4T1S2, L3T2S2, L3T1S2, and L1T2S2 sprouts, respectively.
Antioxidative activity enhancement in the different new sprouts was dramatically affected by root cutting diameters and the concentration of hormones (Figure 8). The results recorded in Table 1 show that antioxidative activity decreased when reducing the diameter of cuttings to values of 2.65, 1.87, and 0.84 (mg EAG/g DW) in L4T2S2, L3T2S2, and L1T1S1, respectively.
Three-way analysis of variance (ANOVA) of certain biochemical factors was per-formed with those as the dependent variables separately for each model (diameter of root cuttings, hormone concentrations of indole-3-butyric acid, IBA), soil types, and their interactive effects) (Table 2). Levene’s test of equality showed a strong significance (p ≤ 0.001) in all parameters except flavonoids (p ≤ 0.01). For F statistics, the diameter of root cuttings was highly significant (p ≤ 0.001) in all the measured biochemical estimates, whereas the soil type variable was not significant in any parameters. However, the hormone concentration variable was highly significant (p ≤ 0.001) in all compared parameters except for total chlorophyll.

Table 2.
Three-way analysis of variance (ANOVA) depicting some biochemical factors as the dependent variables separately for each model (diameter of root cuttings, hormone concentrations of indole-3-butyric acid, IBA), and soil types and their interactive effects).
According to the interactive factors, the diameter of cuttings along with the hormone concentrations was aligned with the effect of the hormone alone. While the diameter of cuttings and soil type interactive effect was highly significant (p ≤ 0.001) for total chlorophyll and proline, we found moderate significance (p ≤ 0.01) for flavonoids and the condensed tannins, and, finally, a non-significant effect on polyphenol and antioxidant activities. In addition, the interactive effect of the hormone concentration with the soil type was not significant in any studied parameters except for proline (p ≤ 0.05). Lastly, the interactive three-factor effect had very high significance (p ≤ 0.001) for proline, moderate significance (p ≤ 0.01) for tannins, and no significance for chlorophyll, polyphenol, and antioxidant parameters.
Independently of our experimental parameters (cutting diameter, hormone concentration, and soil type), all P. tomentosa leaves were rich in secondary metabolites. Schneiderova and Smejkal [53] demonstrated a similar richness in biologically active molecules. However, the dramatic reality is that the sprouts grown on the S2 soil type and derived from treated cuttings with a hormone presented the major sources of secondary metabolites and proline. It is well demonstrated that flavonoids constitute the most numerous groups of secondary metabolites in P. tomentosa leaves [53]. This distinction in containing such phenolic compounds explains the biological activity (antioxidant) detected in P. tomentosa [54,55,56]. Precisely, antiradical activity in the anti-DPPH assay was detected in the various leaves samples, independently of the studied parameters.
3.3. IBA-Derived Auxin Drives Aspects of P. tomentosa Root Development
Indole-3-butyric acid (IBA) is a key phytohormone necessary for multiple plant growth and developmental processes, especially for organogenesis, various plant metabolism, and environmental responses [57,58,59,60,61]. Indole-3-butyric acid (IBA) is a type of auxin precursor, which can convert to indole-3-acetic acid (IAA) by a peroxisomal β-oxidation process [62]. IBA and IAA are structurally similar except for the side chains of these hormones; IBA has a four-carbon side chain while the IAA has two carbons. Unlike IAA, which can bind to TIR1/AFB-Aux/IAA (Transport Inhibitor Responses 1/Auxin Signaling F-Box Protein-Auxin/indole-3-acetic acid) to initiate downstream auxin-responsive gene expression, IBA is unable to bind and form the auxin co-receptor complex because of its lengthened side chain [2,63].
Auxin is biologically synthesized through two sub-pathways starting from tryptophan. The first sub-pathway starts from the following genes: AMI1 (KEGG; K01426), LAX3 (KEGG; K13946), AFB3 (KEGG; K27740), ARF19 (KEGG; K14486), IAA10 (KEGG; K14484), GH3 (KEGG; K14487), and SAUR (KEGG; K14488), which encode amidase, auxin influx carrier (AUX1 LAX family), protein auxin signaling F-box 2/3, auxin response factor, auxin-responsive protein IAA, the auxin-responsive GH3 gene family, and SAUR family protein, respectively (see Figure 9). At the end of the first sub-pathway, the last three genes (IAA10, GH3, and SAUR) have an effect on the cell DNA molecule, which leads to cell control through cell enlargement or/and plant growth, as shown in Figure 9 (https://www.kegg.jp/pathway/ath04075, accessed on 21 April 2025).
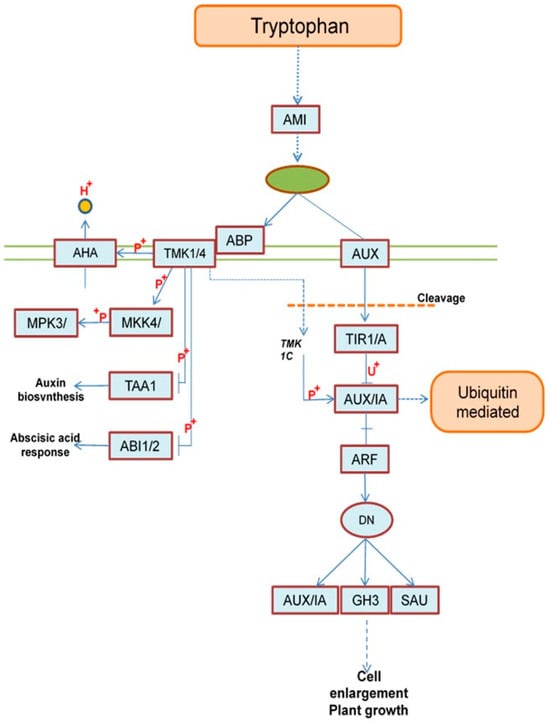
Figure 9.
Overview of auxin metabolism pathways. AMI1 (amidase), LAX3 (auxin influx carrier (AUX1 LAX family)), AFB3 (protein auxin signaling F-box 2/3), ARF19 (auxin response factor), IAA10 (auxin-responsive protein IAA), GH3 (auxin responsive GH3 gene family), SAUR (SAUR family protein), ABP1 (auxin-binding protein), TMK (receptor protein kinase TMK), AHA10 (H+-transporting ATPase), MKK4 (mitogen-activated protein kinase 4/5), MPK6 (mito-gen-activated protein kinase 6), TAR1 (L-tryptophan-pyruvate aminotransferase), and HAI2 (protein phosphatase 2C).
Meanwhile, the second sub-pathway starts from the following genes; ABP1 (KEGG; K27415), TMK (KEGG; K00924), AHA10 (KEGG; K01535), MKK4 (KEGG; K13413), MPK6 (KEGG; K14512), TAR1 (KEGG; K16903), and HAI2 (KEGG; K14497), which encode auxin-binding protein, receptor protein kinase TMK, H+-transporting ATPase, mitogen-activated protein kinase 4/5, mitogen-activated protein kinase 6, L-tryptophan-pyruvate aminotransferase, and protein phosphatase 2C, respectively (Figure 9). Also, at the end of this second sub-pathway, the last two genes are related with the auxin biosynthesis and abscisic acid response (see Figure 9 and https://www.kegg.jp/pathway/ath04075, accessed on 21 April 2025).
In this context, based on the statistical components, accompanied by biochemical and morphological data, the previous data show the effect of different concentrations of indole-3-butyric acid on the various in morphological and chemical data, individually or in combination with other factors such as the soil type and the root cutting diameter. The behavior or effect of the IBA hormone was not the dominant factor improving the morphological traits (e.g., stem height and leaf number) and various biochemical compounds (e.g., total chlorophyll, proline, total polyphenol compounds, total flavonoids, condensed tannins, and antioxidant activity), as the soil type and root cutting diameter could produce comparable results as well. These results are in line with Sevik and Guney [64] and Korasick et al. [65], who found that IBA could be used as a suitable hormone for many biological processes (e.g., stem bending, rooting of stem cuttings, leaf epinasty, root initiation, and root generation and elongation) in various plants, such as lemon balm, sunflower, tomato, pea, buckwheat, tobacco, bean, Arabidopsis, and potato, and across species.
On the other hand, many scientists have pointed out in their studies that the success of propagating plants from stems or root cutting largely depends on the capacity of the explant to form adventitious roots (ARs), which is controlled by the type and concentration of the endogenous or/and exogenous hormones from one side, and from the other side, the interaction between the endogenous or exogenous and various metabolic compounds’ pathways [66,67,68]. Furthermore, the effects of different types of auxins, especially IBA, through the interactions with various metabolic compounds in adventitious root formation, lateral root initiation, root elongation, root gravity response, various plant tissues’ development, and plant growth have been reported in several studies. In addition, Sevik and Guney [64] studied the relationship between the level of IBA and the accumulation of flavones and flavanols in various tissues from two Melissa officinalis L. genotypes and their roles in rooting formation on stem cuttings. Also, Uğur Tan [69] analyzed the effectiveness of IBA for enhancing the chlorophyll content, phenol content, flavonoid content, and antioxidant traits of Salvia fruticosa cuttings and for mitigating the adverse effects of salinity stress.
4. Conclusions
To conclude, independently of the hormone concentration (IBA: 0, 0.1%, and 0.3%), root cutting diameter (0.5, 0.8, 1.25, and 2 cm) and soil type (S1: 50% silt + 50% potting soil, and S2: 43% potting soil + 43% silt + 14% sandy soil), all cuttings were successfully regenerated and resulted in new sprouts of Paulownia tomentosa. Nevertheless, the S2 soil type presented favorable substrate conditions for Paulownia sprouts propagated from root cuttings. At the juvenile step of new sprouting, there was a notable reverse relationship between the diameter of root cuttings and the growth in the height of stems. The smallest diameter (0.5 cm) resulted in a taller stem. In contrast, the number of leaves was greatest in sprouts derived from a root cutting with a diameter of 1.25 cm. According to statistical tests, stems were taller under hormone treatment conditions in both 2023 and 2024. Also, Paulownia leaves’ richness in several bioactive molecules reflected their antioxidant properties. IBA conversion to IAA served as the primary source of auxin in root tissues. As a result, this conversion may be crucial for plant propagation in some species. Other enquiries about the roles of IBA-derived auxin will undoubtedly help us better comprehend this crucial auxin. In future work, further supplementation of plant growth regulators should be performed in order to detect the best concentration of those, for greater enhancement of Paulownia tomentosa regeneration and propagation.
Author Contributions
Conceptualization, A.H.N. and Y.A.; Methodology, A.H.N. and Y.A.; Software, A.H.N. and Y.M.H.; Validation, A.H.N., Y.M.H., M.A. and Y.A.; Formal Analysis, A.H.N. and Y.M.H.; Investigation, A.H.N., Y.M.H. and Y.A.; Resources, Y.A.; Data Curation, A.H.N., Y.M.H. and M.A.; Writing—Original Draft Preparation, A.H.N., Y.M.H. and M.A.; Writing—Review and Editing, A.H.N., Y.M.H., M.A., C.A. and Y.A.; Visualization, A.H.N. and Y.A.; Supervision, A.H.N., Y.M.H. and Y.A.; Project Administration, A.H.N. and Y.A.; Funding Acquisition, Y.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Forest Ecology Laboratory, National Research Institute in Rural Engineering, Water and Forestry, Tunisia.
Data Availability Statement
Data can be made available on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
IBA (indole-3-butyric acid), IAA (indole-3-acetic acid), AMI1 (amidase), LAX3 (auxin influx carrier (AUX1 LAX family)), AFB3 (protein auxin signaling F-box 2/3), ARF19 (auxin response factor), IAA10 (auxin-responsive protein IAA), GH3 (auxin responsive GH3 gene family), SAUR (SAUR family protein), ABP1 (auxin-binding protein), TMK (receptor protein kinase TMK), AHA10 (H+-transporting ATPase), MKK4 (mitogen-activated protein kinase 4/5), MPK6 (mitogen-activated protein kinase 6), TAR1 (L-tryptophan-pyruvate aminotransferase), and HAI2 (protein phosphatase 2C).
References
- Ting, H.; Brajesh, N.V.; Zachary, D.; Perry, P.P.; Nirmal, J. Paulownia as a medicinal Tree: Traditional uses and current advances. Eur. J. Med. Plants 2016, 14, 1–15. [Google Scholar]
- Fahmy, A.; Gendy, A. In vitro propagation of Paulownia hybrid (P. elongata x P. fortunei) tree. Zagazig J. Agric. Res. 2018, 45, 1633–1643. [Google Scholar] [CrossRef]
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Anthony, S. Paulownia imperialis. Agrofor. Syst. 2009, 179–185. Available online: https://apps.worldagroforestry.org/treedb/AFTPDFS/Paulownia_imperialis.PDF (accessed on 25 May 2025).
- Johnson, D.V. Use of Paulownia for Forest Plantations in the Leon Region of Nicaragua, Nicaragua Agriculture Reconstruction Assistance Program, Nicaragua; Chemnoics International, Inc.: Washington, DC, USA, 2000; 802; pp. 1–15. [Google Scholar]
- Info Flora. Paulownia tomentosa (Thunb.) Steud. 2019. Available online: https://www.infoflora.ch/en/flora/4747-paulownia-tomentosa.html (accessed on 25 May 2025).
- Danciu, A.; Vlădut, V.; Grigore, I.; Sorică, C.; Cristea, M.A.; Muscalu, A.; Pruteanu, A.; Marin, E.; Usenko, M. Considerations on the Importance of the Paulownia Trees Planting, Annals of Faculty Engineering Hunedoara. Int. J. Eng. 2016, 4, 73–80. [Google Scholar]
- Flynn, H.; Holder, C. A Guide to Useful Woods of the World; Flynn, J.H., Jr., Holder, C.D., Eds.; Forest Products Society: Madison, WI, USA, 2001. [Google Scholar]
- Caparros, S.; Diaz, M.J.; Ariza, J.; Lopez, F.; Jimenez, L. New perspectives for Paulownia valorization of the auto hydrolysis and pulping processes. Bioresour. Technol. 2008, 99, 741–749. [Google Scholar] [CrossRef]
- ELShowk, S.; Nabil, E.S. The Paulownia Tree. An alternative for sustainable forestry. Farm 2008. Available online: https://docslib.org/doc/11683191/the-paulownia-tree-an-alternative-for-sustainable-forestry (accessed on 25 May 2025).
- Jiang, T.F.; Du, X.; Shi, Y.P. Determination of flavonoids from Paulownia tomentosa (Thunb) Steud by micellar electro kinetic capillary electrophoresis. Chromatographia 2007, 59, 255–258. [Google Scholar] [CrossRef]
- Si, C.L.; Wu, L.; Zhu, Z.Y.; Kin, J.K.; Kwon, D.J.; Bae, Y.S. Apigenin derivatives from Paulownia tomentosa var. tomentosa stem barks. Holzforschung 2009, 63, 440–442. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; EL Hawary, S.S.; Mohamed, M.M.D.; Faraid, M.A.; Nayera, A.M.A.; Refaat, E.S. Chemical composition: Antimicrobial activity of the essential oil of the flowers of Paulownia tomentosa growing in Egypt. J. Appl. Sci. Res. 2013, 9, 3228–3232. [Google Scholar]
- Bahri, B. In vitro propagation of a forest tree Paulownia tomentosa (Thunb.) Steud. A valuable medicinal tree species. Albanian J. Agric. Sci. 2013, 12, 37–42. [Google Scholar]
- Graves, D.H.; Stringer, J.W.; Lexington, K.Y. Paulownia: A Guide to Establishment and Cultivation. FOR-39. ISSUED: 9-89. Available online: http://www2.ca.uky.edu/agcomm/pubs/for/for39/for39.htm (accessed on 25 May 2025).
- Crișan, L.R.; Petruș-Vancea, A. Paulownia tomentosa L. in vitro propagation. Nat. Resour. Sustain. Dev. 2016, 6, 30–37. [Google Scholar]
- Bochnia, E.; Litwińczuk, W. Development of royal paulownia (Paulownia tomentosa Steud.) in vitro shoot cultures under the in-fluence of different saccharides. Acta Sci. Pol. Hortorum Cultus 2012, 11, 3–13. [Google Scholar]
- Barton, I.; Nicholas, I.; Ecroyd, C. Paulownia. For. Res. Bull. 2007, 231, 5–68. [Google Scholar]
- Takoutsing, B.; Tsobeng, A.; Tchoundjeu, Z.; Degrande, A.; Asaah, E. Vegetative Propagation of Garcinia lucida Vesque (Clusiaceae) using leafy stem cuttings and grafting. Afr. Focus 2014, 27, 57–71. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis: A simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Mol. Plant. 2012, 5, 334–338. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef]
- Frick, E.M.; Strader, L.C. Roles for IBA derived auxin in plant development. J. Exp. Bot. 2018, 69, 169–177. [Google Scholar] [CrossRef]
- Małgorzata, W.; Małgorzata, W.; Magdalena, F. Can the Biological Activity of Abandoned Soils Be Changed by the Growth of Paulownia elongata × Paulownia fortunei? Preliminary Study on a Young Tree Plantation. Agriculture 2022, 12, 128. [Google Scholar] [CrossRef]
- Lichenthaler, H.K.; Llbrun, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Romani, P.; Pinelli, C.; Cantini: Cimato, A.; Heimler, D. Characterization of Violetto di Toscana, a typical Italian variety of artichoke (Cynara scolymus L.). J. Food Chem. 2006, 95, 221–225. [Google Scholar] [CrossRef]
- Li, J.W.; Ding, S.D.; Ding, X.L. Comparison of antioxidant capacities of extracts from five cultivars of Chinese jujube. Process Biochem. 2005, 40, 3607–3613. [Google Scholar] [CrossRef]
- Dehpour, A.A.; Ebrahimzadeh, M.A.; Fazel, N.S.; Mohammad, N.S. Antioxidant activity of the methanol extract of Ferula assafoetida and its essential oil composition. Grasas Y Aceites 2009, 60, 405–412. [Google Scholar] [CrossRef]
- Ba, K.; Tine, E.; Destain, J.; Cisse, N.; Thonart, P. Étude comparative des composés phénoliques, du pouvoir. IOP Conf. Ser. Earth Environ. Sci. 2021, 939, 012059. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Swelum, A.A.; Shafi, M.E.; Albaqami, N.M.; El-Saadony, M.T.; El-sify, A.; Abdo, M.; Mohamed, E. COVID-19 in human, animal, and environment: A review. Front. Vet. Sci. 2020, 7, 578. [Google Scholar] [CrossRef]
- Mohamad, M.E.; Awad, A.A.; Majrashi, A.; Abd Esadek, O.A.; El-Saadony, M.T.; Saad, A.M.; Gendy, A.S. In vitro study on the effect of cytokines and auxins addition to growth medium on the micropropagation and rooting of Paulownia species (Paulownia hybrid and Paulownia tomentosa). Saudi J. Biol. Sci. 2022, 29, 1598–1603. [Google Scholar] [CrossRef]
- Pożoga, M.; Ede Olewnicki, D.; Jabłońska, L. In Vitro Propagation Protocols and Variable Cost Comparison in Commercial Production for Paulownia tomentosa × Paulownia fortunei Hybrid as a Renewable Energy Source. Appl. Sci. 2019, 9, 2272. [Google Scholar] [CrossRef]
- Antwi, W.A.; Patience, M.; Gakpetor, R.T.G.; Benezer, O.; Daniel, A.O. Vegetative propagation technologies using stem and root cuttings of Paulownia tree species for mass production. J. Biodivers. Environ. Sci. 2021, 18, 67–76. [Google Scholar]
- Magar, L.B.; Shrestha, N.; Khadka, S.; Joshi, J.R.; Acharya, J.; Gyanwa-li, G.C.; Marasini, B.P.; Rajbahak, S.; Parajuli, N. Challenges and opportunity of in vitro propagation of Paulownia tomentosa steud for commercial production in nepal. Int. J. Appl. Sci. Biotechnol. 2016, 4, 155–160. [Google Scholar] [CrossRef]
- Hassan, H.; Moubarak, M. Micropropagation of yucca plant by using guar and locust bean seed powder as an alternative cheap gelling agent. Sci. J. Flowers Ornam. Plants 2020, 7, 239–246. [Google Scholar] [CrossRef]
- Bhojwani, S.S.; Dantu, P.K. Plant tissue culture. In Plant Tissue Culture: An Introductory Text; Springer: New Delhi, India, 2013. [Google Scholar] [CrossRef]
- Maqbool, M.N.; Aftab, F. An Efficient Propagation Approach to Forcing Softwood Shoots from Epicormic Buds and Subsequent Rooting of Paulownia elongate. Science 2024, 2024, 1515489. [Google Scholar] [CrossRef]
- Preece, J.E.; Ledbetter, D.I.; Zaczek, J.J. Rooting softwood cuttings collected from forced large stems of Oakleaf hydrangea and American chestnut. Comb. Proc. Int. Plant Prop. Soc. 2001, 51, 267–270. [Google Scholar]
- Ede, F.J.; Auger, M.; Green, T.G.A. Optimizing root cut-ting success in Paulownia spp. J. Hortic. Sci. 1997, 72, 179–185. [Google Scholar] [CrossRef]
- Antwi-Wiredu, A.; Amiteye, S.; Diawuoh, R.G.; Klu, G.Y.P. Ex Vitro propagation of rubber tree (Hevea brasiliensis) using stem cuttings. Int. J. Agric. Environ. Biotechnol. 2018, 3, 846–854. [Google Scholar] [CrossRef]
- Stenvall, T.; Haapala, P.; Pulkkinen, A. The role of a root cutting’s diameter and location on the regeneration ability of hybrid aspen. For. Ecol. Manag. 2006, 237, 150–155. [Google Scholar] [CrossRef]
- Gerson, R.; Muñoz, F.; Uribe, M.; Rubilar, R.A. Macropropagation of Paulownia elongata x fortunei from root cuttings in the Biobío Region, Chile. Gayana Bot. 2015, 72, 70–75. [Google Scholar] [CrossRef]
- Dubova, O.; Olena, V.; Olena, B. Paulownia Tomentosa—New species for the industrial land-scaping. Curr. Trends Nat. Sci. 2019, 8, 19–24. [Google Scholar]
- Shu, K.; Liu, X.D.; Xie, Q.; He, Z.H. Two Faces of One Seed: Hormonal Regulation of Dormancy and Germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Fadon, E.; Fernandez, E.; Behn, H.; Luedeling, E. A conceptual framework for winter dormancy in deciduous trees. Agronomy 2020, 10, 241. [Google Scholar] [CrossRef]
- Andrés, F.; Coupland, G. The genetic basis of flowering responses to seasonal cues. Nat. Rev. Genet. 2012, 13, 627–639. [Google Scholar] [CrossRef]
- Gaudinier, A.; Blackman, B.K. Evolutionary processes from the perspective of flowering time diversity. New Phytol. 2020, 225, 1883–1981. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, J. Molecular mechanisms of flowering phenology in tree. For. Res. 2023, 3, 2. [Google Scholar] [CrossRef]
- Wang, J.W. Regulation of flowering time by the miR156-mediated age pathway. J. Exp. Bot. 2014, 65, 4723–4730. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Czech, B.; Weigel, D. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 2009, 138, 738–749. [Google Scholar] [CrossRef]
- Vergun, O.; Dzhamal, R.; Svitlana, R.; Valentyna, F. Comparative study of biochemical composition of Paulownia tomentosa (Thunb.) Steud. Agrobiodivers. Improv. Nutr. Health Life Qual. 2022, 2, 180–190. [Google Scholar] [CrossRef]
- Vergun, O.; Rakhmetov, D.; Bondarchuk, O.; Rakhmetova, S.; Shymanska, O.; Fishchenko, V. Biochemical composition of Vigna spp. genotypes. Agrobiodivers. Improv. Nutr. Health Life Qual. 2022, 6, 41–48. [Google Scholar] [CrossRef]
- Schneiderova, K.; Šmejkal, K. Phytochemical profile of Paulownia tomentosa (Thunb). Steud. Phytochem. Rev. 2015, 14, 799–833. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Marzocchella, L.; Fantini, M. Dietary flavonoids: Molecular mechanisms of action as anti-inflammatory agents. Recent Pat. Inflamm. Allergy Drug Discov. 2011, 5, 200–220. [Google Scholar] [CrossRef]
- Romano, B.; Pagano, E. Novel insights into the pharmacology of flavonoids. Phytother. Res. 2013, 27, 1588–1596. [Google Scholar] [CrossRef]
- Damodaran, S.; Strader, L.C. Indole 3-Butyric Acid Metabolism and Transport in Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 851. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A. In vitro propagation of F1 male hybrid lines in Asparagus officinalus. Egypt. J. Desert Res. 2019, 69, 67–86. [Google Scholar] [CrossRef]
- Sallam, S. In vitro propagation and secondary metabolites production in the wild rare Asparagus apphylus L. plant. Egypt. J. Desert Res. 2021, 2, 149–161. [Google Scholar] [CrossRef]
- Ahmed, M.; Abo El-Fadl, R.; Suliman, M.; Abd Elaziem, T. Effect of micropropagation conditions on adventitious buds formation and the circadian expression of the ACO013229.1 gene in Ananas cosmosus. Egypt. J. Desert Res. 2021, 71, 191–208. [Google Scholar] [CrossRef]
- Ghareb, H. In vitro preservation of the Egyptian endemic Silene schimperiana Boiss. Plant via encapsulation. Egypt. J. Desert Res. 2021, 69, 19–35. [Google Scholar] [CrossRef]
- Brumos, J.; Robles, L.M.; Yun, J.; Vu, T.C.; Jackson, S.; Alonso, J.M. Local auxin biosynthesis is a key regulator of plant development. Dev. Cell 2018, 47, 306–318. [Google Scholar] [CrossRef]
- Uzunova, V.V.; Quareshy, M.; Del Genio, C.I.; Napier, R.M. Tomographic docking suggests the mechanism of auxin receptor TIR1 selectivity. Open Biol. 2016, 6, 160139. [Google Scholar] [CrossRef]
- Sevik, H.; Guney, K. Effects of IAA, IBA, NAA, and GA3 on rooting and morphological features of Melissa officinalis L. stem cuttings. Sci. World J. 2013, 2013, 909507. [Google Scholar] [CrossRef]
- Korasick, D.A.; Enders, T.A.; Strader, L.C. Auxin biosynthesis and storage forms. J. Exp. Bot. 2013, 64, 2541–2555. [Google Scholar] [CrossRef]
- Brown, D.E.; Rashotte, A.M.; Murphy, A.S.; Normanly, J.; Tague, B.W.; Peer, W.A.; Taiz, L.; Muday, G.K. Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol. 2001, 126, 524–535. [Google Scholar] [CrossRef]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Teale, W.D.; Ditengou, F.A.; Dovzhenko, A.D.; Li, X.; Molendijk, A.M.; Ruperti, B.; Paponov, I.; Palme, K. Auxin as a model for the integration of hormonal signal processing and transduction. Mol. Plant 2008, 1, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Ugur, T. Application of indole-3-butyric acid (IBA) enhances agronomic, physiological and antioxidant traits of Salvia fruticosa under saline conditions: A practical approach. PeerJ 2025, 13, e18846. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).