An Effective Protocol for Callus Induction and Plant Regeneration in an Indica Rice Cultivar RD43
Abstract
1. Introduction
2. Materials and Methods
2.1. Explant Preparation and Sterilization
2.2. Callus Induction
2.3. Callus Proliferation
2.4. Plant Regeneration from Callus
2.5. Statistical Analysis
3. Results
3.1. Callus Induction of Rice Cv. RD43 Using 2,4-D
3.2. Callus Proliferation of Rice Cv. RD43 Callus
3.3. Plant Regeneration of Rice Cv. RD43 Callus
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanpawithayakul, K.; Kaewprasert, N.; Tantiyavarong, P.; Wichansawakun, S.; Somprasit, C.; Tanathornkeerati, N.; Srichan, C.; Tharavanij, T. Effects of the consumption of low to medium glycemic index–based rice on the rate of insulin initiation in patients with gestational diabetes: A triple-blind, randomized, controlled trial. Clin. Ther. 2023, 45, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Tisarum, R.; Theerawitaya, C.; Samphumphung, T.; Takabe, T.; Cha-Um, S. Exogenous foliar application of glycine betaine to alleviate water deficit tolerance in two Indica rice genotypes under greenhouse conditions. Agronomy 2019, 9, 138. [Google Scholar] [CrossRef]
- Wijerathna-Yapa, A.; Hiti-Bandaralage, J. Tissue culture—A sustainable approach to explore plant stresses. Life 2023, 13, 780. [Google Scholar] [CrossRef] [PubMed]
- Binte Mostafiz, S.; Wagiran, A. Efficient callus induction and regeneration in selected indica rice. Agronomy 2018, 8, 77. [Google Scholar] [CrossRef]
- Afrasiab, H.; Jafar, R. Effect of different media and solidifying agents on callogenesis and plant regeneration from different explants of rice (Oryza sativa L) varieties Super Basmati and IRRI-6. Pak. J. Bot. 2011, 43, 487–501. [Google Scholar]
- Ikeuchi, M.; Favero, D.S.; Sakamoto, Y.; Iwase, A.; Coleman, D.; Rymen, B.; Sugimoto, K. Molecular mechanisms of plant regeneration. Annu. Rev. Plant Biol 2019, 70, 377–406. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Sumi, M.A.; Jahan, N.; Rana, M.S.; Uddin, M.I.; Alam Khan, N. Callus induction, regeneration and establishment of rice plant from mature embryo. J. Adv. Biol. Biotechnol. 2021, 24, 10–18. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 19 April 2025).
- Thadavong, S.; Sripichitt, P.; Wongyai, W.; Jompuk, P. Callus induction and plant regeneration from mature embryos of glutinous rice (Oryza sativa L.) cultivar TDK1. Agric. Nat. Res. 2002, 36, 334–344. [Google Scholar]
- Noor, W.; Lone, R.; Kamili, A.N.; Husaini, A.M. Callus induction and regeneration in high-altitude Himalayan rice genotype SR4 via seed explant. Biotechnol. Rep. 2022, 36, e00762. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Singh, R.; Bose, B. Standardization of culture medium for somatic embryogenesis of rice var. MTU 7029. Int. J. Bio-Resour. Stress Manag. 2013, 4, 500–505. [Google Scholar]
- Tariq, M.; Ali, G.; Hadi, F.; Ahmad, S.; Ali, N.; Shah, A.A. Callus induction and in vitro plant regeneration of rice (Oryza sativa L.). Pak. J. Biol. Sci. 2008, 11, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Morini, S.; D’Onofrio, C.; Bellocchi, G.; Fisichella, M. Effect of 2,4-D and light quality on callus production and differentiation from in vitro cultured quince leaves. Plant Cell Tissue Organ. Cult. 2000, 63, 47–55. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.J. Plant Propagation by Tissue Culture: Volume 1: The Background; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Kakani, A.; Li, G.; Peng, Z. Role of AUX1 in the control of organ identity during in vitro organogenesis and in mediating tissue specific auxin and cytokinin interaction in Arabidopsis. Planta 2009, 229, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Dhamotharan, P.; Saraswathi, R.; Gnanam, R. Callus induction and regeneration in selected indica genotypes of rice (Oryza sativa L.). Electron. J. Plant Breed. 2021, 12, 1022–1028. [Google Scholar]
- Sawant, G.; Sawardekar, S.; Patil, M.; Jadhav, S. Surface sterilization and callus induction potential of various indica rice (Oryza sativa L.) varieties. Appl. Biol. Res. 2021, 23, 147–156. [Google Scholar] [CrossRef]
- Taratima, W.; Chomarsa, T.; Maneerattanarungroj, P. Salinity stress response of rice (Oryza sativa L. cv. Luem Pua) calli and seedlings. Scientifica 2022, 2022, 5616683. [Google Scholar] [CrossRef] [PubMed]
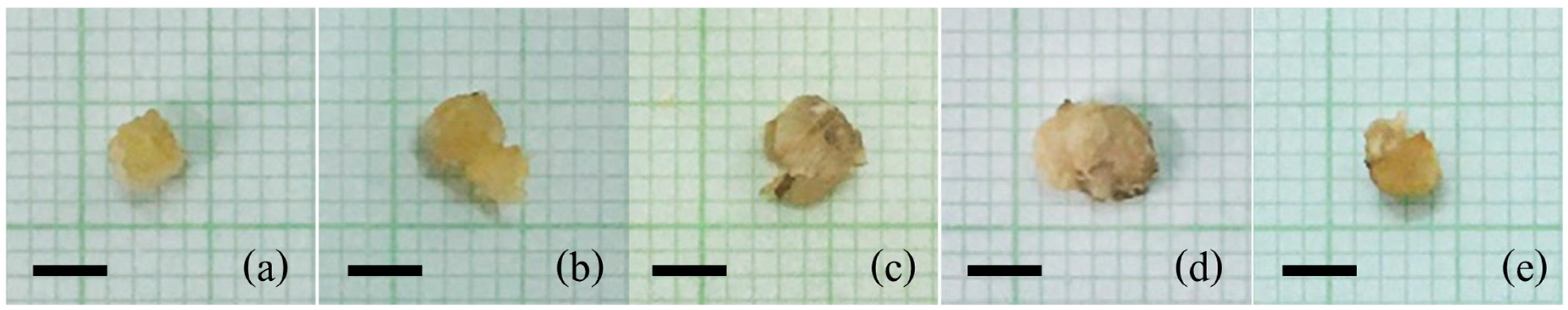
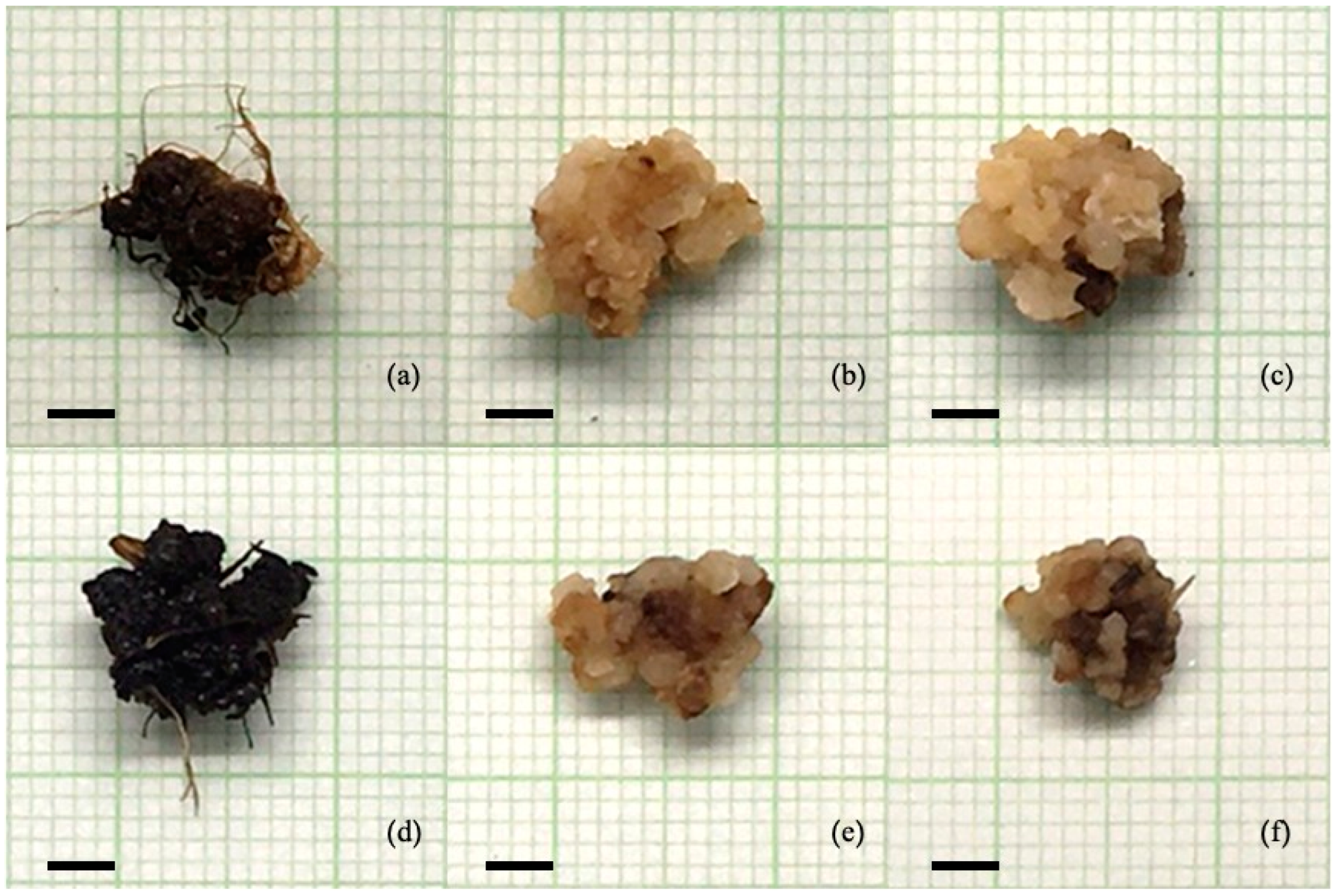
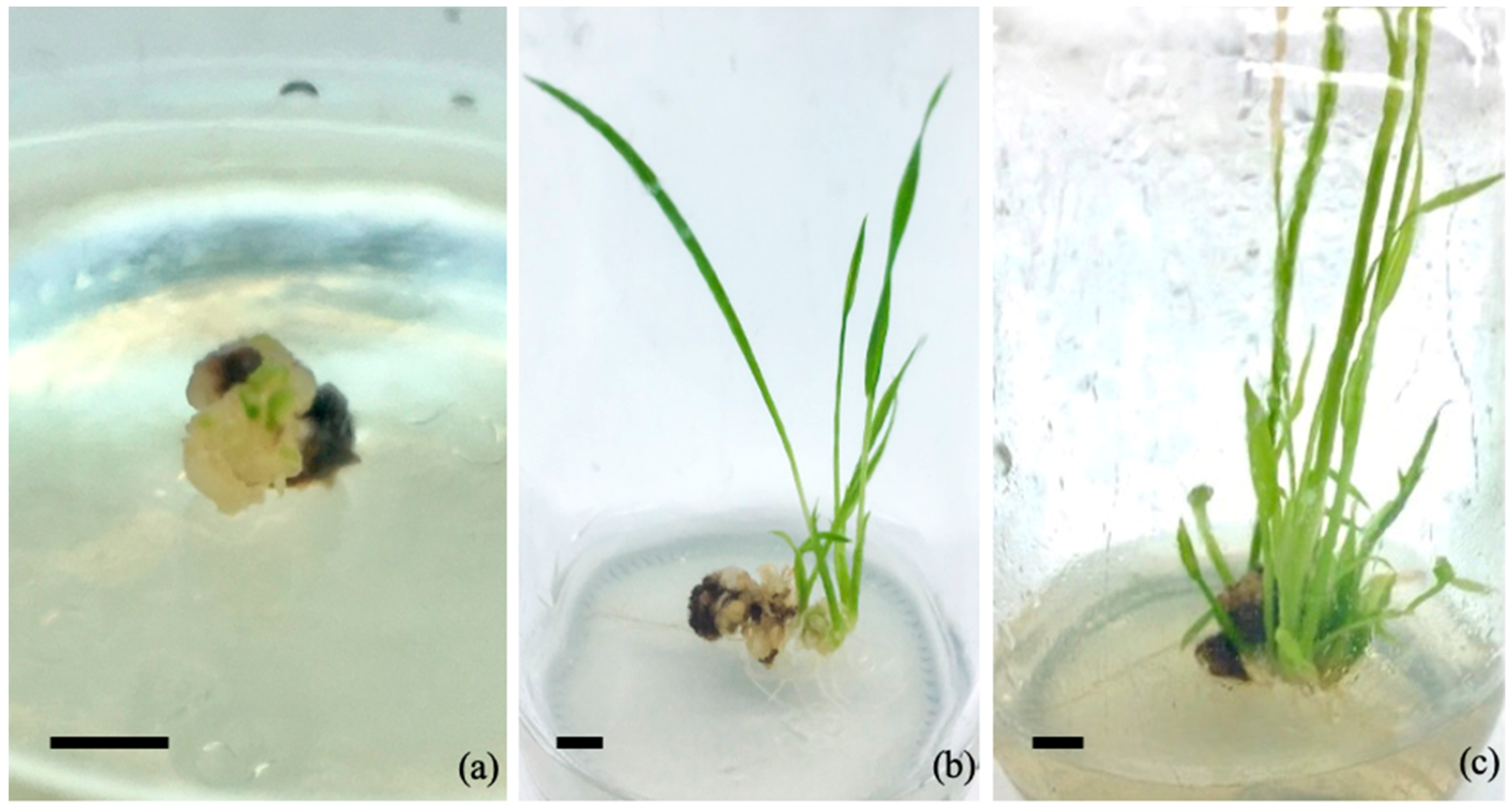
| MS Medium Supplemented with 2,4-D (mg/L) | Callus Induction Frequency (%) | Callus Diameter (mm) | Fresh Weight (mg) | Dry Weight (mg) |
|---|---|---|---|---|
| 0 (control) | 00.00 ± 0.00 c | - | - | - |
| 1.0 | 75.00 ± 9.93 b | 2.72 ± 0.20 c | 3.80 ± 0.75 c | 2.21 ± 0.45 ab |
| 2.0 | 100.00 ± 0.00 a | 3.45 ± 0.17 ab | 7.51 ± 0.94 ab | 2.33 ± 0.24 a |
| 3.0 | 100.00 ± 0.00 a | 3.66 ± 0.15 a | 8.13 ± 0.80 ab | 2.48 ± 0.21 a |
| 4.0 | 100.00 ± 0.00 a | 3.77 ± 0.18 a | 9.98 ± 1.25 a | 2.85 ± 0.31 a |
| 5.0 | 100.00 ± 0.00 a | 3.04 ± 0.18 bc | 5.48 ± 0.73 bc | 1.33 ± 0.16 b |
| Kruskal–Wallis test | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| MS Medium with | Callus Diameter (mm) | Fresh Weight (mg) | Characteristics | Color of Callus | |||
|---|---|---|---|---|---|---|---|
| 2,4-D (mg/L) | BA (mg/L) | ||||||
| 0 | 0 | 9.65 ± 0.26 | abc | 156.98 ± 8.80 | b | friable callus with numerous roots | Dark brown–Black |
| 2 | 0 | 10.70 ± 0.23 | a | 250.95 ± 9.66 | a | friable callus | Yellow–Pale yellow |
| 4 | 0 | 10.41 ±0.14 | ab | 231.68 ± 6.91 | a | friable callus | Yellow–Pale yellow Some parts brown |
| 0 | 1 | 9.08 ± 0.06 | abc | 160.45 ± 3.54 | b | friable callus with roots | Dark brown–Black |
| 2 | 1 | 8.76 ± 0.02 | bc | 124.95 ± 2.10 | b | friable callus | Yellow–Dark brown |
| 4 | 1 | 8.18 ± 0.26 | c | 133.68 ± 6.10 | b | friable callus | Yellow–Dark brown |
| Kruskal–Wallis test | <0.001 * | <0.001 * | |||||
| MS Medium Supplemented with | Plant Regeneration (%) | The Number of Shoots Per Callus | The Number of Roots Per Callus | Number of Calli Tested | |
|---|---|---|---|---|---|
| NAA (mg/L) | BA (mg/L) | ||||
| 0 | 0 | 18.75 ± 10.08 ab | 3.67 ± 0.14 b | 11.33 ± 0.14 | 16 |
| 0 | 1.0 | 30.00 ± 15.28 ab | 3.00 ± 0.84 b | 7.67 ± 0.48 | 10 |
| 0 | 2.0 | 50.00 ± 16.67 a | 9.60 ± 0.28 a | 10.00 ± 0.50 | 10 |
| 0 | 3.0 | 0.00 ± 0.00 b | - | - | 16 |
| 0 | 4.0 | 44.44 ± 17.57 ab | 5.25 ± 1.10 ab | 8.00 ± 1.19 | 9 |
| 0 | 5.0 | 12.5 ± 12.50 ab | 1.00 ± 0.00 b | 4.00 ± 0.00 | 8 |
| 1.0 | 0 | 0.00 ± 0.00 b | - | - | 16 |
| 1.0 | 1.0 | 14.29 ± 9.71 ab | 1.00 ± 0.00 b | 5.50 ± 0.57 | 14 |
| 1.0 | 2.0 | 6.67 ± 6.67 ab | 1.00 ± 0.00 b | 5.00 ± 0.00 | 15 |
| 1.0 | 3.0 | 18.18 ± 12.2 ab | 7.50 ± 0.21 ab | 4.00 ± 0.43 | 11 |
| 1.0 | 4.0 | 28.57 ± 18.44 ab | 1.00 ± 0.00 b | 4.50 ± 0.27 | 7 |
| 1.0 | 5.0 | 0.00 ± 0.00 b | - | - | 12 |
| Kruskal–Wallis test | <0.05 * | <0.05 * | NS | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chitphet, P.; Sanevas, N.; Vuttipongchaikij, S.; Wongkantrakorn, N. An Effective Protocol for Callus Induction and Plant Regeneration in an Indica Rice Cultivar RD43. Int. J. Plant Biol. 2025, 16, 48. https://doi.org/10.3390/ijpb16020048
Chitphet P, Sanevas N, Vuttipongchaikij S, Wongkantrakorn N. An Effective Protocol for Callus Induction and Plant Regeneration in an Indica Rice Cultivar RD43. International Journal of Plant Biology. 2025; 16(2):48. https://doi.org/10.3390/ijpb16020048
Chicago/Turabian StyleChitphet, Pundanai, Nuttha Sanevas, Supachai Vuttipongchaikij, and Narong Wongkantrakorn. 2025. "An Effective Protocol for Callus Induction and Plant Regeneration in an Indica Rice Cultivar RD43" International Journal of Plant Biology 16, no. 2: 48. https://doi.org/10.3390/ijpb16020048
APA StyleChitphet, P., Sanevas, N., Vuttipongchaikij, S., & Wongkantrakorn, N. (2025). An Effective Protocol for Callus Induction and Plant Regeneration in an Indica Rice Cultivar RD43. International Journal of Plant Biology, 16(2), 48. https://doi.org/10.3390/ijpb16020048







