Abstract
Drought and salinity are major factors that hinder crop cultivation and significantly impair agricultural productivity, particularly in (semi)arid regions. These two abiotic constraints cause deterioration in soil structure and reduced fertility and hamper plant growth by limiting access to mineral elements and water, thereby threatening global food security. What’s more, the excessive, long-term use of chemical fertilizers to boost crop productivity can disrupt the balance of agricultural ecosystems, particularly soil health. Faced with these challenges, the sustainable exploitation of natural resources, in particular rhizospheric microorganisms, is an environmentally friendly solution. Arbuscular mycorrhizal fungi play an important role as biofertilizers due to their symbiotic relationship with the roots of nearly 80% of plants. They promote not only the growth of host plants but also their resistance to abiotic stresses. Among these fungi, the Glomus genus stands out for its predominance in plants’ rhizosphere thanks to its richness in high-performance species and ecological adaptability. This review highlights the importance of species within this genus in soils, particularly in terrestrial ecosystems subject to (semi-)arid climates. Molecular mechanisms underlying plant tolerance to drought and salt stress in symbiosis with species of the Glomus genus are also explored.
1. Introduction
The impact of climate change on food security is profound, as it exacerbates hunger, inequality, and economic instability, worsening the challenges of ensuring access to sufficient and nutritious food [1]. The global population will continue to increase in the future and could exceed 9 billion by 2050 [2]. This population growth leads to significant challenges, particularly in arid and semi-arid regions, where it increases pressure on resources and complicates climate change adaptation [3]. This concern is well-founded, as a cursory analysis of the available evidence indicates a clear correlation between population growth and the degradation of environmental health [4]. This population growth took place in parallel with remarkable economic expansion, accelerated industrialization, and rapid urbanization. However, due to economic development strategies, serious environmental problems have accompanied population growth and industrialization [5]. In addition to climate change, the rapidly growing population, the intensification of agricultural practices, and the resulting degradation of soil health for crop production are critical factors undermining the sustainability of agriculture [6]. Indeed, the excessive use of chemical fertilizers and pesticides leads to a fundamental decline in the diversity of beneficial soil microbes and a reduction in organic matter, causing nutrient imbalances, soil salinization and the alteration in biogeochemical cycles, thereby compromising agricultural sustainability [7,8]. The constant effect of abiotic and biotic stresses on the agroecosystem directly alters the characteristics of soil health, fertility, and crop productivity [9,10,11]. Salinity and drought have become some of the major abiotic constraints affecting the agricultural sector and thus food security through the dysfunction of plants’ morphological, physiological, water, and molecular processes [12,13,14]. Salinity stress affects approximately 20% of cultivated land and 50% of irrigated systems worldwide, rendering many of these saline-affected areas unproductive, mainly due to the reduction in plant establishment and growth [13]. Drought is seen as the main threat to global food security in the future and a trigger for the great famines of the past [15,16]. Moreover, drought is intensifying agricultural challenges due to the depletion of water resources and increasing food demand driven by the rapid and concerning growth of the global population [17,18,19,20]. The unpredictable nature of drought depends on various factors, such as the irregular and uncertain distribution of precipitation, variations in evapotranspiration rates, and the water-holding capacity around the soil rhizosphere [21,22,23]. Drought influences soil nutrient transport and availability, leading to alterations in the morphological, physiological, and nutritional traits of plants. These effects are particularly pronounced in parameters like leaf water potential, water content, stomatal conductance, photosynthetic pigment content, and the uptake of essential nutrients such as phosphorus (P) and nitrogen (N) [24,25,26,27,28,29]. Drought also impairs the antioxidant defense system, leading to oxidative stress characterized by the accumulation of reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), and elevated levels of malondialdehyde (MDA), a marker of lipid peroxidation [30,31,32].
The preservation of agricultural ecosystems, especially in these times of climate change, requires the management of water, soil and plant species [33]. Each component interacts dynamically, and their joint management is crucial to ensure the sustainability and resilience of production systems [34]. Water management involves implementing conservation techniques to prevent erosion and salinization, which can compromise soil quality [35]. Soils require special attention to maintain their structure, fertility, and capacity to retain water, all of which are essential to support plant growth [36]. As for plant species, their selection and adaptation to local conditions are key aspects. Plants must not only be able to withstand abiotic stresses, such as drought or salinity but also contribute to the overall health of the ecosystem by, for instance, promoting biodiversity and beneficial interactions with soil microorganisms [37]. It is essential to devise effective, economical and easily applicable methods for dealing with salt stress and drought, which represent a major challenge for the sustainable management of contemporary agriculture. In terms of the soil component, one of the recommended approaches is to explore the potential of biological candidates that govern the rhizosphere of living plants in arid and semi-arid regions. Among these, arbuscular mycorrhizal fungi (AMF) belonging to the order Glomerales and the family Glomeraceae are notable, including genera such as Funneliformis, Rhizophagus, Sclerocystis, Septoglomus, Dominikia, Kamienskia, and Glomus. The latter has recently become the most famous genus of AMF owing to its ability to enhance the growth of a wide range of crops, including legumes, cereals, vegetables, and fruit species [38,39,40,41,42,43]. The Glomus genus, a key player in AMF, has significant diversity and ecological importance worldwide. Studies have identified several Glomus species associated with different plants. For example, the cannabis varieties Khardala and Critical showed a predominance of Glomus among 12 AMF species, with spore densities of 200 and 243 spores/100 g of soil, respectively [44]. Another study on Cytisus monspessulanus revealed a high mycorrhizal spore density of 3773 spores/100 g of soil, with Glomus identified as one of six genera present [45]. Recent studies identified, in the rhizosphere of sugarcane and date palm, AMF dominated by species of the Glomus (Glomus sp.) genus [40,41]. Kachkouch et al. [46] established a consortium of AMF in the rhizosphere of olive containing a mixture of 26 species belonging to 8 genera of Glomales. Similarly, Lahbouki et al. [47] showed a dominance of the same genus in the rhizosphere of Opuntia ficus-indica. Research indicates that Glomus species are widespread in diverse ecosystems, contributing to plant health and soil fertility. Glomus species are found in a variety of environments. Recent discoveries include Glomus highlandensis and G. mongioie, which have been identified in Europe and are thought to have a worldwide distribution [48]. In addition, two new species, G. africanum and G. iranicum, have been described, underlining the continuing discovery of Glomus diversity across continents, including Africa [49]. According to Stürmer and Kemmelmeier [50], Glomus was among the most species-rich genera, representing 29 species, particularly in the Neotropics. Many studies have proven its mycorrhizogenic power in the soil, as was shown by Arif et al. [51] in Sumatra, Indonesia. Glomus is the most widespread genus at the level of soils of (arid)semi-arid areas of Morocco, more so than the genera Sclerocystis and Acaulospora; its power of infection is reflected in a high intensity of mycorrhization, which can reach about 60% [41]. As attention to Glomus in the world increases, the global landscape reveals a rich tapestry of mycorrhizal fungi that play a crucial role in ecosystem functioning, suggesting the need for further exploration and conservation efforts.
This review highlights the phylogenetic relationships within the Glomus genus and explores the molecular mechanisms underlying its symbiosis with plants. The study discusses state of the art advances and knowledge on the role and importance of the genus Glomus in enhancing plant growth and tolerance in stressful environments. The mechanisms (physiological, biochemical, and molecular) involved by Glomus in helping plants cope with stressful environmental conditions are reviewed and discussed in detail.
2. Glomus Genus
2.1. Phylogenetic Relationships
Over the last 40 years, the classification of the AMF has evolved from a strictly descriptive approach based solely on phenotypic traits, such as spore morphology [52,53,54] to a more elaborate approach incorporating other criteria based on cladistic analysis of genetic traits (SSU rDNA sequence variation) [55,56,57]. Until 2001, these fungi were grouped into a single class, one order, three families, and six genera [58]. Ten years later, the use of genetic characters has enabled them to be divided into one to three classes, four to five orders, eleven to fourteen families, and eighteen to twenty-nine genera, according to the classification system adopted by Stürmer et al. [59]. Glomus is a genus of AMF, all species of which establish symbiotic relationships (mycorrhizae) with plant roots. It is the largest AMF genus, with around 85 described species. However, it is currently considered non-monophyletic [30,57]. Species producing glomoid spores form the main group of the Glomeromycota phylum. They were formerly classified in the family Glomeraceae, belonging to the suborder Glomerineae, within the order Glomerales [53,60]. Phylogenetic analyses of rDNA have shown that the Glomus genus is polyphyletic on several occasions [30]. Species of AMF producing Glomus-like spores fall into six distinct lineages within the Glomeromycota.
Glomalean fungi are thought to have arisen over 450 million years ago, and arbuscular mycorrhiza-like symbioses with the earliest land plants are hypothesized from the discovery of morphologically identical arbuscular structures in Aglaophyton fossils dating back 400 million years [61,62]. Recent studies have shown that spores of the Glomus genus have morphological characteristics including being globose, sometimes ovoid, orange-yellow, orange-brown, and light to dark brown in color. In terms of size, spores of Glomus have a diameter between 20 and 200 µm, and the spore wall consists of four layers [54,63,64,65] (Figure 1).
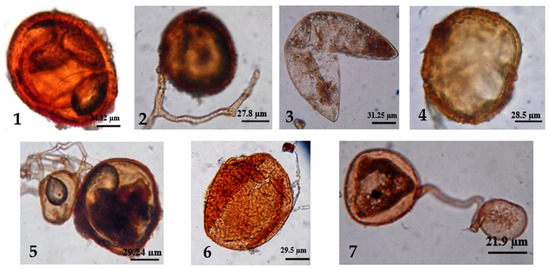
Figure 1.
Spores of (1) Glomus heterosporum, (2) G. microcarpum, (3) G. sp., (4) G. rubiforme, (5) G. multicaule, (6) G. globiferum, and (7) G. microcarpum [47].
2.2. Life Cycle
Arbuscular mycorrhizal fungi are widespread obligate symbionts, requiring a close mutualistic association with plant roots to ensure growth and complete their life cycle. Glomus species produce spores representing the fungus’s dormant and resistant phase. These spores, found in the soil, can remain viable for extended periods, awaiting favorable conditions for germination, such as the presence of chemical signals from plant roots [66,67]. Until recently, species of the Glomus genus were considered strictly asexual. Their spores are formed at the tips of hyphae, either inside the host’s roots or outside, in the soil. These spores, known as chlamydospores, germinate to produce a germ tube that grows in the soil until it comes into contact with the roots of the host plant. Once contact has been made, the fungus colonizes the root, progressing between its cells. Inside the root, it develops arbuscules, highly branched hyphal structures that play a crucial role in the exchange of nutrients between the fungus and the plant. Although these arbuscules develop inside the plant’s cell walls, they remain localized in the apoplast, surrounded by an invaginated cell membrane. At the end of the cycle, when conditions become unfavorable (such as resource depletion or the end of the growing season), the fungus produces new spores to ensure its survival. These spores are dispersed in the soil and restart the cycle when a new host plant becomes available [67].
2.3. Molecular Signaling Pathways in Plant–Glomus Symbiosis
Species of the Glomus genus are among the most dominant and widely distributed in plant roots during symbiosis [68]. This genus is highly diversified, including numerous species that form effective symbioses with a wide variety of plants [69]. In this context, a study evaluated the influence of AMF diversity associated with various legumes (green bean, mung bean, kidney bean, pea, soybean, peanut, and gram). After spore morphological characterization and AMF rDNA gene sequencing, the study confirmed that there are 10 different AMF taxa. The Glomus genus was the most dominant among the different genera, with 30% of species [70]. Several steps involving a complex molecular dialogue are required before establishing symbiosis between the two partners. Recently, strigolactones (SLs) have been shown to be important for successful root colonization by AMF; these signaling molecules characterize the so-called pre-symbiotic stages [71,72] (Figure 2). SL secretion is stimulated by a lack of phosphorus (P) [73]. Indeed, Yolanda et al. [74] suggested that the P uptake efficiency of Maradol papayas was enhanced by association with Glomus intraradices, resulting in improved growth and photosynthesis under low-P conditions. A study has revealed that certain flavonoid molecules, such as chrysin, isorhamnetin, kaempferol, luteolin, morin, and rutin, exert a species-specific effect on the pre-symbiotic growth of the genus Glomus (G. mosseae and G. intraradices). These effects relate in particular to spore germination, hyphal elongation and branching, and the formation of auxiliary cells and secondary spores [75]. A study has confirmed that SLs stimulate spore germination of G. intraradices and G. claroideum [76]. N-acetylglucosamine (GlcNAc) is the main component of the fungal cell wall and plays a key role as a signal in the induction of plant defense mechanisms and the establishment of symbiotic relationships [77]. Symbiosis between the host plant and AMF relies on the exchange of signaling molecules, such as carbon (C) and P. The results of Azmat et al. [78] demonstrated that Glomus fasciculatum acts as a bio-converter and bio-activator of soil nutrients, particularly P, and that their hyphal interaction absorbs soil nutrients and activates insoluble P into soluble P for plant development. During the establishment of the symbiosis, metabolic events and the expression of genes involved in lipid-synthesizing enzymes were detected in Glomus intraradices associated with Lotus japonicus, as revealed by chromatographic analysis. Glycosylated sphingolipids and inositolphosphorylceramide were among the lipids detected [79]. When the fungus comes into contact with the root of the host plant, it germinates, and two acids secreted by the root, 2-hydroxy-tetradecanoic acid and fatty acid, trigger the formation of elongated hyphal side branches [76,80,81]. A study on the symbiosis between Lotus japonicus and Rhizophagus irregularis (formerly Glomus intraradices) demonstrated that when the hyphae approach the root, they detect SLs and respond by branching more intricately, allowing them to grow closer to the root and produce Myc factors (lipochitooligosaccharides) and chitin (short-chain chitin oligomers) [82,83,84]. Before the recognition of GlcNAc from Myc factors by lysine-motif (LysM) proteins (LYK3 and NFR1) located in the plant plasma membrane, the root secretes cutin monomers to induce hyphae penetration into the root epidermis, which activate the common symbiosis signaling pathway (CSSP) and the formation of arbuscules [85]. This pathway includes several genes encoding receptor kinases, cation channels, nucleoporins, calcium- and calmodulin-dependent kinases, and CYCLOPS/IPD3 [86,87,88]. The formation of arbuscules is regulated by specific phytohormones. For instance, in Oryza sativa, Medicago truncatula, and Lotus japonicus, mycorrhizal symbiosis is negatively impacted by gibberellic acid (GA). On the other hand, auxin is required in the early stages of fungal growth and to differentiate arbuscules [89] (Figure 2). Inoculation of Solanum lycopersicum, Medicago truncatula, and Oryza sativa with Glomus intraradices induced the formation of arbuscules in the cortical cells of auxin-treated roots. Similarly, abscisic acid (ABA) plays a primordial role in the differentiation and function of arbuscules in tomato plants [82,90]. It has been suggested that the development and branching of Glomus intraradices arbuscules in the cortical cells of three plant species was stimulated by the expression of the “Required for Arbuscular Mycorrhiza1” gene (RAM1) [91] (Figure 2). The symbiosis between AMF, such as Glomus, and plants is pivotal in enhancing nutrient uptake, particularly phosphorus, while enhancing plant resilience to abiotic stresses such as drought and salinity. This mutualistic relationship supports sustainable agriculture and natural ecosystems’ resilience by improving soil structure, promoting plant growth and health, and reducing dependency on chemical inputs.
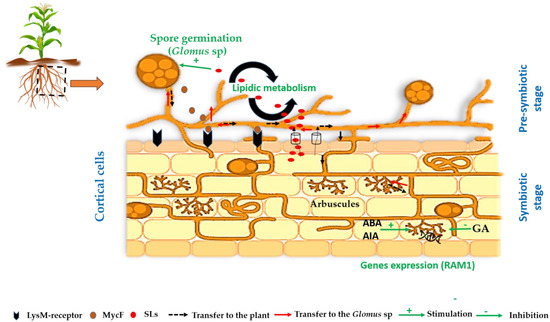
Figure 2.
Stages in establishing symbiosis between Glomus sp. and plant root. SLs: strigolactone; AIA: indole-3-acetic acid; ABA: abscisic acid; GA: gebirilic acid; MycF: myc factors; RAM1: Required for arbuscular mycorrhiza1.
3. Effect of Salt and Drought Stress on Crop Production
Mediterranean agricultural ecosystems, characterized by their semi-arid to arid climate, are particularly vulnerable to abiotic stresses such as salinity and drought. These environmental factors, exacerbated by climate change, pose significant challenges to the sustainability of agricultural production and food security in this region. Soil salinization disrupts the production and quality of cereal and vegetable crops by causing a wide range of physiological imbalances. It negatively impacts morphological and biochemical traits, inhibits plant growth and development, and directly affects seed germination, ultimately reducing yield [14,92]. This constraint disrupts the photosynthetic apparatus [93], transpiration, and gas exchange by reducing chlorophyll and carotenoid levels, altering chloroplast structure, and impairing the photosystem II (PSII) [94,95,96]. High levels in leaves increase the synthesis of ROS, which can change cell behavior by disturbing its redox homeostasis [97,98]. Excess ROS in cells leads to protein and enzyme degradation, as well as lipid peroxidation, further deteriorating the electron transport system, PSII system, and the structure of various membranes. It also damages the cell’s genetic material (DNA) [99,100] (Figure 3). However, extended salinity durations in soil degradation scenarios (including dispersion of aggregates and soil crusting, stable soil structures, flooding, and desertification) are realistic options, but their feasibility depends on the geographical, hydrological, morphological, and climatic conditions of areas exposed to salinity [99,100]. Similarly, drought ranks among the most severe environmental stresses that hinder plant productivity, since its effects range from morphological to molecular levels. This stress is associated with salinity caused by high temperatures and drought, which accelerate soil evaporation and lead to salt accumulation, particularly in regions with arid and semi-arid climates. In the same way, Sahin et al. [101] reported that the combined effect of salt and drought exerts more negative impact on plant growth than their individual effects. A large portion of fresh biomass (approximately 80–95% of the plant body) is composed of water, which is required for the optimal performance of various physiological processes, encompassing multiple facets of plant growth, development, and metabolism. Plant exposure to drought stress negatively affects the plant’s water balance, and normal physiological processes are affected [102]. The plant’s relative water content (RWC) and leaf water potential decrease significantly in soil-drying conditions [103]. In addition, severe drought causes stomata closure to reduce water loss, which can limit CO2 supply and lead to photosynthesis reduction [104]. The response through the production of ROS represents the primary response of plants to stress [105]. Drought stress can interfere with the production and elimination of ROS in plants, resulting in their excessive accumulation and subsequent oxidative stress [105,106] (Figure 3).
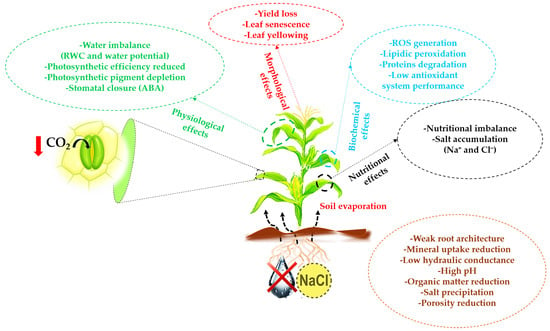
Figure 3.
Effects of drought and salinity on plant growth, physiology, biochemistry, and soil properties. ROS: reactive oxygen species; RWC: relative water content; ABA: abscisic acid, downward-curving arrow: assimilation, downward red arrow: low CO2 assimilation.
4. Interaction of Glomus and Abiotic Stress
4.1. Drought
4.1.1. Glomus and Drought Stress
Several scientific reports have shown that species of the Glomus genus are the most dominant in rhizosphere soils of areas affected by drought and salinity (Tables S1 and S2). The dominance of the Glomus genus in the soils of different ecosystems in China [107], Kenya [108], Sudan [109], Tunisia [110], Morocco [111], and Burkina Faso [47,112,113,114] has been reported. This genus stands out for its ability to produce large numbers of spores in a short span of time, surpassing other genera such as Gigaspora and Scutellospora. This dominance is also explained by its remarkable adaptation to conditions of drought, salinity, and soil degradation [115,116,117]. Because of their mycorrhizogenicity, species of this genus are able to enter into symbiosis with several plant species, in particular trees (olive, date palm, black locust, citrus, and carob), cereals (maize, barley, quinoa, sorghum, and sugarcane), and legumes (lettuce and bean) in environments exposed to drought [118,119,120,121,122,123,124,125,126,127,128]. For example, the Super 2 sorghum variety showed a more marked response to inoculation by Glomus mosseae than the Konawe Selatan variety [119] (Table S1).
Research suggests that, under drought-induced stress conditions, the symbiotic association between AMF and plants helps mitigate the effects of stress by improving osmotic adjustment, leaf gas exchange, and leaf water relations, as well as stomatal conductance and transpiration rates [129]. It has recently been shown that the symbiosis of sesame plants with two species of Glomus improved the lipid (oleic and linoleic acids) content of sesame seed oil [130]. Mycorrhizal associations also contribute significantly to the decomposition and mineralization of soil organic matter while promoting the mobilization of poorly mobile nutrients, such as phosphorus, for the benefit of the host plant [131].
4.1.2. Mechanisms of Tolerance to Drought Stress
Drought tolerance induced by AMFs is achieved by the synergy of several submechanisms, including pigment protection, regulation of water balance, maintenance of proline levels, and detoxification of reactive oxygen species. These processes enhance photosynthetic characteristics and osmotic adjustments, particularly through the accumulation of compounds such as proline, chlorophyll, and carotenoids [128,132,133] (Table S1). Recent studies have demonstrated that Glomus mosseae enhances sorghum’s drought tolerance by improving water and nutrient uptake via extraradical hyphae, reducing water loss, and safeguarding photosynthetic efficiency through the stabilization of pigments. The fungus also plays a critical role in maintaining proline balance and boosting antioxidant activity to eliminate reactive oxygen species [119]. Glomus mosseae is one of the AMF species known to have beneficial effects on a wide range of plant species. It can enhance plant growth by improving nutrient uptake and increasing drought resistance [134]. The positive contribution of AMF is not only limited to the aerial part of the plant but also includes the improvement of the nutritional status of the soil. Several scientific reports showed that species of the Glomus genus positively affected the physicochemical properties of the soil [70]. The liberation of phosphorus from its bound form to its free form from other molecules is the main characteristic of this genus [135,136]. Indeed, thanks to the extensions of the hyphae, the species of this genus are able to feed the plant with several mineral elements, in particular macroelements (N and K) and trace elements (Fe, Mg, Mn, Ni, Si, and Zn). The work of Meddich et al. [126] showed that the symbiosis between the roots of barley and three species of the genus glomus (Glomus mosseae, G. monosporus, and G. versiforme) improved the content of P, Ca, Mg, and K under water stress. This nutritional feeding has repercussions on the good development of the photosynthetic apparatus, especially the synthesis of photosynthetic pigments [137,138].
Previous studies have demonstrated the positive impact of AMF inoculation on various plants, including Medicago nolana, Populus cathayana, Allium cepa, Abelmoschus esculentus, and Phoenix dactylifera. AMF application led to improved drought tolerance in the host plants, as indicated by reduced malondialdehyde and proline accumulation, increased sugar and P uptake, enhanced leaf photosynthesis, and modifications to the hydraulic properties of the roots [139,140]. These results suggest that AMF plays a significant role in improving plant resilience to drought through mechanisms such as optimizing nutrient uptake, stabilizing cellular processes, and enhancing water use efficiency.
A key factor in drought tolerance is the higher P content observed in AMF plants under drought stress, contributing to their enhanced resilience compared to non-AMF plants. The PHOSPHATE TRANSPORTER1 (PHT1) gene family is crucial for P uptake and translocation within the plant, including the uptake of Pi from AMF [141]. In tomato plants, AMF induced the expression of phosphate transporter (PT) genes, such as LePT4 and LePT5, improving tolerance to water deficit with species-specific effects [142]. Similarly, in Populus trichocarpa, drought conditions upregulated certain PT genes, like PHT1.2 and PHO9, independently of Pi levels, while others were Pi-dependent [143]. Additionally, Glomus intraradices inoculation under drought stress led to the upregulation of ammonium and K transporter genes, which are vital for N and K uptake in arid environments [144]. Together, these findings highlight the multifaceted role of AMF in improving nutrient uptake, stabilizing metabolic processes, and enhancing drought tolerance. In addition to these benefits, AMF inoculation upregulates the expression of key metabolites, including amino acids (such as proline) and phytohormones, further strengthening the plant’s capacity to endure stress and optimize growth under challenging conditions. The elevated levels of proline, glucose, and total soluble proteins in AMF-inoculated E. foliate plants significantly contributed to their enhanced drought tolerance, as demonstrated by increased sucrose phosphate synthase activity. Moreover, AMF notably improved the uptake of essential nutrients like K, Mg, and Ca. Importantly, higher concentrations of plant hormones—such as IAA, GA, and ABA—were sustained in AMF (Glomus etunicatum, G. intraradices, and G. mosseae)-inoculated E. foliate plants, helping to regulate both stress responses and growth [145]. This hormone regulation is further supported by exogenous IAA treatment, which significantly increased relative water content and chlorophyll levels, contributing to enhanced drought tolerance. IAA treatment significantly increased the content of ABA and jasmonic acid (JA), upregulated the expression of drought-responsive genes (such as bZIP11, DREB2, MYB14, MYB48, WRKY2, WRKY56, WRKY108715, and RD22), and promoted the expression of auxin-responsive genes (GH3.1, GH3.9, and IAA8) [146]. These changes, in turn, help to mitigate ROS accumulation and fine-tune the expression of several key genes involved in stress responses and cellular protection [146]. Moreover, the regulators in mycorrhizal plants play a crucial role in enhancing drought tolerance by facilitating signaling crosstalk between the host plant and AMF, which helps to optimize stress responses and improve nutrient and water uptake. This communication aids in regulating various physiological processes, contributing to the plant’s overall resilience to drought stress. Additionally, AMF influences the expression of aquaporin genes, which are crucial for regulating water transport across plant membranes [147,148,149]. These genes, such as those identified in species like Glomus intraradices, play a key role in enhancing water uptake, especially under drought conditions, by facilitating the movement of water molecules through the plant’s root system. Inoculation with AMF has been shown to upregulate the expression of specific aquaporin genes, enhancing the plant’s ability to absorb and retain water more efficiently, thus contributing to its improved drought tolerance. Recent studies have revealed that the expression of the ZmTIP2 gene in maize plants inoculated with Glomus intraradices plays an important role in boosting maize’s tolerance to drought stress. This phenomenon is related to the regulation of water absorption through aquaporins, thereby enabling better water management during periods of water stress. Similar studies have shown that inoculation with mycorrhizal fungi, such as Glomus mosseae and G. intraradices, induces the expression of aquaporin genes in other plant species, also contributing to their drought tolerance [150,151,152]. More specifically, the GintAQPF1 and GintAQPF2 genes, encoding aquaporins identified in Glomus intraradices, showed a positive correlation with the survival of plants subjected to drought conditions. [153]. This suggests that the positive effects of mycorrhizal inoculation on drought tolerance are, at least in part, mediated by the upregulation of these aquaporin genes, which facilitate more efficient water transport and retention in plants during water-limiting conditions. Furthermore, such mechanisms highlight the importance of AMF in improving plant tolerance to drought stress by boosting both nutrient and water uptake through the modulation of specific gene expression related to water homeostasis. Drought stress tolerance by enhancing the water status of lettuce plants was due to overexpression of aquaporin genes induced by inoculation with Glomus intraradices [123]. Species of the Glomus tend to give many plants a certain resilience to drought stress by increasing the performance of antioxidant systems (enzymatic and non-enzymatic) [154]. In their work, Benaffari et al. [120] showed that inoculation of quinoa plants with a consortium based on species of the genus Glomus in the presence of vermicompost increased their resistance to water stress by improving soil fertility and maintaining water balance in the cells. Phaseolus vulgaris plants mycorrhized by Glomus mosseae showed high activity of antioxidant enzymes like polyphenol oxidase (PPO), superoxide dismutase (SOD), and peroxidase (POX) under water stress conditions [155,156,157]. This was explained by the biostimulatory effect on the overexpression of genes coding for antioxidant enzymes (APX, SOD, and glutathione reductase (GR), as noted in the case of Robinia pseudoacacia L. inoculated with Glomus intraradices (Rhizophagus irregularis) [156,157,158]. The modulation of the stomata dynamics and the improvement of the gas exchanges are among the advantages associated with colonization with AMF, especially the species of the Glomus genus [159]. Under water-stressed conditions, inoculation of maize with Glomus mosseae significantly reduced leaf ABA content and delayed the decline in photosynthetic rate, stomatal conductance and osmotic adjustment [90]. In 25% field capacity conditions, mycorrhizal bean plants showed higher CO2 assimilation, water relative content, and transpiration rate and lower stomatal resistance [122].
Many studies have shown that the presence of AMF in the soil improves its structure [160,161]. This is due to the secretion, by the hyphae or spores, of glycoproteic substances (Glomalin), allowing a better stabilization of the soil by forming much more stable aggregates [162,163]. Glomus is among the genera recognized by Glomalin [164]. This molecule increases in the soil structure under extreme environmental conditions such as drought [165,166] (Figure 4, Table S1).
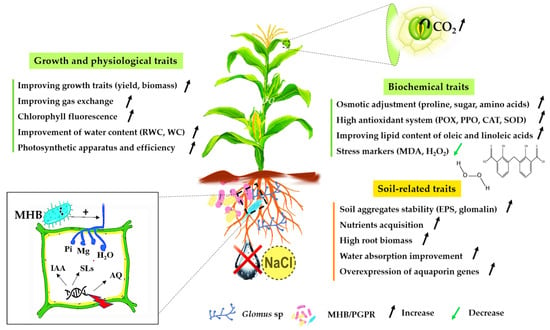
Figure 4.
Impact of Glomus sp. on plant traits under drought and salt stress. AMF: arbuscular mycorrhizal fungi; PGPR: plant growth-promoting rhizobacteria; MHB: mycorrhiza helper bacteria; RWC: relative water content; WC: water content; MDA: malondialdehyde; H2O2: hydrogen peroxide; POX: peroxidase; PPO: polyphenoloxidase; CAT: catalase: SOD: superoxide dismutase; EPS: exopolysaccharides; downward-curving arrow: assimilation.
4.2. Salinity
4.2.1. Glomus and Salt Stress
High concentrations of Na+ and Cl− cause an imbalance in the plant’s ionic composition, which affect its physiological characteristics. Owing to the resemblance in structure of Na+ and K+, these two elements compete for access to the binding site. As a result, the K+/Na+ ratio becomes low in the cytosol in the case of saline soils with very high Na+ levels [167,168,169]. This leads to the disruption of protein, enzyme activity, photosynthesis, turgidity, and stomatal movement. However, the application of AMFs like species of Glomus can mitigate these adverse effects of salinity through various mechanisms: (i) nutrient uptake and maintaining ion homeostasis; (ii) improving water uptake and maintaining osmotic balance in plants; (iii) inducing an antioxidant system to prevent ROS damage; (iv) protecting the photosynthetic apparatus and improving photosynthetic efficiency; and (v) modulating phytohormone profiles to minimize salt-induced damages to growth and development (Figure 4).
4.2.2. Salt Stress Tolerance Mechanisms
Plants naturally possess a defense system against salt stress, although prolonged stress adversely affects plants’ physiological, biochemical, nutritional, and growth traits [168,169]. Nevertheless, mycorrhizae allow plants exposed to high doses of salt to tolerate salinity stress [170,171,172,173]. Na translocation to aerial parts is the primary mechanism enhanced in mycorrhized plants, particularly species of the Glomus genus [174]. Ye et al. [175] explain this by stating that tomato plants inoculated with Glomus geosporum and G. intraradices consortium tend to sequester Na+ in the vacuoles or exclude it from the cytosol. The symbiosis of AMF with host plants leads to the formation of a vast network of hyphae that allows plants to absorb water and nutrients, thereby improving the rate of photosynthesis and osmotic potential of water. Tolerance to salt stress is also due to the accumulation of certain osmolyte molecules in mycorrhized plants compared to their controls. Proline, betaine, polyamines, sugars, organic acids, amino acids, and trehalose are substances that accumulate during salinity stress [176]. Their role is to reduce the potency of water in order to maintain a gradient favoring a call for water from the soil to the roots [177]. Tolerance to salt stress also results in an increase in the antioxidant activity of certain enzymes in AMF plants [175]. It is well known that mycorrhizal plants protect themselves from damage caused by this oxidative stress by ROS detoxification mechanisms, which may be enzymatic (SOD, CAT, APX, GR, and monodehydroascorbate reductase (MDHAR)) and non-enzymatic (flavanones, anthocyanins, carotenoids, and ascorbic acid) [136]. These two systems are the predominant ones used to combat high salinity concentrations in plants inoculated with species of the Glomus genus. Among the species that enhance the antioxidant enzyme system under salt stress conditions are Glomus intraradices, G. mosseae, and G. versiforme [136]. The increase in antioxidant activity is due to the fact that AMF plants cause the overexpression of the genes encoding antioxidant enzymes [12]. The beneficial effects of species from the Glomus genus are not confined to plants’ aerial and root parts but also extend to improving soil components impacted by salinity. Several species of the Glomus genus are known for their positive contribution to the physico-chemical properties of soils impacted by salinity. The stability of saline soil aggregates through certain glycoproteins, such as glomalin, is among the assets of the Glomus genus [51,138]. Ouhaddou et al. [178] reported that inoculation of lettuce with a consortium rich in Glomus species increased glomalin contents in salt-stress environments. The advantage of this genus, as well, is its broad response to several plant species that suffer from salinity (50 to 200 mM NaCl) [179,180,181,182,183,184,185,186] (Table S2). Evaluation of the influence of five Glomus strains (G. aggregatum, G. Intraradices, G. Verriculosum, G. Mosseae, and G. fasciculatum) on the behavior of Nakhla hamra and Tijib cultivars subjected to salt stress (0, 1, 2, 4, 6, 8, and 16 mg L−1) showed that G. intraradices and G. fasciculatum were the best performers in terms of growth and osmoregulation, indicating that symbiosis also depends on the fungal species [185] (Figure 4, Table S2).
5. Interaction of Glomus and PGPR Under Abiotic Stresses
Building on the previous discussion of Glomus, it is important to highlight the additional role of plant growth-promoting rhizobacteria (PGPR) in enhancing plant resilience under salinity and drought stress. While Glomus mycorrhizal fungi contribute to improved nutrient uptake and water retention, PGPR further strengthens plant growth by producing growth hormones, enhancing nutrient availability, and alleviating the effects of environmental stress. Together, these microorganisms form a synergistic partnership that improves plant survival and productivity in challenging conditions, making them a viable solution for promoting sustainable agriculture in saline and drought-affected regions. In addition, PGPR facilitate the establishment of mycorrhizal associations with plants by stimulating spore germination and enhancing hyphal penetration into host plant roots [187,188,189]. Synergistic interaction between Glomus and PGPR’s role in promoting plant growth has been reported compared to single inoculation with either of them. This is due to improved nutrient uptake, better protection against plant pathogens, and a reduction in the effects of abiotic stresses (such as lack of water, salinity, and heavy metals) made possible by double inoculation, compared to single inoculation with Glomus or PGPR [190,191,192]. Several scientific studies have demonstrated the positive effect of applying PGPRs and AMFs in synergy under extreme environmental conditions [190,191,193]. The dual inoculation of PGPR and Glomus versiforme enhances plant nutrition status and productivity and attenuates the impairment of photosynthesis, resulting in an increase in secondary metabolism, osmolyte accumulation, and the antioxidant system compared to a single inoculation in stressed environments [42]. In addition, the Glomus versiforme and PGPR inoculation also boosted IAA and ABA concentrations [194]. Furthermore, the plants inoculated with Glomus intraradices and PGPR performed best and significantly enhanced the plant growth parameters and nutrient absorption under both abiotic and biotic stress [194]. In a study by Sharma et al. [195], phosphate-solubilizing bacteria belonging to the genus Pseudomonas and closely associated with the hyphae of Rhizoglomus irregulare were isolated. The interaction between Rhizoglomus irregulare and these phosphate-solubilizing bacteria (PSB) was then examined. Plants inoculated with the combination of Glomus and bacteria showed significantly higher plant biomass than those inoculated individually, highlighting synergistic interactions within the bacterial–fungal consortium (Figure 4).
6. Factors Affecting Glomus Responsiveness to Mycorrhizal Symbiosis
Plant response to AMF is not always positive but can become neutral or even negative. Several factors are linked to successful colonization of roots by AMF, including the genetic material of the plant and fungus and abiotic and biotic factors [196]. The response to mycorrhization also depends on the duration of the symbiotic process, which varies from one species to another, as recently demonstrated by Boutasknit et al. [26] on carob plants mycorrhized by a consortium based on Glomus species. From a genetic aspect, it was suggested that the response to mycorrhization of the roots of two genotypes (B73 and W22) was different since the density and branching of the root system increased under the effect of Glomus intraradices (Rhizophagus irregularis) more strongly in W22 than in B73. Mycorrhizal symbiosis can be inhibited or blocked depending on the soil’s mineral and organic matter content [197]. For instance, soils with high P content can block mycorrhization of roots with Glomus, while phosphorus-deficient soils also hinder this process [198]. Furthermore, among the six geographically distinct varieties of Medicago truncatula, only some showed a response to mycorrhization by modifying stomatal conductance and root hydraulic conductivity [199]. Additionally, one study revealed that the phylogenetic position of the fungus did not predict the plant’s growth response. Indeed, after comparing the effect of 56 AMF isolates on three different hosts, the researchers observed that most of the variation in plant growth was linked to AMF species belonging to the same family or to isolates of the same species, such as those of the Glomus genus [200].
7. Conclusions
The Glomus genus is representative of AMF in the soils of arid and semi-arid zones thanks to its form of tolerance and its adaptations to extreme conditions such as drought and salinity. Its ecological elasticity enables it to play a crucial role in plant tolerance to environmental stress, promoting growth. Although the applications of AMF based on Glomus species in the form of consortiums present a positive contribution to plants due to their synergetic effect, we should still seek to understand the engineering of the rhizosphere and study all its facets as well as the factors positively or negatively influencing root colonization by Glomus species. A proper understanding of metabolism in Glomus species during symbiosis would also be of significant interest to enhance their potential under changing climatic conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijpb16010032/s1, Table S1: Effects of Glomus species on different plants under drought stress; Table S2: Effects of Glomus species on different plants under salt stress.
Author Contributions
Conceptualization, A.M., R.O., R.B.-L., A.B. and M.A.; methodology, A.M., R.O., R.B.-L., A.B. and M.A.; validation, A.M.; investigation, A.M., R.O., R.B.-L., A.B. and M.A.; writing—original draft preparation, R.O.; writing—review and editing, A.M., R.O., R.B.-L., A.B., M.A. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This project received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement N 862555. The project “Sus-Agri-CC” was carried out under the ERA-Net Cofund FOSC (Grant N 862555).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Saleem, A.; Anwar, S.; Nawaz, T.; Fahad, S.; Saud, S.; Ur Rahman, T.; Khan, M.N.R.; Nawaz, T. Securing a Sustainable Future: The Climate Change Threat to Agriculture, Food Security, and Sustainable Development Goals. J. Umm Al-Qura Univ. Appl. Sci. 2024, 1–17. [Google Scholar] [CrossRef]
- FAOSTAT. FAO Statistical Yearbook 2020—World Food and Agriculture. In Food and Agriculture Organization of the United Nations; FAOSTAT: Rome, Italy, 2022; ISBN 9789251333945. [Google Scholar]
- Biswas, A.; Sarkar, S.; Das, S.; Dutta, S.; Roy Choudhury, M.; Giri, A.; Bera, B.; Bag, K.; Mukherjee, B.; Banerjee, K.; et al. Water Scarcity: A Global Hindrance to Sustainable Development and Agricultural Production—A Critical Review of the Impacts and Adaptation Strategies. Camb. Prism. Water 2025, 3, e4. [Google Scholar] [CrossRef]
- Haseeb, M.; Zandi, G.; Hartani, N.H.; Pahi, M.H.; Nadeem, S.; Kedah, S. Environmental Analysis of the Effect of Population Growth Rate on Supply Chain Performance and Economic Growth of Indonesia. Ekoloji Derg. 2019, 28, 417–426. [Google Scholar]
- Zhang, X.; Han, L.; Wei, H.; Tan, X.; Zhou, W.; Li, W.; Qian, Y. Linking Urbanization and Air Quality Together: A Review and a Perspective on the Future Sustainable Urban Development. J. Clean. Prod. 2022, 346, 130988. [Google Scholar] [CrossRef]
- Ekka, P.; Patra, S.; Upreti, M.; Kumar, G.; Kumar, A.; Saikia, P. Land Degradation and Its Impacts on Biodiversity and Ecosystem Services. In Land and Environmental Management Through Forestry; Wiley Online Library: Hoboken, NJ, USA, 2023; pp. 77–101. [Google Scholar] [CrossRef]
- Dincă, L.C.; Grenni, P.; Onet, C.; Onet, A. Fertilization and Soil Microbial Community: A Review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Bai, Y.C.; Chang, Y.Y.; Hussain, M.; Lu, B.; Zhang, J.P.; Song, X.B.; Lei, X.S.; Pei, D. Soil Chemical and Microbiological Properties Are Changed by Long-Term Chemical Fertilizers That Limit Ecosystem Functioning. Microorganisms 2020, 8, 694. [Google Scholar] [CrossRef]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.A.; Baenziger, P.S.; Börner, A. Drought Stress Tolerance in Wheat and Barley: Advances in Physiology, Breeding and Genetics Research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef] [PubMed]
- Ait-Rahou, Y.; Boutaj, H.; Boutasknit, A.; Douira, A.; Benkirane, R.; El Modafar, C.; Abdelilah, M. Colonization of Tomato Roots with Arbuscular Mycorrhizal Fungi Changes of Antioxidative Activity and Improves Tolerance to Verticillium dahliae. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 65–81. [Google Scholar]
- Ouhaddou, R.; Ech-chatir, L.; Anli, M.; Ben-Laouane, R.; Boutasknit, A.; Meddich, A. Secondary Metabolites, Osmolytes and Antioxidant Activity as the Main Attributes Enhanced by Biostimulants for Growth and Resilience of Lettuce to Drought Stress. Gesunde Pflanz. 2023, 75, 1737–1753. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Plant Physiology and Biochemistry Salinity Induced Physiological and Biochemical Changes in Plants: An Omic Approach towards Salt Stress Tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Majidian, P.; Ghorbani, H. Salinity Stress in Plants: Challenges in View of Physiological Aspects. In Abiotic Stress in Crop Plants—Ecophysiological Responses and Molecular Approaches; IntechOpen: Boston, MA, USA, 2024. [Google Scholar]
- Secomandi, E.; De Gregorio, M.A.; Castro-Cegrí, A.; Lucini, L. Biochemical, Photosynthetic and Metabolomics Insights of Single and Combined Effects of Salinity, Heat, Cold and Drought in Arabidopsis. Physiol. Plant. 2025, 177, e70062. [Google Scholar] [CrossRef]
- Fischer, S.; Wilckens, R.; Jara, J.; Aranda, M.; Valdivia, W.; Bustamante, L.; Graf, F.; Obal, I. Protein and Antioxidant Composition of Quinoa (Chenopodium quinoa Willd.) Sprout from Seeds Submitted to Water Stress, Salinity and Light Conditions. Ind. Crops Prod. 2017, 107, 558–564. [Google Scholar] [CrossRef]
- Anwaar, H.A.; Perveen, R.; Mansha, M.Z.; Abid, M.; Sarwar, Z.M.; Aatif, H.M.; Umar, U.U.D.; Sajid, M.; Aslam, H.M.U.; Alam, M.M.; et al. Assessment of Grain Yield Indices in Response to Drought Stress in Wheat (Triticum aestivum L.). Saudi J. Biol. Sci. 2020, 27, 1818–1823. [Google Scholar] [CrossRef]
- Chakhchar, A.; Haworth, M.; El Modafar, C.; Lauteri, M.; Mattioni, C.; Wahbi, S.; Centritto, M. An Assessment of Genetic Diversity and Drought Tolerance in Argan Tree (Argania spinosa) Populations: Potential for the Development of Improved Drought Tolerance. Front. Plant Sci. 2017, 8, 276. [Google Scholar] [CrossRef]
- Arenas-Castro, S.; Gonçalves, J.F.; Moreno, M.; Villar, R. Projected Climate Changes Are Expected to Decrease the Suitability and Production of Olive Varieties in Southern Spain. Sci. Total Environ. 2020, 709, 136161. [Google Scholar] [CrossRef] [PubMed]
- Zaib, M.; Zeeshan, A.; Aslam, S.; Bano, S.; Ilyas, A.; Abbas, Z.; Nazar, A.; Mumtaz, S. Drought Stress and Plants Production: A Review with Future Prospects. Int. J. Sci. Res. Eng. Dev. 2023, 6, 1278–1293. [Google Scholar]
- Janni, M.; Maestri, E.; Gullì, M.; Marmiroli, M.; Marmiroli, N. Plant Responses to Climate Change, How Global Warming May Impact on Food Security: A Critical Review. Front. Plant Sci. 2023, 14, 1297569. [Google Scholar] [CrossRef]
- Ayed, S.; Othmani, A.; Bouhaouel, I.; Teixeira da Silva, J.A. Multi-Environment Screening of Durum Wheat Genotypes for Drought Tolerance in Changing Climatic Events. Agronomy 2021, 11, 875. [Google Scholar] [CrossRef]
- Del Pozo, A.; Brunel-Saldias, N.; Engler, A.; Ortega-Farias, S.; Acevedo-Opazo, C.; Lobos, G.A.; Jara-Rojas, R.; Molina-Montenegro, M.A. Climate Change Impacts and Adaptation Strategies of Agriculture in Mediterranean-Climate Regions (MCRs). Sustainability 2019, 11, 2769. [Google Scholar] [CrossRef]
- Zaremehrjardy, M.; Victor, J.; Park, S.; Smerdon, B.; Alessi, D.S.; Faramarzi, M. Assessment of Snowmelt and Groundwater-Surface Water Dynamics in Mountains, Foothills, and Plains Regions in Northern Latitudes. J. Hydrol. 2022, 606, 127449. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Emam, Y.; Pessarakli, M. Effect of Silicon on Photosynthetic Gas Exchange, Photosynthetic Pigments, Cell Membrane Stability and Relative Water Content of Different Wheat Cultivars under Drought Stress Conditions. J. Plant Nutr. 2016, 39, 1001–1015. [Google Scholar] [CrossRef]
- Soussani, F.E.; Boutasknit, A.; Ben-Laouane, R.; Benkirane, R.; Baslam, M.; Meddich, A. Arbuscular Mycorrhizal Fungi and Compost-Based Biostimulants Enhance Fitness, Physiological Responses, Yield, and Quality Traits of Drought-Stressed Tomato Plants. Plants 2023, 12, 1856. [Google Scholar] [CrossRef]
- Boutasknit, A.; Baslam, M.; Anli, M.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Ait-Rahou, Y.; El Modafar, C.; Douira, A.; Wahbi, S.; Meddich, A. Impact of Arbuscular Mycorrhizal Fungi and Compost on the Growth, Water Status, and Photosynthesis of Carob (Ceratonia siliqua) under Drought Stress and Recovery. Plant Biosyst. 2021, 156, 994–1010. [Google Scholar] [CrossRef]
- Boutasknit, A.; Baslam, M.; Ait-El-mokhtar, M.; Anli, M.; Ben-Laouane, R.; Douira, A.; Modafar, C.E.; Mitsui, T.; Wahbi, S.; Meddich, A. Arbuscular Mycorrhizal Fungi Mediate Drought Tolerance and Recovery in Two Contrasting Carob (Ceratonia siliqua L.) Ecotypes by Regulating Stomatal, Water Relations, and (in)Organic Adjustments. Plants 2020, 9, 80. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS Metabolism in Plants under Environmental Stress: A Review of Recent Experimental Evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef]
- He, J.D.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Mycorrhizas Enhance Drought Tolerance of Trifoliate Orange by Enhancing Activities and Gene Expression of Antioxidant Enzymes. Sci. Hortic. 2020, 262, 108745. [Google Scholar] [CrossRef]
- Schwarzott, D.; Walker, C.; Schu, A. Glomus, the Largest Genus of the Arbuscular Mycorrhizal Fungi (Glomales), Is Nonmonophyletic. Mol. Phylogenet. Evol. 2001, 21, 190–197. [Google Scholar] [CrossRef]
- Singh, G.; Kaur, M.; Kaushik, P.; Nasser, M.; Abdullah, A.; Ahmad, P. Arbuscular Mycorrhiza in Combating Abiotic Stresses in Vegetables: An Eco-Friendly Approach. Saudi J. Biol. Sci. 2021, 28, 1465–1476. [Google Scholar] [CrossRef]
- Sarkodee-addo, E.; Yasuda, M.; Lee, C.G.; Kanasugi, M.; Fujii, Y.; Omari, R.A.; Abebrese, S.O.; Bam, R.; Asuming-brempong, S.; Mohammad, K.; et al. Arbuscular Mycorrhizal Fungi Associated with Rice (Oryza sativa L.) in Ghana: Effet of Regional Locations and Soil Factors on Diversity and Community Assembly. Agronomy 2020, 10, 559. [Google Scholar] [CrossRef]
- Datta, K.; Chakraborty, S.; Roychoudhury, A. Management of Soil, Waste and Water in the Context of Global Climate Change. In Environmental Nexus for Resource Management; CRC Press: Boca Raton, FL, USA, 2024; pp. 1–26. [Google Scholar]
- Ikehi, M.E.; Ifeanyieze, F.O.; Onu, F.M.; Ejiofor, T.E.; Nwankwo, C.U. Assessing Climate Change Mitigation and Adaptation Strategies and Agricultural Innovation Systems in the Niger Delta. GeoJournal 2023, 88, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Basche, A.; Traylor, E.; Roy, T. The Efficacy of Conservation Practices in Reducing Floods and Improving Water Quality. Front. Environ. Sci. 2023, 11, 1136989. [Google Scholar] [CrossRef]
- Khan, S.; Irshad, S.; Mehmood, K.; Hasnain, Z.; Nawaz, M.; Rais, A.; Gul, S.; Wahid, M.A.; Hashem, A.; Abd_Allah, E.F.; et al. Biochar Production and Characteristics, Its Impacts on Soil Health, Crop Production, and Yield Enhancement: A Review. Plants 2024, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Pal, T.; Yadav, N.; Singh, V.K.; Tripathi, V.; Choudhary, K.K.; Shukla, A.K.; Sunita, K.; Kumar, A.; Bontempi, E.; et al. Current Scenario and Future Prospects of Endophytic Microbes: Promising Candidates for Abiotic and Biotic Stress Management for Agricultural and Environmental Sustainability; Springer: Berlin/Heidelberg, Germany, 2023; Volume 86, ISBN 0123456789. [Google Scholar]
- Abbaspour, H.; Saeidi-Sar, S.; Afshari, H.; Abdel-Wahhab, M.A. Tolerance of Mycorrhiza Infected Pistachio (Pistacia vera L.) Seedling to Drought Stress under Glasshouse Conditions. J. Plant Physiol. 2012, 169, 704–709. [Google Scholar] [CrossRef]
- Meddich, A.; Oihabi, A.; Abbas, Y.; Bizid, E. Rôle des Champignons Mycorhiziens à Arbuscules de Zones Arides dans La Résistance du Trèfle (Trifolium alexandrinum L.) au Déficit Hydrique. Agronomie 2000, 20, 283–295. [Google Scholar] [CrossRef]
- Hartoyo, B.; Trisilawati, O. Diversity of Arbuscular Mycorrhiza Fungi (AMF) in the Rhizosphere of Sugarcane. IOP Conf. Ser. Earth Environ. Sci. 2021, 653, 012066. [Google Scholar] [CrossRef]
- Meddich, A.; El Mokhtar, M.A.; Wahbi, S.; Boumezzough, A. Évaluation des Potentialités Mycorhizogènes en Lien Avec les Paramètres Physico-Chimiques des Sols de Palmeraies du Maroc (Marrakech et Tafilalet). Cah. Agric. 2017, 26, 45012. [Google Scholar] [CrossRef]
- Afrangan, F.; Kazemeini, S.A.; Alinia, M.; Mastinu, A. Glomus versiforme and Micrococcus yunnanensis Reduce the Negative Effects of Salinity Stress by Regulating the Redox State and Ion Homeostasis in Brassica napus L. Crops. Biologia 2023, 78, 3049–3061. [Google Scholar] [CrossRef]
- Alarcón-Zayas, A.; Hernández-Montiel, L.G.; Medina-Hernández, D.; Rueda-Puente, E.O.; Ceiro-Catasú, W.G.; Holguín-Peña, R.J. Effects of Glomus fasciculatum, Azotobacter chroococcum and Vermicompost Leachate on the Production and Quality of Tomato Fruit. Microbiol. Res. 2024, 15, 187–195. [Google Scholar] [CrossRef]
- Msairi, S.; Rais, C.; Maazouzi, S.; Artib, M.; El Gabardi, S.; Mouden, N.; Selmaoui, K.; Benkirane, R.; Ouazzani Touhami, A.; Douira, A. Arbuscular Mycorrhizal Symbiosis in Two Cannabis Varieties (Khardala and Critical) in Morocco. Ecol. Eng. Environ. Technol. 2023, 24, 30–35. [Google Scholar] [CrossRef]
- Belechheb, T. Mycorrhizal Fungi Status Associated with the Rhizosphere of Cytisus monspessulanus in the North West of Morocco. Int. J. Pure Appl. Biosci. 2016, 4, 1–8. [Google Scholar] [CrossRef]
- Kachkouch, W.; Touhami, A.O.; Filali-maltouf, A.; El Modafar, C.; Moukhli, A.; Oukabli, A.; Benkirane, R.; Douira, A. Diversity of Arbuscular Mycorrhizal Fungi in the Rhizosphere of Olea europaea in Three Regions of Morocco (Tafilalt, Zagora and Taounate). Int. J. Pure Appl. Biosci. 2014, 2, 178–195. [Google Scholar]
- Lahbouki, S.; Anli, M.; El Gabardi, S.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Boutasknit, A.; Ait-Rahou, Y.; Outzourhit, A.; Wahbi, S.; Douira, A.; et al. Evaluation of Arbuscular Mycorrhiost Supplementation on Growth, Phenolic Content and Antioxidant Activity of Prickly Pear Cactus (Opuntia ficus-indica). Plant Biosyst. 2021, 156, 882–892. [Google Scholar] [CrossRef]
- Magurno, F.; Uszok, S.; Bierza, K.; Bakr, J.; Kende, Z.; Bessa de Queiroz, M.; Goto, B.T. Glomus highlandensis and G. mongioie, Two New Arbuscular Mycorrhizal Fungi from Saltmarshes, Dunes and Mountains of Europe. Ecol. Evol. Behav. Syst. 2024. [Google Scholar] [CrossRef]
- Błaszkowski, J.; Kovács, G.M.; Balázs, T.K.; Orłowska, E.; Sadravi, M.; Wubet, T.; Buscot, F. Glomus africanum and G. iranicum, Two New Species of Arbuscular Mycorrhizal Fungi (Glomeromycota). Mycologia 2010, 102, 1450–1462. [Google Scholar] [CrossRef]
- Stürmer, S.L.; Kemmelmeier, K. The Glomeromycota in the Neotropics. Front. Microbiol. 2021, 11, 553679. [Google Scholar] [CrossRef]
- Arif, S.; YusnainI, S.; Niswati, A.; Setiawan, A.; Tuchida, K.; Katou, T.; Touji, Y.; Nonaka, M. Population of Arbuscular Mycorrhizal Fungi (AMF) by Different Land Use in Sumatra, Indonesia. Comparison of AMF Spore Numbers in Primary Forest, Secondary Forest, Fields Growing Coffee and Native Grass. Microbes Environ. 1999, 14, 9–17. [Google Scholar] [CrossRef]
- Gerdemann, J.W.; Trappe, J.M. The Endogonaceae in the Pacific Northwest. Mycol. Mem. 1974, 5, 76. [Google Scholar]
- Morton, J.B.; Benny, G.L. Revised Classification of Arbuscular Mycorrhizal Fungi (Zygomycetes): A New Order, Glomales, Two New Suborders, Glomineae and Gigasporineae, and Two New Families, Acaulosporaceae and Gigasporaceae, with an Emendation of Glomaceae. Mycotaxon 1990, 37, 471–491. [Google Scholar]
- Droh, G.; Meliton Djezou, K.; Tuo, S.; Touré, M.; Kouassi, A.-B. Morphometric Characterization of Endomycorrhizal Fungi (Glomeraceae and Acaulosporaceae) from the Bouaflé and Niellé Areas in Côte D'Ivoire. Am. J. Biosci. 2023, 11, 1–10. [Google Scholar] [CrossRef]
- Schüßler, A.; Walker, C. The Glomeromycota: A Species List with New Families and Genera; CreateSpace Independent Publishing Platform: North Charleston, SC, USA, 2010. [Google Scholar]
- Oehl, F.; Goto, B.T.; Sieverding, E. Glomeromycota: Three New Genera and Glomoid Species Reorganized. Mycotaxon 2011, 116, 75–120. [Google Scholar] [CrossRef]
- Magurno, F.; Uszok, S.; Bierza, K.; Bakr, J.; Kende, Z.; Bessa de Queiroz, M.; Casieri, L. Glomus mongioiense, a New Species of Arbuscular Mycorrhizal Fungi from Italian Alps and the Phylogeny-Spoiling Issue of Ribosomal Variants in the Glomus Genus. Agronomy 2024, 14, 1350. [Google Scholar] [CrossRef]
- Souza, T. An Old Relationship. Handbook of Arbuscular Mycorrhizal Fungi; Springer: Cham, Switzerland, 2015; pp. 9–41. ISBN 9783319248509. [Google Scholar]
- Stürmer, S.L. A History of the Taxonomy and Systematics of Arbuscular Mycorrhizal Fungi Belonging to the Phylum Glomeromycota. Mycorrhiza 2012, 22, 247–258. [Google Scholar] [CrossRef]
- Pirozynski, K.A.; Dalpe, Y. Geological History of the Glomaceae with Particular Reference to Mycorrhizal Symbiosis. Symbiosis 1989, 7, 1–36. [Google Scholar]
- Taylor, A.T.N.; Remy, W.; Hass, H.; Kerp, H.; Mycologia, S.; Aug, J.; Aug, N.J. Fossil Arbuscular Mycorrhizae from the Early Devonian. Mycologia 1995, 87, 560–573. [Google Scholar] [CrossRef]
- Redecker, D.; Redecker, D.; Kodner, R.; Graham, L.E. Glomalean Fungi from the Ordovician. Science 2000, 289, 1920–1921. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.; Roux, C.; Lopez-raez, J.A. Rhizosphere Communication of Plants, Parasitic Plants and AM Fungi. Trends Plant Sci. 2007, 12, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, U.; Miranda-Apodaca, J.; Muñoz-Rueda, A.; Mena-Petite, A. Lettuce Production and Antioxidant Capacity Are Differentially Modified by Salt Stress and Light Intensity under Ambient and Elevated CO2. J. Plant Physiol. 2013, 170, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Błaszkowski, J.; Czerniawska, B. Glomus eburneum and Scutellospora fulgida, Species of Arbuscular Mycorrhizal Fungi (Glomeromycota) New for Europe. Acta Mycol. 2013, 43, 57–65. [Google Scholar] [CrossRef]
- Priyadharsini, P.; Muthukumar, T.; Pandey, R.R. Arbuscular Mycorrhizal and Dark Septate Fungal Associations in Shallot (Allium cepa L. Var. Aggregatum) under Conventional Agriculture. Acta Bot. Croat. 2012, 71, 159–175. [Google Scholar] [CrossRef]
- Dalpé, Y.; de Souza, F.A.; Declerck, S. Life Cycle of Glomus Species in Monoxenic Culture. In In Vitro Culture of Mycorrhizas; Springer: Berlin/Heidelberg, Germany, 2005; pp. 49–71. [Google Scholar] [CrossRef]
- Acoltzi-conde, M.C.; Chimal-sánchez, E.; Tovar-soto, A.; Díaz-reyes, J. Arbuscular Mycorrhizal Fungi Consortia in Six Vegetable Crops in the Tepeaca Valley, Puebla, Mexico. Rev. Terra Latinoam. 2024, 42, 1–11. [Google Scholar] [CrossRef]
- Retama-Ortiz, Y.; Ávila-Bello, C.H.; Alarcón, A.; Ferrera-Cerrato, R. Effectiveness of Native Arbuscular Mycorrhiza on the Growth of Four Tree Forest Species from the Santa Marta Mountain, Veracruz (Mexico). For. Syst. 2017, 26, e001. [Google Scholar] [CrossRef]
- Noreen, S.; Yaseen, T.; Iqbal, J.; Abbasi, B.A.; Farouk Elsadek, M.; Eldin, S.M.; Ijaz, S.; Ali, I. Morphological and Molecular Characterizations of Arbuscular Mycorrhizal Fungi and Their Influence on Soil Physicochemical Properties and Plant Nutrition. ACS Omega 2023, 8, 32468–32482. [Google Scholar] [CrossRef]
- Scervino, J.M.; Ponce, M.A.; Erra-bassells, R.; Vierheilig, H. Flavonoids Exhibit Fungal Species and Genus Specific Effects on the Presymbiotic Growth of Gigaspora and Glomus. Mycol. Res. 2005, 109, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Sun, C.; Li, S.; Tauqeer, A.; Bian, X.; Shen, J.; Wu, S. The Utilization and Molecular Mechanism of Arbuscular Mycorrhizal Symbiosis in Vegetables. Veg. Res. 2023, 3, 1–7. [Google Scholar] [CrossRef]
- Naseer, M.A.; Zhang, Z.Q.; Mukhtar, A.; Asad, M.S.; Wu, H.Y.; Yang, H.; Zhou, X.B. Strigolactones: A Promising Tool for Nutrient Acquisition through Arbuscular Mycorrhizal Fungi Symbiosis and Abiotic Stress Tolerance. Plant Physiol. Biochem. 2024, 215, 109057. [Google Scholar] [CrossRef] [PubMed]
- Yolanda, N.-G.; Ferrera-Cerrato, R.; Santamaría, J.M. Glomus intraradices Attenuates the Negative Effect of Low Pi Supply on Photosynthesis and Growth of Papaya Maradol Plants. J. Bot. 2012, 2012, 129591. [Google Scholar] [CrossRef]
- Steinkellner, S.; Lendzemo, V.; Langer, I.; Schweiger, P.; Khaosaad, T.; Toussaint, J.P.; Vierheilig, H. Flavonoids and Strigolactones in Root Exudates as Signals in Symbiotic and Pathogenic Plant-Fungus Interactions. Molecules 2007, 12, 1290–1306. [Google Scholar] [CrossRef]
- Gust, A.A.; Willmann, R.; Desaki, Y.; Grabherr, H.M.; Nu, T. Plant LysM Proteins: Modules Mediating Symbiosis and Immunity. Trends Plant Sci. 2012, 17, 495–502. [Google Scholar] [CrossRef]
- Nagahashi, G.; Douds, D.D. The Effects of Hydroxy Fatty Acids on the Hyphal Branching of Germinated Spores of AM Fungi. Fungal Biol. 2011, 115, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Azmat, R.; Hamid, N.; Moin, S.; Saleem, A. Glomus fasciculatum Fungi as a Bio-Convertor and Bio-Activator of Inorganic and Organic P in Dual Symbiosis. Recent Pat. Biotechnol. 2015, 9, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Wewer, V.; Brands, M.; Dörmann, P. Fatty Acid Synthesis and Lipid Metabolism in the Obligate Biotrophic Fungus Rhizophagus irregularis during Mycorrhization of Lotus japonicus. Plant J. 2014, 79, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, A.M.; Harrison, M.J. Signaling Events during Initiation of Arbuscular Mycorrhizal Symbiosis. J. Integr. Plant Biol. 2014, 56, 250–261. [Google Scholar] [CrossRef]
- Nadal, M.; Sawers, R.; Naseem, S.; Bassin, B.; Kulicke, C.; Sharman, A.; An, G.; An, K.; Ahern, K.R.; Romag, A.; et al. An N -Acetylglucosamine Transporter Required for Arbuscular Mycorrhizal Symbioses in Rice and Maize. Nat. Plants. 2017, 3, 17073. [Google Scholar] [CrossRef] [PubMed]
- Steinkellner, S.; Vierheilig, H.; Antonio, J.; Bote, O. Abscisic Acid Determines Arbuscule Development and Functionality in the Tomato Arbuscular Mycorrhiza. New Phytol. 2007, 175, 554–564. [Google Scholar] [CrossRef]
- Feng, F.; Sun, J.; Radhakrishnan, G.V.; Lee, T.; Bozsóki, Z.; Fort, S.; Gavrin, A.; Gysel, K.; Thygesen, M.B.; Andersen, K.R.; et al. A Combination of Chitooligosaccharide and Lipochitooligosaccharide Recognition Promotes Arbuscular Mycorrhizal Associations in Medicago truncatula. Nat. Commun. 2019, 10, 5047. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.R.; Füchtbauer, W.; Novero, M.; Volpe, V.; Malkov, N.; Genre, A.; Bonfante, P.; Stougaard, J.; Radutoiu, S. Intraradical Colonization by Arbuscular Mycorrhizal Fungi Triggers Induction of a Lipochitooligosaccharide Receptor. Sci. Rep. 2016, 6, 29733. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, W.; Sun, J.; Feng, F.; Deng, Y.; He, Z.; Oldroyd, G.E.D.; Wang, E. The Receptor Kinase CERK1 Has Dual Functions in Symbiosis and Immunity Signalling. Plant J. 2015, 81, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Stracke, S.; Kistner, C.; Yoshida, S.; Mulder, L.; Sato, S.; Kaneko, T.; Tabata, S.; Sandal, N.; Stougaard, J.; Szczyglowski, K.; et al. A Plant Receptor-like Kinase Required for Both Bacterial and Fungal Symbiosis. Nature 2002, 417, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, M.; Sun, J.; Martins, T.V.; Radhakrishnan, G.V.; Findlay, K.; Soumpourou, E.; Thouin, J.; Véry, A.; Sanders, D.; Morris, R.J.; et al. Nuclear-Localized Cyclic Nucleotide–Gated Channels Mediate Symbiotic Calcium Oscillations. Science 2016, 352, 1102–1105. [Google Scholar] [CrossRef]
- Yano, K.; Yoshida, S.; Müller, J.; Singh, S.; Banba, M.; Vickers, K.; Markmann, K.; White, C.; Schuller, B.; Sato, S.; et al. CYCLOPS, a Mediator of Symbiotic Intracellular Accommodation. Proc. Natl. Acad. Sci. USA 2008, 105, 20540–20545. [Google Scholar] [CrossRef]
- Etemadi, M.; Gutjahr, C.; Couzigou, J.M.; Zouine, M.; Lauressergues, D.; Timmers, A.; Audran, C.; Bouzayen, M.; Bécard, G.; Combier, J.P. Auxin Perception Is Required for Arbuscule Development in Arbuscular Mycorrhizal Symbiosis. Plant Physiol. 2014, 166, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.T.; Zhu, Y.; Chen, Y.L.; Ren, H.X.; Li, J.Y.; Kay Abbott, L.; Xiong, Y.C. Arbuscular Mycorrhizal Fungus Alters Root-Sourced Signal (Abscisic Acid) for Better Drought Acclimation in Zea mays L. Seedlings. Environ. Exp. Bot. 2019, 167, 103824. [Google Scholar] [CrossRef]
- Rich, M.K.; Schorderet, M.; Bapaume, L.; Falquet, L.; Morel, P.; Vandenbussche, M.; Reinhardt, D. The Petunia GRAS Transcription Factor ATA/RAM1 Regulates Symbiotic Gene Expression and Fungal Morphogenesis in Arbuscular Mycorrhiza. Plant Physiol. 2015, 168, 788–797. [Google Scholar] [CrossRef]
- Hossain, M.S.; Dietz, K.J. Tuning of Redox Regulatory Mechanisms, Reactive Oxygen Species and Redox Homeostasis under Salinity Stress. Front. Plant Sci. 2016, 7, 548. [Google Scholar] [CrossRef] [PubMed]
- Ouhaddou, R.; Meddich, A.; Ikan, C.; Lahlali, R.; Ait Barka, E.; Hajirezaei, M.R.; Duponnois, R.; Baslam, M. Enhancing Maize Productivity and Soil Health under Salt Stress through Physiological Adaptation and Metabolic Regulation Using Indigenous Biostimulants. Plants 2023, 12, 3703. [Google Scholar] [CrossRef] [PubMed]
- Kreslavski, V.D.; Los, D.A.; Allakhverdiev, S.I.; Kuznetsov, V.V. Signaling Role of Reactive Oxygen Species in Plants under Stress. Russ. J. Plant Physiol. 2012, 59, 141–154. [Google Scholar] [CrossRef]
- Babar, M.; Saif-ur-Rehman; Rasul, S.; Aslam, K.; Abbas, R.; Athar, H.U.R.; Manzoor, I.; Kashif Hanif, M.; Naqqash, T. Mining of Halo-Tolerant Plant Growth Promoting Rhizobacteria and Their Impact on Wheat (Triticum aestivum L.) under Saline Conditions. J. King Saud Univ.-Sci. 2021, 33, 101372. [Google Scholar] [CrossRef]
- Lu, C.; Li, L.; Liu, X.; Chen, M.; Wan, S.; Li, G. Salt Stress Inhibits Photosynthesis and Destroys Chloroplast Structure by Downregulating Chloroplast Development–Related Genes in Robinia pseudoacacia Seedlings. Plants 2023, 12, 1283. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, H.; Xue, L.; Nie, N.; Zhang, H.; Zhao, N.; He, S.; Liu, Q.; Gao, S.; Zhai, H. IbMYC2 Contributes to Salt and Drought Stress Tolerance via Modulating Anthocyanin Accumulation and ROS-Scavenging System in Sweet Potato. Int. J. Mol. Sci. 2024, 25, 2096. [Google Scholar] [CrossRef]
- Sairam, R.K.; Rao, K.; Srivastava, G.C. Differential Response of Wheat Genotypes to Long Term Salinity Stress in Relation to Oxidative Stress, Antioxidant Activity and Osmolyte Concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Sahab, S.; Suhani, I.; Srivastava, V.; Chauhan, P.S.; Singh, R.P.; Prasad, V. Potential Risk Assessment of Soil Salinity to Agroecosystem Sustainability: Current Status and Management Strategies. Sci. Total Environ. 2020, 764, 144164. [Google Scholar] [CrossRef]
- Sahin, U.; Ekinci, M.; Ors, S.; Turan, M.; Yildiz, S.; Yildirim, E. Effects of Individual and Combined Effects of Salinity and Drought on Physiological, Nutritional and Biochemical Properties of Cabbage (Brassica oleracea Var. Capitata). Sci. Hortic. 2018, 240, 196–204. [Google Scholar] [CrossRef]
- Marzec, M.; Daszkowska-Golec, A.; Collin, A.; Melzer, M.; Eggert, K.; Szarejko, I. Barley Strigolactone Signalling Mutant Hvd14.d Reveals the Role of Strigolactones in Abscisic Acid-Dependent Response to Drought. Plant Cell Environ. 2020, 43, 2239–2253. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Reichheld, J.P.; Foyer, C.H. ROS-Related Redox Regulation and Signaling in Plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Panda, S.K.; Gupta, D.; Patel, M.; Vyver, C.V.D.; Koyama, H. Functionality of Reactive Oxygen Species (ROS) in Plants: Toxicity and Control in Poaceae Crops Exposed to Abiotic Stress. Plants 2024, 13, 2071. [Google Scholar] [CrossRef]
- Jafari, M.; Shahsavar, A.R.; Talebi, M.; Hesami, M. Exogenous Melatonin Protects Lime Plants from Drought Stress-Induced Damage by Maintaining Cell Membrane Structure, Detoxifying ROS and Regulating Antioxidant Systems. Horticulturae 2022, 8, 257. [Google Scholar] [CrossRef]
- Dandan, Z.; Zhiwei, Z. Biodiversity of Arbuscular Mycorrhizal Fungi in the Hot-Dry Valley of the Jinsha River, Southwest China. Appl. Soil Ecol. 2007, 37, 118–128. [Google Scholar] [CrossRef]
- Jefwa, J.M.; Mung’atu, J.; Okoth, P.; Muya, E.; Roimen, H.; Njuguini, S. Influence of Land Use Types on Occurrence of Arbuscular Mycorrhizal Fungi in the High Altitude Regions of Mt. Kenya. Trop. Subtrop. Agroecosyst. 2009, 11, 277–290. [Google Scholar]
- Abdelhalim, T.S.; Finckh, M.R.; Babiker, A.G.; Oehl, F. Species Composition and Diversity of Arbuscular Mycorrhizal Fungi in White Nile state, Central Sudan. Arch. Agron. Soil Sci. 2014, 60, 37–41. [Google Scholar] [CrossRef]
- Mahmoudi, N.; Cruz, C.; Mahdhi, M.; Mars, M.; Caeiro, M.F. Arbuscular Mycorrhizal Fungi in Soil, Roots and Rhizosphere of Medicago truncatula: Diversity and Heterogeneity under Semi-Arid Conditions. PeerJ 2019, 7, e6401. [Google Scholar] [CrossRef] [PubMed]
- Sellal, Z.; Ouazzani, T.A.; Dahmani, J.; Maazouzi, S.; Najoua, M.; Mohamed, C.; Karima, S.; Rachid, B.; Cherkaoui, E.M.; Douira, A. Diversity of Arbuscular Mycorrhizal Fungi in the Rhizosphere of Argania spinosa in Morocco. In Arbuscular Mycorrhizal Fungi in Agriculture-New Insights; IntechOpen: Boston, MA, USA, 2022. [Google Scholar] [CrossRef]
- Bâ, A.M.; Guissou, T.; Dalpé, Y. Les Glomales d’Acacia Holosericea et d’Acacia Mangium. Bois For. Trop. 1996, 250, 5–17. [Google Scholar]
- Yooyongwech, S.; Phaukinsang, N.; Cha-um, S.; Supaibulwatana, K. Arbuscular Mycorrhiza Improved Growth Performance in Macadamia tetraphylla L. Grown under Water Deficit Stress Involves Soluble Sugar and Proline Accumulation. Plant Growth Regul. 2013, 69, 285–293. [Google Scholar] [CrossRef]
- Mena-Violante, H.G.; Ocampo-Jiménez, O.; Dendooven, L.; Martínez-soto, G.; González-castañeda, J.; Fred, T.D.J.; Olalde-Portugal, V. Arbuscular Mycorrhizal Fungi Enhance Fruit Growth and Quality of Chile Ancho (Capsicum annuum L. Cv San Luis) Plants Exposed to Drought. Mycorrhiza 2006, 16, 261–267. [Google Scholar] [CrossRef]
- Gobat, J.M.; Aragno, M.; Matthey, W. The Living Soil: Basic Pedology-Soil Biology; Presses Polytechniques et Universitaires Romandes: Lausanne, Switzerland, 2003; p. 569. ISBN 2-88074-501-2. [Google Scholar]
- Frey, S.D. Mycorrhizal Fungi as Mediators of Soil Organic Matter Dynamics. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 237–259. [Google Scholar] [CrossRef]
- Al-Maliki, S.; Al-Amery, A.; Sallal, M.; Radhi, A.; Al-Taey, D.K.A. Effects of Arbuscular Mycorrhiza and Organic Wastes on Soil Carbon Mineralisation, Actinomycete s and Nutrient Content in Maize Plants (Zea mays L.). Malays. J. Soil Sci. 2021, 25, 107–124. [Google Scholar]
- Boutasknit, A.; Ait-El-Mokhtar, M.; Fassih, B.; Ben-Laouane, R.; Wahbi, S.; Meddich, A. Effect of Arbuscular Mycorrhizal Fungi and Rock Phosphate on Growth, Physiology, and Biochemistry of Carob under Water Stress and after Rehydration in Vermicompost-Amended Soil. Metabolites 2024, 14, 202. [Google Scholar] [CrossRef]
- Putri, D.A.L.P.; Widyastuti, R.; Idris, I.; Ikhwani, A.Z.N.; Nugroho, S.; Sudiana, I.M.; Kanti, A.; Purnaningsih, I.; Ochiai, K.; Kobayashi, M.; et al. Unraveling the Mechanisms of Drought Tolerance Enhancement in Sorghum Bicolor through Glomus mosseae Inoculation: Insights from Comparative Analysis of Super 2 and Konawe Selatan Accessions. S. Afr. J. Bot. 2023, 161, 293–304. [Google Scholar] [CrossRef]
- Benaffari, W.; Boutasknit, A.; Anli, M.; Ait-el-mokhtar, M.; Ait-Rahou, Y.; Ben-Laouane, R.; Ahmed, H.B.; Mitsui, T.; Baslam, M.; Meddich, A. The Native Arbuscular Mycorrhizal Fungi and Vermicompost-Based Organic Amendments Enhance Soil Fertility, Growth Performance, and the Drought Stress Tolerance of Quinoa. Plants 2022, 11, 393. [Google Scholar] [CrossRef] [PubMed]
- Spinoso-Castillo, J.L.; Moreno-Hernández, M.D.R.; Mancilla-Álvarez, E.; Sánchez-Segura, L.; Sánchez-Páez, R.; Bello-Bello, J.J. Arbuscular Mycorrhizal Symbiosis Improves Ex Vitro Acclimatization of Sugarcane Plantlets (Saccharum spp.) under Drought Stress Conditions. Plants 2023, 12, 687. [Google Scholar] [CrossRef] [PubMed]
- Ganjeali, A.; Ashiani, E.; Zare, M.; Tabasi, E. Influences of the Arbuscular Mycorrhizal Fungus Glomus mosseae on Morphophysiological Traits and Biochemical Compounds of Common Bean (Phaseolus vulgaris) under Drought Stress. S. Afr. J. Plant Soil. 2018, 35, 121–127. [Google Scholar] [CrossRef]
- He, F.; Sheng, M.; Tang, M. Effects of Rhizophagus irregularis on Photosynthesis and Antioxidative Enzymatic System in Robinia pseudoacacia L. under Drought Stress. Front. Plant Sci. 2017, 8, 183. [Google Scholar] [CrossRef]
- Meddich, A.; Jaiti, F.; Bourzik, W.; Asli, A.E.; Hafidi, M. Use of Mycorrhizal Fungi as a Strategy for Improving the Drought Tolerance in Date Palm (Phoenix dactylifera). Sci. Hortic. 2015, 192, 468–474. [Google Scholar] [CrossRef]
- Ouledali, S.; Ennajeh, M.; Ferrandino, A.; Khemira, H.; Schubert, A.; Secchi, F. Influence of Arbuscular Mycorrhizal Fungi Inoculation on the Control of Stomata Functioning by Abscisic Acid (ABA) in Drought-Stressed Olive Plants. S. Afr. J. Bot. 2019, 121, 152–158. [Google Scholar] [CrossRef]
- Meddich, A.; Ouhaddou, R.; Anli, M.; Boutasknit, A. Role of Phosphorus and Arbuscular Mycorrhizal Fungi in the Growth Performances and Tolerance of Barley to Water Stress. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 45–67. [Google Scholar]
- Baslam, M.; Goicoechea, N. Water Deficit Improved the Capacity of Arbuscular Mycorrhizal Fungi (AMF) for Inducing the Accumulation of Antioxidant Compounds in Lettuce Leaves. Mycorrhiza 2012, 22, 347–359. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N.; Xia, R.X. Effects of Water Stress and Arbuscular Mycorrhizal Fungi on Reactive Oxygen Metabolism and Antioxidant Production by Citrus (Citrus tangerine) Roots. Eur. J. Soil Biol. 2006, 42, 166–172. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Zhang, L. AMF Inoculation and Phosphorus Supplementation Alleviates Drought Induced Growth and Photosynthetic Decline in Nicotiana tabacum by Up-Regulating Antioxidant Metabolism and Osmolyte Accumulation. Environ. Exp. Bot. 2020, 176, 104088. [Google Scholar] [CrossRef]
- Ghasemi, M.; Zahedi, M.; Gheysari, M.; Sabzalian, M.R. Effects of Inoculation with Four Mycorrhizal Species on Seed Phenolic and Fatty Acids of Sesame Plants Grown under Different Irrigation Regimes. Sci. Rep. 2023, 13, 16482. [Google Scholar] [CrossRef] [PubMed]
- Del Mar Alguacil, M.; Kohler, J.; Caravaca, F.; Roldán, A. Differential Effects of Pseudomonas mendocina and Glomus intraradices on Lettuce Plants Physiological Response and Aquaporin PIP2 Gene Expression under Elevated Atmospheric CO2 and Drought. Microb. Ecol. 2009, 58, 942–951. [Google Scholar] [CrossRef]
- Das, S.; Sarkar, S. Arbuscular Mycorrhizal Fungal Contribution towards Plant Resilience to Drought Conditions. Front. Fungal Biol. 2024, 5, 1355999. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhou, L.J.; Xu, Y.J.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.S. Growth Performance and Osmolyte Regulation of Drought-Stressed Walnut Plants Are Improved by Mycorrhiza. Agriculture 2024, 14, 367. [Google Scholar] [CrossRef]
- Chen, M.; Yang, G.; Sheng, Y.; Li, P.; Qiu, H.; Zhou, X.; Huang, L.; Chao, Z. Glomus mosseae Inoculation Improves the Root System Architecture, Photosynthetic Efficiency and Flavonoids Accumulation of Liquorice under Nutrient Stress. Front. Plant Sci. 2017, 8, 931. [Google Scholar] [CrossRef] [PubMed]
- Sagar, A.; Rathore, P.; Ramteke, P.W.; Ramakrishna, W.; Reddy, M.S.; Pecoraro, L. Plant Growth Promoting Rhizobacteria, Arbuscular Mycorrhizal Fungi and Their Synergistic Interactions to Counteract the Negative Effects of Saline Soil on Agriculture: Key Macromolecules and Mechanisms. Microorganisms 2021, 9, 1491. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.L.; Mooney, S.J.; Dickinson, M.J.; West, H.M. The Effects of Simultaneous Root Colonisation by Three Glomus Species on Soil Pore Characteristics. Soil Biol. Biochem. 2012, 49, 167–173. [Google Scholar] [CrossRef]
- Lei, A.Q.; Yang, Q.H.; Zhang, Y.; Liao, W.Y.; Xie, Y.C.; Srivastava, A.K.; Hashem, A.; Alqahtani, M.D.; Abd_Allah, E.F.; Wu, Q.S.; et al. Agronomic Practices Alter Regulated Effects of Easily Extractable Glomalin-Related Soil Protein on Fruit Quality and Soil Properties of Satsuma Mandarin. Agronomy 2023, 13, 881. [Google Scholar] [CrossRef]
- Singh, A.K.; Zhu, X.; Chen, C.; Wu, J.; Yang, B.; Zakari, S.; Jiang, X.J.; Singh, N.; Liu, W. The Role of Glomalin in Mitigation of Multiple Soil Degradation Problems. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1604–1638. [Google Scholar] [CrossRef]
- El-Sherbeny, T.M.S.; Mousa, A.M.; El-Sayed, E.S.R. Use of Mycorrhizal Fungi and Phosphorus Fertilization to Improve the Yield of Onion (Allium cepa L.) Plant. Saudi J. Biol. Sci. 2022, 29, 331–338. [Google Scholar] [CrossRef]
- Jabborova, D.; Annapurna, K.; Al-Sadi, A.M.; Alharbi, S.A.; Datta, R.; Zuan, A.T.K. Biochar and Arbuscular Mycorrhizal Fungi Mediated Enhanced Drought Tolerance in Okra (Abelmoschus esculentus) Plant Growth, Root Morphological Traits and Physiological Properties. Saudi J. Biol. Sci. 2021, 28, 5490–5499. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xu, Y.; Han, G.; Wang, W.; Li, X.; Cheng, B. Identification and Functional Characterization of a Maize Phosphate Transporter Induced by Mycorrhiza Formation. Plant Cell Physiol. 2018, 59, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Volpe, V.; Chitarra, W.; Cascone, P.; Volpe, M.G.; Bartolini, P.; Moneti, G.; Pieraccini, G.; Serio, C.D.; Maserti, B.; Guerrieri, E.; et al. The Association With Two Different Arbuscular Mycorrhizal Fungi Differently Affects Water Stress Tolerance in Tomato. Front. Plant Sci. 2018, 9, 1480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Meng, S.; Li, M.; Zhao, Z. Genomic Identification and Expression Analysis of the Phosphate Transporter Gene Family in Poplar. Front. Plant Sci. 2016, 7, 1398. [Google Scholar] [CrossRef]
- Balestrini, R.; Rosso, L.C.; Veronico, P.; Melillo, M.T.; De Luca, F.; Fanelli, E.; Colagiero, M.; Di Fossalunga, A.S.; Ciancio, A.; Pentimone, I. Transcriptomic Responses to Water Deficit and Nematode Infection in Mycorrhizal Tomato Roots. Front. Microbiol. 2019, 10, 1807. [Google Scholar] [CrossRef]
- Al-Arjani, A.B.F.; Hashem, A.; Abd_Allah, E.F. Arbuscular Mycorrhizal Fungi Modulates Dynamics Tolerance Expression to Mitigate Drought Stress in Ephedra foliata Boiss. Saudi J. Biol. Sci. 2020, 27, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhao, Q.; Zaman, S.; Anwar, A.; Li, S. The Transcriptomic Analysis Revealed the Molecular Mechanism of Arbuscular Mycorrhizal Fungi (AMF) Inoculation in Watermelon. Sci. Hortic. 2024, 332, 113184. [Google Scholar] [CrossRef]
- Bahadur, A.; Batool, A.; Nasir, F.; Jiang, S.; Mingsen, Q.; Zhang, Q.; Pan, J.; Liu, Y.; Feng, H. Mechanistic Insights into Arbuscular Mycorrhizal Fungi-Mediated Drought Stress Tolerance in Plants. Int. J. Mol. Sci. 2019, 20, 4199. [Google Scholar] [CrossRef]
- Xie, W.; Hao, Z.; Zhou, X.; Jiang, X.; Xu, L.; Wu, S.; Zhao, A.; Zhang, X.; Chen, B. Arbuscular Mycorrhiza Facilitates the Accumulation of Glycyrrhizin and Liquiritin in Glycyrrhiza uralensis under Drought Stress. Mycorrhiza 2018, 28, 285–300. [Google Scholar] [CrossRef]
- Sharma, K.; Gupta, S.; Thokchom, S.D.; Jangir, P.; Kapoor, R. Arbuscular Mycorrhiza-Mediated Regulation of Polyamines and Aquaporins During Abiotic Stress: Deep Insights on the Recondite Players. Front. Plant Sci. 2021, 12, 642101. [Google Scholar] [CrossRef]
- El-Mesbahi, M.N.; Azcón, R.; Ruiz-Lozano, J.M.; Aroca, R. Plant Potassium Content Modifies the Effects of Arbuscular Mycorrhizal Symbiosis on Root Hydraulic Properties in Maize Plants. Mycorrhiza 2012, 22, 555–564. [Google Scholar] [CrossRef]
- Gong, M.; Bai, N.; Wang, P.; Su, J.; Chang, Q.; Zhang, Q. Co-Inoculation with Arbuscular Mycorrhizal Fungi and Dark Septate Endophytes under Drought Stress: Synergistic or Competitive Effects on Maize Growth, Photosynthesis, Root Hydraulic Properties and Aquaporins? Plants 2023, 12, 2596. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, G.; Erice, G.; Aroca, R.; Delgado-Huertas, A.; Ruiz-Lozano, J.M. Elucidating the Possible Involvement of Maize Aquaporins and Arbuscular Mycorrhizal Symbiosis in the Plant Ammonium and Urea Transport under Drought Stress Conditions. Plants 2020, 9, 148. [Google Scholar] [CrossRef]
- Li, T.; Hu, Y.; Hao, Z.; Li, H.; Chen, B. Aquaporin Genes GintAQPF1 and GintAQPF2 from Glomus intraradices Contribute to Plant Drought Tolerance. Plant Signal. Behav. 2013, 8, e24030. [Google Scholar] [CrossRef]
- Gong, M.; Tang, M.; Chen, H.; Zhang, Q.; Feng, X. Effects of Two Glomus Species on the Growth and Physiological Performance of Sophora davidii Seedlings under Water Stress. New For. 2013, 44, 399–408. [Google Scholar] [CrossRef]
- Wu, H.; Xiang, W.; Chen, L.; Ouyang, S.; Xiao, W.; Li, S.; Forrester, D.I.; Lei, P.; Zeng, Y.; Deng, X.; et al. Soil Phosphorus Bioavailability and Recycling Increased with Stand Age in Chinese Fir Plantations. Ecosystems 2020, 23, 973–988. [Google Scholar] [CrossRef]
- Wahid, F.; Sharif, M.; Fahad, S.; Ali, A.; Adnan, M.; Rafiullah; Saud, S.; Danish, S.; Arif Ali, M.; Ahmed, N.; et al. Mycorrhiza and Phosphate Solubilizing Bacteria: Potential Bioagents for Sustainable Phosphorus Management in Agriculture. Phyton 2022, 91, 257–278. [Google Scholar] [CrossRef]
- Alonso, L.M.; Kleiner, D. Spores of the Mycorrhizal Fungus Glomus mosseae Host Yeasts That Solubilize Phosphate and Accumulate Polyphosphates. Mycorrhiza 2008, 18, 197–204. [Google Scholar] [CrossRef]
- Shi, Q.; Pang, J.; Yong, J.W.H.; Bai, C.; Pereira, C.G.; Song, Q.; Wu, D.; Dong, Q.; Cheng, X.; Wang, F.; et al. Phosphorus-Fertilisation Has Differential Effects on Leaf Growth and Photosynthetic Capacity of Arachis hypogaea L. Plant Soil 2020, 447, 99–116. [Google Scholar] [CrossRef]
- Salamatullah, A.M.; Soufan, W.; Nafidi, H.; Ouahmane, L. Mycorrhizal Fungi Inoculation Improves Capparis spinosa’s Yield, Nutrient Uptake and Photosynthetic Efficiency under Water Deficit. Agronomy 2022, 12, 149. [Google Scholar] [CrossRef]
- Hammer, E.C.; Rillig, M.C. The Influence of Different Stresses on Glomalin Levels in an Arbuscular Mycorrhizal Fungus-Salinity Increases Glomalin Content. PLoS ONE 2011, 6, 28426. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Ma, S.; Liu, J.; Qin, S.; Liu, X.; Li, T.; Liao, Y.; Shi, Y.; Zhang, J. Organic Materials and AMF Addition Promote Growth of Taxodium ‘Zhongshanshan’ by Improving Soil Structure. Forests 2023, 14, 731. [Google Scholar] [CrossRef]
- Channavar, V.R.; Hussain, K.N.J.; Vyas, R.D.V.; Malappannavar, N.; Radder, V.S.; Jagadeesh, B.R. The Hidden Powers of Glomalin: Insights into Soil Health and Functionality. Arch. Curr. Res. Int. 2024, 24, 469–479. [Google Scholar] [CrossRef]
- Serrano, R.; Rodriguez-navarro, A. Ion Homeostasis during Salt Stress in Plants. Curr. Opin. Cell Biol. 2001, 13, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Samiappan, S.C.; Mahalakshmi, P.; Pandiyan, R. Impact of Mycorrhizal Soil Fertility Proteins and Arbuscular Mycorrhizal Application to Combat Drought Stress in Maize Plants. J. Plant Biochem. Biotechnol. 2021, 30, 906–917. [Google Scholar] [CrossRef]
- Prasad, K.; Khare, A.; Rawat, P. Glomalin Arbuscular Mycorrhizal Fungal Reproduction, Lifestyle and Dynamic Role in Global Sustainable Agriculture for Future Generation. In Fungal Reproduction and Growth; IntechOpen: Boston, MA, USA, 2022; pp. 1–22. [Google Scholar]
- Gao, W.Q.; Wang, P.; Wu, Q.S. Functions and Application of Glomalin-Related Soil Proteins: A Review. Sains Malays. 2019, 48, 111–119. [Google Scholar] [CrossRef]
- Nawaz, A.; Qamar, Z.U.; Marghoob, M.U.; Imtiaz, M.; Imran, A.; Mubeen, F. Contribution of Potassium Solubilizing Bacteria in Improved Potassium Assimilation and Cytosolic K+/Na+ Ratio in Rice (Oryza sativa L.) under Saline-Sodic Conditions. Front. Microbiol. 2023, 14, 1196024. [Google Scholar] [CrossRef]
- Evelin, H.; Giri, B.; Kapoor, R. Contribution of Glomus intraradices Inoculation to Nutrient Acquisition and Mitigation of Ionic Imbalance in NaCl-Stressed Trigonella foenum-graecum. Mycorrhiza 2012, 22, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Giri, B.; Kapoor, R.; Mukerji, K.G. Improved Tolerance of Acacia nilotica to Salt Stress by Arbuscular Mycorrhiza, Glomus fasciculatum May Be Partly Related to Elevated K/Na Ratios in Root and Shoot Tissues. Microb. Ecol. 2007, 54, 753–760. [Google Scholar] [CrossRef]
- Karimi, R.; Noori, A. Streptomyces rimosus Rhizobacteria and Glomus mosseae Mycorrhizal Fungus Inoculation Alleviate Salinity Stress in Grapevine through Morphophysiological Changes and Nutritional Balance. Sci. Hortic. 2022, 305, 111433. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, Q. Sodium Chloride Stress Induced Changes in Leaf Osmotic Adjustment of Trifoliate Orange (Poncirus trifoliata) Seedlings Inoculated with Mycorrhizal Fungi. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 64–69. [Google Scholar] [CrossRef]
- Ouhaddou, R.; Anli, M.; Ech-Chatir, L.; Ben-Laouane, R.; Boutasknit, A.; Benaffari, W.; Meddich, A. Boosting Agricultural Productivity in the Mediterranean: Harnessing Natural Biostimulants to Alleviate Abiotic Constraints. In Agricultural Research Updates; Nova Science Publishers: Hauppauge, NY, USA, 2023; Volume 46, pp. 181–216. ISBN 9798891133785. [Google Scholar]
- Sharma, V.; Sharma, D.P.; Salwan, R. Surviving the Stress: Understanding the Molecular Basis of Plant Adaptations and Uncovering the Role of Mycorrhizal Association in Plant Abiotic Stresses. Microb. Pathog. 2024, 193, 106772. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Wani, K.I.; Naeem, M.; Khan, M.M.A.; Aftab, T. Cellular Responses, Osmotic Adjustments, and Role of Osmolytes in Providing Salt Stress Resilience in Higher Plants: Polyamines and Nitric Oxide Crosstalk. J. Plant Growth Regul. 2023, 42, 539–553. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, X.; Bao, E.; Cao, K.; Zou, Z. Effects of Arbuscular Mycorrhizal Fungi on Watermelon Growth, Elemental Uptake, Antioxidant, and Photosystem Ii Activities and Stress-Response Gene Expressions under Salinity-Alkalinity Stresses. Front. Plant Sci. 2019, 10, 863. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R. Arbuscular Mycorrhizal Symbiosis Modulates Antioxidant Response in Salt-Stressed Trigonella foenum-graecum Plants. Mycorrhiza 2014, 24, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Kohler, J.; Hernández, J.A.; Caravaca, F.; Roldán, A. Induction of Antioxidant Enzymes Is Involved in the Greater Effectiveness of a PGPR versus AM Fungi with Respect to Increasing the Tolerance of Lettuce to Severe Salt Stress. Environ. Exp. Bot. 2009, 65, 245–252. [Google Scholar] [CrossRef]
- Ouhaddou, R.; Ben-Laouane, R.; Lahlali, R.; Anli, M.; Ikan, C.; Boutasknit, A.; Slimani, A.; Oufdou, K.; Baslam, M.; Ait Barka, E.; et al. Application of Indigenous Rhizospheric Microorganisms and Local Compost as Enhancers of Lettuce Growth, Development, and Salt Stress Tolerance. Microorganisms 2022, 10, 1625. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.R.Z.; Seifi, E.; Varasteh, F.; Akbarpour, V. Mitigating Salinity Stress in Pomegranate: Effects of Pseudomonas fluorescens and Glomus mosseae on Stress Responses of Red Angel and Wonderful Cultivars. Sci. Hortic. 2024, 330, 113036. [Google Scholar] [CrossRef]
- Yilmaz, A.; Yildirim, E.; Yilmaz, H.; Soydemir, H.E.; Güler, E.; Ciftci, V.; Yaman, M. Use of Arbuscular Mycorrhizal Fungi for Boosting Antioxidant Enzyme Metabolism and Mitigating Saline Stress in Sweet Basil (Ocimum basilicum L.). Sustainability 2023, 15, 5982. [Google Scholar] [CrossRef]
- Huang, S.; Gill, S.; Ramzan, M.; Ahmad, M.Z.; Danish, S.; Huang, P.; Al Obaid, S.; Alharbi, S.A. Uncovering the Impact of AM Fungi on Wheat Nutrient Uptake, Ion Homeostasis, Oxidative Stress, and Antioxidant Defense under Salinity Stress. Sci. Rep. 2023, 13, 8249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shi, F.; Zheng, X.; Pan, H.; Wen, Y.; Song, F. Effects of AMF Compound Inoculants on Growth, Ion Homeostasis, and Salt Tolerance-Related Gene Expression in Oryza sativa L. Under Salt Treatments. Rice 2023, 16, 18. [Google Scholar] [CrossRef]
- Garg, N.; Baher, N. Role of Arbuscular Mycorrhizal Symbiosis in Proline Biosynthesis and Metabolism of Cicer arietinum L. (Chickpea) Genotypes Under Salt Stress. J. Plant Growth Regul. 2013, 32, 767–778. [Google Scholar] [CrossRef]
- Altuntas, O.; Dasgan, H.Y.; Akhoundnejad, Y.; Nas, Y. Unlocking the Potential of Pepper Plants under Salt Stress: Mycorrhizal Effects on Physiological Parameters Related to Plant Growth and Gas Exchange across Tolerant and Sensitive Genotypes. Plants 2024, 13, 1380. [Google Scholar] [CrossRef] [PubMed]
- Léopold, I.; Diatta, D.; Kane, A.; Agbangba, C.E.; Sagna, M. Inoculation with Arbuscular Mycorrhizal Fungi Improves Seedlings Growth of Two Sahelian Date Palm Cultivars (Phoenix dactylifera L., Cv. Nakhla Hamra and Cv. Tijib) under Salinity Stresses. Adv. Biosci. Biotechnol. 2014, 5, 64–72. [Google Scholar] [CrossRef]
- Estrada, B.; Barea, J.M.; Aroca, R.; Ruiz-Lozano, J.M. A Native Glomus intraradices Strain from a Mediterranean Saline Area Exhibits Salt Tolerance and Enhanced Symbiotic Efficiency with Maize Plants under Salt Stress Conditions. Plant Soil 2013, 366, 333–349. [Google Scholar] [CrossRef]
- Ikan, C.; Ben-Laouane, R.; Ouhaddou, R.; Ghoulam, C.; Meddich, A. Co-Inoculation of Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria Can Mitigate the Effects of Drought in Wheat Plants (Triticum durum). Plant Biosyst. 2023, 157, 907–919. [Google Scholar] [CrossRef]
- Akensous, F.; Anli, M.; Meddich, A. Biostimulants as Innovative Tools to Boost Date Palm (Phoenix dactylifera L.) Performance under Drought, Salinity, and Heavy Metal(Oid)s’ Stresses: A Concise Review. Sustainability 2022, 14, 15484. [Google Scholar] [CrossRef]
- Ghadbane, M.; Medjekal, S.; Benderradji, L.; Belhadj, H.; Daoud, H. Assessment of Arbuscular Mycorrhizal Fungi Status and Rhizobium on Date Palm (Phoenix dactylifera L.) Cultivated in a Pb Contaminated Soil. In Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions, 2nd ed.; Environmental Science and Engineering; Springer: Cham, Switzerland, 2019; pp. 703–707. [Google Scholar] [CrossRef]
- Begum, N.; Wang, L.; Ahmad, H.; Akhtar, K.; Roy, R.; Khan, M.I.; Zhao, T. Co-Inoculation of Arbuscular Mycorrhizal Fungi and the Plant Growth-Promoting Rhizobacteria Improve Growth and Photosynthesis in Tobacco Under Drought Stress by Up-Regulating Antioxidant and Mineral Nutrition Metabolism. Microb. Ecol. 2022, 83, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Soni, S.K.; Bajpai, A. Cooperative Interaction of Glomus intraradices with Plant Growth-Promoting Rhizobacteria Promotes Plant Development and Essential Oil Yield of Pogostemon Cablin and Reduces Disease Occurrence under Organic Field Conditions. Australas. Plant Pathol. 2023, 52, 595–607. [Google Scholar] [CrossRef]
- Li, W.; Li, W.; Xing, L.; Guo, S. Effect of Arbuscular Mycorrhizal Fungi (AMF) and Plant Growth-Promoting Rhizobacteria (PGPR) on Microorganism of Phenanthrene and Pyrene Contaminated Soils. Int. J. Phytoremediat. 2022, 25, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Tong, C.L.; Sun, M.F. Effects and Molecular Mechanism of Mycorrhiza on the Growth, Nutrient Absorption, Quality of Fresh Leaves, and Antioxidant System of Tea Seedlings Suffering from Salt Stress. Agronomy 2022, 12, 1163. [Google Scholar] [CrossRef]
- Postgraduados, C.; Montecillo, C.; Postgraduados, C.; Montecillo, C.; Texcoco, M.; Postgraduados, C.; Hidalgo, S.; Cotaxtla-inifap, C.E. Quality of Floral Stems of Lisianthus (Eustoma grandiflorum Raf.) Inoculated with Bacillus subtilis and Glomus intraradices. Ornam. Hortic. 2022, 28, 414–422. [Google Scholar]
- Sharma, S.; Compant, S.; Ballhausen, M.B.; Ruppel, S.; Franken, P. The Interaction between Rhizoglomus irregulare and Hyphae Attached Phosphate Solubilizing Bacteria Increases Plant Biomass of Solanum lycopersicum. Microbiol. Res. 2020, 240, 126556. [Google Scholar] [CrossRef]
- Berger, F.; Gutjahr, C. Factors Affecting Plant Responsiveness to Arbuscular Mycorrhiza. Curr. Opin. Plant Biol. 2021, 59, 101994. [Google Scholar] [CrossRef]
- Ikan, C.; Ben-Laouane, R.; Ouhaddou, R.; Anli, M.; Boutasknit, A.; Lahbouki, S.; Benchakour, A.; Jaouad, A.; Bouchdoug, M.; El Moatasime, A.; et al. Interactions between Arbuscular Mycorrhizal Fungus and Indigenous Compost Improve Salt Stress Tolerance in Wheat (Triticum durum). S. Afr. J. Bot. 2023, 158, 417–428. [Google Scholar] [CrossRef]
- Yazici, M.A.; Asif, M.; Tutus, Y.; Ortas, I.; Ozturk, L.; Lambers, H.; Cakmak, I. Reduced Root Mycorrhizal Colonization as Affected by Phosphorus Fertilization Is Responsible for High Cadmium Accumulation in Wheat. Plant Soil 2021, 468, 19–35. [Google Scholar] [CrossRef]
- Watts-Williams, S.J.; Cavagnaro, T.R.; Tyerman, S.D. Variable Effects of Arbuscular Mycorrhizal Fungal Inoculation on Physiological and Molecular Measures of Root and Stomatal Conductance of Diverse Medicago truncatula Accessions. Plant. Cell Environ. 2018, 42, 285–294. [Google Scholar] [CrossRef]
- Koch, A.M.; Antunes, P.M.; Maherali, H.; Hart, M.M.; Klironomos, J.N. Evolutionary Asymmetry in the Arbuscular Mycorrhizal Symbiosis: Conservatism in Fungal Morphology Does Not Predict Host Plant Growth. New Phytol. 2017, 214, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).