Micropropagation of ‘Manacá-de-Cheiro’ (Brunfelsia uniflora (Pohl) D. Don), an Ornamental Species Native to Brazil
Abstract
1. Introduction
2. Results
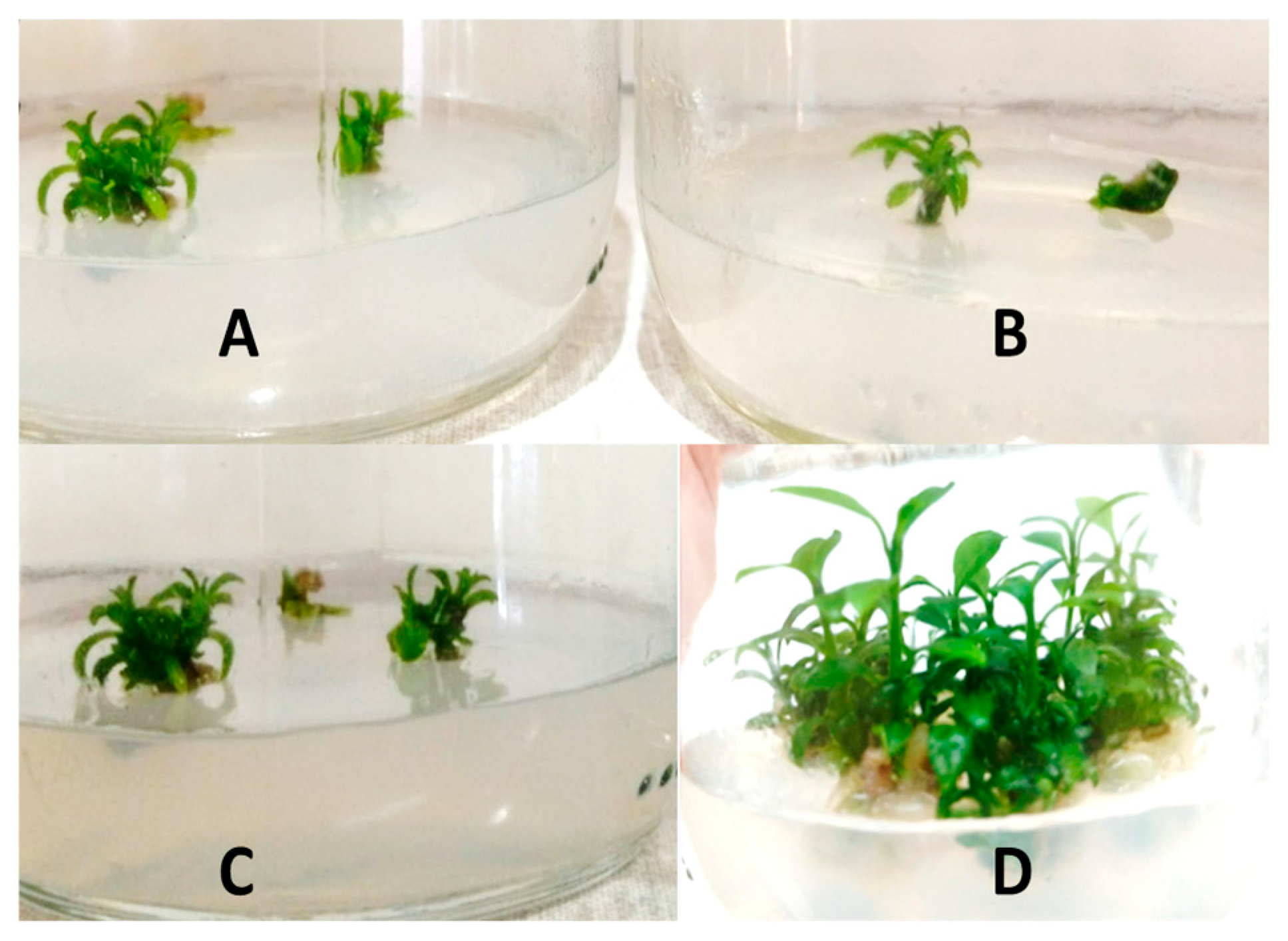
2.1. In Vitro Establishment
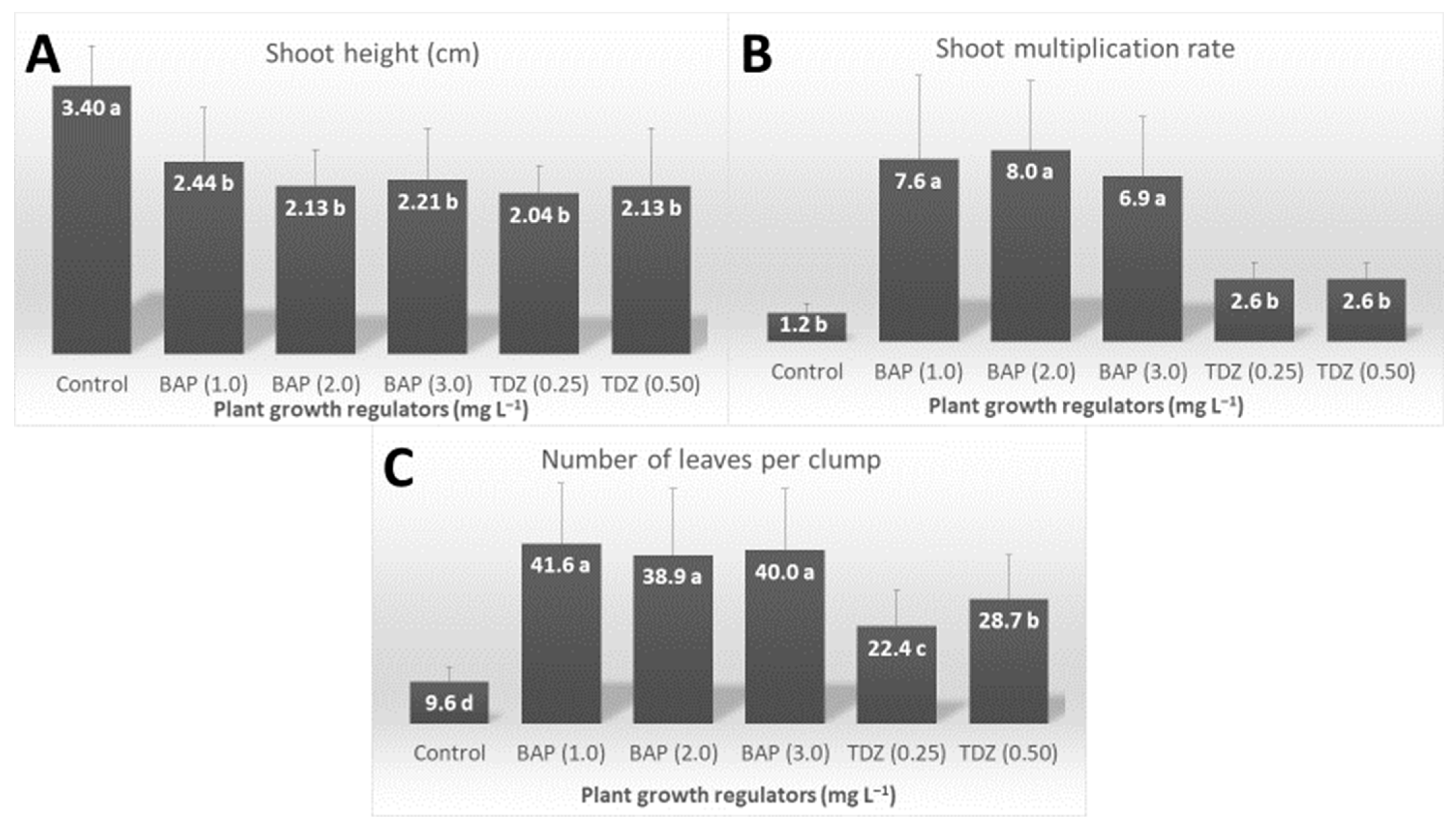
2.2. Effects of Culture Medium and Plant Growth Regulators on the Shoot Proliferation Phase
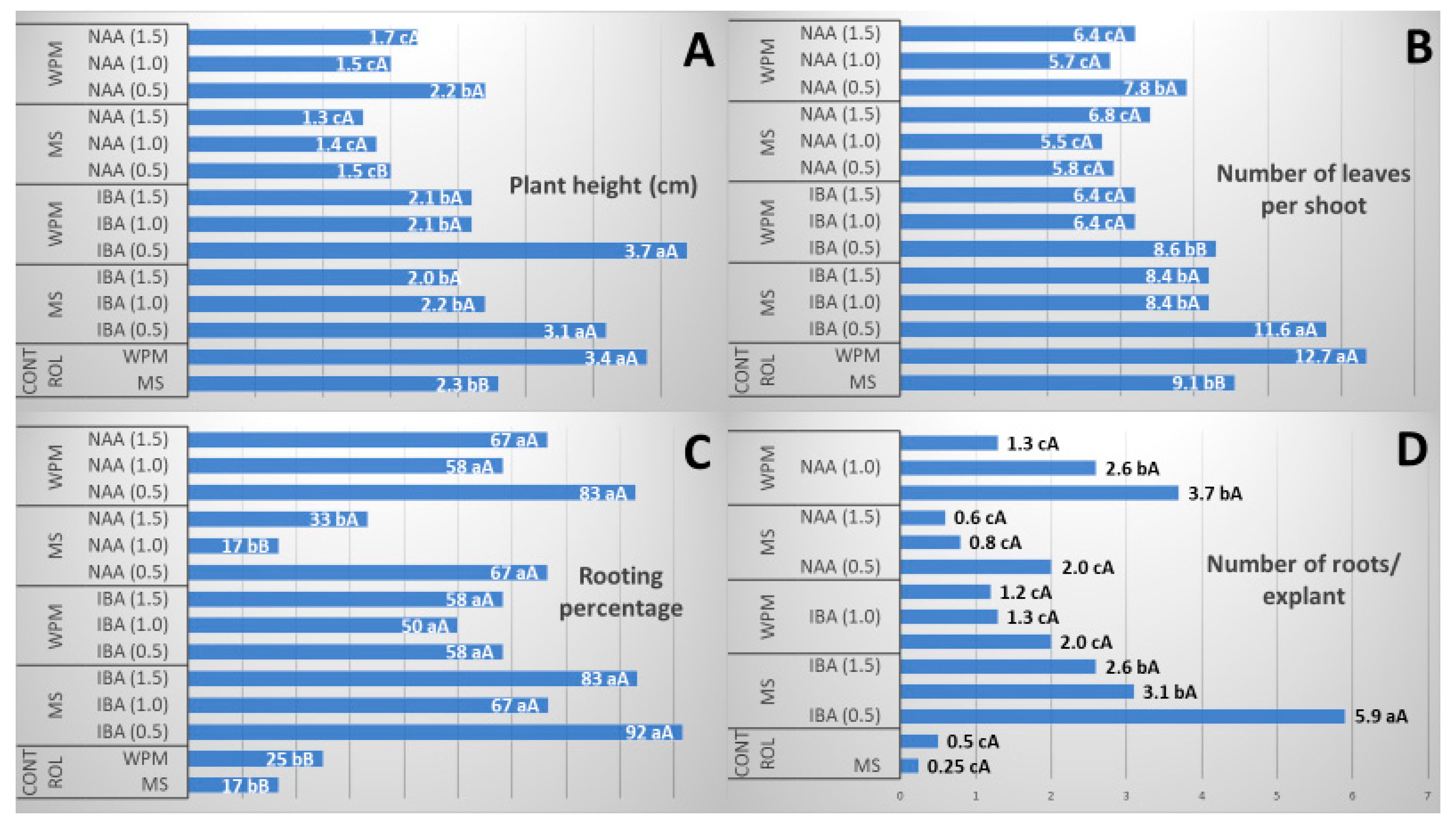
2.3. Effects of Culture Medium and Plant Growth Regulatorss in the Rooting Phase and Acclimatization
3. Discussion
3.1. In Vitro Establishment
3.2. Effects of Culture Medium and Plant Growth Regulators on the Shoot Proliferation Phase
3.3. Effects of Culture Medium and Auxins on Rooting and Acclimatization
4. Materials and Methods
4.1. Plant Material
4.2. In Vitro Establishment
4.3. Effects of Culture Media and Plant Growth Regulators on Shoot Proliferation
4.4. Effects of Culture Medium and Auxins on Rooting and Plantlets’ Acclimatization
4.5. Experiment to Improve In Vitro Rooting Performance
4.6. Experimental Design and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- REFLORA. Available online: https://reflora.jbrj.gov.br/reflora/herbarioVirtual/ConsultaPublicoHVUC/ConsultaPublicoHVUC.do (accessed on 25 October 2017).
- Griffiths, M. Index of Garden Plants; Timber Press: Portland, OR, USA, 1994. [Google Scholar]
- Dias, P.C.; Oliveira, L.S.; Xavier, A.; Wendling, I. Estaquia e miniestaquia de espécies florestais lenhosas do Brasil. Pesqui. Florest. Bras. 2012, 32, 453–462. [Google Scholar] [CrossRef]
- Simão, E.; Nakamura, A.T.; Takaki, M. Época de colheita e capacidade germinativa de sementes de Tibouchina mutabilis (Vell.) Cogn. (Melastomataceae). Biota Neotrop. 2007, 7, 67–73. [Google Scholar] [CrossRef]
- Verma, V.; Katoch, M.; Kapoor, P.; Misra, A.; Bhargava, B. Domestication of ornamental plants: Breeding innovations and molecular breakthroughs to bring wild into limelight. S. Afr. J. Bot. 2024, 175, 436–452. [Google Scholar] [CrossRef]
- Liberman, R.; Shahar, L.; Nissim-Levi, A.; Evenor, D.; Reuveni, M.; Oren-Shamir, M. Shoot regeneration from leaf explants of Brunfelsia calycina. Plant Cell Tissue Organ Cult. (PCTOC) 2010, 100, 345–348. [Google Scholar] [CrossRef]
- Tarek, A.A.; Shaaban, S.A.; Taha, L.S.; Hashish, K.I.; Gabr, A.M.; Ali, A.I. Encapsulation of Brunfelsia pauciflora micropropagated shoots as a suitable conservation protocol. J. Pharm. Negat. Results 2022, 13, 1864–1878. [Google Scholar] [CrossRef]
- Khaliluev, M.R.; Bogoutdinova, L.R.; Baranova, G.B.; Baranova, E.N.; Kharchenko, P.N.; Dolgov, S.V. Influence of genotype, explant type, and component of culture medium on in vitro callus induction and shoot organogenesis of tomato (Solanum lycopersicum L.). Biol. Bull. 2014, 41, 512–521. [Google Scholar] [CrossRef]
- Guo, X.; Chen, G.; Zhang, Y.; Shen, X. Effect of genotype on callus induction and plant regeneration of Calibrachoa hybrida. In Vitro Cell. Dev. Biol.-Plant. 2025, 61, 117–126. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot tip culture. Plant Prop. Soc. 1981, 30, 421–427. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lameira, O.A.; Pinto, J.E.B.P. In vitro propagation of Cordia verbenaceae L. (Boraginaceae). Rev. Bras. Plantas Med. 2006, 8, 102–104. [Google Scholar]
- Victório, C.P.; Lage, C.L.S.; Sato, A. Tissue culture techniques in the proliferation of shoots and roots of Calendula officinalis. Rev. Ciência Agron. 2012, 43, 539–545. [Google Scholar] [CrossRef]
- Sulava, S.; Andia, S.; Bhol, R.; Jena, A.; Jena, S.; Bidyadhar, B. In vitro micropropagation of Asparagus racemosus by using of nodal explants. J. Cell Tissue Res. 2020, 20, 6883–6888. [Google Scholar]
- Jadid, N.; Anggraeni, S.; Ramadani, M.R.N.; Arieny, M.; Mas’ud, F. In vitro propagation of Indonesian stevia (Stevia rebaudiana) genotype using axenic nodal segments. BMC Res. Notes 2024, 17, 45. [Google Scholar] [CrossRef]
- Nazir, U.; Gul, Z.; Shah, G.; Khan, N. Interaction Effect of Auxin and Cytokinin on In Vitro Shoot Regeneration and Rooting of Endangered Medicinal Plant Valeriana jatamansi Jones Through Tissue Culture. Am. J. Plant Sci. 2022, 13, 223–240. [Google Scholar] [CrossRef]
- Akram, M.S.; Alvi, A.K.; Iqbal, J. Enhanced in vitro regeneration in sugarcane (Saccharum officinarum L.) by use of alternate high-low picloram doses and thidiazuron supplementation. Cytol. Genet. 2021, 55, 566–575. [Google Scholar] [CrossRef]
- Schuchovski, C.; Sant’Anna-Santos, B.; Marra, R.; Biasi, L. Morphological and anatomical insights into de novo shoot organogenesis of in vitro ‘Delite rabbiteye’ blueberries. Heliyon 2020, 6, e05468. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Nurmansiah; Naidoo, Y.; Teixeira da Silva, J.A. Thidiazuron-induced abnormalities in plant tissue cultures. Plant Cell Rep. 2018, 37, 1451–1470. [Google Scholar] [CrossRef]
- Jan, T.; Gul, S.; Khan, A.; Pervez, S.; Noor, A.; Amin, H.; Bibi, S.; Nawaz, M.A.; Rahim, A.; Ahmad, M.S.; et al. Range of factors in the reduction of hyperhydricity associated with in vitro shoots of Salvia santolinifolia Bioss. Braz. J. Biol. 2021, 83, e246904. [Google Scholar] [CrossRef]
- Ragonezi, C.; Klimaszewsa, K.; Castro, M.R.; Lima, M.; Oliveira, P.; Zavattieri, M.A. Adventitious rooting of conifers: Influence of physical and chemical factors. Trees. 2010, 24, 975–992. [Google Scholar] [CrossRef]
- Hesami, M.; Yoosefzadeh-Najafabadi, M.; Jones, A.M.P. Challenges and opportunities in in vitro rooting of recalcitrant plant species. Front. Plant Sci. 2023, 14, 1210346. [Google Scholar]
- Ancasi-Espejo, R.G.; Vivado, J.A.A.; Guzmán, I.M. Concentraciones de acido indolbutirico para la formacion de raices en condiciones in vitro de castaña (Bertholletia excelsa bonpl., Lecythidaceae). UNESUM-Cienc. Rev. Cient. Mult. 2023, 7, 17–22. [Google Scholar] [CrossRef]
- Elmongy, M.S.; Cao, Y.; Zhou, H.; Xia, Y. Root development enhanced by using indole-3-butyric acid and naphthalene acetic acid and associated biochemical changes of in vitro azalea microshoots. J. Plant Growth Regul. 2018, 37, 813–825. [Google Scholar] [CrossRef]
- Qarachoboogh, A.F.; Alijanpour, A.; Hosseini, B.; Shafiei, A.B. Efficient and reliable propagation and rooting of foetid juniper (Juniperus foetidissima Willd.), as an endangered plant under in vitro condition. Vitr. Cell. Dev. Biol.-Plant 2022, 58, 399–406. [Google Scholar] [CrossRef]
- Kaviani, B.; Barandan, A.; Tymoszuk, A.; Kulus, D. Optimization of In Vitro Propagation of Pear (Pyrus communis L.) ‘Pyrodwarf®(S)’ Rootstock. Agronomy 2023, 13, 268. [Google Scholar] [CrossRef]
- Bhojwani, S.S.; Dantu, P.K. Tissue and cell culture. In Plant Tissue Culture: An Introductory Text, 1st ed.; Bhojwani, S.S., Dantu, P.K., Eds.; Springer: Delhi, India, 2013; pp. 39–50. [Google Scholar]
- Arab, M.M.; Yadollahi, A.; Eftekhari, M.; Akbari, M.; Khorami, S.S. Modeling and Optimizing a New Culture Medium for In Vitro Rooting of G×N15 Prunus Rootstock using Artificial Neural Network-Genetic Algorithm. Sci. Rep. 2018, 8, 9977. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Elhiti, M.; Wang, H.; Xu, A.; Brown, D.; Wang, A. Adventitious root formation of in vitro peach shoots is regulated by auxin and ethylene. Sci. Hortic. 2017, 226, 250–260. [Google Scholar] [CrossRef]
- Santana-Buzzy, N.; Canto-Flick, A.; Iglesias-Andreu, L.G.; Montalvo-Peniche, M.C.; López-Puc, G.; Barahona-Pérez, F. Improvement of in vitro culturing of habanero pepper by inhibition of ethylene effects. HortScience 2006, 41, 405–409. [Google Scholar] [CrossRef]
- Yue, P.; Lu, Q.; Liu, Z.; Lv, T.; Li, X.; Bu, H.; Liu, W.; Xu, Y.; Yuan, H.; Wang, A. Auxin-activated MdARF5 induces the expression of ethylene biosynthetic genes to initiate apple fruit ripening. New Phytol. 2020, 226, 1781–1795. [Google Scholar] [CrossRef]
- Chandra, S.; Bandopadhyay, R.; Kumar, V.; Chandra, R. Acclimatization of tissue cultured plantlets: From laboratory to land. Biotechnol. Lett. 2010, 32, 1199–1205. [Google Scholar] [CrossRef]
- Mohammed, M.; Munir, M.; Ghazzawy, H.S. Design and Evaluation of a Smart Ex Vitro Acclimatization System for Tissue Culture Plantlets. Agronomy 2023, 13, 78. [Google Scholar] [CrossRef]
- Matos, A.V.C.D.S.D.; Oliveira, B.S.D.; Oliveira, M.E.B.S.D.; Cardoso, J.C. AgNO3 improved micropropagation and stimulate in vitro flowering of rose (Rosa × hybrida) cv. Sena. Ornam. Hortic. 2020, 27, 33–40. [Google Scholar] [CrossRef]
- Woodward, A.J.; Bennett, I.J.; Pusswonge, S. The effect of nitrogen source and concentration, medium pH and buffering on in vitro shoot growth and rooting in Eucalyptus marginata. Sci. Hortic. 2006, 110, 208–213. [Google Scholar] [CrossRef]
- Pai, S.R.; Desai, N.S. Effect of TDZ on Various Plant Cultures. In Thidiazuron: From Urea Derivative to Plant Growth Regulator; Ahmad, N., Faisal, M., Eds.; Springer: Singapore, 2018; pp. 439–454. [Google Scholar]
- RSTUDIO TEAM. Available online: http://www.rstudio.com/ (accessed on 12 September 2018).





| Auxins | Survival in | Number of Leaves | Chlorophyll Content Index | |||
|---|---|---|---|---|---|---|
| (mg L−1) | Acclimatization (%) | /Plantlet | Chl A | Chl B | Chl A + B | Chl A/B |
| 0.0 | 81.3 | 5.0 ± 1.4 | 28.6 ± 0.6 | 5.7 ± 0.2 | 34.2 ± 0.8 | 5.0 ± 0.4 |
| 0.5 | 68.8 | 7.0 ± 1.7 | 31.5 ± 3.8 | 7.0 ± 1.7 | 38.5 ± 5.3 | 4.5 ± 0.1 |
| 1.0 | 31.3 | 4.5 ± 1.4 | 28.4 ± 0.5 | 5.6 ± 0.2 | 34.0 ± 0.6 | 5.1 ± 0.3 |
| 1.5 | 31.3 | 5.5 ± 1.4 | 25.6 ± 5.9 | 4.7 ± 1.8 | 30.3 ± 7.8 | 5.4 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Broek, A.V.C.; Leite, M.P.; Cardoso, J.C. Micropropagation of ‘Manacá-de-Cheiro’ (Brunfelsia uniflora (Pohl) D. Don), an Ornamental Species Native to Brazil. Int. J. Plant Biol. 2025, 16, 69. https://doi.org/10.3390/ijpb16020069
van den Broek AVC, Leite MP, Cardoso JC. Micropropagation of ‘Manacá-de-Cheiro’ (Brunfelsia uniflora (Pohl) D. Don), an Ornamental Species Native to Brazil. International Journal of Plant Biology. 2025; 16(2):69. https://doi.org/10.3390/ijpb16020069
Chicago/Turabian Stylevan den Broek, Ana Victória Conde, Mariana Pelais Leite, and Jean Carlos Cardoso. 2025. "Micropropagation of ‘Manacá-de-Cheiro’ (Brunfelsia uniflora (Pohl) D. Don), an Ornamental Species Native to Brazil" International Journal of Plant Biology 16, no. 2: 69. https://doi.org/10.3390/ijpb16020069
APA Stylevan den Broek, A. V. C., Leite, M. P., & Cardoso, J. C. (2025). Micropropagation of ‘Manacá-de-Cheiro’ (Brunfelsia uniflora (Pohl) D. Don), an Ornamental Species Native to Brazil. International Journal of Plant Biology, 16(2), 69. https://doi.org/10.3390/ijpb16020069






