Mitigating Salinity Stress in Pea Plants with Titanium Oxide Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Characterization of TiO2 NPs
2.3. Growth Parameters
2.4. Pigment Analysis
2.5. Determination of Markers of Oxidative Stress
2.6. Measurements of Electrolyte Leakage
2.7. Chlorophyll a Fluorescence at Room Temperature
2.8. P700 Photooxidation
2.9. Statistical Analysis
3. Results
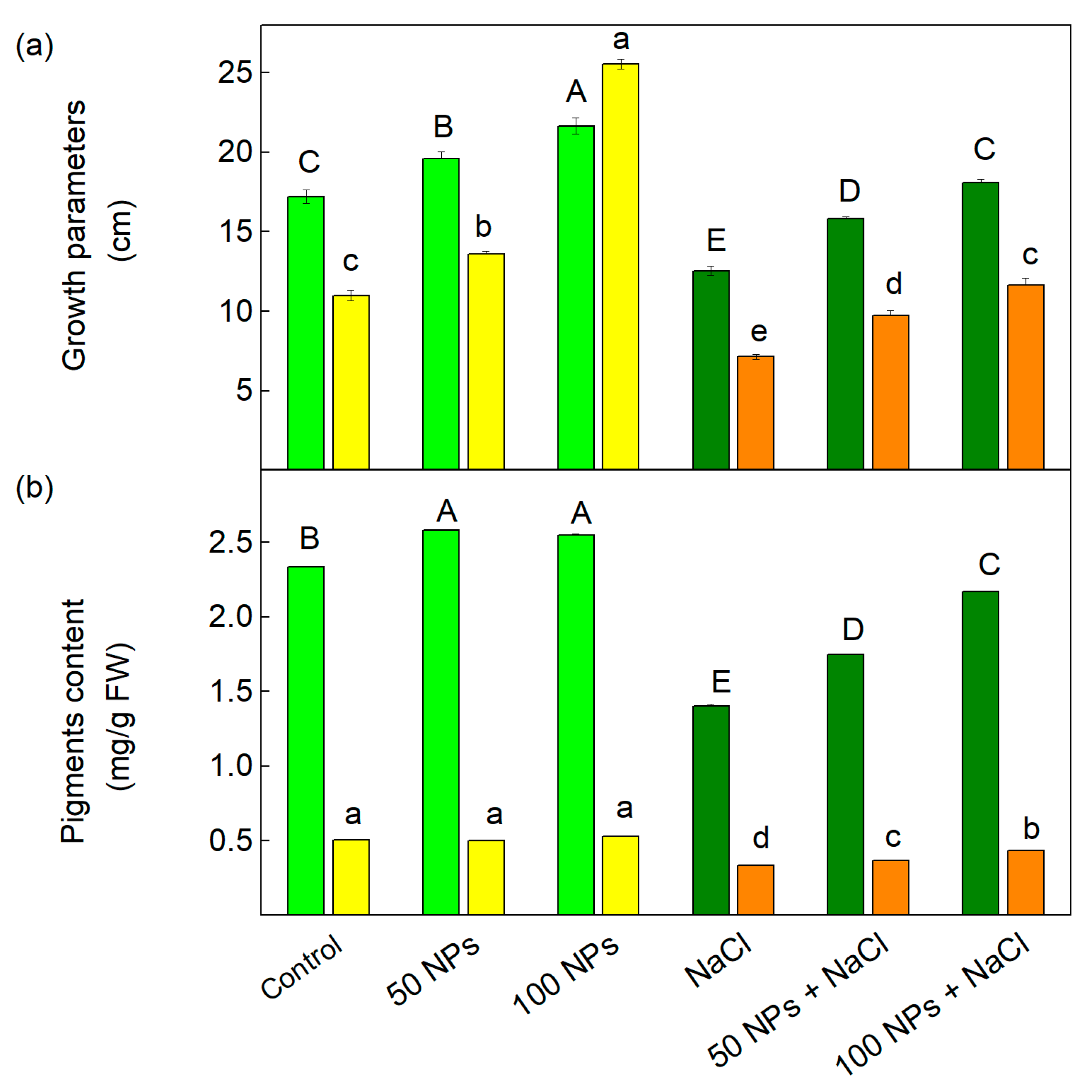
3.1. Growth Parameters and Pigment Analysis
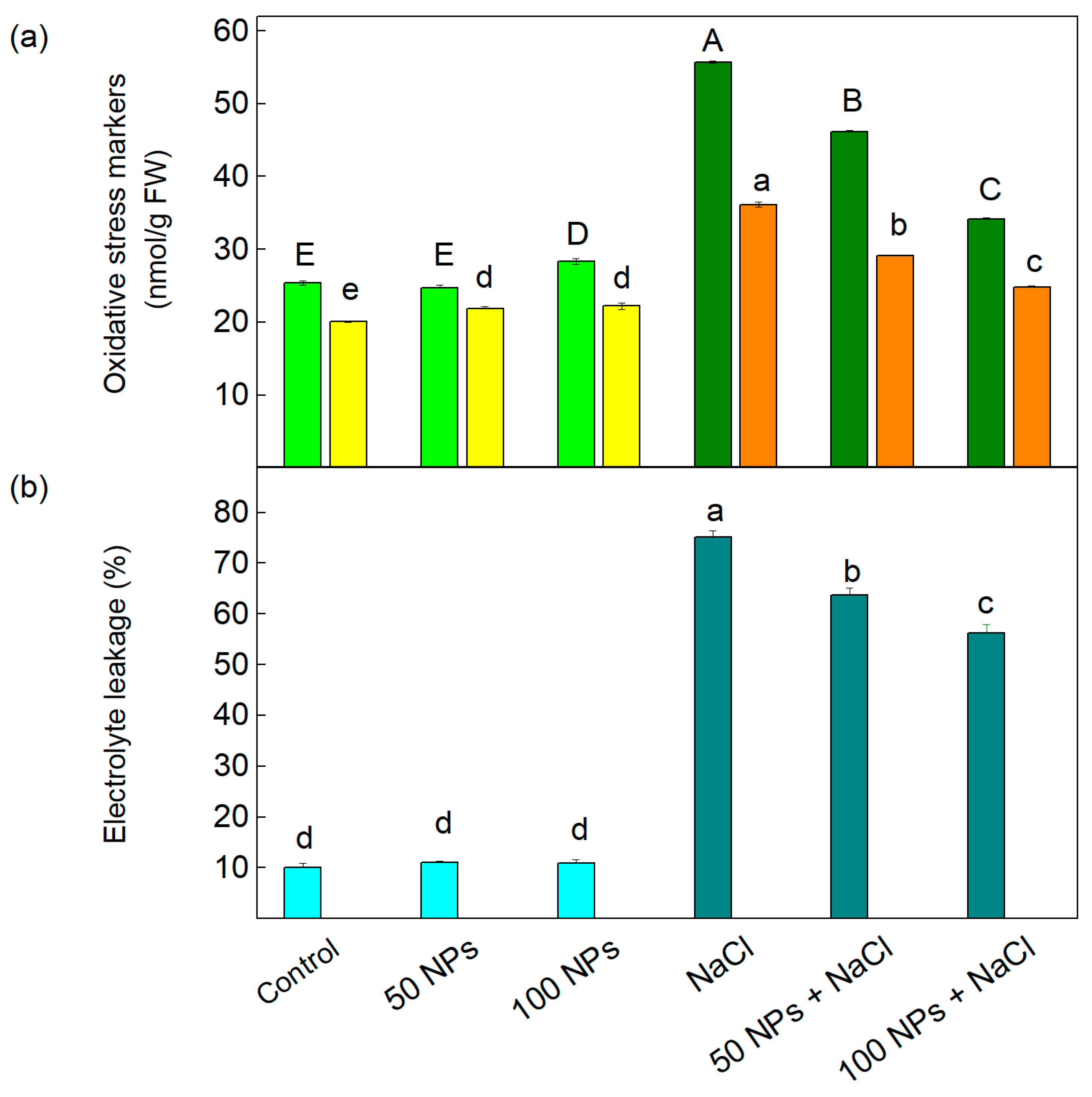
3.2. Oxidative Stress Markers and Electrolyte Leakage
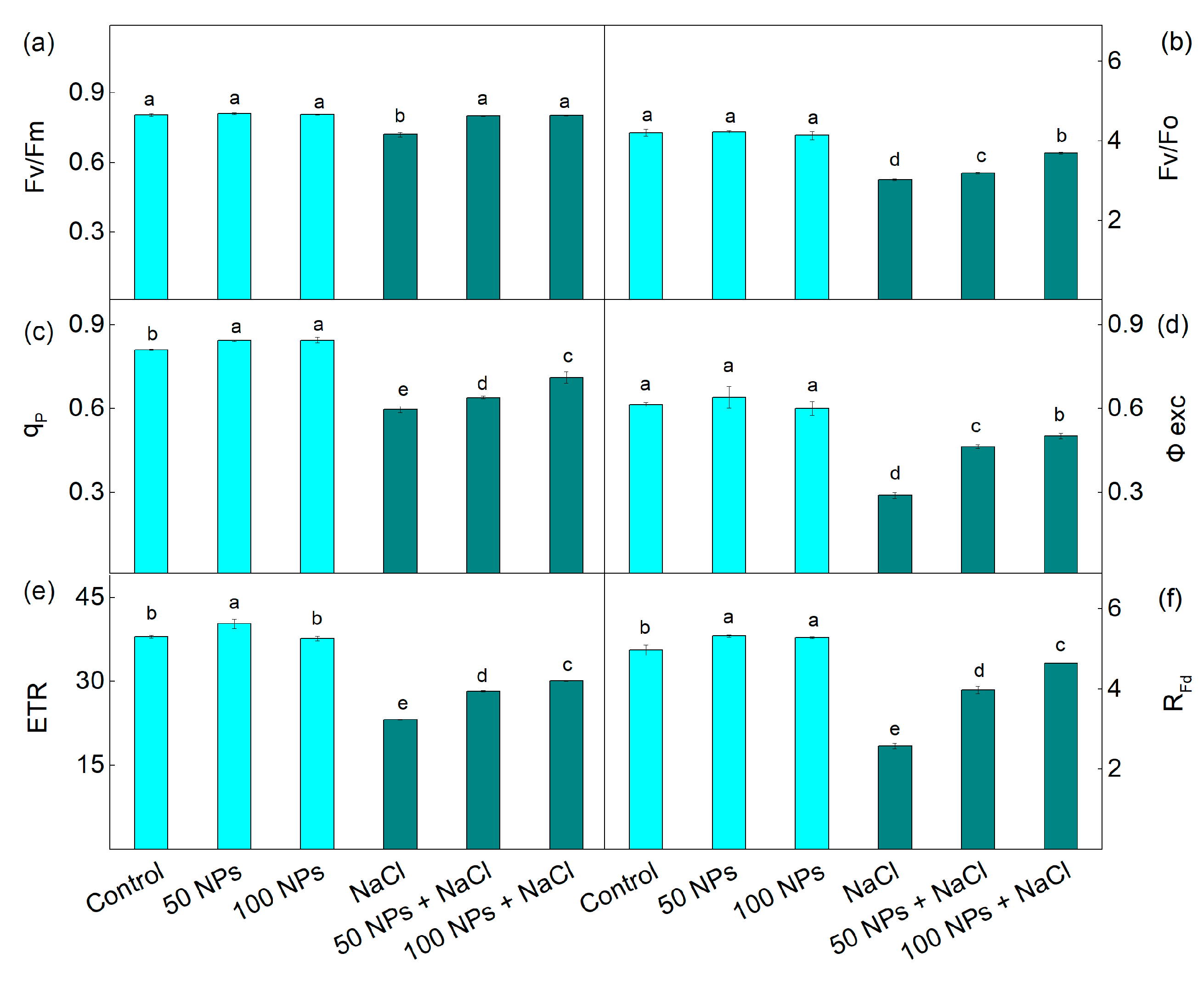
3.3. Chlorophyll Fluorescence Parameters
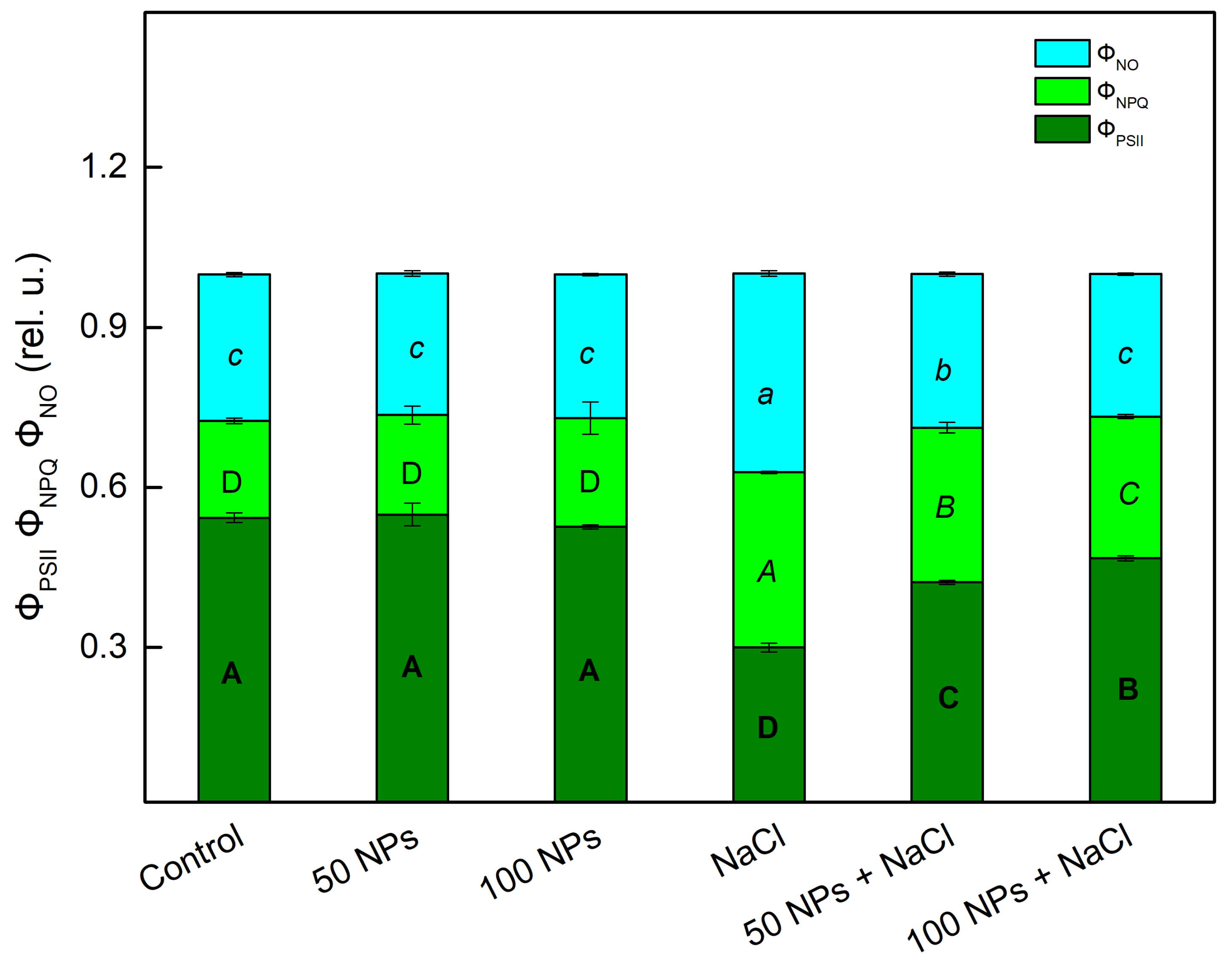
3.4. Oxidation-Reduction Properties of P700
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Chl | chlorophyll |
| Car | carotenoids |
| MDA | malondialdehyde |
| EL | electrolyte leakage |
| NPs | nanoparticles |
| PAM | pulse-amplitude-modulated chlorophyll fluorescence |
| PSI | photosystem I |
| PSII | photosystem II |
| OEC | oxygen evolving complex |
| Fv/Fm | maximum quantum efficiency of PSII |
| Fv/Fo | quantum yield ratio of photochemical and non-photochemical processes |
| qp | photochemical quenching |
| ΦPSII | effective quantum yield of PSII |
| ΦNO | non-regulated energy loss in PSII |
| ΦNPQ | regulated energy loss in PSII |
| Φexc | excitation efficiency of open PSII centers |
| ETR | electron transport rate |
| k1 | decay rate constant for the fast component A1 of dark relaxation of chlorophyll fluorescence |
| k2 | decay rate constant for the slow component A2 of dark relaxation of chlorophyll fluorescence |
| t1 | time for the fast component A1 of dark relaxation of chlorophyll fluorescence |
| t2 | time for the slow component A2 of dark relaxation of chlorophyll fluorescence |
| RFd | chlorophyll fluorescence decay ratio |
| A1/A2 | the ratio of two pathways for QA− re-oxidation |
| ∆A/A | photo-oxidation of P700+ |
References
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Li, G.; Yang, J.; Huang, X.; Ji, Q.; Liu, Z.; Ke, W.; Hou, H. Effect of salt stress on growth, physiological parameters, and ionic concentration of water dropwort (Oenanthe javanica) cultivars. Front. Plant Sci. 2021, 12, 660409. [Google Scholar] [CrossRef] [PubMed]
- Munawar, W.; Hameed, A.; Khan, M.K.R. Differential morphophysiological and biochemical responses of cotton genotypes under various salinity stress levels during early growth stage. Front. Plant Sci. 2021, 12, 622309. [Google Scholar] [CrossRef] [PubMed]
- Stefanov, M.A.; Rashkov, G.D.; Yotsova, E.K.; Borisova, P.B.; Dobrikova, A.G.; Apostolova, E.L. Different sensitivity levels of the photosynthetic apparatus in Zea mays L. and Sorghum bicolor L. under salt Stress. Plants 2021, 10, 1469. [Google Scholar] [CrossRef]
- Zeeshan, M.; Lu, M.; Sehar, S.; Holford, P.; Wu, F. Comparison of biochemical, anatomical, morphological, and physiological responses to salinity stress in wheat and barley genotypes deferring in salinity tolerance. Agronomy 2020, 10, 127. [Google Scholar] [CrossRef]
- Hameed, A.; Ahmed, M.Z.; Hussain, T.; Aziz, I.; Ahmad, N.; Gul, B.; Nielsen, B.L. Effects of salinity stress on chloroplast structure and function. Cells 2021, 10, 2023. [Google Scholar] [CrossRef]
- Mitsuya, S.; Takeoka, Y.; Miyake, H. Effects of sodium chloride on foliar ultrastructure of sweet potato (Ipomoea batatas Lam.) plantlets grown under light and dark conditions in vitro. J. Plant Physiol. 2000, 157, 661–667. [Google Scholar] [CrossRef]
- Li, W.; Li, Q. Effect of environmental salt stress on plants and the molecular mechanism of salt stress tolerance. Int. J. Environ. Sci. Nat. Resour. 2017, 7, 555714. [Google Scholar] [CrossRef]
- Papadakis, I.E.; Sotiras, M.I.; Landi, M.; Ladikou, E.; Oikonomou, A.; Psychoyou, M.; Fasseas, C. Salinity alters plant’s allometry and sugar metabolism, and impairs the photosynthetic process and photosystem II efficiency in Eriobotrya japonica plants. Agrochimica 2019, 63, 27–42. [Google Scholar] [CrossRef]
- Stefanov, M.; Biswal, A.K.; Misra, M.; Misra, A.N.; Apostolova, E.L. Responses of Photosynthetic Apparatus to Salt Stress: Structure, Function, and Protection. In Handbook of Plant and Crop Stress, 4th ed.; Pessarakli, M., Ed.; Taylor & Francis CRC Press: New York, NY, USA, 2019; pp. 233–250. ISBN 9781351104609. [Google Scholar]
- Barhoumi, Z.; Djebali, W.; Chaïbi, W.; Abdelly, C.; Smaoui, A. Salt impact on photosynthesis and leaf ultrastructure of Aeluropus littoralis. J. Plant Res. 2007, 120, 529–537. [Google Scholar] [CrossRef]
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.-H.; Ahmad, H.; Li, F.B. Mechanisms regulating the dynamics of photosynthesis under abiotic stresses. Front. Plant Sci. 2021, 11, 615942. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Cheng, J.; Gong, D.; Zhao, X.; Qi, Y.; Su, Y.; Ma, W. The effect of NaCl stress on photosynthetic efficiency and lipid production in freshwater microalga—Scenedesmus obliquus XJ002. Sci. Total Environ. 2018, 633, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, P.; Baczewska, A.H.; Pawluśkiewicz, B.; Paunov, M.; Alexantrov, V.; Goltsev, V.; Kalaji, M.H. Prompt chlorophyll a fluorescence as a rapid tool for diagnostic changes in PSII structure inhibited by salt stress in Perennial ryegrass. J. Photochem. Photobiol. B. 2016, 157, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Cavaliere, C.; Guarino, C.; Gubbiotti, R.; Foglia, P.; Laganà, A. Identification of changes in Triticum durum L. leaf proteome in response to salt stress by two-dimensional electrophoresis and MALDI-TOF mass Spectrom. Anal. Bioanal. Chem. 2008, 391, 381–390. [Google Scholar] [CrossRef]
- Huang, L.; Li, Z.; Liu, Q.; Pu, G.; Zhang, Y.; Li, J. Research on the adaptive mechanism of photosynthetic apparatus under salt stress: New directions to increase crop yield in saline soils. Ann. Appl. Biol. 2019, 175, 1–17. [Google Scholar] [CrossRef]
- Pang, Q.; Chen, S.; Dai, S.; Chen, Y.; Wang, Y.; Yan, X. Comparative proteomics of salt tolerance in Arabidopsis thaliana and Thellungiella halophila. J. Proteome Res. 2010, 9, 2584–2599. [Google Scholar] [CrossRef]
- Liu, Z.; Zou, L.; Chen, C.; Zhao, H.; Yan, Y.; Wang, C.; Liu, X. ITRAQ-based quantitative proteomic analysis of salt stress in Spica Prunellae. Sci. Rep. 2019, 9, 9590. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Pandey, D.M.; Choi, I.; Yeo, U.-D. Photosystem 2-activity and thylakoid membrane polypeptides of in vitro cultured Chrysanthemum as affected by NaCl. Biol. Plant. 2009, 53, 329–333. [Google Scholar] [CrossRef]
- Mishra, S.K.; Subrahmanyam, D.; Singhal, G.S. Interrelationship between salt and light stress on primary processes of photosynthesis. J. Plant Physiol. 1991, 138, 92–96. [Google Scholar] [CrossRef]
- Rashkov, G.D.; Stefanov, M.A.; Yotsova, E.K.; Borisova, P.B.; Dobrikova, A.G.; Apostolova, E.L. Exploring nitric oxide as a regulator in salt tolerance: Insights into photosynthetic efficiency in maize. Plants 2024, 13, 1312. [Google Scholar] [CrossRef] [PubMed]
- Stefanov, M.A.; Rashkov, G.D.; Borisova, P.B.; Apostolova, E.L. Changes in photosystem II complex and physiological activities in pea and maize plants in response to salt stress. Plants 2024, 13, 1025. [Google Scholar] [CrossRef] [PubMed]
- Bora, K.A.; Hashmi, S.; Zulfiqar, F.; Abideen, Z.; Ali, H.; Siddiqui, Z.S.; Siddique, K.H.M. Recent progress in bio-mediated synthesis and applications of engineered nanomaterials for sustainable agriculture. Front. Plant Sci. 2022, 13, 999505. [Google Scholar] [CrossRef]
- Singh, S.P.; Keswani, C.; Minkina, T.; Ortiz, A.; Sansinenea, E. Nano-inputs: A next-generation solution for sustainable crop production. J. Plant Growth Regul. 2023, 42, 5311–5324. [Google Scholar] [CrossRef]
- Dilnawaz, F.; Kalaji, M.H.; Misra, A.N. Nanotechnology in improving photosynthesis under adverse climatic conditions: Cell to Canopy action. Plant Nano Biol. 2023, 4, 100035. [Google Scholar] [CrossRef]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of metal and metal oxide nanoparticles on plant: A critical review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef]
- Masarovičová, E.; Kráľová, K. Metal nanoparticles and plants / nanocząstki metaliczne I rośliny. Ecol. Chem. Eng. S 2013, 20, 9–22. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; De Silva, K.; Biris, A.S.; Dervishi, E.; Villagarcia, H. Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano 2012, 6, 2128–2135. [Google Scholar] [CrossRef]
- Sheikhalipour, M.; Esmaielpour, B.; Behnamian, M.; Gohari, G.; Giglou, M.T.; Vachova, P.; Rastogi, A.; Brestic, M.; Skalicky, M. Chitosan–selenium nanoparticle (Cs–Se NP) foliar spray alleviates salt stress in bitter melon. Nanomaterials 2021, 11, 684. [Google Scholar] [CrossRef]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, W.; Zhu, G.; Wang, Q.; Zhang, P.; Rui, Y. Recent trends in foliar nanofertilizers: A review. Nanomaterials 2023, 13, 2906. [Google Scholar] [CrossRef] [PubMed]
- Nemček, L.; Šebesta, M.; Urík, M.; Bujdoš, M.; Dobročka, E.; Vávra, I. Impact of bulk ZnO, ZnO nanoparticles and dissolved Zn on early growth stages of barley—A pot experiment. Plants 2020, 9, 1365. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, N.B.; Afzal, S.; Singh, T.; Hussain, I. Zinc oxide nanoparticles: A review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J. Mater. Sci. 2018, 53, 185–201. [Google Scholar] [CrossRef]
- Rossi, L.; Fedenia, L.N.; Sharifan, H.; Ma, X.; Lombardini, L. Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee (Coffea arabica L.) plants. Plant Physiol. Biochem. 2019, 135, 160–166. [Google Scholar] [CrossRef]
- Jampílek, J.; Král’Ová, K. Application of nanotechnology in agriculture and food industry, its prospects and risks. Ecol. Chem. Eng. S 2015, 22, 321–361. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Rizwan, M.; Zia ur Rehman, M.; Javed, M.R.; Imran, M.; Chatha, S.A.S.; Nazir, R. Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ. Pollut. 2018, 242, 1518–1526. [Google Scholar] [CrossRef]
- Shafiq, T.; Yasmin, H.; Shah, Z.A.; Nosheen, A.; Ahmad, P.; Kaushik, P.; Ahmad, A. Titanium oxide and zinc oxide nanoparticles in combination with cadmium tolerant Bacillus pumilus ameliorates the cadmium toxicity in maize. Antioxidants 2022, 11, 2156. [Google Scholar] [CrossRef]
- Mahawar, L.; Živčák, M.; Barboricova, M.; Kovár, M.; Filaček, A.; Ferencova, J.; Vysoká, D.M.; Brestič, M. Effect of copper oxide and zinc oxide nanoparticles on photosynthesis and physiology of Raphanus sativus L. under salinity stress. Plant Physiol. Biochem. 2024, 206, 108281. [Google Scholar] [CrossRef]
- Faraz, A.; Faizan, M.; Hayat, S.; Alam, P. Foliar application of copper oxide nanoparticles increases the photosynthetic efficiency and antioxidant activity in Brassica juncea. J. Food Qual. 2022, 2022, 5535100. [Google Scholar] [CrossRef]
- Pérez-Labrada, F.; López-Vargas, E.R.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Responses of tomato plants under saline stress to foliar application of copper nanoparticles. Plants 2019, 8, 151. [Google Scholar] [CrossRef]
- Elshoky, H.A.; Yotsova, E.; Farghali, M.A.; Farroh, K.Y.; El-Sayed, K.; Elzorkany, H.E.; Rashkov, G.; Dobrikova, A.; Borisova, P.; Stefanov, M.; et al. Impact of foliar spray of zinc oxide nanoparticles on the photosynthesis of Pisum sativum L. under salt stress. Plant Physiol. Biochem. 2021, 167, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Faisal, M.; Al Sahli, A.A. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 2014, 33, 2429–2437. [Google Scholar] [CrossRef]
- Raliya, R.; Nair, R.; Chavalmane, S.; Wang, W.-N.; Biswas, P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 2015, 7, 1584–1594. [Google Scholar] [CrossRef]
- Du, W.; Tan, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; Ji, R.; Yin, Y.; Guo, H. Interaction of metal oxide nanoparticles with higher terrestrial plants: Physiological and biochemical aspects. Plant Physiol. Biochem. 2017, 110, 210–225. [Google Scholar] [CrossRef]
- Jampílek, J.; Kráľová, K. Impact of Nanoparticles on Photosynthesizing Organisms and Their Use in Hybrid Structures with Some Components of Photosynthetic Apparatus. In Nanotechnology in the Life Sciences; Prasad, R., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; ISBN 978-3-030-12495-3. [Google Scholar]
- Jampílek, J.; Král’ová, K. Nanomaterials for Delivery of Nutrients and Growth-Promoting Compounds to Plants. In Nanotechnology: An Agricultural Paradigm; Prasad, R., Kumar, M., Kumar, V., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2017. [Google Scholar]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M.; Ibrahim, M.; Zia-ur-Rehman, M.; Farid, M.; Abbas, F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard. Mater. 2017, 322, 2–16. [Google Scholar] [CrossRef]
- Zuverza-Mena, N.; Martínez-Fernández, D.; Du, W.; Hernandez-Viezcas, J.A.; Bonilla-Bird, N.; López-Moreno, M.L.; Komárek, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Exposure of engineered nanomaterials to plants: Insights into the physiological and biochemical responses-A review. Plant Physiol. Biochem. 2017, 110, 236–264. [Google Scholar] [CrossRef] [PubMed]
- Malea, P.; Charitonidou, K.; Sperdouli, I.; Mylona, Z.; Moustakas, M. Zinc uptake, photosynthetic efficiency and oxidative stress in the seagrass Cymodocea nodosa exposed to ZnO nanoparticles. Materials 2019, 12, 2101. [Google Scholar] [CrossRef]
- Bacilieri, F.S.; de Vasconcelos, A.C.P.; Lana, R.M.Q.; Mageste, J.G.; Torres, J.L.R. Titanium (Ti) in plant nutrition—A review. Aust. J. Crop Sci. 2017, 11, 382–386. [Google Scholar] [CrossRef]
- Chaudhary, I.; Singh, V. Titanium dioxide nanoparticles and its impact on growth, biomass and yield of agricultural crops under environmental stress: A review. Res. J. Nanosci. Nanotechnol. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Li, X.; Ghanizadeh, H.; Han, Z.; Wang, Q.; Li, F.; Qiu, Y.; Zhang, Y.; Chen, X.; Liu, J.; Wang, A. Metabolic profile and physiological mechanisms underlying the promoting effects of TiO2NPs on the photosynthesis and growth of tomato. Sci. Hortic. 2023, 322, 112394. [Google Scholar] [CrossRef]
- Higashimoto, S. Titanium-dioxide-based visible-light-sensitive photocatalysis: Mechanistic insight and applications. Catalysts 2019, 9, 201. [Google Scholar] [CrossRef]
- Sang, L.; Zhao, Y.; Burda, C. TiO2 Nanoparticles as functional building blocks. Chem. Rev. 2014, 114, 9283–9318. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.R.; Chen, H.; Cui, H. Nanotechnology Applications and Implications of Agrochemicals Toward Sustainable Agriculture and Food Systems. Proc. J. Agric. Food Chem. 2018, 66, 6451–6456. [Google Scholar] [CrossRef] [PubMed]
- Morteza, E.; Moaveni, P.; Farahani, H.A.; Kiyani, M. Study of photosynthetic pigments changes of maize (Zea mays L.) under nano TiO2 spraying at various growth stages. Springerplus 2013, 2, 247. [Google Scholar] [CrossRef]
- Raliya, R.; Biswas, P.; Tarafdar, J.C. TiO2 nanoparticle biosynthesis and its physiological effect on mung bean (Vigna radiata L.). Biotechnol. Rep. 2015, 5, 22–26. [Google Scholar] [CrossRef]
- Kamal, R.; Mogazy, A.M. Effect of doping on TiO2 nanoparticles characteristics: Studying of fertilizing effect on cowpea plant growth and yield. J. Soil Sci. Plant Nutr. 2023, 23, 338. [Google Scholar] [CrossRef]
- Lyu, S.; Wei, X.; Chen, J.; Wang, C.; Wang, X.; Pan, D. Titanium as a beneficial element for crop production. Front. Plant Sci. 2017, 8, 597. [Google Scholar] [CrossRef]
- Mohajjel Shoja, H.; Ahmadi, L.; Kolahi, M.; Mohajel Kazemi, E. Effect of TiO2 NPs on the growth, anatomic features and biochemistry parameters of Baby sun rose (Aptenia cordifolia). Physiol. Mol. Biol. Plants 2021, 27, 2071–2081. [Google Scholar] [CrossRef]
- Fraceto, L.F.; Grillo, R.; de Medeiros, G.A.; Scognamiglio, V.; Rea, G.; Bartolucci, C. Nanotechnology in agriculture: Which innovation potential does it have? Front. Environ. Sci. 2016, 4, 20. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of nanomaterials in agricultural production and crop protection: A review. Crop Prot. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Farooqui, A.; Tabassum, H.; Ahmad, A.; Mabood, A.; Ahmad, A.; Zareeen Ahmad, I. Role of nanoparticles in growth and development ofplants: A review. Int. J. Pharma Bio Sci. 2016, 7, P22–P37. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; Lahiani, M.H. Nanoparticles and Plants: From Toxicity to Activation of Growth. In Handbook of Nanotoxicology, Nanomedicine and Stem Cell Use in Toxicology; Sahu, S.C., Casciano, D.A., Eds.; Wiley: Hoboken, NJ, USA, 2014; pp. 121–130. [Google Scholar]
- Chen, H.; Seiber, J.N.; Hotze, M. ACS select on nanotechnology in food and agriculture: A perspective on implications and applications. J. Agric. Food Chem. 2014, 62, 1209–1212. [Google Scholar] [CrossRef]
- Owolade, O.; Ogunleti, D. Effects of titanium dioxide on the diseases, development and yield of edible cowpea. J. Plant Prot. Res. 2008, 48, 329–336. [Google Scholar] [CrossRef]
- Tighe-Neira, R.; Reyes-Díaz, M.; Nunes-Nesi, A.; Recio, G.; Carmona, E.; Corgne, A.; Rengel, Z.; Inostroza-Blancheteau, C. Titanium dioxide nanoparticles provoke transient increase in photosynthetic performance and differential response in antioxidant system in Raphanus sativus L. Sci. Hortic. 2020, 269, 109418. [Google Scholar] [CrossRef]
- Ahmad, B.; Shabbir, A.; Jaleel, H.; Khan, M.M.A.; Sadiq, Y. Efficacy of titanium dioxide nanoparticles in modulating photosynthesis, peltate glandular trichomes and essential oil production and quality in Mentha piperita L. Curr. Plant Biol. 2018, 13, 6–15. [Google Scholar] [CrossRef]
- Hussain, S.; Iqbal, N.; Brestic, M.; Raza, M.A.; Pang, T.; Langham, D.R.; Safdar, M.E.; Ahmed, S.; Wen, B.; Gao, Y.; et al. Changes in morphology, chlorophyll fluorescence performance and Rubisco activity of soybean in response to foliar application of ionic titanium under normal light and shade environment. Sci. Total Environ. 2019, 658, 626–637. [Google Scholar] [CrossRef]
- Qi, M.; Liu, Y.; Li, T. Nano-TiO2 Improve the photosynthesis of tomato leaves under mild heat stress. Biol. Trace Elem. Res. 2013, 156, 323–328. [Google Scholar] [CrossRef]
- Skiba, E.; Pietrzak, M.; Michlewska, S.; Gruszka, J.; Malejko, J.; Godlewska-Żyłkiewicz, B.; Wolf, W.M. Photosynthesis governed by nanoparticulate titanium dioxide. The Pisum sativum L. case study. Environ. Pollut. 2024, 340, 122735. [Google Scholar] [CrossRef]
- Waghmode, M.S.; Gunjal, A.B.; Mulla, J.A.; Patil, N.N.; Nawani, N.N. Studies on the titanium dioxide nanoparticles: Biosynthesis, applications and remediation. SN Appl. Sci. 2019, 1, 310. [Google Scholar] [CrossRef]
- Shah, T.; Latif, S.; Saeed, F.; Ali, I.; Ullah, S.; Abdullah Alsahli, A.; Jan, S.; Ahmad, P. Seed priming with titanium dioxide nanoparticles enhances seed vigor, leaf water status, and antioxidant enzyme activities in maize (Zea mays L.) under salinity stress. J. King Saud. Univ.—Sci. 2021, 33, 101207. [Google Scholar] [CrossRef]
- Yang, F.; Hong, F.; You, W.; Liu, C.; Gao, F.; Wu, C.; Yang, P. Influences of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol. Trace Elem. Res. 2006, 110, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Mishra, R.K.; Dikshit, A.; Pandey, A.C. Interactions of nanoparticles with plants. Emerg. Technol. Manag. Crop Stress Toler. 2014, 1, 159–180. [Google Scholar] [CrossRef]
- Hasanpour, H.; Maali-Amir, R.; Zeinali, H. Effect of TiO2 nanoparticles on metabolic limitations to photosynthesis under cold in chickpea. Russ. J. Plant Physiol. 2015, 62, 779–787. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Srivastava, A.K.; El-sadek, M.S.A.; Kordrostami, M.; Tran, L.S.P. Titanium dioxide nanoparticles improve growth and enhance tolerance of broad bean plants under saline soil conditions. Land Degrad. Dev. 2018, 29, 1065–1073. [Google Scholar] [CrossRef]
- Khan, M.N. Nano-titanium dioxide (Nano-TiO2) mitigates NaCl stress by enhancing antioxidative enzymes and accumulation of compatible solutes in tomato (Lycopersicon Esculentum Mill.). J. Plant Sci. 2016, 11, 1–11. [Google Scholar] [CrossRef]
- Gohari, G.; Mohammadi, A.; Akbari, A.; Panahirad, S.; Dadpour, M.R.; Fotopoulos, V.; Kimura, S. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 2020, 10, 912. [Google Scholar] [CrossRef]
- Jaberzadeh, A.; Moaveni, P.; Tohidi Moghadam, H.R.; Zahedi, H. Influence of bulk and nanoparticles titanium foliar application on some agronomic traits, seed gluten and starch contents of wheat subjected to water deficit stress. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 201–207. [Google Scholar] [CrossRef]
- Morales, F.; Ancín, M.; Fakhet, D.; González-Torralba, J.; Gámez, A.L.; Seminario, A.; Soba, D.; Ben Mariem, S.; Garriga, M.; Aranjuelo, I. Photosynthetic metabolism under stressful growth conditions as a bases for crop Breeding and yield improvement. Plants 2020, 9, 88. [Google Scholar] [CrossRef]
- Baslam, M.; Mitsui, T.; Hodges, M.; Priesack, E.; Herritt, M.T.; Aranjuelo, I.; Sanz-Sáez, Á. Photosynthesis in a changing global climate: Scaling up and scaling down in crops. Front. Plant Sci. 2020, 11, 882. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R.; Maas, E.V. Plant Salt Tolerance. In Agricultural Salinity Assessment and Management; Wallender, W.W., Tanji, K.K., Eds.; American Society of Civil Engineers: Reston, VA, USA, 2011; pp. 405–459. [Google Scholar]
- Stefanov, M.A.; Rashkov, G.D.; Apostolova, E.L. Assessment of the photosynthetic apparatus functions by chlorophyll fluorescence and P700 absorbance in C3 and C4 plants under physiological conditions and under salt stress. Int. J. Mol. Sci. 2022, 23, 3768. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Yotsova, E.K.; Dobrikova, A.G.; Stefanov, M.A.; Kouzmanova, M.; Apostolova, E.L. Improvement of the rice photosynthetic apparatus defence under cadmium stress modulated by salicylic acid supply to roots. Theor. Exp. Plant Physiol. 2018, 30, 57–70. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Dankov, K.; Busheva, M.; Stefanov, D.; Apostolova, E.L. Relationship between the degree of carotenoid depletion and function of the photosynthetic apparatus. J. Photochem. Photobiol. B Biol. 2009, 96, 49–56. [Google Scholar] [CrossRef]
- Mansour, M.M.F.; Salama, K.H.A. Cellular basis of salinity tolerance in plants. Environ. Exp. Bot. 2004, 52, 113–122. [Google Scholar] [CrossRef]
- Lei, Z.; Mingyu, S.; Xiao, W.; Chao, L.; Chunxiang, Q.; Liang, C.; Hao, H.; Xiaoqing, L.; Fashui, H. Antioxidant stress is promoted by nano-anatase in spinach chloroplasts under UV-B radiation. Biol. Trace Elem. Res. 2008, 121, 69–79. [Google Scholar] [CrossRef]
- Yang, X.; Cao, C.; Erickson, L.; Hohn, K.; Maghirang, R.; Klabunde, K. Synthesis of visible-light-active TiO2-based photocatalysts by carbon and nitrogen doping. J. Catal. 2008, 260, 128–133. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Siddiqui, H.; Sami, F.; Zaidi, R.; Azam, A.; Alam, P.; Hayat, S. Nanoparticles enhances the salinity toxicity tolerance in Linum usitatissimum L. by modulating the antioxidative enzymes, photosynthetic efficiency, redox status and cellular damage. Ecotoxicol. Environ. Saf. 2021, 213, 112020. [Google Scholar] [CrossRef]
- Edge, R.; McGarvey, D.J.; Truscott, T.G. The carotenoids as anti-oxidants—A review. J. Photochem. Photobiol. B Biol. 1997, 41, 189–200. [Google Scholar] [CrossRef]
- Stefanov, M.; Yotsova, E.; Gesheva, E.; Dimitrova, V.; Markovska, Y.; Doncheva, S.; Apostolova, E.L. Role of flavonoids and proline in the protection of photosynthetic apparatus in Paulownia under salt stress. South Afr. J. Bot. 2021, 139, 246–253. [Google Scholar] [CrossRef]
- Moustakas, M.; Bayçu, G.; Sperdouli, I.; Eroğlu, H.; Eleftheriou, E.P. Arbuscular mycorrhizal symbiosis enhances photosynthesis in the medicinal herb Salvia fruticosa by improving photosystem II photochemistry. Plants 2020, 9, 962. [Google Scholar] [CrossRef] [PubMed]
- Stefanov, M.A.; Rashkov, G.D.; Yotsova, E.K.; Dobrikova, A.G.; Apostolova, E.L. Impact of salinity on the energy transfer between pigment–protein complexes in photosynthetic apparatus, functions of the oxygen-evolving complex and photochemical activities of photosystem II and photosystem I in two Paulownia lines. Int. J. Mol. Sci. 2023, 24, 3108. [Google Scholar] [CrossRef]
- Trela-Makowej, A.; Orzechowska, A.; Szymańska, R. Less is more: The hormetic effect of titanium dioxide nanoparticles on plants. Sci. Total Environ. 2024, 910, 168669. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Zhou, J.; Liu, C.; Yang, F.; Wu, C.; Zheng, L.; Yang, P. Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol. Trace Elem. Res. 2005, 105, 269–280. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.; Iavicoli, I.; Calabrese, E.J. The two faces of nanomaterials: A quantification of hormesis in algae and plants. Environ. Int. 2019, 131, 105044. [Google Scholar] [CrossRef]
- Albertsson, P.Å. The structure and function of the chloroplast photosynthetic membrane—A model for the domain organization. Photosynth. Res. 1995, 46, 141–149. [Google Scholar] [CrossRef]
- Bukhov, N.; Egorova, E.; Carpentier, R. Electron flow to photosystem I from stromal reductants in vivo: The size of the pool of stromal reductants controls the rate of electron donation to both rapidly and slowly reducing photosystem I units. Planta 2002, 215, 812–820. [Google Scholar] [CrossRef]
- Takahashi, S.; Milward, S.E.; Milward, D.Y.F.; Chow, W.S.; Badger, M.R. How does cyclic electron flow alleviate photoinhibition in arabidopsis? Plant Physiol. 2009, 149, 1560–1567. [Google Scholar] [CrossRef]

| Variants | k1 (s−1) | k2 (s−1) | A1/A2 |
|---|---|---|---|
| Control | 2.212 ± 0.015 b | 0.061 ± 0.006 a | 7.194 ± 0.719 a |
| 50 mg/L TiO2 NPs | 2.202 ± 0.066 b | 0.066 ± 0.006 a | 7.486 ± 0.746 a |
| 100 mg/L TiO2 NPs | 2.293 ± 0.028 b | 0.073 ± 0.007 a | 6.992 ± 0.540 a |
| 100 mM NaCl | 1.890 ± 0.021 d | 0.031 ± 0.009 c | 3.261 ± 0.288 c |
| 50 mg/L TiO2 NPs + NaCl | 2.136 ± 0.056 c | 0.044 ± 0.004 b | 4.304 ± 0.250 b |
| 100 mg/L TiO2 NPs + NaCl | 2.386 ± 0.029 a | 0.064 ± 0.023 a | 4.241 ± 0.131 b |
| Variants | t1 (s) | t2 (s) | ΔA/A |
|---|---|---|---|
| Control | 1.38 ± 0.11 c | 10.13 ± 0.40 c | 11.81 ± 0.12 c |
| 50 mg/L TiO2 NPs | 1.47 ± 0.06 c | 9.98 ± 0.28 c | 16.91 ± 0.19 b |
| 100 mg/L TiO2 NPs | 1.52 ± 0.08 c | 9.856± 0.39 c | 15.35 ± 0.26 b |
| 100 mM NaCl | 2.74 ± 0.03 a | 16.28 ± 0.12 a | 5.89 ± 0.12 e |
| 50 mg/L TiO2 NPs + NaCl | 1.99 ± 0.04 b | 14.80 ± 0.21 b | 7.01 ± 0.14 d |
| 100 mg/L TiO2 NPs + NaCl | 1.05 ± 0.05 d | 10.67 ± 0.36 c | 11.78 ± 0.23 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yotsova, E.; Stefanov, M.; Rashkov, G.; Dobrikova, A.; Apostolova, E. Mitigating Salinity Stress in Pea Plants with Titanium Oxide Nanoparticles. Int. J. Plant Biol. 2025, 16, 34. https://doi.org/10.3390/ijpb16010034
Yotsova E, Stefanov M, Rashkov G, Dobrikova A, Apostolova E. Mitigating Salinity Stress in Pea Plants with Titanium Oxide Nanoparticles. International Journal of Plant Biology. 2025; 16(1):34. https://doi.org/10.3390/ijpb16010034
Chicago/Turabian StyleYotsova, Ekaterina, Martin Stefanov, Georgi Rashkov, Anelia Dobrikova, and Emilia Apostolova. 2025. "Mitigating Salinity Stress in Pea Plants with Titanium Oxide Nanoparticles" International Journal of Plant Biology 16, no. 1: 34. https://doi.org/10.3390/ijpb16010034
APA StyleYotsova, E., Stefanov, M., Rashkov, G., Dobrikova, A., & Apostolova, E. (2025). Mitigating Salinity Stress in Pea Plants with Titanium Oxide Nanoparticles. International Journal of Plant Biology, 16(1), 34. https://doi.org/10.3390/ijpb16010034








