Deciphering Arabidopsis Aquaporin Networks: Comparative Analysis of the STRING and BioGRID Interactomes
Abstract
1. Introduction
2. Materials and Methods
2.1. A. thaliana L. Aquaporins Analysis
2.2. Interactome Analysis with STRING
2.3. Interactome Analysis with BioGRID
2.4. AlphaFold and ChimeraX for Structure Prediction and Protein Interaction
3. Results
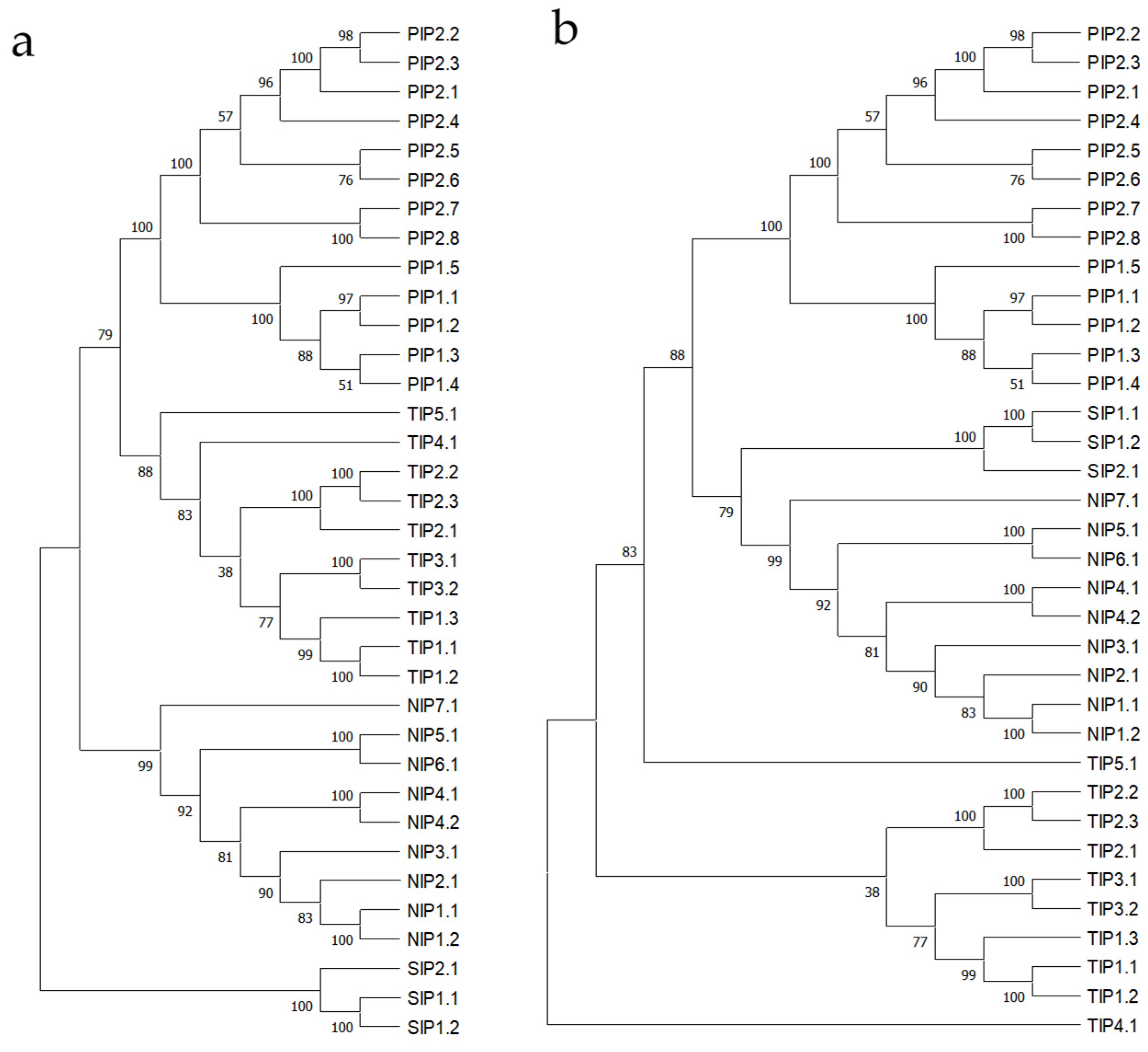
3.1. A. thaliana L. Aquaporins, Multiple Sequence Alignment, and Phylogenetic Tree
3.2. A. thaliana L. Aquaporins Interactome by STRING
3.3. Aquaporins Interactome by BioGRID
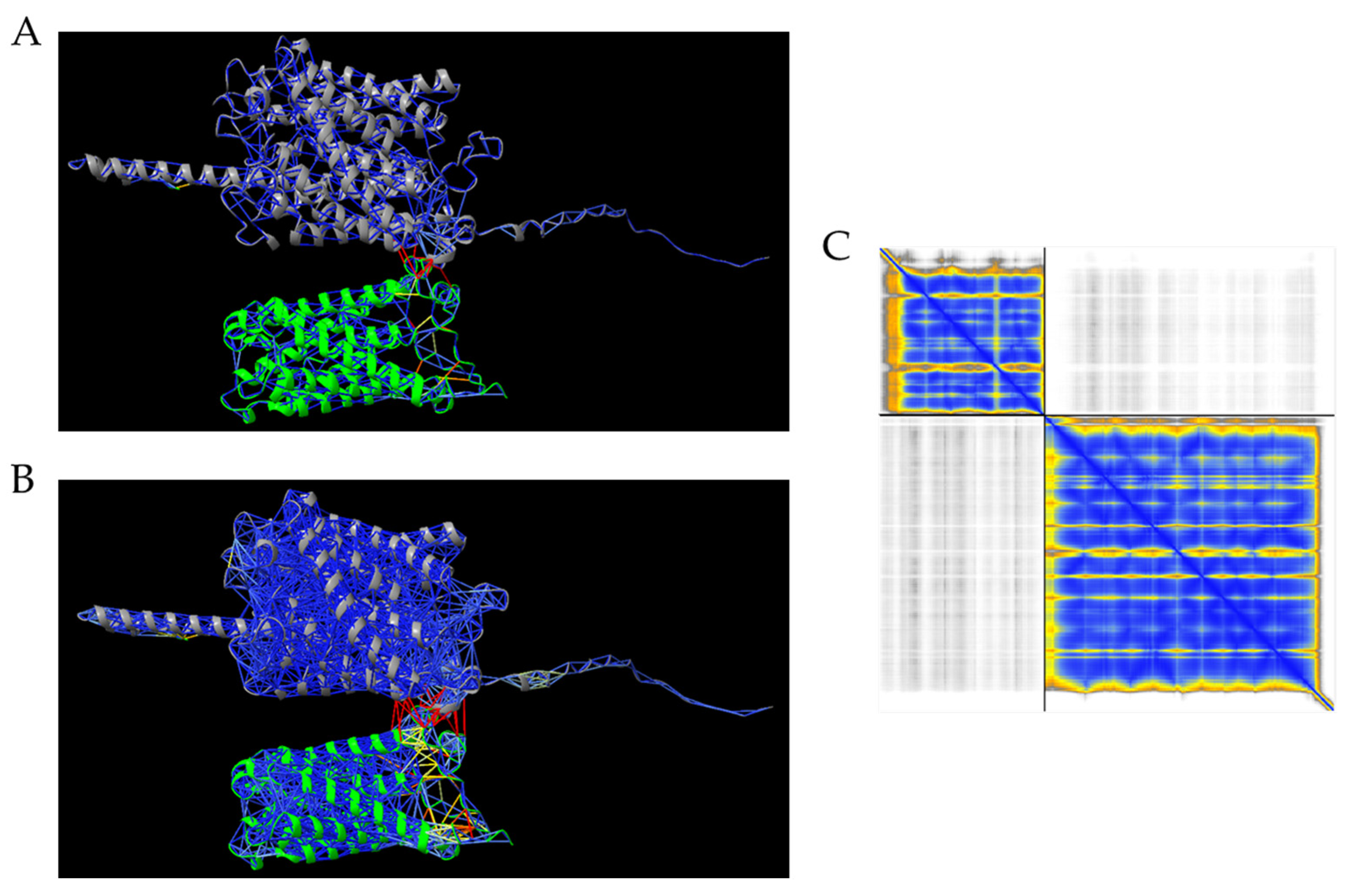
3.4. Aquaporin-Nutrient Transporter Interaction by AlphaFold
4. Discussion
4.1. Aquaporins Interact with Other Aquaporins
4.2. Aquaporins’ Redistribution and Activation in Response to Stress
4.3. Aquaporins Interact with Vesicle-Trafficking Proteins
4.4. Aquaporins Interact with Nutrients Transporters
4.5. Protein–Protein Interaction Between AtPIP1;1 and AtAMT1;3
4.6. Comparison Between STRING and BioGRID
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maurel, C.; Boursiac, Y.; Luu, D.T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in Plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.M.; Rivers, R.L.; Zeidel, M.L.; Roberts, D.M. Purification and Functional Reconstitution of Soybean Nodulin 26. An Aquaporin with Water and Glycerol Transport Properties†. Biochemistry 1998, 38, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Gerbeau, P.; Güçlü, J.; Ripoche, P.; Maurel, C. Aquaporin Nt-TIPa Can Account for the High Permeability of Tobacco Cell Vacuolar Membrane to Small Neutral Solutes. Plant J. 1999, 18, 577–587. [Google Scholar] [CrossRef]
- Bertl, A.; Kaldenhoff, R. Function of a Separate NH3-Pore in Aquaporin TIP2;2 from Wheat. FEBS Lett. 2007, 581, 5413–5417. [Google Scholar] [CrossRef]
- Kaldenhoff, R. Mechanisms Underlying CO2 Diffusion in Leaves. Curr. Opin. Plant Biol. 2012, 15, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Chaumont, F. Aquaporin-Facilitated Transmembrane Diffusion of Hydrogen Peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef]
- Takano, J.; Wada, M.; Ludewig, U.; Schaaf, G.; Von Wirén, N.; Fujiwara, T. The Arabidopsis Major Intrinsic Protein NIP5;1 Is Essential for Efficient Boron Uptake and Plant Development under Boron Limitation. Plant Cell 2006, 18, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Chiba, Y.; Mitani, N.; Yamaji, N.; Ma, J.F. HvLsi1 Is a Silicon Influx Transporter in Barley. Plant J. 2009, 57, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Q.; Mitani, N.; Yamaji, N.; Shen, R.F.; Ma, J.F. Involvement of Silicon Influx Transporter OsNIP2;1 in Selenite Uptake in Rice. Plant Physiol. 2010, 153, 1871–1877. [Google Scholar] [CrossRef]
- Bienert, G.P.; Jahn, T.P. Major Intrinsic Proteins and Arsenic Transport in Plants: New Players and Their Potential Role. Adv. Exp. Med. Biol. 2010, 679, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Thorsen, M.; Schüssler, M.D.; Nilsson, H.R.; Wagner, A.; Tamás, M.J.; Jahn, T.P. A Subgroup of Plant Aquaporins Facilitate the Bi-Directional Diffusion of As(OH)3 and Sb(OH)3 across Membranes. BMC Biol. 2008, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.T.H.; Imran, S.; Horie, T.; Qiu, J.; McGaughey, S.; Byrt, C.S.; Tyerman, S.D.; Katsuhara, M. A Survey of Barley PIP Aquaporin Ionic Conductance Reveals Ca2+-Sensitive HvPIP2;8 Na+ and K+ Conductance. Int. J. Mol. Sci. 2020, 21, 7135. [Google Scholar] [CrossRef] [PubMed]
- Tyerman, S.D.; McGaughey, S.A.; Qiu, J.; Yool, A.J.; Byrt, C.S. Adaptable and Multifunctional Ion-Conducting Aquaporins. Annu. Rev. Plant Biol. 2021, 72, 703–736. [Google Scholar] [CrossRef]
- Noronha, H.; Araújo, D.; Conde, C.; Martins, A.P.; Soveral, G.; Chaumont, F.; Delrot, S.; Gerós, H. The Grapevine Uncharacterized Intrinsic Protein 1 (VvXIP1) Is Regulated by Drought Stress and Transports Glycerol, Hydrogen Peroxide, Heavy Metals but Not Water. PLoS ONE 2016, 11, e0160976. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Mitsuoka, K.; Hiral, T.; Walz, T.; Agre, P.; Heymann, J.B.; Engel, A.; Fujiyoshi, Y. Structural Determinants of Water Permeation through Aquaporin-1. Nature 2000, 407, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Andoo, A.; Shimono, M.; Takamatsu, N.; Taki, A.; Muta, K.; Matsushita, W.; Uechi, T.; Matsuzaki, T.; Kenmochi, N.; et al. The NPC Motif of Aquaporin-11, Unlike the NPA Motif of Known Aquaporins, Is Essential for Full Expression of Molecular Function. J. Biol. Chem. 2011, 286, 3342–3350. [Google Scholar] [CrossRef] [PubMed]
- Ozu, M.; Galizia, L.; Acuña, C.; Amodeo, G. Aquaporins: More Than Functional Monomers in a Tetrameric Arrangement. Cells 2018, 7, 209. [Google Scholar] [CrossRef]
- Gonen, T.; Walz, T. The Structure of Aquaporins. Q. Rev. Biophys. 2006, 39, 361–396. [Google Scholar] [CrossRef] [PubMed]
- Besserer, A.; Burnotte, E.; Bienert, G.P.; Chevalier, A.S.; Errachid, A.; Grefen, C.; Blatt, M.R.; Chaumont, F. Selective Regulation of Maize Plasma Membrane Aquaporin Trafficking and Activity by the SNARE SYP121. Plant Cell 2012, 24, 3463–3481. [Google Scholar] [CrossRef] [PubMed]
- Hachez, C.; Laloux, T.; Reinhardt, H.; Cavez, D.; Degand, H.; Grefen, C.; De Rycke, R.; Inzé, D.; Blatt, M.R.; Russinova, E.; et al. Arabidopsis SNAREs SYP61 and SYP121 Coordinate the Trafficking of Plasma Membrane Aquaporin PIP2;7 to Modulate the Cell Membrane Water Permeability. Plant Cell 2014, 26, 3132–3147. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ding, L.; Gao, L.; Li, Y.; Shen, Q.; Guo, S. The Interactions of Aquaporins and Mineral Nutrients in Higher Plants. Int. J. Mol. Sci. 2016, 17, 1229. [Google Scholar] [CrossRef]
- Barzana, G.; Rios, J.J.; Lopez-Zaplana, A.; Nicolas-Espinosa, J.; Yepes-Molina, L.; Garcia-Ibañez, P.; Carvajal, M. Interrelations of Nutrient and Water Transporters in Plants under Abiotic Stress. Physiol. Plant. 2021, 171, 595–619. [Google Scholar] [CrossRef]
- Wang, F.; Miao, H.; Zhang, S.; Hu, X.; Chu, Y.; Yang, W.; Wang, H.; Wang, J.; Shan, S.; Chen, J. Weighted Gene Co-Expression Network Analysis Reveals Hub Genes Regulating Response to Salt Stress in Peanut. BMC Plant Biol. 2024, 24, 1–19. [Google Scholar] [CrossRef]
- Garcia-Gomez, P.; Olmos-Ruiz, R.; Nicolas-Espinosa, J.; Carvajal, M. Effects of Low Nitrogen Supply on Biochemical and Physiological Parameters Related to Nitrate and Water, Involving Nitrate Transporters and Aquaporins in Citrus Macrophylla. Plant Biol. 2023, 25, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Uehleln, N.; Lovisolo, C.; Siefritz, F.; Kaldenhoff, R. The Tobacco Aquaporin NtAQP1 Is a Membrane CO2 Pore with Physiological Functions. Nature 2003, 425, 734–737. [Google Scholar] [CrossRef]
- Bellati, J.; Champeyroux, C.; Hem, S.; Rofidal, V.; Krouk, G.; Maurel, C.; Santoni, V. Novel Aquaporin Regulatory Mechanisms Revealed by Interactomics. Mol. Cell. Proteomics 2016, 15, 3473–3487. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ballesta, M.d.C.; Carvajal, M. Mutual Interactions between Aquaporins and Membrane Components. Front. Plant Sci. 2016, 7, 206371. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, B.A.; Panchenko, A.R. Deciphering Protein–Protein Interactions. Part I. Experimental Techniques and Databases. PLoS Comput. Biol. 2007, 3, e42. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING Database in 2017: Quality-Controlled Protein-Protein Association Networks, Made Broadly Accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef] [PubMed]
- Oughtred, R.; Stark, C.; Breitkreutz, B.J.; Rust, J.; Boucher, L.; Chang, C.; Kolas, N.; O’Donnell, L.; Leung, G.; McAdam, R.; et al. The BioGRID Interaction Database: 2019 Update. Nucleic Acids Res. 2019, 47, D529–D541. [Google Scholar] [CrossRef]
- Stark, C.; Breitkreutz, B.J.; Reguly, T.; Boucher, L.; Breitkreutz, A.; Tyers, M. BioGRID: A General Repository for Interaction Datasets. Nucleic Acids Res. 2006, 34, D535–D539. [Google Scholar] [CrossRef]
- Johanson, U.; Karlsson, M.; Johansson, I.; Gustavsson, S.; Sjövall, S.; Fraysse, L.; Weig, A.R.; Kjellbom, P. The Complete Set of Genes Encoding Major Intrinsic Proteins in Arabidopsis Provides a Framework for a New Nomenclature for Major Intrinsic Proteins in Plants. Plant Physiol. 2001, 126, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Paulic, M.; Seidel, T. Interactome of Arabidopsis thaliana. Plants 2022, 11, 350. [Google Scholar] [CrossRef]
- Lopez-Zaplana, A.; Nicolas-Espinosa, J.; Carvajal, M.; Bárzana, G. Genome-Wide Analysis of the Aquaporin Genes in Melon (Cucumis Melo L.). Sci. Rep. 2020, 10, 22240. [Google Scholar] [CrossRef]
- Chou, K.C.; Shen, H. Bin Plant-MPLoc: A Top-Down Strategy to Augment the Power for Predicting Plant Protein Subcellular Localization. PLoS ONE 2010, 5, e11335. [Google Scholar] [CrossRef] [PubMed]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein Localization Predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- Franceschini, A.; Szklarczyk, D.; Frankild, S.; Kuhn, M.; Simonovic, M.; Roth, A.; Lin, J.; Minguez, P.; Bork, P.; Von Mering, C.; et al. STRING v9.1: Protein-Protein Interaction Networks, with Increased Coverage and Integration. Nucleic Acids Res. 2013, 41, D808–D815. [Google Scholar] [CrossRef] [PubMed]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making Protein Folding Accessible to All. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for Structure Building and Analysis. Protein Sci. 2023, 32, e4792. [Google Scholar] [CrossRef] [PubMed]
- Preston, G.M.; Agre, P. Isolation of the CDNA for Erythrocyte Integral Membrane Protein of 28 Kilodaltons: Member of an Ancient Channel Family. Proc. Natl. Acad. Sci. USA 1991, 88, 11110–11114. [Google Scholar] [CrossRef]
- Agre, P.; Saboori, A.M.; Asimos, A.; Smith, B.L. Purification and Partial Characterization of the Mr 30,000 Integral Membrane Protein Associated with the Erythrocyte Rh(D) Antigen. J. Biol. Chem. 1987, 262, 17497–17503. [Google Scholar] [CrossRef]
- Agre, P.; Sasaki, S.; Chrispeels, M.J. Aquaporins: A Family of Water Channel Proteins. Am. J. Physiol. 1993, 265, F461. [Google Scholar] [CrossRef] [PubMed]
- Yepes-Molina, L.; Bárzana, G.; Carvajal, M. Controversial Regulation of Gene Expression and Protein Transduction of Aquaporins under Drought and Salinity Stress. Plants 2020, 9, 1662. [Google Scholar] [CrossRef] [PubMed]
- Ariani, A.; Barozzi, F.; Sebastiani, L.; di Toppi, L.S.; di Sansebastiano, G.P.; Andreucci, A. AQUA1 Is a Mercury Sensitive Poplar Aquaporin Regulated at Transcriptional and Post-Translational Levels by Zn Stress. Plant Physiol. Biochem. 2019, 135, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Filippou, P.; Manganaris, G.A.; Fotopoulos, V. Sodium Hydrosulfide Induces Systemic Thermotolerance to Strawberry Plants through Transcriptional Regulation of Heat Shock Proteins and Aquaporin. BMC Plant Biol. 2014, 14, 42. [Google Scholar] [CrossRef]
- Lopez-Zaplana, A.; Martinez-Garcia, N.; Carvajal, M.; Bárzana, G. Relationships between Aquaporins Gene Expression and Nutrient Concentrations in Melon Plants (Cucumis melo L.) during Typical Abiotic Stresses. Environ. Exp. Bot. 2022, 195, 104759. [Google Scholar] [CrossRef]
- Jozefkowicz, C.; Berny, M.C.; Chaumont, F.; Alleva, K. Heteromerization of Plant Aquaporins. In Plant Aquaporins. Signaling and Communication in Plants; Chaumont, F., Tyerman, S., Eds.; Springer: Cham, Switzerland, 2017; Volume 29, pp. 29–46. [Google Scholar] [CrossRef]
- Berny, M.C.; Gilis, D.; Rooman, M.; Chaumont, F. Single Mutations in the Transmembrane Domains of Maize Plasma Membrane Aquaporins Affect the Activity of Monomers within a Heterotetramer. Mol. Plant 2016, 9, 986–1003. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-H.; Kim, H.-S.; Kim, S.; Ahn, S.-J. Osmotic Stress-Induced CsRCI2E Endosomal Trafficking Promotes the Redistribution of Aquaporin CsPIP2 at the Plasma Membrane of Camelina sativa L. 2023; preprint. [Google Scholar] [CrossRef]
- Hachez, C.; Veljanovski, V.; Reinhardt, H.; Guillaumot, D.; Vanhee, C.; Chaumont, F.; Batoko, H. The Arabidopsis Abiotic Stress-Induced TSPO-Related Protein Reduces Cell-Surface Expression of the Aquaporin PIP2;7 through Protein-Protein Interactions and Autophagic Degradation. Plant Cell 2015, 26, 4974–4990. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Tian, N.; She, F.; Cao, A.; Wu, W.; Zheng, S.; Yang, N. Characteristics Analysis of Early Responsive to Dehydration Genes in Arabidopsis thaliana (AtERD). Plant Signal Behav. 2023, 18, 2105021. [Google Scholar] [CrossRef] [PubMed]
- Van Wilder, V.; Miecielica, U.; Degand, H.; Derua, R.; Waelkens, E.; Chaumont, F. Maize Plasma Membrane Aquaporins Belonging to the PIP1 and PIP2 Subgroups Are in Vivo Phosphorylated. Plant Cell Physiol. 2008, 49, 1364–1377. [Google Scholar] [CrossRef] [PubMed]
- Tarutani, Y.; Sasaki, A.; Yasuda, M.; Nakashita, H.; Yoshida, S.; Yamaguchi, I.; Suzuki, Y. Identification of Three Clones Which Commonly Interact with the Kinase Domains of Highly Homologous Two Receptor-like Kinases, RLK902 and RKL1. Biosci. Biotechnol. Biochem. 2004, 68, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.; Aalen, R.B.; Audenaert, D.; Beeckman, T.; Broadley, M.R.; Butenko, M.A.; Caño-Delgado, A.I.; de Vries, S.; Dresselhaus, T.; Felix, G.; et al. Tackling Drought SECEPTOR-LIKE KINASES Present New Approaches. Plant Cell 2012, 24, 2262. [Google Scholar] [CrossRef]
- Haruta, M.; Sabat, G.; Stecker, K.; Minkoff, B.B.; Sussman, M.R. A Peptide Hormone and Its Receptor Protein Kinase Regulate Plant Cell Expansion. Science 2014, 343, 408–411. [Google Scholar] [CrossRef]
- Israel, D.; Lee, S.H.; Robson, T.M.; Zwiazek, J.J. Plasma Membrane Aquaporins of the PIP1 and PIP2 Subfamilies Facilitate Hydrogen Peroxide Diffusion into Plant Roots. BMC Plant Biol. 2022, 22, 566. [Google Scholar] [CrossRef] [PubMed]
- Dynowski, M.; Schaaf, G.; Loque, D.; Moran, O.; Ludewig, U. Plant Plasma Membrane Water Channels Conduct the Signalling Molecule H2O2. Biochem. J. 2008, 414, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, M.; Shi, D.; Zhou, G.; Niu, T.; Hahn, M.G.; O’Neill, M.A.; Kong, Y. DGE-Seq Analysis of MUR3-Related Arabidopsis Mutants Provides Insight into How Dysfunctional Xyloglucan Affects Cell Elongation. Plant Sci. 2017, 258, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ganguly, A.; Baik, S.; Cho, H.T. Calcium-Dependent Protein Kinase 29 Modulates PIN-FORMED Polarity and Arabidopsis Development via Its Own Phosphorylation Code. Plant Cell 2021, 33, 3513–3531. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.; Liu, F.; Sun, L.; Hao, F. Versatile Roles of Aquaporins in Plant Growth and Development. Int. J. Mol. Sci. 2020, 21, 9485. [Google Scholar] [CrossRef]
- He, W.D.; Gao, J.; Dou, T.X.; Shao, X.H.; Bi, F.C.; Sheng, O.; Deng, G.M.; Li, C.Y.; Hu, C.H.; Liu, J.H.; et al. Early Cold-Induced Peroxidases and Aquaporins Are Associated with High Cold Tolerance in Dajiao (Musa Spp. ‘Dajiao’). Front. Plant Sci. 2018, 9, 323137. [Google Scholar] [CrossRef]
- Hsu, J.L.; Wang, L.Y.; Wang, S.Y.; Lin, C.H.; Ho, K.C.; Shi, F.K.; Chang, I.F. Functional Phosphoproteomic Profiling of Phosphorylation Sites in Membrane Fractions of Salt-Stressed Arabidopsis thaliana. Proteome Sci. 2009, 7, 42. [Google Scholar] [CrossRef]
- Laloux, T.; Matyjaszczyk, I.; Beaudelot, S.; Hachez, C.; Chaumont, F. Interaction Between the SNARE SYP121 and the Plasma Membrane Aquaporin PIP2;7 Involves Different Protein Domains. Front. Plant Sci. 2021, 11, 631643. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Xuan, Y.; Xu, M.; Wang, R.S.; Ho, C.H.; Lalonde, S.; You, C.H.; Sardi, M.I.; Parsa, S.A.; Smith-Valle, E.; et al. Border Control—A Membrane-Linked Interactome of Arabidopsis. Science 2014, 344, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Dreze, M.; Carvunis, A.-R.; Charloteaux, B.; Galli, M.; Pevzner, S.J.; Tasan, M.; Ahn, Y.-Y.; Balumuri, P.; Barabási, A.-L.; Bautista, V.; et al. Evidence for Network Evolution in an Arabidopsis Interactome Map. Science 2011, 333, 601–607. [Google Scholar] [CrossRef]
- Roche, J.V.; Törnroth-Horsefield, S. Aquaporin Protein-Protein Interactions. Int. J. Mol. Sci. 2017, 18, 2255. [Google Scholar] [CrossRef]
- Pommerrenig, B.; Diehn, T.A.; Bienert, G.P. Metalloido-Porins: Essentiality of Nodulin 26-Like Intrinsic Proteins in Metalloid Transport. Plant Sci. 2015, 238, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Li, Y.; Gao, L.; Lu, Z.; Wang, M.; Ling, N.; Shen, Q.; Guo, S. Aquaporin Expression and Water Transport Pathways inside Leaves Are Affected by Nitrogen Supply through Transpiration in Rice Plants. Int. J. Mol. Sci. 2018, 19, 256. [Google Scholar] [CrossRef]
- Di Pietro, M.; Vialaret, J.; Hem, S.; Prado, K.; Rossignol, M.; Maurel, C.; Santoni, V. Coordinated Post-Translational Responses of Aquaporins to Abiotic and Nutritional Stimuli in Arabidopsis Roots. Mol. Cell. Proteom. 2013, 12, 3886–3897. [Google Scholar] [CrossRef] [PubMed]
- Bohner, A.; Kojima, S.; Hajirezaei, M.; Melzer, M.; Von Wirén, N. Urea Retranslocation from Senescing Arabidopsis Leaves Is Promoted by DUR3-Mediated Urea Retrieval from Leaf Apoplast. Plant J. 2015, 81, 377–387. [Google Scholar] [CrossRef]
- Wakuta, S.; Fujikawa, T.; Naito, S.; Takano, J. Tolerance to Excess-Boron Conditions Acquired by Stabilization of a BOR1 Variant with Weak Polarity in Arabidopsis. Front. Cell Dev. Biol. 2016, 4, 176044. [Google Scholar] [CrossRef]
- Huai, Z.; Peng, L.; Wang, S.; Zhao, H.; Shi, L.; Xu, F. Identification and Characterization of an Arabidopsis thaliana Mutant Lbt with High Tolerance to Boron Deficiency. Front. Plant Sci. 2018, 9, 362361. [Google Scholar] [CrossRef]
- Jothi, M.; Takano, J. Understanding the Regulatory Mechanisms of B Transport to Develop Crop Plants with B Efficiency and Excess B Tolerance. Plant Soil 2023, 487, 1–20. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, W.; Cao, Y.; Cai, Y.; Lyi, S.M.; Wu, W.; Kang, Y.; Liang, C.; Liu, J. An Exclusion Mechanism Is Epistatic to an Internal Detoxification Mechanism in Aluminum Resistance in Arabidopsis. BMC Plant Biol. 2020, 20, 122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, R.; Li, D.; Jia, X.; Zhou, D.; Li, J.; Lyi, S.M.; Hou, S.; Huang, Y.; Kochian, L.V.; et al. NIP1;2 Is a Plasma Membrane-Localized Transporter Mediating Aluminum Uptake, Translocation, and Tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, 5047–5052. [Google Scholar] [CrossRef] [PubMed]
- Johanson, U.; Gustavsson, S. A New Subfamily of Major Intrinsic Proteins in Plants. Mol. Biol. Evol. 2002, 19, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.P.; Jiang, T.; Sun, C.; Lihan, M.; Pant, S.; Mahinthichaichan, P.; Trifan, A.; Tajkhorshid, E. Characterization of Lipid-Protein Interactions and Lipid-Mediated Modulation of Membrane Protein Function through Molecular Simulation. Chem. Rev. 2019, 119, 6086–6161. [Google Scholar] [CrossRef]
- Duart, G.; Grau, B.; Mingarro, I.; Martinez-Gil, L. Methodological Approaches for the Analysis of Transmembrane Domain Interactions: A Systematic Review. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183712. [Google Scholar] [CrossRef] [PubMed]
- Teese, M.G.; Langosch, D. Role of GxxxG Motifs in Transmembrane Domain Interactions. Biochemistry 2015, 54, 5125–5135. [Google Scholar] [CrossRef]
- Brown, D.; Katsura, T.; Kawashima, M.; Verkman, A.S.; Sabolic, I. Cellular Distribution of the Aquaporins: A Family of Water Channel Proteins. Histochem. Cell Biol. 1995, 104, 1–9. [Google Scholar] [CrossRef]

| Name a | TAIR Locus b | Accession No c | NPA Motifs d | Location e (mPlot/WOLF) |
|---|---|---|---|---|
| PIP1;1 | AT3G61430 | CAB71073 | NPA/NPA | PM/PM |
| PIP1;2 | AT2G45960 | AAC28529 | NPA/NPA | PM/PM |
| PIP1;3 | AT1G01620 | AAF81320 | NPA/NPA | PM/PM |
| PIP1;4 | AT4G00430 | AAF02782 | NPA/NPA | PM/PM |
| PIP1;5 | AT4G23400 | CAA20461 | NPA/NPA | PM/PM |
| PIP2;1 | AT3G53420 | CAB67649 | NPA/NPA | PM/PM |
| PIP2;2 | AT2G37170 | AAD18142 | NPA/NPA | PM/C |
| PIP2;3 | AT2G37180 | AAD18141 | NPA/NPA | PM/PM |
| PIP2;4 | AT5G60660 | BAB09839 | NPA/NPA | PM/PM |
| PIP2;5 | AT3G54820 | CAB41102 | NPA/NPA | PM/PM |
| PIP2;6 | AT2G39010 | AAC79629 | NPA/NPA | PM/PM |
| PIP2;7 | AT4G35100 | CAA17774 | NPA/NPA | PM/PM |
| PIP2;8 | AT2G16850 | AAC64216 | NPA/NPA | PM/PM |
| TIP1;1 | AT2G36830 | AAD31569 | NPA/NPA | V/V |
| TIP1;2 | AT3G26520 | BAB01832 | NPA/NPA | V/V |
| TIP1;3 | AT4G01470 | AAC62778 | NPA/NPA | V/PM |
| TIP2;1 | AT3G16240 | BAB01264 | NPA/NPA | V/V |
| TIP2;2 | AT4G17340 | CAB10515 | NPA/NPA | V/V |
| TIP2;3 | AT5G47450 | BAB09071 | NPA/NPA | V/V |
| TIP3;1 | AT1G73190 | AAG52132 | NPA/NPA | V/V |
| TIP3;2 | AT1G17810 | AAF97261 | NPA/NPA | V/V |
| TIP4;1 | AT2G25810 | AAC42249 | NPA/NPA | V/V |
| TIP5;1 | AT3G47440 | CAB51216 | NPA/NPA | PM/C |
| NIP1;1 | AT4G19030 | CAA16760 | NPA/NPG | PM/PM |
| NIP1;2 | AT4G18910 | CAA16748 | NPA/NPG | PM/PM |
| NIP2;1 | AT2G34390 | AAC26712 | NPA/NPA | PM/PM |
| NIP3;1 | AT1G31885 | AAG50717 | NPA/NPA | PM/V |
| NIP4;1 | AT5G37810 | BAB10360 | NPA/NPA | PM/PM |
| NIP4;2 | AT5G37820 | BAB10361 | NPA/NPA | PM/PM |
| NIP5;1 | AT4G10380 | CAB39791 | NPS/NPV | PM/PM |
| NIP6;1 | AT1G80760 | AAF14664 | NPA/NPV | PM/PM |
| NIP7;1 | AT3G06100 | AAF30303 | NPS/NPA | PM/PM |
| SIP1;1 | AT3G04090 | AAF26804 | NPT/NPA | PM/V |
| SIP1;2 | AT5G18290 | BAB09487 | NPC/NPA | PM and V/V |
| SIP2;1 | AT3G56950 | CAB72165 | NPL/NPA | PM/ER |
| Name | STRING Interactors |
|---|---|
| PIP1;1 | PIP2;3, PIP1 * |
| PIP1;2 | PIP2;1, SIP2;1, PIP1 * |
| PIP1;3 | SIP2;1, PIP1 * |
| PIP1;4 | SIP2;1, PIP1 *, NPSN12 |
| PIP1;5 | PIP1 * |
| PIP2;1 | PIP1;2, PIP2;2, SIP1;1, SIP2;1, SIP2;2, PIP1 *, RCI2A, RCI2B |
| PIP2;2 | PIP2;1, SIP1;1, SIP1;2, SIP2;1, PIP1 *, T20K18.110 *, F11A12.3 *, dl3910c *, MTG13.3 * |
| PIP2;3 | PIP1;1, PIP2;8; SIP1;1 |
| PIP2;4 | NIP2, SIP1;1, SIP1;2, SIP2;1, PIP1 * |
| PIP2;5 | PIP2;7, PIP2;8, SIP1;1, SIP1;2, SIP2;1, SYP121, PIP1 * |
| PIP2;6 | SIP2;1, TEL1 *, F18A8.10 * |
| PIP2;7 | PIP2;5, PIP2;8, SIP1;2, SIP2;1, SYP121, TSPO, MTG13.3, SYP61, PIP1 * |
| PIP2;8 | PIP2;3, PIP2;5, PIP2;7, SIP1;2, SIP2;1, PIP1 * |
| TIP1;1 | SIP1;1, SIP1;2, SIP2;1, PAT24, NAC091, PIP1 * |
| TIP1;2 | SIP1;1, PAT24, NAC091, PIP1 *, |
| TIP1;3 | SIP1;1, SIP1;2, SIP2;1, PAT24, T21P5.16, PIP1 * |
| TIP2;1 | SIP2;1, PAT24, NAC091, F4J3I0_ARATH |
| TIP2;2 | SIP1;1, SIP1;2, SIP2;1, PAT24, NAC091 |
| TIP2;3 | SIP1;1, SIP1;2, SIP2;1, PAT24, NAC091, K22F20.5 |
| TIP3;1 | SIP1;1, SIP2;1, PAT24, NAC091, VHA-a3, T13K14.180, CBL6 |
| TIP3;2 | PAT24, NAC091 |
| TIP4;1 | ACT2, GAPC1, GAPC2, MON1, SKIP16, YLS8, AP2M, F17M5.140, PP2AA3, TIP41L |
| TIP5;1 | SIP1;1, SIP1;2, SIP2;1, NIP2, BOR4, DUR3, AA1, AGD13, PAT24, NAC091 |
| NIP1;1 | SIP1;1, SIP1;2, SIP2;1, F7K2.10, CPK31, MLP36, F7F1.4 |
| NIP1;2 | SIP1;1, SIP1;2, SIP2;1, ACR3, SDP6, ALMT1, T9J23.9, T7I23.21 |
| NIP2;1 | NIP2, SIP1;1, SIP1;2, SIP2;1 |
| NIP3;1 | SIP1;1, SIP1;2, SIP2;1, ACR3 |
| NIP4;1 | NIP2, SIP1;1, SIP1;2, SIP2;1, CPK34, NAC091, PPAN, F26K9_220, Q6NLB7_ARATH * |
| NIP4;2 | SIP1;1, SIP1;2, SIP2;1, CPK34, NAC091 |
| NIP5;1 | SIP1;1, SIP1;2, SIP2;1, NIP2, BOR1, BOR2, BOR3, BOR4, BOR7, AAA1 * |
| NIP6;1 | SIP1;1, SIP1;2, SIP2;1, NIP2, BOR1, BOR3, BOR4, BOR7, DUR7, ACR3 |
| NIP7;1 | SIP1;1, SIP1;2, SIP2;1, NIP2, ACR3, SDP6, BOR4, F21O3.16 * |
| SIP1;1 | PIP2;2, PIP2;5, TIP1;1, TIP2;2, TIP3;1, NIP1;2, NIP2;1, NIP4;1, NIP4;2, NIP5;1 |
| SIP1;2 | PIP2;4, PIP2;8, TIP2;2, TIP5;1, NIP1;2, NIP4;1, NIP4;2, NIP5;1, NIP6;1. NIP7;1 |
| SIP2;1 | PIP2;4, PIP2;8, TIP3;1, TIP5;1, NIP1;2, NIP4;1, NIP4;2, NIP5;1, NIP6;1. NIP7;1 |
| Name | BioGRID Interactors |
|---|---|
| PIP1;1 | PIP1;2, PIP2;1, PIP2;3, PIP2;5, PIP2;7, SYP132, UBQ3, RLK7 *, ABCB4, ABCB19, AMT1;3 (12) |
| PIP1;2 | PIP1;1, PIP1;2, PIP1;3, PIP1;5, PIP2s, ABCB4, ABCB19, PEN3, PDR6, PDR7, PIN5, AMT1;1/1;2/1;3, COPT2, AKT1, SULTR1;2, PHT1;1, ERD4, STP1/4/7, RKL1 (376) |
| PIP1;3 | PIP1;2, PIP2;5, PIP1;4, PIP1;5, PIP2;1, PIP2;3, PIP2;7, PIP2;8, ABC4, ABC19, AMT1;3, ATBET12, PIN5, UTR2, VP2 (66) |
| PIP1;4 | PIP1;2, PIP1;3, PIP2;1, PIP2;2, PIP2;3, PIP2;5, PIP2;6, PIP2;7, ABC4, ABC19, AMT1;3, T1F15.6, FACE2, FMA, UBQ3 (17) |
| PIP1;5 | PIP1;2, PIP1;3, PIP1;5, PIP2;1, PIP2;2, PIP2;3, PIP2;5, PIP2;6, PIP2;7, PIP2;8, NIP1;1, NIP7;1, ABC4/19, AMT1;3, ATVEX1, UTR2/3, UBC34, SEC61β, HHP4, CEV1, BCB (99) |
| PIP2;1 | PIP1s, PIP2s except PIP2;8, FER, NHL3, PLDδ, PLDγ, RKL1, RKL902, ABC4/19, ADK1, ACA8, ACA10, PDR6/7/9, ALA1, ACA8, AMT1;1/1;2/1;3, SULTR1;2 (306) |
| PIP2;2 | PIP1;2, PIP1;3PIP1;4, PIP1;5, PIP2;1, PIP2;7, ABC4, ABC19, AMT1;3, CHAL, CHX9SYP132 (14) |
| PIP2;3 | PIP1s, PIP2;5, PIP2;1, PIP2;5,PIP2;8, NIP1;1, VAP, NTL9, LPAT4, ABCB4, AMT1;3 (17) |
| PIP2;4 | PIP1;2, PIP2;1, ABCB4, ABCB19, AMT1;3, SYP132, UBQ3 (7) |
| PIP2;5 | PIPs1, PIP2;3, PIP2;7, PIP2;8, CHX9, NTL9 (13) |
| PIP2;6 | PIP1;2, PIP1;3, PIP1;5, PIP2;1, PIP2;7, AMT1;3, TSPO (7) |
| PIP2;7 | PIP1s, PIP2s except PIP2;4, NIP1;1, NIP2;1, SYP61, SYP121, TSPO, ABCB19, ABCB4, AMT1;3, ACBP6, AT2G28315, CERK1, ELIP2, RD28, SYP132, UTR2, VAP (49) |
| PIP2;8 | PIP1;2, PIP1;3, PIP1;4, PIP1;5, PIP2;3, PIP2;5, PIP2;7, NIP1;1, KUP7, UBQ3, VAP (18) |
| TIP1;1 | TIP3;1, HHP2, IQD6, NHL3, PAP3, TSPO, AT1G22570, AT1G34640, AT5G49540 (12) |
| TIP1;2 | UBQ3, TSPO (2) |
| TIP1;3 | TIP2;2 (1) |
| TIP2;1 | NIP1;1, NAC089, NTL9, TSPO, UBQ3 (6) |
| TIP2;2 | TIP1;3, NIP1;1, NAC089, TSPO, UPS1, VAP, AT1G34640, AT3G16310 (12) |
| TIP2;3 | AT1G63110, TSPO (2) |
| TIP3;1 | TIP1;1, NIP1;1, NAC089, AT3G16310 (5) |
| TIP3;2 | CAX7, CNGC18, GCL1, NHX8 (4) |
| TIP4;1 | AGP27, AT1G29060, HHP2, NAC089, NHL3, NTL9, UBC34, VPS60.1, WAK3 (16) |
| TIP5;1 | AT3G49190 (O-acyltransferase family protein) (1) |
| NIP1;1 | PIP1;5, PIP2;3, PIP2;7, PIP2;8, TIP2;2, TIP3;1, SIP1;2, ANTR2, VIT1,UTR1, UTR3, NPSN12, NPSN13, ATBET12, PYR6, SAR1, SEP1, SYP31, SY51, SYP121, SYP132, (184) |
| NIP1;2 | GRF3 (1) |
| NIP2;1 | PIP2;7, AT1G29060, FACE2, HHP2, HHP4, IQD6, PAP3, SK42, TBL18, UBC34 (24) |
| NIP3;1 | No curated interaction data for this protein (0) |
| NIP4;1 | ATBET12, HHP2, HHP4, IQD6, MAPR3, NHL3, TBL18, UBC32, UBC34 (17) |
| NIP4;2 | No curated interaction data for this protein (0) |
| NIP5;1 | No curated interaction data for this protein (0) |
| NIP6;1 | AT3G17620 (putative F-box protein) (1) |
| NIP7;1 | PIP1;5, CNGC10, CNGC18, GDU4, GONST1, MSBP1, PIN4, SUT2 (12) |
| SIP1;1 | No curated interaction data for this protein (0) |
| SIP1;2 | NIP1;1, AT3G26110 (anther-specific agp1-like protein) (2) |
| SIP2;1 | AT4G29450, CB5-D, CB5-E, CYP81D8, HHP2, HHP4, NAC089, NHL3, VPS60.1 (11) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Zaplana, A. Deciphering Arabidopsis Aquaporin Networks: Comparative Analysis of the STRING and BioGRID Interactomes. Int. J. Plant Biol. 2025, 16, 28. https://doi.org/10.3390/ijpb16010028
Lopez-Zaplana A. Deciphering Arabidopsis Aquaporin Networks: Comparative Analysis of the STRING and BioGRID Interactomes. International Journal of Plant Biology. 2025; 16(1):28. https://doi.org/10.3390/ijpb16010028
Chicago/Turabian StyleLopez-Zaplana, Alvaro. 2025. "Deciphering Arabidopsis Aquaporin Networks: Comparative Analysis of the STRING and BioGRID Interactomes" International Journal of Plant Biology 16, no. 1: 28. https://doi.org/10.3390/ijpb16010028
APA StyleLopez-Zaplana, A. (2025). Deciphering Arabidopsis Aquaporin Networks: Comparative Analysis of the STRING and BioGRID Interactomes. International Journal of Plant Biology, 16(1), 28. https://doi.org/10.3390/ijpb16010028






