Lotus tenuis in Association with Arbuscular Mycorrhizal Fungi Is More Tolerant to Partial Submergence than to High-Intensity Defoliation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set Up
2.2. Growing Conditions
2.3. Plant Yield and Analytical Determinations in Tissue
2.4. Arbuscular Mycorrhizal Fungi Colonization and Root Nodulation
2.5. Statistical Analyses
3. Results
3.1. Plant Yield
3.2. P in Tissue
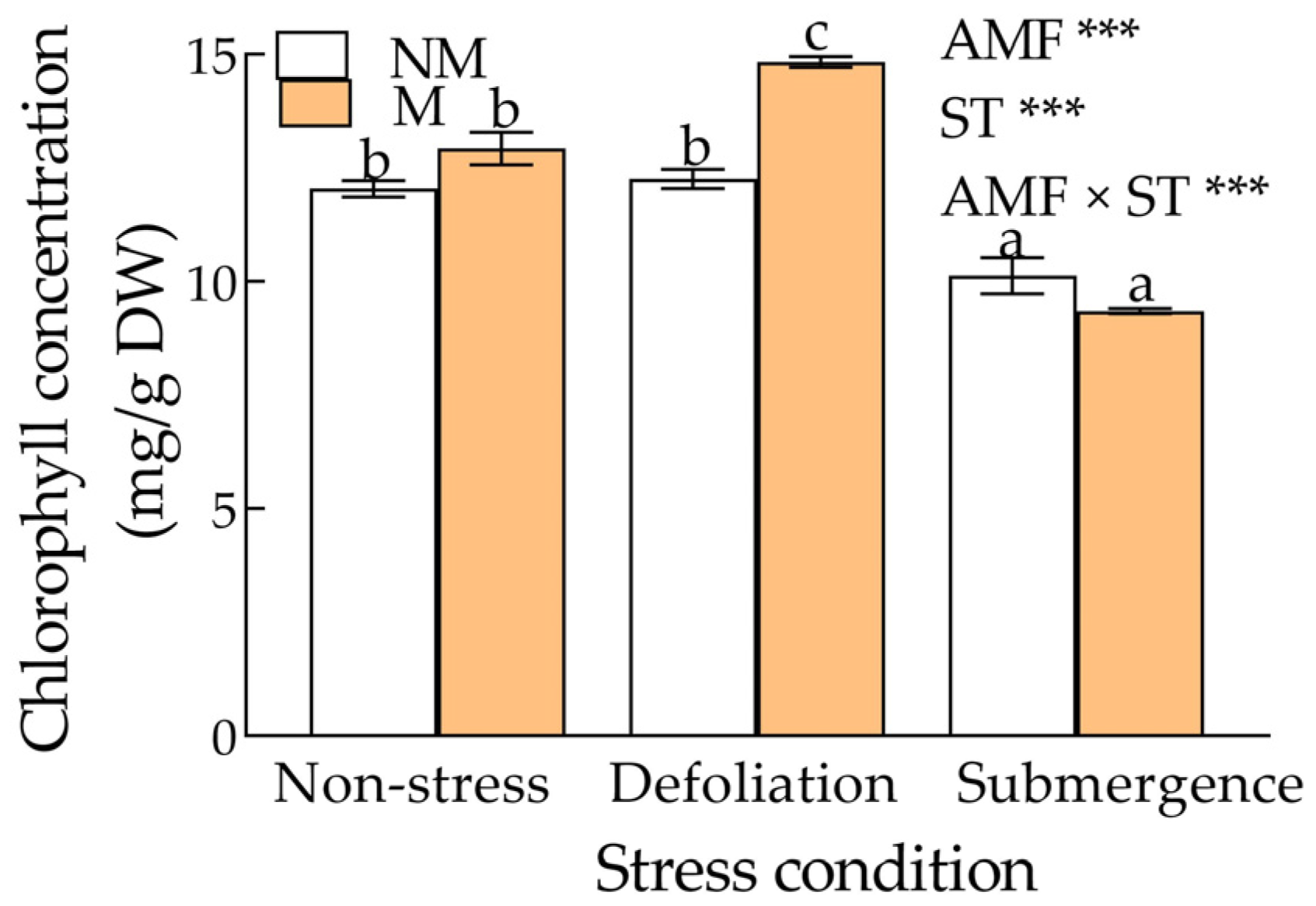
3.3. Photosynthetic Pigments
3.4. Mycorrhizal Growth and P Response
3.5. Mycorrhizal Root Colonization and Nodulation
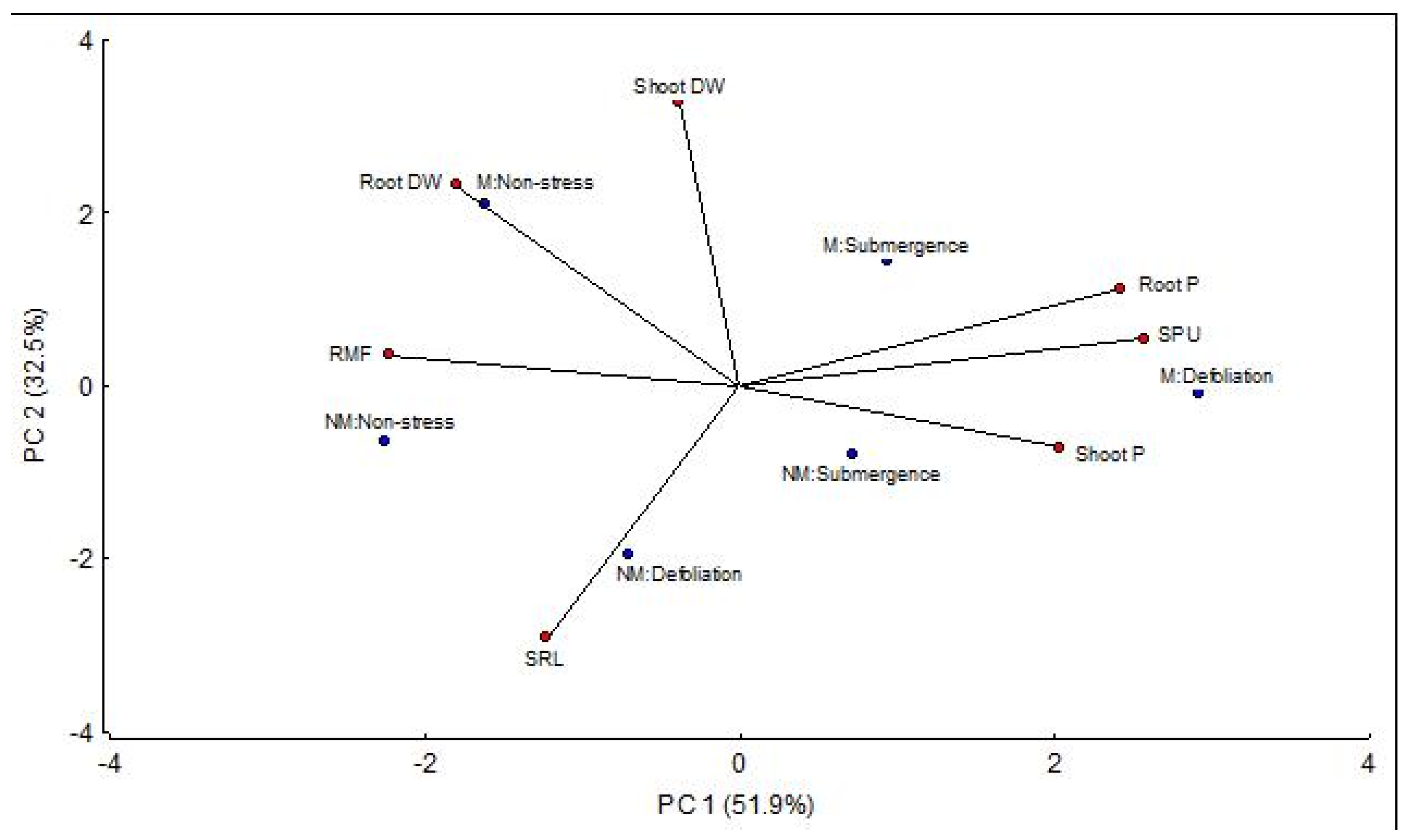
3.6. Relationships Between Mycorrhizal and Non-Mycorrhizal Plants Under Severe Stress Conditions
4. Discussion
4.1. Mycorrhizal Growth and P Response
4.2. Photosynthetic Pigments
4.3. Mycorrhizal Root Colonization and Nodulation
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMF | Arbuscular mycorrhizal fungi |
| M | With arbuscular mycorrhizal fungi |
| NM | Without arbuscular mycorrhizal fungi |
| ST | Stress treatment |
| DW | Dry weight |
| RMF | Root mass fraction |
| SRL | Specific root length |
| SPU | Specific P uptake |
| MGR | Mycorrhizal growth response |
| MPR | Mycorrhizal P response |
References
- Muir, J.P.; Pitman, W.D.; Foster, J.L. Sustainable, low input, warm season, grass–legume grassland mixtures: Mission (nearly) impossible? Grass Forage Sci. 2011, 66, 301–315. [Google Scholar] [CrossRef]
- García, I.; Mendoza, R. Relationships among soil properties, plant nutrition and arbuscular mycorrhizal fungi–plant symbioses in a temperate grassland along hydrologic, saline and sodic gradients. FEMS Microbiol. Ecol. 2008, 63, 359–371. [Google Scholar] [CrossRef]
- Sainz Rozas, H.; Echeverria, H.; Angelini, H. Fósforo disponible en suelos agrícolas de la región Pampeana y ExtraPampeana argentina. Rev. Investig. Agropecu. 2012, 38, 33–39. [Google Scholar]
- Antonelli, C.J.; Calzadilla, P.I.; Vilas, J.M.; Campestre, M.P.; Escaray, F.J.; Ruiz, O.A. Physiological and anatomical traits associated with tolerance to long-term partial submergence stress in the Lotus genus: Responses of forage species, a model and an interspecific hybrid. J. Agron. Crop Sci. 2019, 205, 65–76. [Google Scholar] [CrossRef]
- Roy, T.; Mandal, U.; Mandal, D.; Yadav, D. Role of arbuscular mycorrhizal fungi in soil and water conservation: A potentially unexplored domain. Curr. Sci. 2021, 120, 1573–1577. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoono, S. Roles of Arbuscular Mycorrhizal Fungi on Plant Growth and Performance: Importance in Biotic and Abiotic Stressed Regulation. Diversity 2020, 12, 370. [Google Scholar] [CrossRef]
- Nie, W.; He, Q.; Guo, H.; Zhang, W.; Ma, L.; Li, J.; Wen, D. Arbuscular Mycorrhizal fungi: Boosting Crop Resilience to Environmental Stresses. Microorganisms 2024, 12, 2448. [Google Scholar] [CrossRef]
- Gai, J.P.; Feng, G.; Cai, X.B.; Christie, P.; Li, X.L. A preliminary survey of the arbuscular mycorrhizal status of grassland plants in southern Tibet. Mycorrhiza 2006, 16, 191–196. [Google Scholar] [CrossRef]
- García, I.V.; Covacevich, F.; Fernández-López, C.; Cabello, M. Lotus tenuis maintains high arbuscular mycorrhizal diversity in grasslands regardless of soil properties or management. Rhizosphere 2023, 27, 100754. [Google Scholar] [CrossRef]
- McSherry, M.E.; Ritchie, M.E. Effects of grazing on grassland soil carbon: A global review. Glob. Change Biol. 2013, 19, 1347–1357. [Google Scholar] [CrossRef]
- Conant, R.T.; Cerri, C.E.P.; Osborne, B.B.; Paustian, K. Grassland management impacts on soil carbon stocks: A new synthesis. Ecol. Appl. 2017, 27, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, Y.; Mahendran, R.; Koirala, S.; Konoshima, L.; Yamazaki, D.; Watanabe, S.; Kanae, S. Global flood risk under climate change. Nat. Clim. Change 2013, 3, 816–821. [Google Scholar] [CrossRef]
- Barcelo, M.; van Bodegom, P.M.; Tedersoo, L.; den Haan, N.; Veen, G.F.; Ostonen, I.; Trimbos, K.; Soudzilovskaia, N.A. The abundance of arbuscular mycorrhiza in soils is linked to the total length of roots colonized at ecosystem level. PLoS ONE 2020, 15, e0237256. [Google Scholar] [CrossRef] [PubMed]
- Baral, N.K.; Giri, A.; Shah, P.K.; Kemmelmeier, K.; Stürmer, S.L.; Gyawali, S.; Raut, J.K. Diversity of arbuscular mycorrhizal fungi (Glomeromycota) in adjacent areas of different land use in Nepal. GSC Biol. Pharm. Sci. 2021, 15, 141–150. [Google Scholar] [CrossRef]
- Colmer, T.D.; Voesenek, L. Flooding tolerance: Suites of plant traits in variable environments. Funct. Plant Biol. 2009, 36, 665–681. [Google Scholar] [CrossRef]
- Barto, E.K.; Rilling, M.C. Does herbivory really suppress mycorrhiza? A meta-analysis. J. Ecol. 2010, 98, 745–753. [Google Scholar] [CrossRef]
- Malik, A.I.; Islam, A.; Colmer, T.D. Transfer of the barrier to radial oxygen loss in roots of Hordeum marinum to wheat (Triticum aestivum): Evaluation of four H. arinum–wheat amphiploids. New Phytol. 2011, 190, 499–508. [Google Scholar] [CrossRef]
- van der Heyde, M.; Abbott, L.K.; Gehring, C.; Kokkoris, V.; Hart, M.M. Reconciling disparate responses to grazing in the arbuscular mycorrhizal symbiosis. Rhizosphere 2019, 11, 100167. [Google Scholar] [CrossRef]
- Faghihinia, M.; Zou, Y.; Chen, Z.; Bai, Y.; Li, W.; Marrs, R.; Staddon, P.L. Environmental drivers of grazing effects on arbuscular mycorrhizal fungi in grasslands. Appl. Soil Ecol. 2020, 153, 103591. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J.; Shen, Y.; Dong, F.; Chen, J. Global negative effects of livestock grazing on arbuscular mycorrhizas: A meta-analysis. Sci. Total Environ. 2020, 708, 134553. [Google Scholar] [CrossRef]
- Dalle Carbonare, L.; Jiménez, J.D.L.C.; Lichtenauer, S.; Van Veen, H. Plant responses to limited aeration: Advances and future challenges. Plant Direct 2023, 7, e488. [Google Scholar] [CrossRef] [PubMed]
- Neto, D.; Carvalho, L.M.; Cruz, C.; Martins-Loução, M.A. How do mycorrhizas affect C and N relationships in flooded Aster tripolium plants? Plant Soil 2006, 279, 51–63. [Google Scholar] [CrossRef]
- Fougnies, L.; Renciot, S.; Muller, F.; Plenchette, C.; Prin, Y.; de Faria, S.M.; Bouvet, J.M.; Sylla, S.N.; Dreyfus, B.; Bâ, A.M. Arbuscular mycorrhizal colonization and nodulation improve flooding tolerance in Pterocarpus officinalis Jacq. seedlings. Mycorrhiza 2007, 17, 159–166. [Google Scholar] [CrossRef]
- Xu, Y.; Tu, Y.; Feng, J.; Peng, Z.; Peng, Y.; Huang, J. Arbuscular Mycorrhizal Fungi Mediate the Acclimation of Rice to Submergence. Plants 2024, 13, 1908. [Google Scholar] [CrossRef]
- Miller, S.P.; Sharitz, R.R. Manipulation of flooding and arbuscular mycorrhiza formation influences growth and nutrition of two semiaquatic grass species. Funct. Ecol. 2000, 14, 738–748. [Google Scholar] [CrossRef]
- Stevens, K.J.; Wall, C.B.; Janssen, J.A. Effects of arbuscular mycorrhizal fungi on seedling growth and development of two wetland plants, Bidens frondosa L., and Eclipta prostrata (L.) L., grown under three levels of water availability. Mycorrhiza 2011, 21, 279–288. [Google Scholar] [CrossRef]
- Nieva, A.S.; Bailleres, M.A.; Llames, M.E.; Taboada, M.A.; Ruiz, O.A.; Menéndez, A. Promotion of Lotus tenuis in the Flooding Pampa (Argentina) increases the soil fungal diversity. Fungal Ecol. 2018, 33, 80–91. [Google Scholar] [CrossRef]
- Xiao, D.; Tan, Y.; Liu, X.; Yang, R.; Zhang, W.; He, X.; Wang, K. Effects of different legume species and densities on arbuscular mycorrhizal fungal communities in a karst grassland ecosystem. Sci. Total Environ. 2019, 678, 551–558. [Google Scholar] [CrossRef]
- Temperton, V.M.; Mwangi, P.N.; Scherer-Lorenzen, M.; Schmid, B.; Buchmann, N. Positive interactions between nitrogen-fixing legumes and four different neighbouring species in a biodiversity experiment. Oecologia 2007, 151, 190–205. [Google Scholar] [CrossRef]
- Cahuépé, M. Does Lotus glaber improve beef production at the Flooding pampas? Lotus Newsl. 2004, 34, 38–43. [Google Scholar]
- García, I.; Mendoza, R.; Pomar, M.C. Deficit and excess of soil water impact on plant growth of Lotus tenuis by affecting nutrient uptake and arbuscular mycorrhizal symbiosis. Plant Soil 2008, 304, 117–131. [Google Scholar] [CrossRef]
- García, I.; Mendoza, R. Impact of defoliation intensities on plant biomass, nutrient uptake and arbuscular mycorrhizal symbiosis in Lotus tenuis growing in a saline-sodic soil. Plant Biol. 2012, 14, 964–971. [Google Scholar] [CrossRef] [PubMed]
- García, I.V. Lotus tenuis and Schedonorus arundinaceus co-culture exposed to defoliation and water stress. Rev. FCA UNCuyo 2021, 53, 100–108. [Google Scholar] [CrossRef]
- Striker, G.G.; Izaguirre, R.F.; Manzur, M.E.; Grimoldi, A.A. Diferent strategies of Lotus japonicus, L. corniculatus and L. tenuis to deal with complete submergence at seedling stage. Plant Biol. 2012, 14, 50–55. [Google Scholar] [CrossRef]
- Di Bella, C.E.; Kotula, L.; Striker, G.G.; Colmer, T.D. Submergence tolerance and recovery in Lotus: Variation among fifteen accessions in response to partial and complete submergence. J. Plant Physiol. 2020, 249, 153180. [Google Scholar] [CrossRef]
- Mendoza, R.; Escudero, V.; García, I. Plant growth, nutrient acquisition and mycorrhizal colonization of a waterlogging tolerant legume (Lotus glaber Mill.) in a saline-sodic soil. Plant Soil 2005, 275, 305–315. [Google Scholar] [CrossRef]
- Chippano, T.; Mendoza, R.; Cofré, N.; García, I. Divergent root P uptake strategies of three temperate grassland forage species. Rhizosphere 2021, 17, 100312. [Google Scholar] [CrossRef]
- Bunn, R.A.; Correa, A.; Joshi, J.; Kaiser, C.; Lekberg, Y.; Prescott, C.E.; Sala, A.; Karst, J. What determines transfer of carbon from plants to mycorrhizal fungi? New Phytol. 2024, 244, 1199–1215. [Google Scholar] [CrossRef]
- Raj, H.; Sharma, S.D. Integration of soil solarization and chemical sterilization with beneficial microorganisms for the control of white root rot and growth of nursery apple. Sci. Hortic. 2009, 119, 126–131. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic membranes. Method Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall: Englewood Cliffs, NJ, USA, 1964. [Google Scholar]
- Phillip, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and VAM fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchaild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Cavagnaro, T.R.; Smith, F.A.; Ayling, S.M.; Smith, S.E. Growth and phosphorus nutrition of a Paris-type arbuscular mycorrhizal symbiosis. New Phytol. 2003, 157, 127–134. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Version. Centro de Transferencia InfoStat, FCA, Universidad Nacional de Cordoba. Argentina. 2019. Available online: http://www.infostat.com.ar (accessed on 1 February 2020).
- Striker, G.G.; Insausti, P.; Grimoldi, A. Flooding Effects on Plants Recovering from Defoliation in Paspalum dilatatum and Lotus tenuis. Ann. Bot. 2008, 102, 247–254. [Google Scholar] [CrossRef]
- Gibbs, J.; Greenway, H. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct. Plant Biol. 2003, 30, 353. [Google Scholar] [CrossRef]
- Manzur, M.E.; Grimoldi, A.A.; Insausti, P.; Striker, G.G. Escape from water or remain quiescent? Lotus tenuis changes its strategy depending on depth of submergence. Ann. Bot. 2009, 104, 1163–1169. [Google Scholar] [CrossRef]
- Tian, J.; Dong, G.; Karthikeyan, R.; Li, L.; Daren Harmel, R. Phosphorus Dynamics in Long-Term Flooded, Drained, and Reflooded Soils. Water 2017, 9, 531. [Google Scholar] [CrossRef]
- Ruíz-Lozano, J.M.; Porcel, R.; Azcón, C.; Aroca, R. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: New challenges in physiological and molecular studies. J. Exp. Bot. 2012, 63, 4033–4044. [Google Scholar] [CrossRef]
- Bompadre, M.J.; Silvani, V.A.; Bidondo, L.F.; Ríos de Molina, M.D.C.; Colombo, R.P.; Pardo, A.G.; Godeas, A.M. Arbuscular mycorrhizal fungi alleviate oxidative stress in pomegranate plants growing under different irrigation conditions. Botany 2014, 92, 187–193. [Google Scholar] [CrossRef]
- Eftekhari, M.; Alizadeh, M.; Mashayekhi, K.; Asghari, H.; Kamkar, B. Integration of arbuscular mycorrhizal fungi to grape vine (Vitis vinifera L.) in nursery stage. J. Adv. Lab. Res. Biol. 2010, 1, 102–111. [Google Scholar]
- Khattab, M.M.; Shaban, A.E.; El-Shrief, A.H.; El-Deen Mohamed, A.S. Growth and productivity of pomegranate trees under different irrigation levels. III: Leaf pigments, proline and mineral content. J. Hort. Sci. Ornamen. Plants 2011, 3, 265–269. [Google Scholar]
- Mishra, S.K.; Patro, L.; Mohapatra, P.K.; Biswal, B. Response of senescing rice leaves to flooding stress. Photosynthetica 2008, 46, 315–317. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology, 4th ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2003. [Google Scholar]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Functional diversity in arbuscular mycorrhizal (AM) symbioses: The contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol. 2004, 162, 511–524. [Google Scholar] [CrossRef]
- Hawkins, H.J.; Cargill, R.I.M.; van Nuland, M.E.; Hagen, S.C.; Field, K.J.; Sheldrake, M.; Soudzilovskaia, N.A.; Kiers, E.T. Mycorrhizal mycelium as a global carbon pool. Curr. Biol. 2023, 33, R560–R573. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol. 2003, 133, 16–20. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Horton, T.R. Socialism in soil? The importance of mycorrhizal fungal networks for facilitation in natural ecosystems. J. Ecol. 2009, 97, 1139–1150. [Google Scholar] [CrossRef]
- Frank, D.A.; Gehring, C.A.; Machut, L.; Phillips, M. Soil community composition and the regulation of grazed temperate grassland. Oecologia 2003, 137, 603–609. [Google Scholar] [CrossRef]
- Saravesi, K.; Ruotsalainen, A.L.; Cahill, J.F. Contrasting impacts of defoliation on root colonization by arbuscular mycorrhizal and dark septate endophytic fungi of Medicago sativa. Mycorrhiza 2014, 24, 239–245. [Google Scholar] [CrossRef]
- Torres, M.J.; Moreira, G.; Bhadha, J.H.; McLamore, E.S. Arbuscular mycorrhizal fungi as inspiration for sustainable technology. Encyclopedia 2024, 4, 1188–1200. [Google Scholar] [CrossRef]
- Miller, S.P. Arbuscular mycorrhizal colonization of semi-aquatic grasses along a wide hydrologic gradient. New Phytol. 2000, 145, 145–155. [Google Scholar] [CrossRef]
- Stevens, K.J.; Spender, S.W.; Peterson, R.L. Phosphorus, arbuscular mycorrhizal fungi and performance of the wetland plant Lythrum salicaria L. under inundated conditions. Mycorrhiza 2002, 12, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.H.; Leonard, R.T.; Menge, J.A. Membrane mediated decrease in root exudation responsible for phosphorus inhibition of vesicular-arbuscular mycorrhiza formation. Plant Physiol. 1981, 68, 548–552. [Google Scholar] [CrossRef]
- Lepetit, M.; Brouquisse, R. Control of the rhizobium–legume symbiosis by the plant nitrogen demand is tightly integrated at the whole plant level and requires interorgan systemic signaling. Front. Plant Sci. 2023, 14, 1114840. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, I. Lotus tenuis in Association with Arbuscular Mycorrhizal Fungi Is More Tolerant to Partial Submergence than to High-Intensity Defoliation. Int. J. Plant Biol. 2025, 16, 47. https://doi.org/10.3390/ijpb16020047
García I. Lotus tenuis in Association with Arbuscular Mycorrhizal Fungi Is More Tolerant to Partial Submergence than to High-Intensity Defoliation. International Journal of Plant Biology. 2025; 16(2):47. https://doi.org/10.3390/ijpb16020047
Chicago/Turabian StyleGarcía, Ileana. 2025. "Lotus tenuis in Association with Arbuscular Mycorrhizal Fungi Is More Tolerant to Partial Submergence than to High-Intensity Defoliation" International Journal of Plant Biology 16, no. 2: 47. https://doi.org/10.3390/ijpb16020047
APA StyleGarcía, I. (2025). Lotus tenuis in Association with Arbuscular Mycorrhizal Fungi Is More Tolerant to Partial Submergence than to High-Intensity Defoliation. International Journal of Plant Biology, 16(2), 47. https://doi.org/10.3390/ijpb16020047




