Real-Time Partitioning of Diurnal Stem CO2 Efflux into Local Stem Respiration and Xylem Transport Processes
Abstract
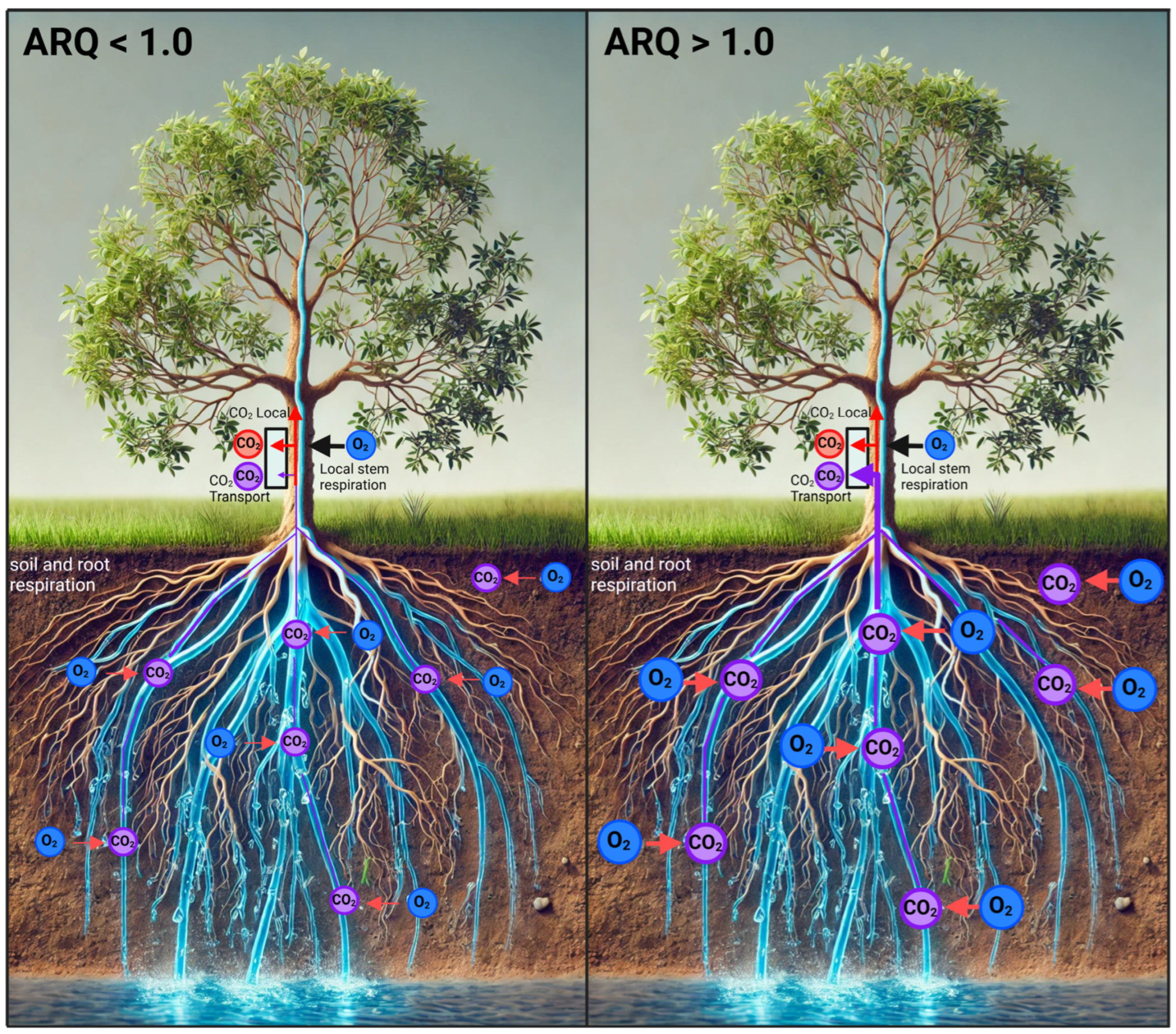
1. Introduction
| Reference | Method Used | Field/Lab, Intact/Cut | Range of ARQ Values | Species | Ecosystem | Diurnal Patterns? |
|---|---|---|---|---|---|---|
| Smart, 2004 [27] | Sealed static chamber | Cut branches vs. [CO2] | Branch: 0.088–0.99 | Vitis rupestris × V. riparia cv. 3309 Couderc | Cultivated vineyards | No |
| Hilman et al., 2019 [17] | Sealed static chamber | Intact stems in the field | Stem: 0.23–0.90 | Pinus halepensis, Quercus calliprinos | Mediterranean | No |
| Helm et al., 2023 [18] | Sealed static chamber | Intact stems in the field | Stem: 0.7 | European beech (Fagus sylvatica) | Temperate | No |
| Helm et al., 2023 [52] | Sealed static chamber | Intact stems in the field | Stem: 0.7- 1.0 | Populus tremula hybrids | Temperate | No |
| Hilman et al., 2022 [21] | Sealed static chamber | Stem cores | Stem: Cores: 0.19–0.70 | Quercus calliprinos | Mediterranean | No |
| Hilman et al., 2019 [22] | Sealed static chamber | Intact stems | Stem: 0.39–0.78 | Cedrela odorata, Swietenia macrophylla, Quercus ilex, Pinus halepensis. | Tropical, temperate, Mediterranean | No |
| Hilman and Angert, 2016 [23] | Sealed static chamber | Intact stems | Stem: 0.23–0.90 | Quercus calliprinos, Pinus halepensis, Tetragastris panamensis (Engl.) Kuntze | Mediterranean | No |
| Patterson et al., 2018 [25] | Sealed static chamber | Intact stems in the field | Stem: 0.7–1.3 | 16 co-occurring temperate tree species, conifers and broadleaf | Temperate | No |
| Angert and Sherer, 2011 [38] | Sealed static chamber | Intact stems in the field | Stem: 0.61–0.84 | Malus domestica, Pinus pinea L., Pinus halepensis Mill. | Mediterranean | No |
| Angert et al., 2012 [24] | Sealed static chamber | Intact stems in the field | Stem: 0.48–0.84 | Hymenaea courbaril, Bertholletia excelsa, Cedrela odorata, Swietenia macrophylla | Tropical | No |
| Current study | Open flow chamber | Intact stems in the field | Stem: 0.9–2.9 | Prunus ilicifolia | Temperate | Yes |
2. Materials and Methods
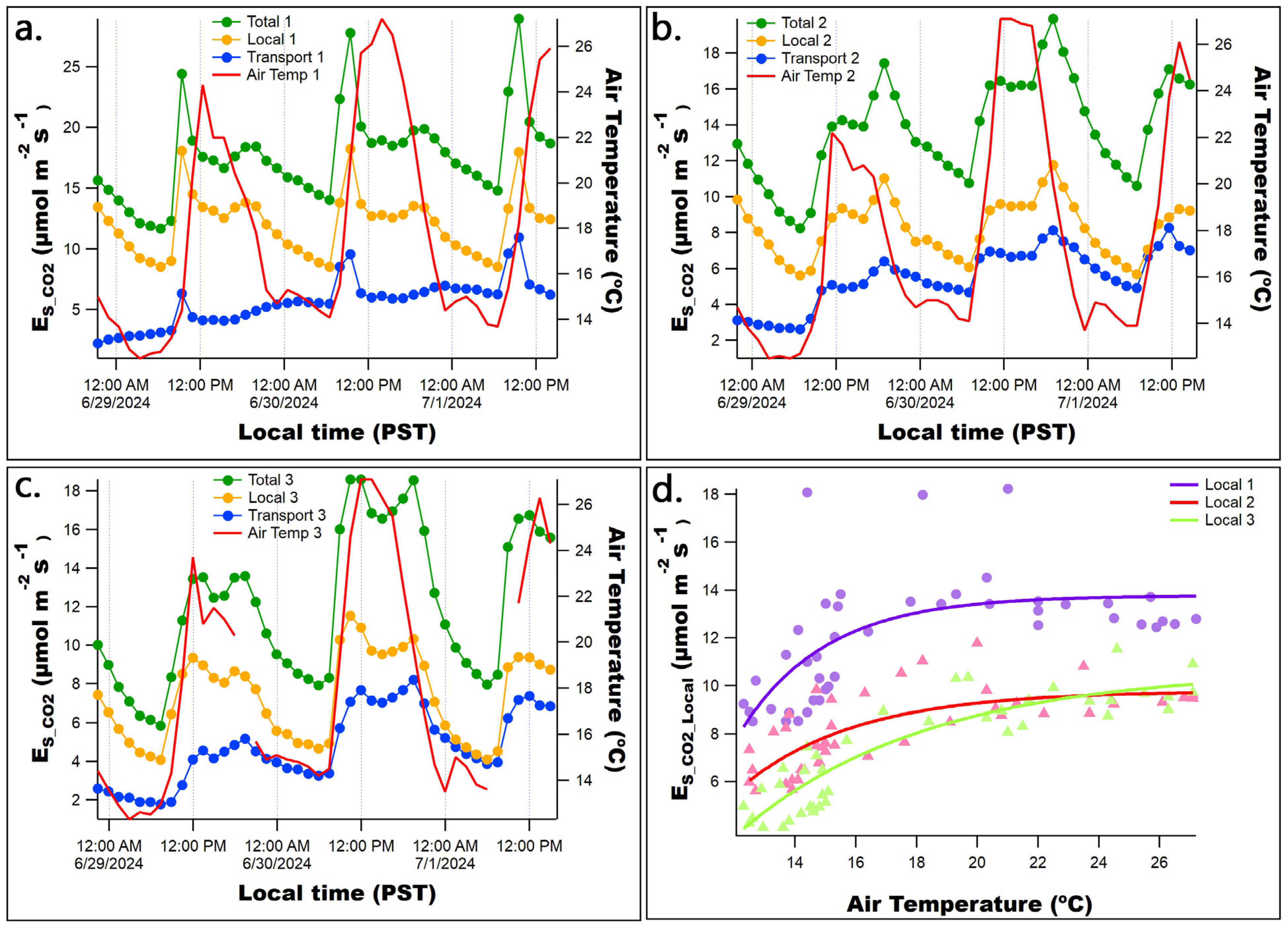
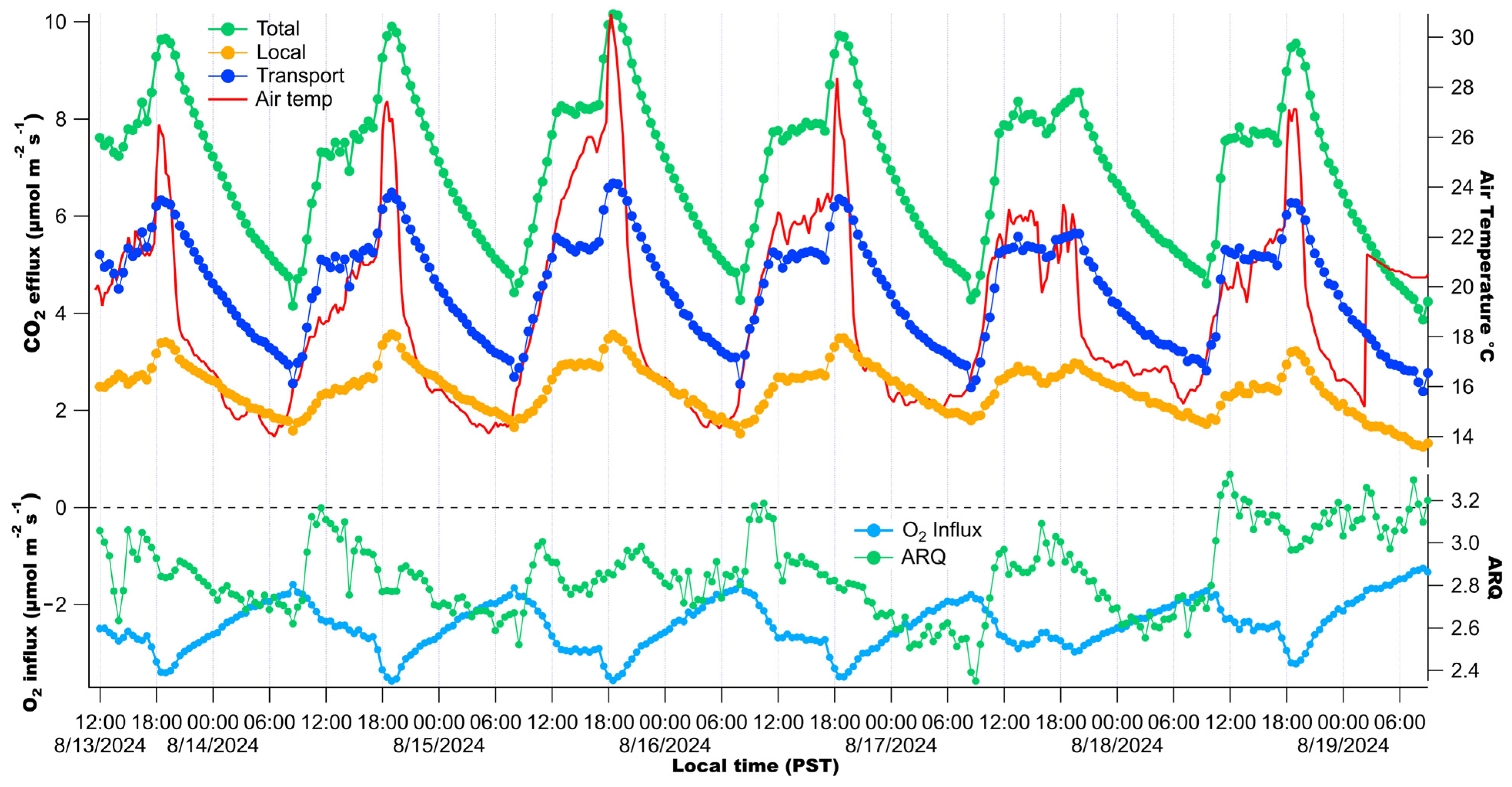
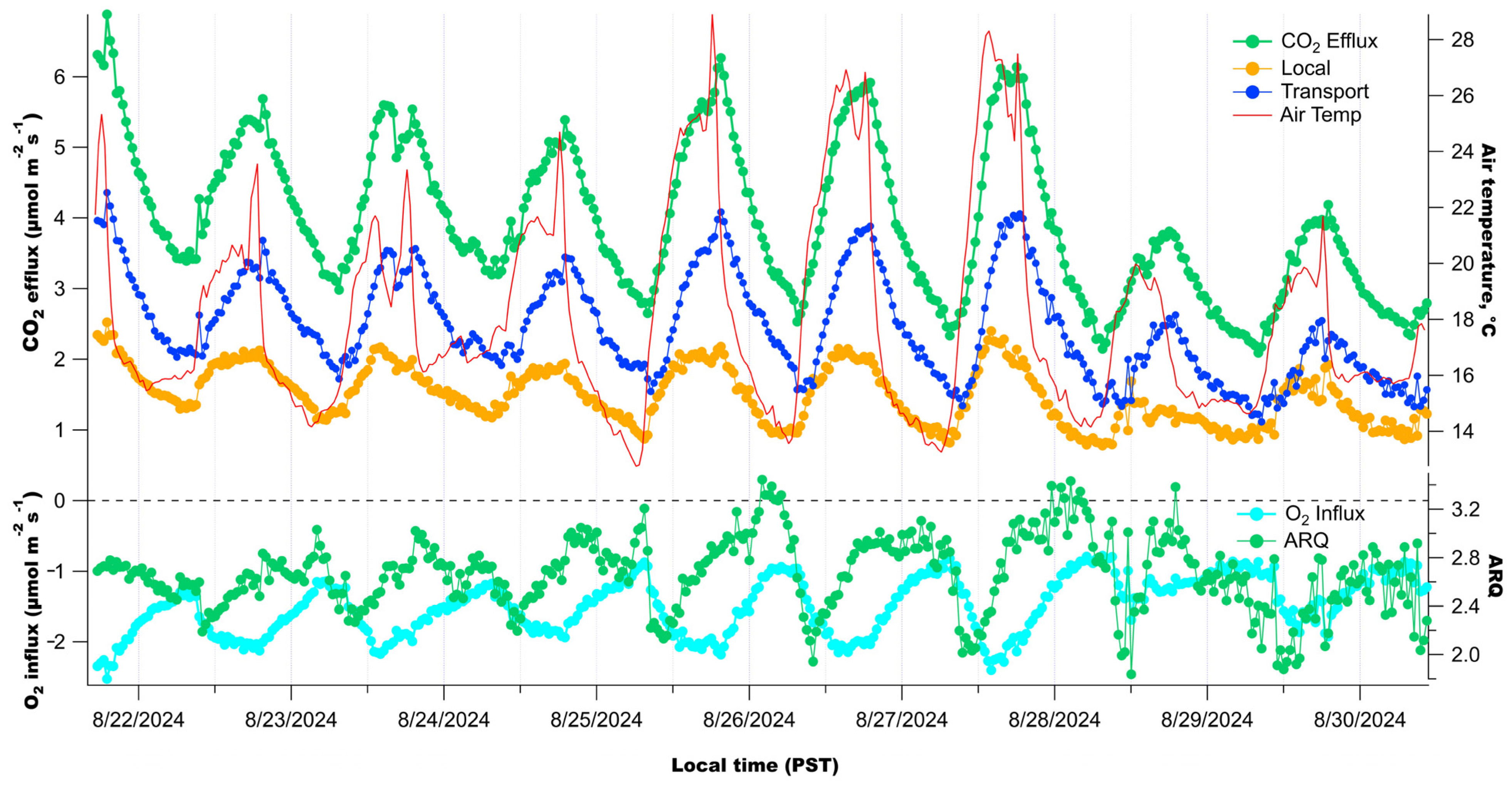
3. Results
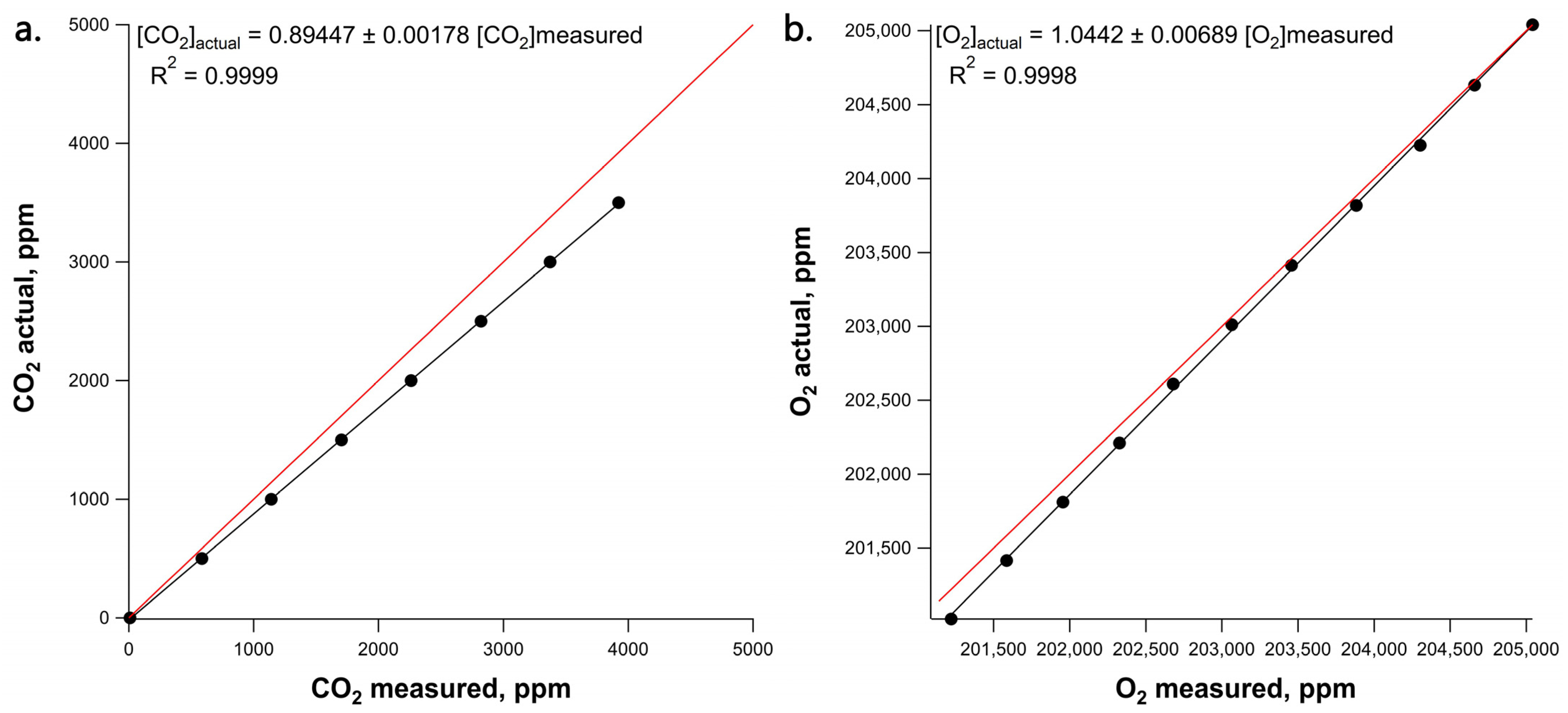
3.1. Calibration of CO2 and O2 CRDS Sensors
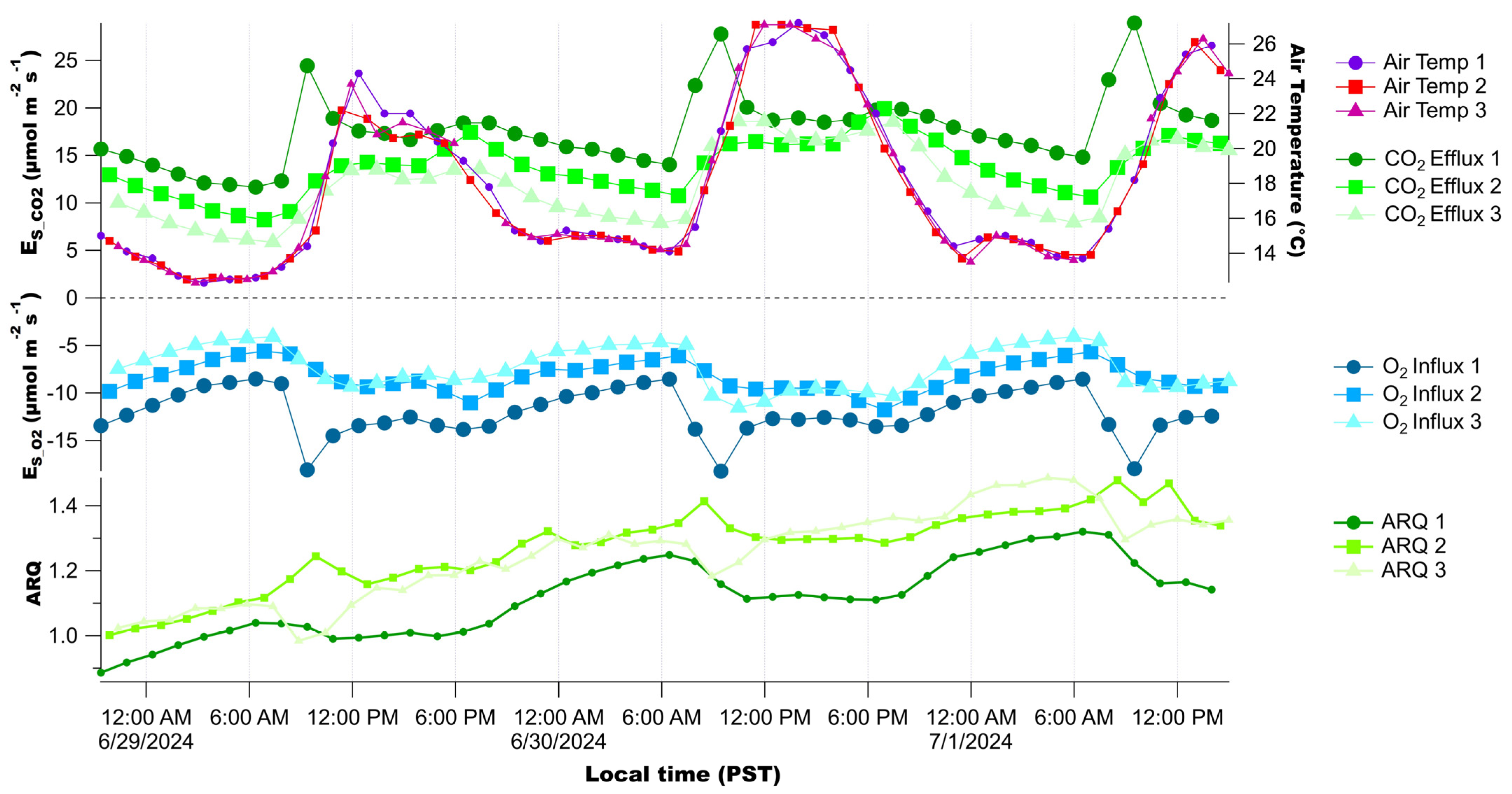
3.2. Diurnal Patterns of Raw Stem CO2 and O2 Concentrations
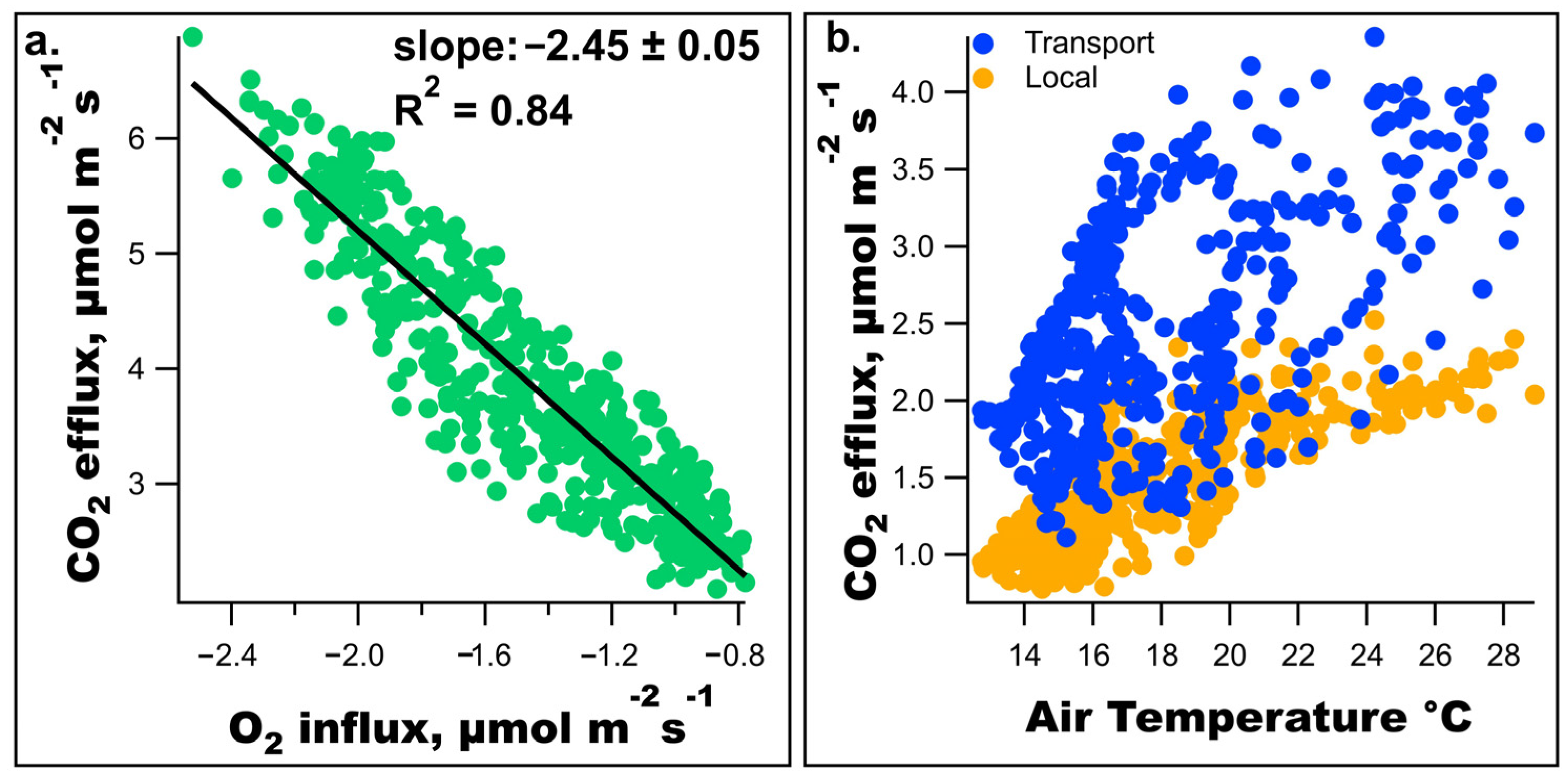
4. Discussion
4.1. Advantages of Real-Time, Continuous Measurements of CO2 Efflux
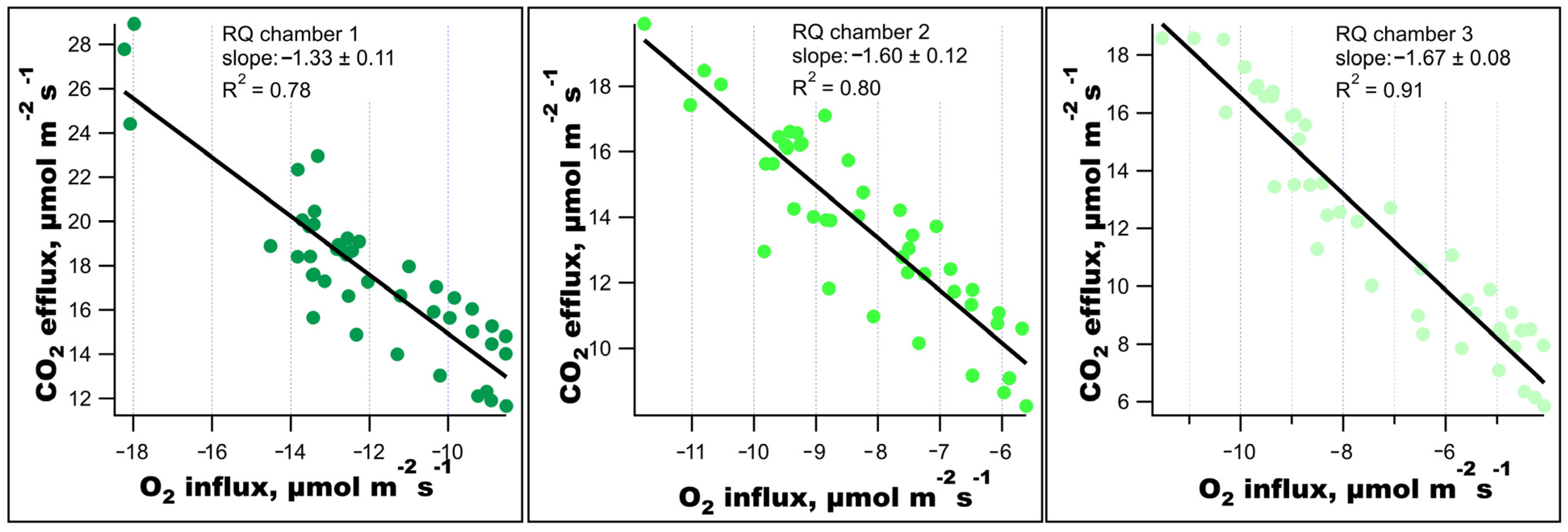
4.2. Significance of Observed Respiratory Quotient
4.3. Physiological Processes and Modeling of CO2 Efflux
4.4. Consequences for Molecular Signaling Pathways
| Measurement | Instrument Needed | What It Reflects | Units/Output | Quantitative Link to Gas Fluxes |
|---|---|---|---|---|
| Mitochondrial O2 Consumption Rate [61] | Clark-type O2 electrode or Seahorse XF Analyzer | Total respiratory activity (cytochrome + AOX pathways) | µmol O2 mg−1 protein h−1 or µmol O2 g−1 FW h−1 | Directly comparable to stem O2 influx (Es_O2) |
| CO2 Production in Isolated Tissues or Mitochondria [62] | Infrared gas analyzer (IRGA), GC, or CRDS | Net decarboxylation rate from respiration | µmol CO2 g−1 FW h−1 | Directly comparable to stem CO2 efflux (Es_CO2) |
| AOX and COX Enzyme Activities [63] | Spectrophotometer or O2 electrode with inhibitors (e.g., SHAM, KCN) | Partitioning of O2 consumption across ETC pathways | nmol O2 min−1 mg−1 protein | Explains variation in RQ and O2 uptake efficiency |
| TCA Cycle Enzyme Activities [64] | Enzymatic activity assay kits with spectrophotometry | Respiratory flux capacity | nmol min−1 mg−1 protein | High activity aligns with elevated CO2 production |
| ROS Production Rates (H2O2, O2−) [65] | Fluorometric ROS assays (e.g., Amplex Red, MitoSOX) or microplate reader | Redox state and mitochondrial efficiency | nmol g−1 FW h−1 | High ROS can suppress respiration and may influence RQ |
| Antioxidant Enzyme Activities (SOD, CAT, APX) [66] | Spectrophotometer or plate reader | ROS detoxification capacity | nmol min−1 mg−1 protein | Reflects ability to maintain respiration under oxidative stress |
| Metabolic Profiling: Sugars, Organic Acids, and Amino Acids [67] | GC-MS, LC-MS, or HPLC | Primary carbon substrates | µmol g−1 FW | Correlate with respiratory fluxes and CO2 efflux with composition shifts potentially impacting RQ |
| ATP/ADP and NADH/NAD⁺ Ratios [68] | Bioluminescence assay kits, HPLC, or LC-MS | Energetic/redox status | Molar ratios | Low ratios often coincide with reduced gas exchange |
| Calorimetry of Stem Segments [69] | Isothermal microcalorimeter (e.g., TAM (Thermal Activity Monitor)) | Total respiratory heat production from all pathways | µW g−1 FW or J h−1 | Quantitatively related to O2 consumption (∼470 kJ/mol O2) and CO2 production |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARQ | Apparent Respiratory Quotient (the ratio of net stem CO2 efflux to net stem O2 influx) |
| ES_CO2 | Net Stem CO2 Efflux (measured in µmol m−2 s−1) |
| ES_O2 | Net Stem O2 Influx (measured in µmol m−2 s−1) |
| Cellular RQ | Respiratory Quotient (ratio of CO2 produced to O2 consumed during cellular respiration) |
| CRDS | Cavity Ring-Down Spectrometry |
| PEPC | Phosphoenolpyruvate Carboxylase |
| TreSpire | A biophysical model for simulating stem respiration and associated CO2 transport processes |
| TBMs | Terrestrial Biosphere Models |
| FATES | Functionally Assembled Terrestrial Ecosystem Simulator |
| Q10 | A coefficient that quantifies the temperature sensitivity of a biological process |
| DOE | U.S. Department of Energy |
| BER | Biological and Environmental Research |
| NGEE-Tropics | Next Generation Ecosystem Experiments—Tropics |
| H2CO3 | Carbonic Acid |
| HCO3− | Bicarbonate |
| CO32− | Carbonate |
| ppm | Parts per Million |
| UHP | Ultra-High Purity |
References
- Keightley, A.T.; Field, C.D.; Rowson, J.G.; Caporn, S.J. Comparative photosynthetic capacity, respiration rates, and nutrient content of micropropagated and wild-sourced Sphagnum. Int. J. Plant Biol. 2024, 15, 959–978. [Google Scholar] [CrossRef]
- Chambers, J.Q.; Tribuzy, E.S.; Toledo, L.C.; Crispim, B.F.; Higuchi, N.; Santos, J.d.; Araújo, A.C.; Kruijt, B.; Nobre, A.D.; Trumbore, S.E. Respiration from a tropical forest ecosystem: Partitioning of sources and low carbon use efficiency. Ecol. Appl. 2004, 14, 72–88. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Wright, I.J.; Prentice, I.C.; Harrison, S.P.; Smith, N.G.; Westerband, A.; Rowland, L.; Plavcova, L.; Morris, H. Thermal acclimation of stem respiration reduces global carbon burden. bioRxiv 2024. [Google Scholar] [CrossRef]
- Tcherkez, G.; Ghashghaie, J. Plant Respiration: Metabolic Fluxes and Carbon Balance; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Jardine, K.J.; Cobello, L.O.; Teixeira, L.M.; East, M.-M.S.; Levine, S.; Gimenez, B.O.; Robles, E.; Spanner, G.; Koven, C.; Xu, C. Stem respiration and growth in a central Amazon rainforest. Trees 2022, 36, 991–1004. [Google Scholar] [CrossRef]
- Gielen, B.; Scarascia-Mugnozza, G.; Ceulemans, R. Stem respiration of Populus species in the third year of free-air CO2 enrichment. Physiol. Plant. 2003, 117, 500–507. [Google Scholar] [CrossRef]
- Teskey, R.O.; McGuire, M.A.; Bloemen, J.; Aubrey, D.P.; Steppe, K. Respiration and CO2 fluxes in trees. In Plant Respiration: Metabolic Fluxes and Carbon Balance; Springer: Berlin/Heidelberg, Germany, 2017; pp. 181–207. [Google Scholar]
- Salomón, R.L.; Helm, J.; Gessler, A.; Grams, T.E.; Hilman, B.; Muhr, J.; Steppe, K.; Wittmann, C.; Hartmann, H. The quandary of sources and sinks of CO2 efflux in tree stems—New insights and future directions. Tree Physiol. 2024, 44, tpad157. [Google Scholar] [CrossRef]
- Mills, M.B.; Both, S.; Jotan, P.; Huaraca Huasco, W.; Cruz, R.; Pillco, M.M.; Burslem, D.F.; Maycock, C.; Malhi, Y.; Ewers, R.M. From tree to plot: Investigating stem CO2 efflux and its drivers along a logging gradient in Sabah, Malaysian Borneo. New Phytol. 2024, 244, 91–103. [Google Scholar] [CrossRef]
- Teskey, R.O.; Saveyn, A.; Steppe, K.; McGuire, M.A. Origin, fate and significance of CO2 in tree stems. New Phytol. 2008, 177, 17–32. [Google Scholar] [CrossRef]
- Salomón, R.L.; De Roo, L.; Bodé, S.; Boeckx, P.; Steppe, K. Efflux and assimilation of xylem-transported CO2 in stems and leaves of tree species with different wood anatomy. Plant Cell Environ. 2021, 44, 3494–3508. [Google Scholar] [CrossRef]
- Stutz, S.S.; Hanson, D.T. Contribution and consequences of xylem-transported CO2 assimilation for C3 plants. New Phytol. 2019, 223, 1230–1240. [Google Scholar] [CrossRef]
- Bloemen, J.; Teskey, R.; McGuire, M.; Aubrey, D.; Steppe, K. Root xylem CO2 flux: An important but unaccounted-for component of root respiration. Trees 2016, 30, 343–352. [Google Scholar] [CrossRef]
- Salomaón, R.L.; De Roo, L.; Oleksyn, J.; De Pauw, D.J.; Steppe, K. TReSpire–a biophysical TRee Stem respiration model. New Phytol. 2020, 225, 2214–2230. [Google Scholar] [CrossRef]
- Salomón, R.L.; De Roo, L.; Oleksyn, J.; Steppe, K. Mechanistic drivers of stem respiration: A modelling exercise across species and seasons. Plant Cell Environ. 2022, 45, 1270–1285. [Google Scholar] [CrossRef]
- Tcherkez, G.; Gauthier, P.; Buckley, T.N.; Busch, F.A.; Barbour, M.M.; Bruhn, D.; Heskel, M.A.; Gong, X.Y.; Crous, K.Y.; Griffin, K. Leaf day respiration: Low CO2 flux but high significance for metabolism and carbon balance. New Phytol. 2017, 216, 986–1001. [Google Scholar] [CrossRef]
- Jardine, K.J.; Som, S.; Gallo, L.B.; Demus, J.; Domingues, T.F.; Wistrom, C.M.; Gu, L.; Tcherkez, G.; Niinemets, Ü. Concurrent Measurement of O2 Production and Isoprene Emission During Photosynthesis: Pros, Cons and Metabolic Implications of Responses to Light, CO2 and Temperature. Plant Cell Environ. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Hilman, B.; Weiner, T.; Haran, T.; Angert, A. Using Coupled CO2/O2 Measurements to Study Respiration in Tree Stems, Soil, and Carbon Fluxes Between Them. Geophys. Res. Abstr. 2019, 21, 1. [Google Scholar]
- Helm, J.; Salomón, R.L.; Hilman, B.; Muhr, J.; Knohl, A.; Steppe, K.; Gibon, Y.; Cassan, C.; Hartmann, H. Differences between tree stem CO2 efflux and O2 influx rates cannot be explained by internal CO2 transport or storage in large beech trees. Plant Cell Environ. 2023, 46, 2680–2693. [Google Scholar] [CrossRef]
- Helm, J. From Theory to Methods and Applications–for a Better Understanding of Stem Respiration and Carbon Dynamics in Mature Trees. Ph.D. Thesis, University of Basel, Basel, Switzerland, 2023. [Google Scholar]
- Helm, J.; Hartmann, H.; Göbel, M.; Hilman, B.; Herrera Ramírez, D.; Muhr, J. Low-cost chamber design for simultaneous CO2 and O2 flux measurements between tree stems and the atmosphere. Tree Physiol. 2021, 41, 1767–1780. [Google Scholar] [CrossRef]
- Hilman, B.; Weiner, T.; Haran, T.; Angert, A. Studying Soil and Tree Stem Respiration in Mediterranean oak forest using the Respiratory Quotient. J. Geophys. Res. Biogeosciences, 2022; submitted. [Google Scholar]
- Hilman, B.; Muhr, J.; Trumbore, S.E.; Kunert, N.; Carbone, M.S.; Yuval, P.; Wright, S.J.; Moreno, G.; Pérez-Priego, O.; Migliavacca, M. Comparison of CO2 and O2 fluxes demonstrate retention of respired CO2 in tree stems from a range of tree species. Biogeosciences 2019, 16, 177–191. [Google Scholar] [CrossRef]
- Hilman, B.; Angert, A. Measuring the ratio of CO2 efflux to O2 influx in tree stem respiration. Tree Physiol. 2016, 36, 1422–1431. [Google Scholar]
- Angert, A.; Muhr, J.; Negron Juarez, R.; Alegria Muñoz, W.; Kraemer, G.; Ramirez Santillan, J.; Barkan, E.; Mazeh, S.; Chambers, J.; Trumbore, S.E. Internal respiration of Amazon tree stems greatly exceeds external CO2 efflux. Biogeosciences 2012, 9, 4979–4991. [Google Scholar] [CrossRef]
- Patterson, A.E.; Arkebauer, R.; Quallo, C.; Heskel, M.A.; Li, X.; Boelman, N.; Griffin, K.L. Temperature response of respiration and respiratory quotients of 16 co-occurring temperate tree species. Tree Physiol. 2018, 38, 1319–1332. [Google Scholar] [CrossRef]
- Hilman, B.; Weiner, T.; Haran, T.; Masiello, C.A.; Gao, X.; Angert, A. The apparent respiratory quotient of soils and tree stems and the processes that control it. J. Geophys. Res. Biogeosciences 2022, 127, e2021JG006676. [Google Scholar] [CrossRef]
- Smart, D.R. Exposure to elevated carbon dioxide concentration in the dark lowers the respiration quotient of Vitis cane wood. Tree Physiol. 2004, 24, 115–120. [Google Scholar] [CrossRef]
- Dukat, P.; Hölttä, T.; Oren, R.; Salmon, Y.; Urbaniak, M.; Vesala, T.; Aalto, J.; Lintunen, A. Partitioning seasonal stem carbon dioxide efflux into stem respiration, bark photosynthesis, and transport-related flux in Scots pine. J. Exp. Bot. 2024, 75, 4944–4959. [Google Scholar] [CrossRef]
- Berveiller, D.; Kierzkowski, D.; Damesin, C. Interspecific variability of stem photosynthesis among tree species. Tree Physiol. 2007, 27, 53–61. [Google Scholar] [CrossRef]
- Bessemans, N.; Verboven, P.; Verlinden, B.; Janssens, M.; Hertog, M.; Nicolaï, B. Apparent respiratory quotient observed in headspace of static respirometers underestimates cellular respiratory quotient of pear fruit. Postharvest Biol. Technol. 2020, 162, 111104. [Google Scholar] [CrossRef]
- Heinemeyer, A.; McNamara, N.P. Comparing the closed static versus the closed dynamic chamber flux methodology: Implications for soil respiration studies. Plant Soil 2011, 346, 145–151. [Google Scholar] [CrossRef]
- Acosta, M.; Pavelka, M.; Pokorný, R.; Janouš, D.; Marek, M.V. Seasonal variation in CO2 efflux of stems and branches of Norway spruce trees. Ann. Bot. 2008, 101, 469–477. [Google Scholar] [CrossRef]
- Ubierna, N.; Kumar, A.S.; Cernusak, L.A.; Pangle, R.E.; Gag, P.J.; Marshall, J.D. Storage and transpiration have negligible effects on δ13C of stem CO2 efflux in large conifer trees. Tree Physiol. 2009, 29, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Araki, M.G.; Utsugi, H.; Kajimoto, T.; Han, Q.; Kawasaki, T.; Chiba, Y. Estimation of whole-stem respiration, incorporating vertical and seasonal variations in stem CO2 efflux rate, of Chamaecyparis obtusa trees. J. For. Res. 2010, 15, 115–122. [Google Scholar] [CrossRef]
- Jardine, K.J.; McDowell, N. Fermentation-mediated growth, signaling, and defense in plants. New Phytol. 2023, 239, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Salomón, R.L.; De Schepper, V.; Valbuena-Carabaña, M.; Gil, L.; Steppe, K. Daytime depression in temperature-normalised stem CO2 efflux in young poplar trees is dominated by low turgor pressure rather than by internal transport of respired CO2. New Phytol. 2018, 217, 586–598. [Google Scholar] [CrossRef]
- Angert, A.; Sherer, Y. Determining the relationship between tree-stem respiration and CO2 efflux by δO2/Ar measurements. Rapid Commun. Mass Spectrom. 2011, 25, 1752–1756. [Google Scholar] [CrossRef]
- Tjoelker, M.G.; Oleksyn, J.; Reich, P.B. Modelling respiration of vegetation: Evidence for a general temperature-dependent Q10. Glob. Change Biol. 2001, 7, 223–230. [Google Scholar] [CrossRef]
- Moorcroft, P.R.; Hurtt, G.C.; Pacala, S.W. A method for scaling vegetation dynamics: The ecosystem demography model (ED). Ecol. Monogr. 2001, 71, 557–586. [Google Scholar] [CrossRef]
- Koven, C.D.; Knox, R.G.; Fisher, R.A.; Chambers, J.Q.; Christoffersen, B.O.; Davies, S.J.; Detto, M.; Dietze, M.C.; Faybishenko, B.; Holm, J. Benchmarking and parameter sensitivity of physiological and vegetation dynamics using the Functionally Assembled Terrestrial Ecosystem Simulator (FATES) at Barro Colorado Island, Panama. Biogeosciences 2020, 17, 3017–3044. [Google Scholar] [CrossRef]
- Fisher, R.A.; Muszala, S.; Verteinstein, M.; Lawrence, P.; Xu, C.; McDowell, N.G.; Knox, R.G.; Koven, C.; Holm, J.; Rogers, B.M. Taking off the training wheels: The properties of a dynamic vegetation model without climate envelopes, CLM4.5 (ED). Geosci. Model Dev. 2015, 8, 3593–3619. [Google Scholar] [CrossRef]
- Levy, P.; Meir, P.; Allen, S.; Jarvis, P. The effect of aqueous transport of CO2 in xylem sap on gas exchange in woody plants. Tree Physiol. 1999, 19, 53–58. [Google Scholar] [CrossRef]
- Tarvainen, L.; Henriksson, N.; Näsholm, T.; Marshall, J.D. Among-species variation in sap pH affects the xylem CO2 transport potential in trees. New Phytol. 2023, 238, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Jardine, K.; Augusto, E.; Levine, S.D.; Sunder, A.; Som, S.; Chambers, J. Development of a lightweight, portable, waterproof, and low power stem respiration system for trees. MethodsX 2023, 10, 101986. [Google Scholar] [CrossRef] [PubMed]
- Morote, F.; Abellán, M.; Rubio, E.; Anta, I.; Serrano, F. Stem CO2 efflux as an indicator of forests’ productivity in Relict Juniper Woodlands (Juniperus thurifera L.) of southern Spain. Forests 2021, 12, 1340. [Google Scholar] [CrossRef]
- Goodger, J.Q.; Sharp, R.E.; Marsh, E.L.; Schachtman, D.P. Relationships between xylem sap constituents and leaf conductance of well-watered and water-stressed maize across three xylem sap sampling techniques. J. Exp. Bot. 2005, 56, 2389–2400. [Google Scholar] [CrossRef]
- Lake, J.A.; Walker, H.J.; Cameron, D.D.; Lomax, B.H. A novel root-to-shoot stomatal response to very high CO2 levels in the soil: Electrical, hydraulic and biochemical signalling. Physiol. Plant. 2017, 159, 433–444. [Google Scholar] [CrossRef]
- Gloser, V.; Korovetska, H.; Martín-Vertedor, A.I.; Hájíčková, M.; Prokop, Z.; Wilkinson, S.; Davies, W. The dynamics of xylem sap pH under drought: A universal response in herbs? Plant Soil 2016, 409, 259–272. [Google Scholar] [CrossRef]
- Wilkinson, S.; Davies, W.J. Xylem sap pH increase: A drought signal received at the apoplastic face of the guard cell that involves the suppression of saturable abscisic acid uptake by the epidermal symplast. Plant Physiol. 1997, 113, 559–573. [Google Scholar] [CrossRef]
- Thomas, D.S.; Eamus, D. Seasonal patterns of xylem sap pH, xylem abscisic acid concentration, leaf water potential and stomatal conductance of six evergreen and deciduous Australian savanna tree species. Aust. J. Bot. 2002, 50, 229–236. [Google Scholar] [CrossRef]
- Helm, J.; Muhr, J.; Hilman, B.; Kahmen, A.; Schulze, E.-D.; Trumbore, S.; Herrera-Ramírez, D.; Hartmann, H. Carbon dynamics in long-term starving poplar trees—The importance of older carbohydrates and a shift to lipids during survival. Tree Physiol. 2023, 44, 173–185. [Google Scholar] [CrossRef]
- Darenova, E.; Knott, R.; Vichta, T. Does lower water availability limit stem CO2 efflux of oak and hornbeam coppices? AoB Plants 2024, 16, plae023. [Google Scholar] [CrossRef]
- Gao, L.; Wang, W.; Xu, C.; Han, X.; Li, Y.; Liu, Y.; Qi, H. Physiological and transcriptomic analyses reveal the effects of elevated root-zone CO2 on the metabolism of sugars and starch in the roots of oriental melon seedlings. Int. J. Mol. Sci. 2022, 23, 12537. [Google Scholar] [CrossRef] [PubMed]
- Pagliarani, C.; Casolo, V.; Ashofteh Beiragi, M.; Cavalletto, S.; Siciliano, I.; Schubert, A.; Gullino, M.L.; Zwieniecki, M.A.; Secchi, F. Priming xylem for stress recovery depends on coordinated activity of sugar metabolic pathways and changes in xylem sap pH. Plant Cell Environ. 2019, 42, 1775–1787. [Google Scholar] [CrossRef]
- Rodríguez-Celma, J.; Ceballos-Laita, L.; Grusak, M.A.; Abadía, J.; López-Millán, A.-F. Plant fluid proteomics: Delving into the xylem sap, phloem sap and apoplastic fluid proteomes. Biochim. Biophys. Acta Proteins Proteom. 2016, 1864, 991–1002. [Google Scholar] [CrossRef]
- Munoz, J.; Garcia-Molina, F.; Varon, R.; Rodriguez-Lopez, J.; Garcia-Ruiz, P.; Garcia-Canovas, F.; Tudela, J. Kinetic characterization of the oxidation of chlorogenic acid by polyphenol oxidase and peroxidase. Characteristics of the o-quinone. J. Agric. Food Chem. 2007, 55, 920–928. [Google Scholar] [CrossRef]
- Zhang, H.; Joseph, J.; Gurney, M.; Becker, D.; Kalyanaraman, B. Bicarbonate enhances peroxidase activity of Cu, Zn-superoxide dismutase: Role of carbonate anion radical and scavenging of carbonate anion radical by metalloporphyrin antioxidant enzyme mimetics. J. Biol. Chem. 2002, 277, 1013–1020. [Google Scholar] [CrossRef]
- Jarin, A.S.; Islam, M.M.; Rahat, A.; Ahmed, S.; Ghosh, P.; Murata, Y. Drought stress tolerance in rice: Physiological and biochemical insights. Int. J. Plant Biol. 2024, 15, 692–718. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I. Generation and Fate of ROS in Mitochondria. In Reactive Oxygen Species in Plants: The Right Balance; Springer: Berlin/Heidelberg, Germany, 2023; pp. 93–106. [Google Scholar]
- Jacoby, R.P.; Millar, A.H.; Taylor, N.L. Assessment of respiration in isolated plant mitochondria using Clark-type electrodes. In Plant Mitochondria: Methods and Protocols; Humana Press: New York, NY, USA, 2015; pp. 165–185. [Google Scholar]
- Bruhn, D.; Wiskich, J.T.; Atkin, O.K. Contrasting responses by respiration to elevated CO2 in intact tissue and isolated mitochondria. Funct. Plant Biol. 2007, 34, 112–117. [Google Scholar] [CrossRef]
- Huang, S.; Lee, C.P.; Millar, A.H. Activity assay for plant mitochondrial enzymes. In Plant Mitochondria: Methods and Protocols; Humana Press: New York, NY, USA, 2015; pp. 139–149. [Google Scholar]
- Omena-Garcia, R.P.; Araújo, W.L.; Gibon, Y.; Fernie, A.R.; Nunes-Nesi, A. Measurement of tricarboxylic acid cycle enzyme activities in plants. In Plant Respiration and Internal Oxygen: Methods and Protocols; Humana Press: New York, NY, USA, 2017; pp. 167–182. [Google Scholar]
- Akter, S.; Khan, M.S.; Smith, E.N.; Flashman, E. Measuring ROS and redox markers in plant cells. RSC Chem. Biol. 2021, 2, 1384–1401. [Google Scholar] [CrossRef]
- Elavarthi, S.; Martin, B. Spectrophotometric assays for antioxidant enzymes in plants. In Plant Stress Tolerance: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2010; pp. 273–280. [Google Scholar]
- Adeeyo, A.O.; Amao, I.O.; Ngandjui, Y.A.; Alabi, M.A.; Msagati, T.A. Metabolic profiling of plant and other natural products: Platforms, applications and quality evaluation. Microchem. J. 2024, 207, 111779. [Google Scholar] [CrossRef]
- Steinbeck, J.; Fuchs, P.; Negroni, Y.L.; Elsässer, M.; Lichtenauer, S.; Stockdreher, Y.; Feitosa-Araujo, E.; Kroll, J.B.; Niemeier, J.-O.; Humberg, C. In vivo NADH/NAD+ biosensing reveals the dynamics of cytosolic redox metabolism in plants. Plant Cell 2020, 32, 3324–3345. [Google Scholar] [CrossRef]
- Hansen, L.D.; Hopkin, M.S.; Rank, D.R.; Anekonda, T.S.; Breidenbach, R.W.; Criddle, R.S. The relation between plant growth and respiration: A thermodynamic model. Planta 1994, 194, 77–85. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jardine, K.J.; Oliveira, R.; Ajami, P.; Knox, R.; Koven, C.; Gimenez, B.; Spanner, G.; Warren, J.; McDowell, N.; Tcherkez, G.; et al. Real-Time Partitioning of Diurnal Stem CO2 Efflux into Local Stem Respiration and Xylem Transport Processes. Int. J. Plant Biol. 2025, 16, 46. https://doi.org/10.3390/ijpb16020046
Jardine KJ, Oliveira R, Ajami P, Knox R, Koven C, Gimenez B, Spanner G, Warren J, McDowell N, Tcherkez G, et al. Real-Time Partitioning of Diurnal Stem CO2 Efflux into Local Stem Respiration and Xylem Transport Processes. International Journal of Plant Biology. 2025; 16(2):46. https://doi.org/10.3390/ijpb16020046
Chicago/Turabian StyleJardine, Kolby J., Regison Oliveira, Parsa Ajami, Ryan Knox, Charlie Koven, Bruno Gimenez, Gustavo Spanner, Jeffrey Warren, Nate McDowell, Guillaume Tcherkez, and et al. 2025. "Real-Time Partitioning of Diurnal Stem CO2 Efflux into Local Stem Respiration and Xylem Transport Processes" International Journal of Plant Biology 16, no. 2: 46. https://doi.org/10.3390/ijpb16020046
APA StyleJardine, K. J., Oliveira, R., Ajami, P., Knox, R., Koven, C., Gimenez, B., Spanner, G., Warren, J., McDowell, N., Tcherkez, G., & Chambers, J. (2025). Real-Time Partitioning of Diurnal Stem CO2 Efflux into Local Stem Respiration and Xylem Transport Processes. International Journal of Plant Biology, 16(2), 46. https://doi.org/10.3390/ijpb16020046







