The Role of Different Rhizobacteria in Mitigating Aluminum Stress in Rice (Oriza sativa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Genetic Material
2.2. Germination Trial for Sensitivity of Oryza sativa L. to Aluminum
Variables to Evaluate in Germination
2.3. Degree of Protection of Rhizobacteria in the Presence of Aluminum
Variables Evaluated
2.4. Effect of Application of Aluminum-Tolerant Rhizobacteria in Acidic Soils with the Presence of Aluminum
Variables Evaluated from the Third Experiment
2.5. Design and Statistical Analysis
3. Results
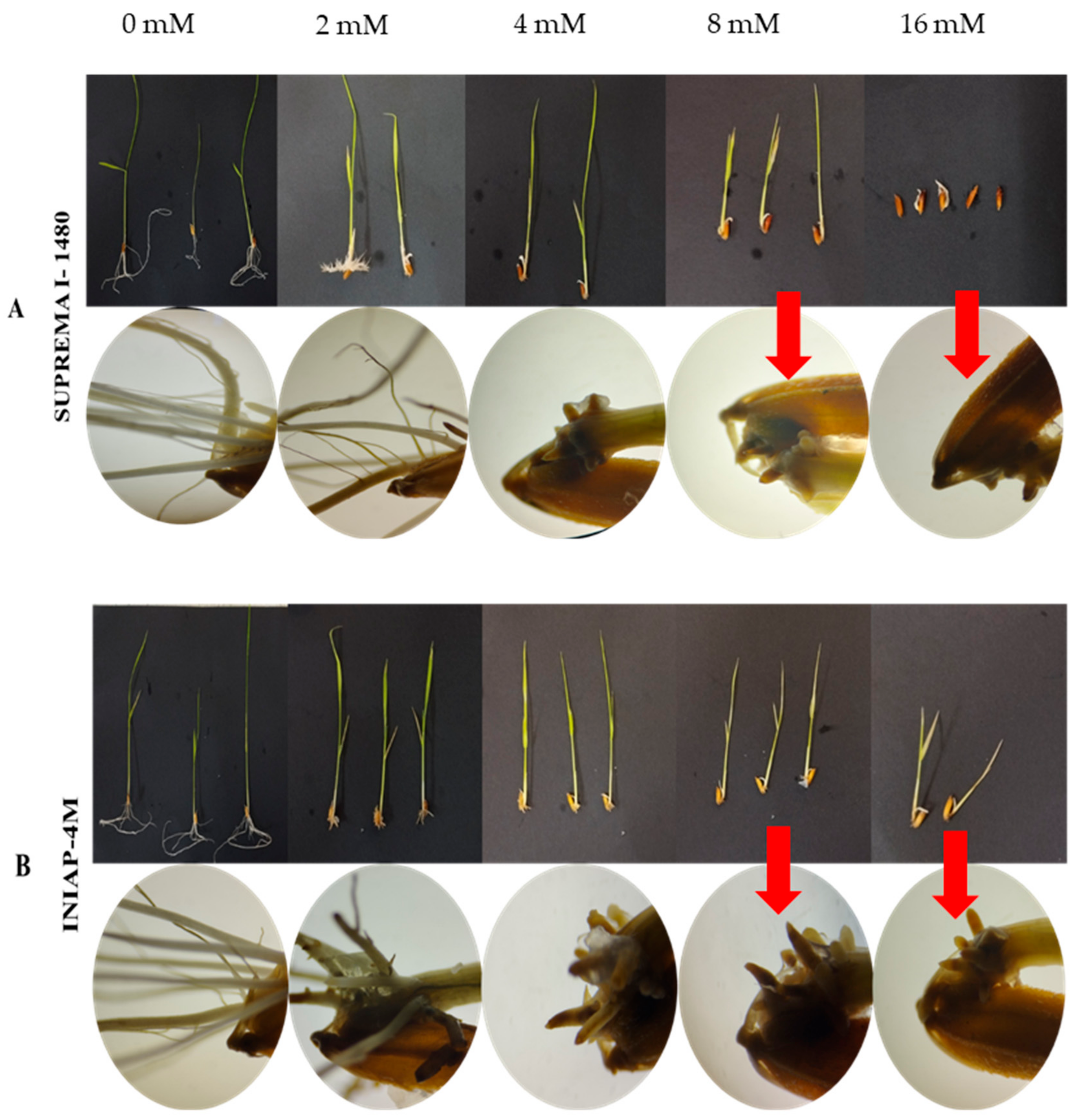
3.1. Degree of Aluminum Tolerance in the Germination Process of Two Rice Varieties
3.2. Degree of Protection of Rhizobacteria to Aluminum
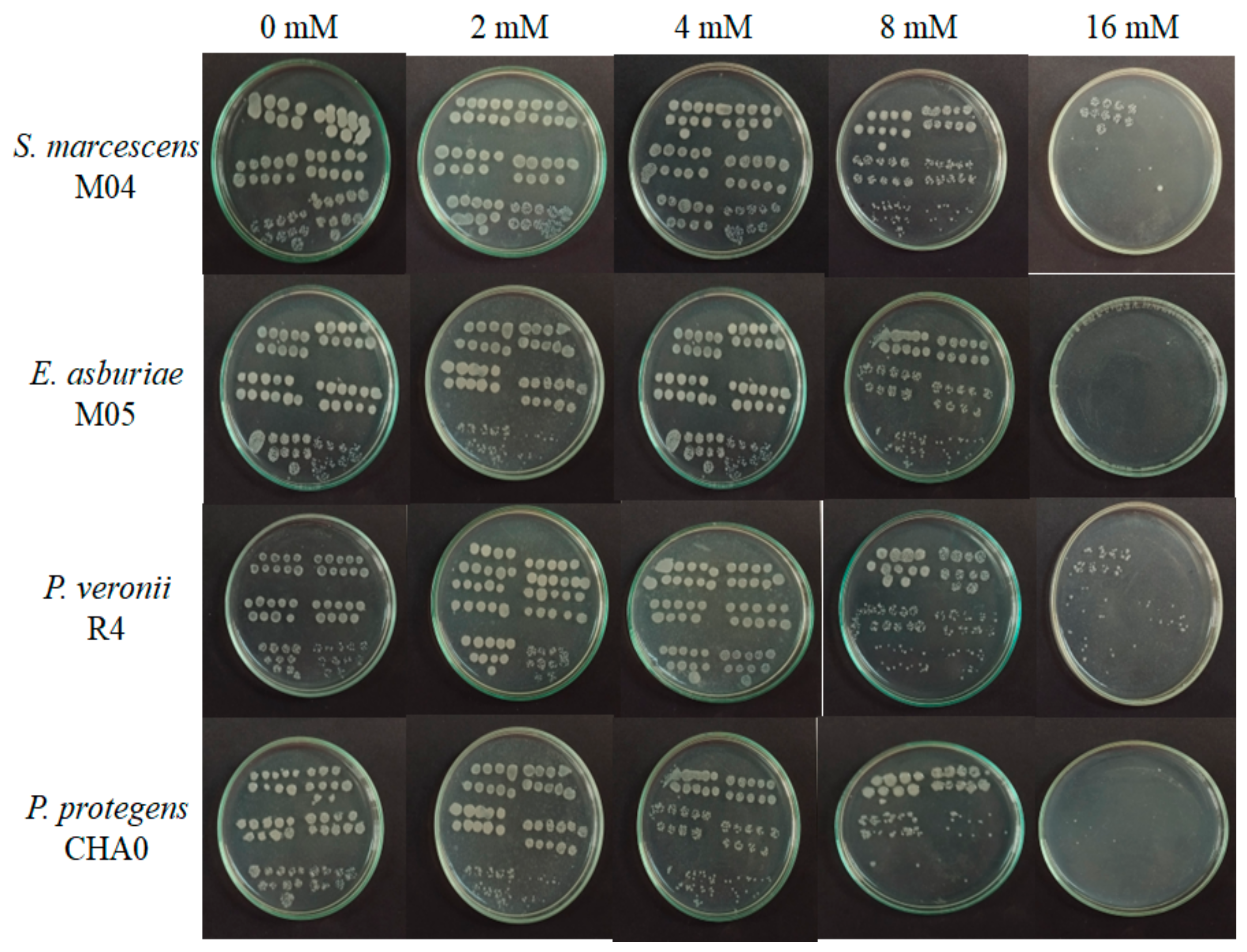
3.2.1. Viability of Rhizobacteria to Aluminum
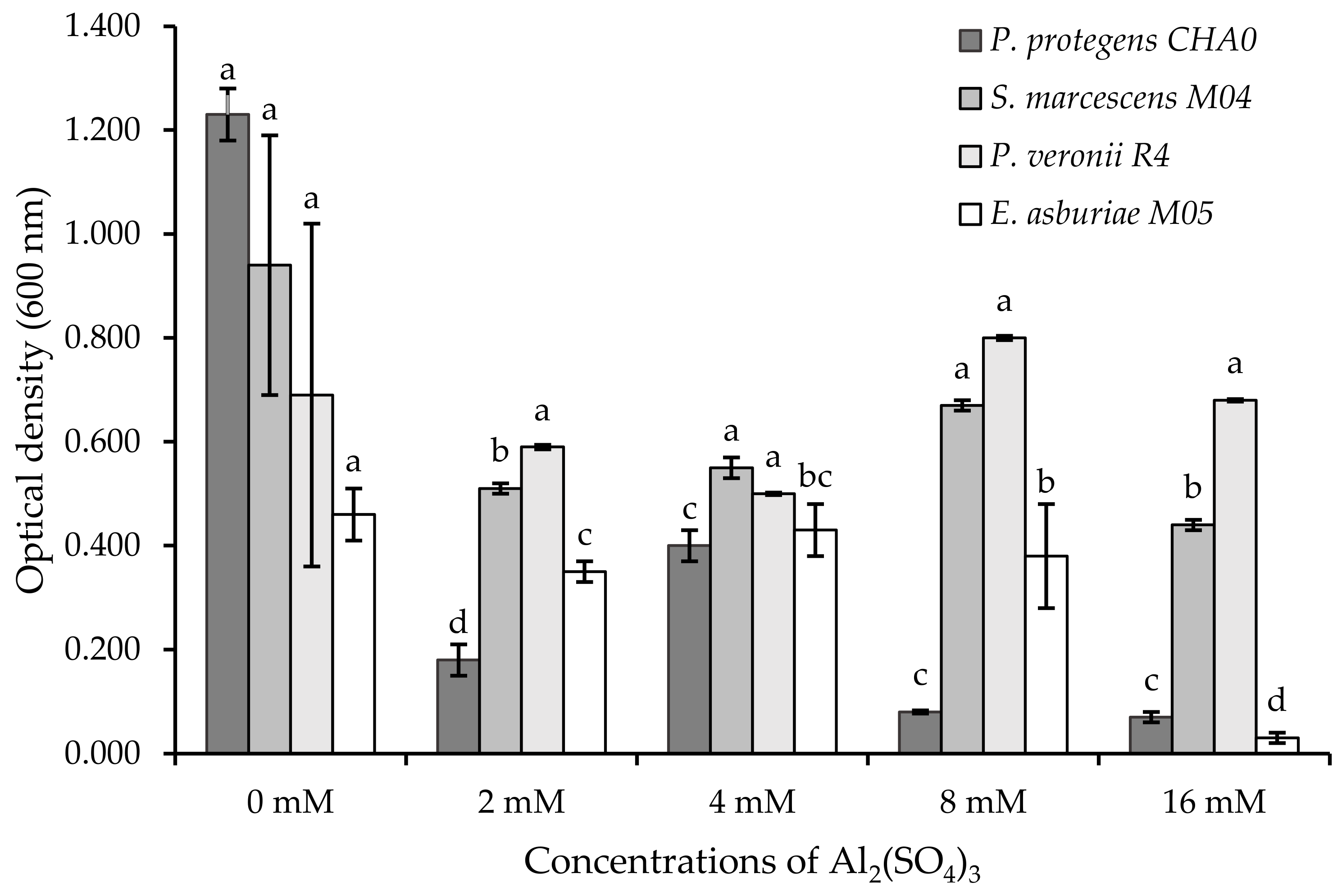
3.2.2. Optical Density Analysis of Tolerant Rhizobacteria
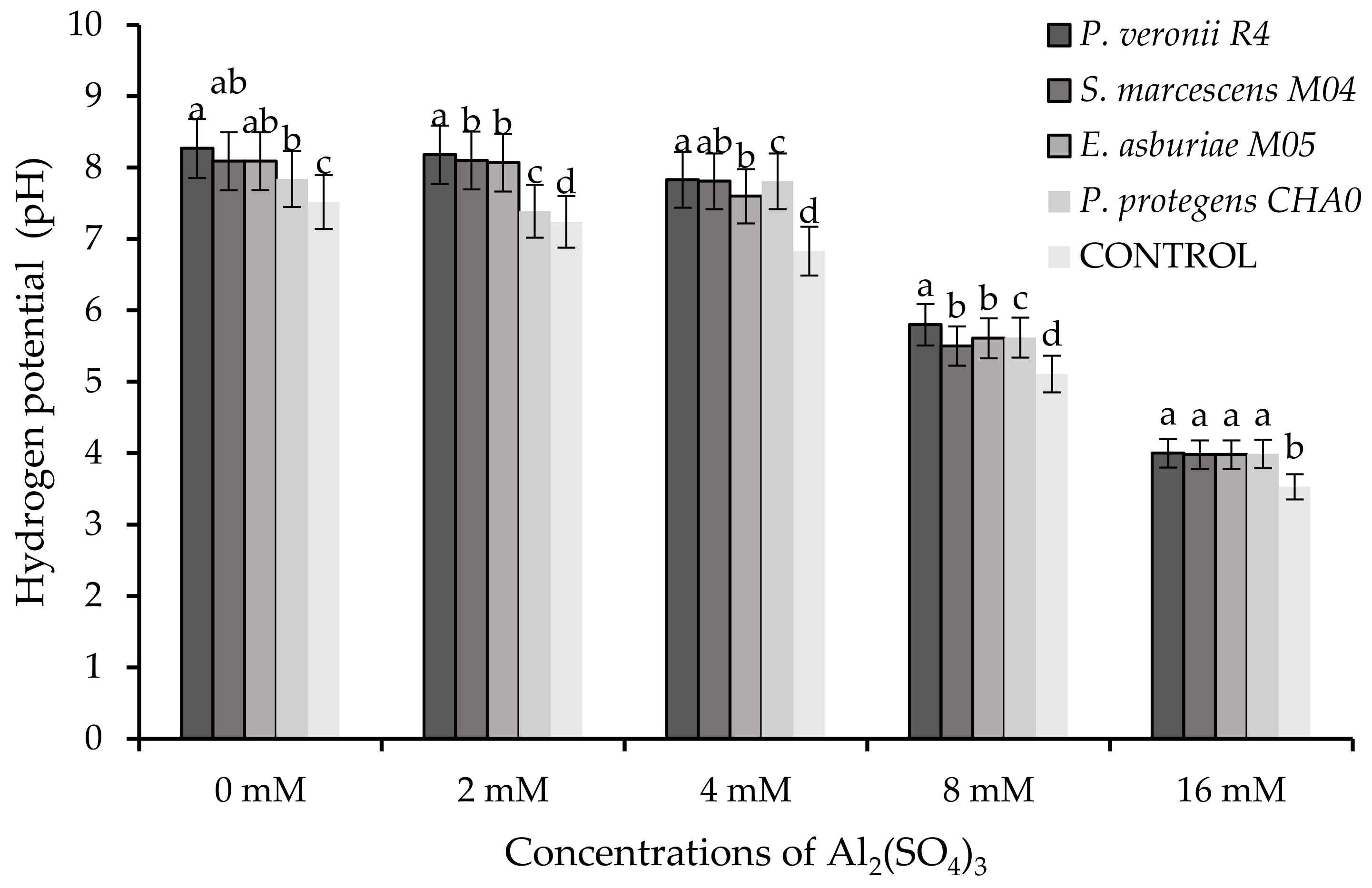
3.2.3. Determination of pH at Aluminum Concentrations Inoculated with Rhizobacteria
3.3. Application of Rhizobacteria in Soil Contaminated with Aluminum on the Vegetative Development of Oryza sativa L.
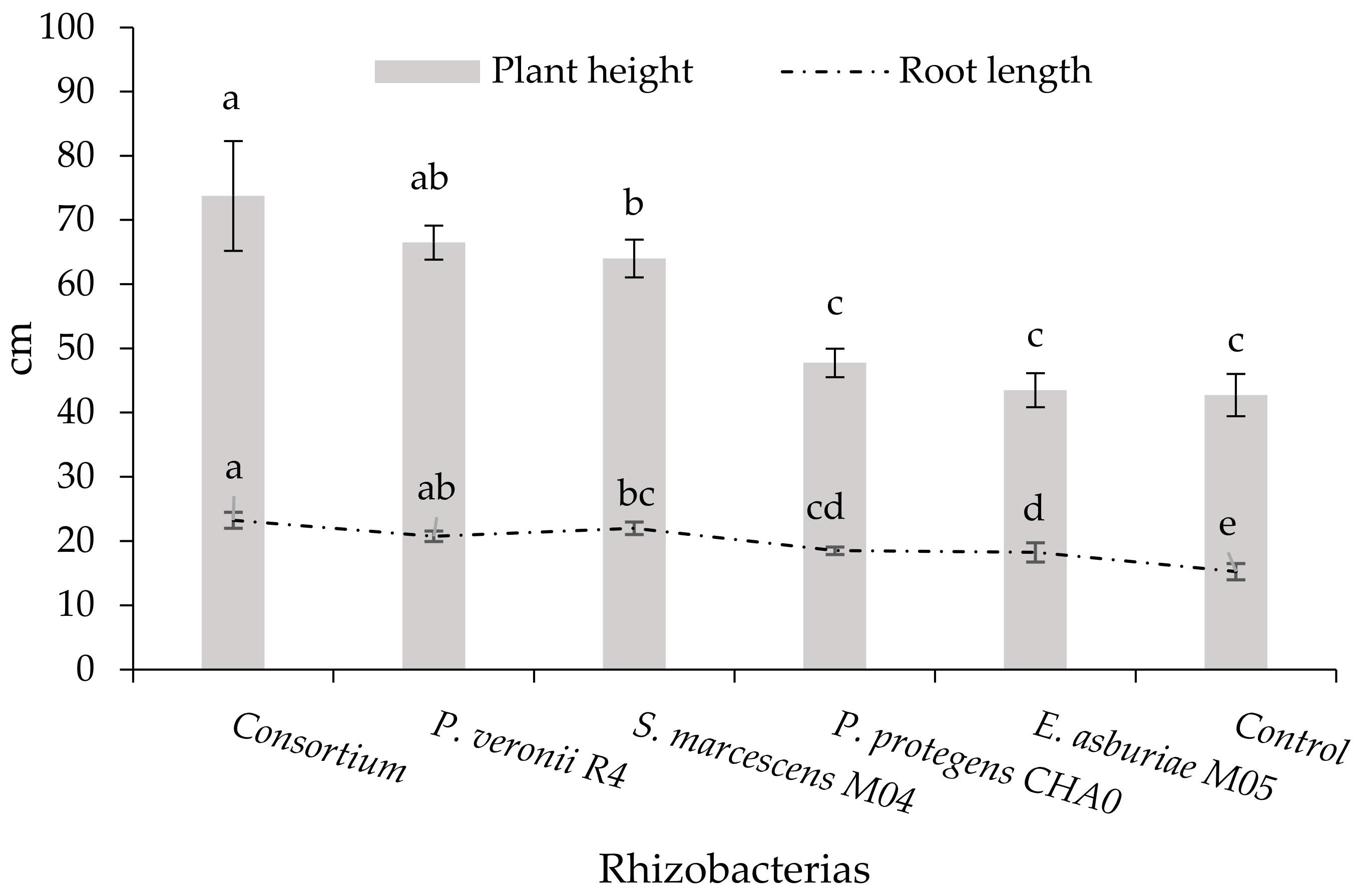
3.3.1. Plant Height and Root Length
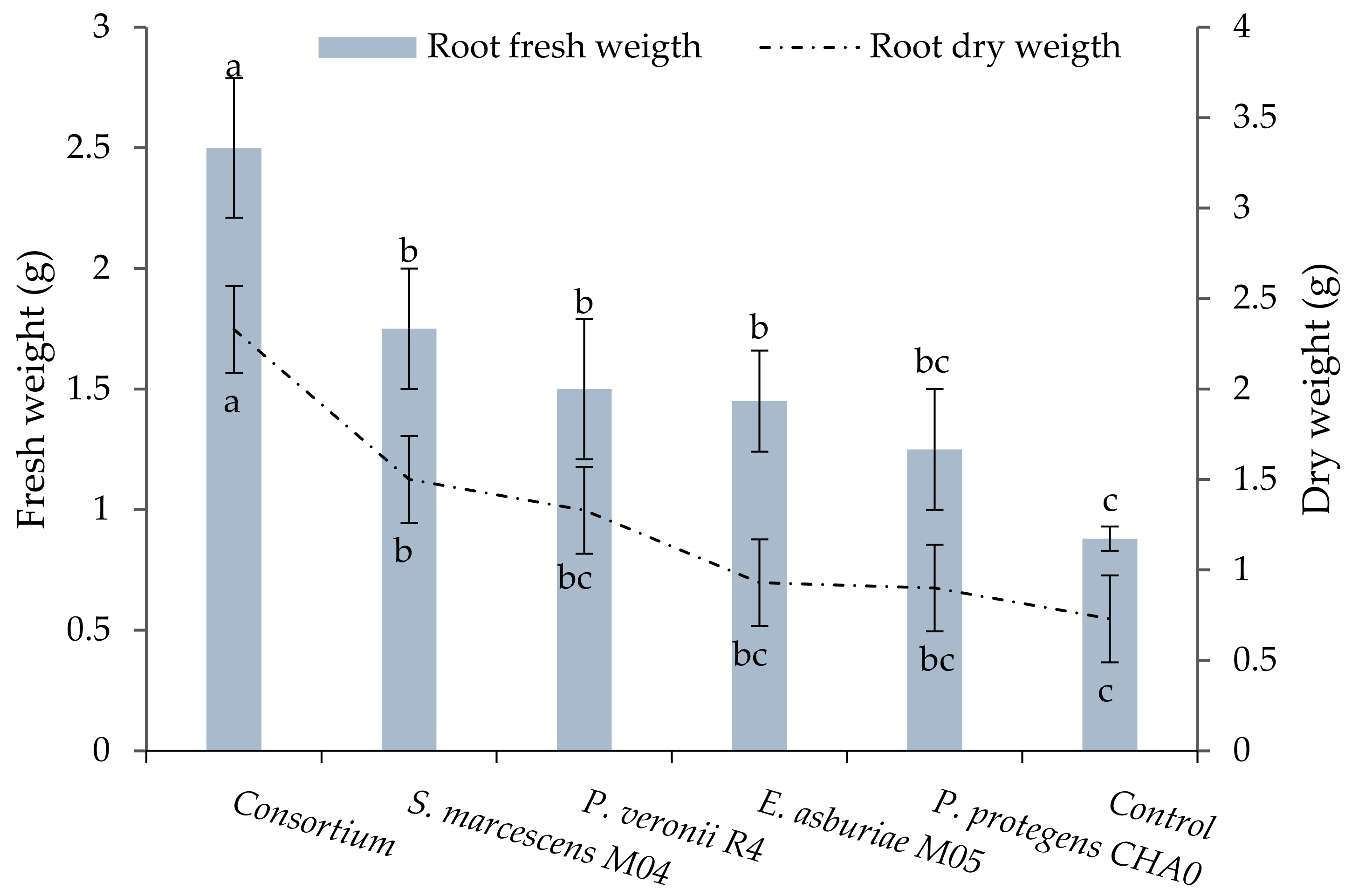
3.3.2. Fresh and Dry Root Weight
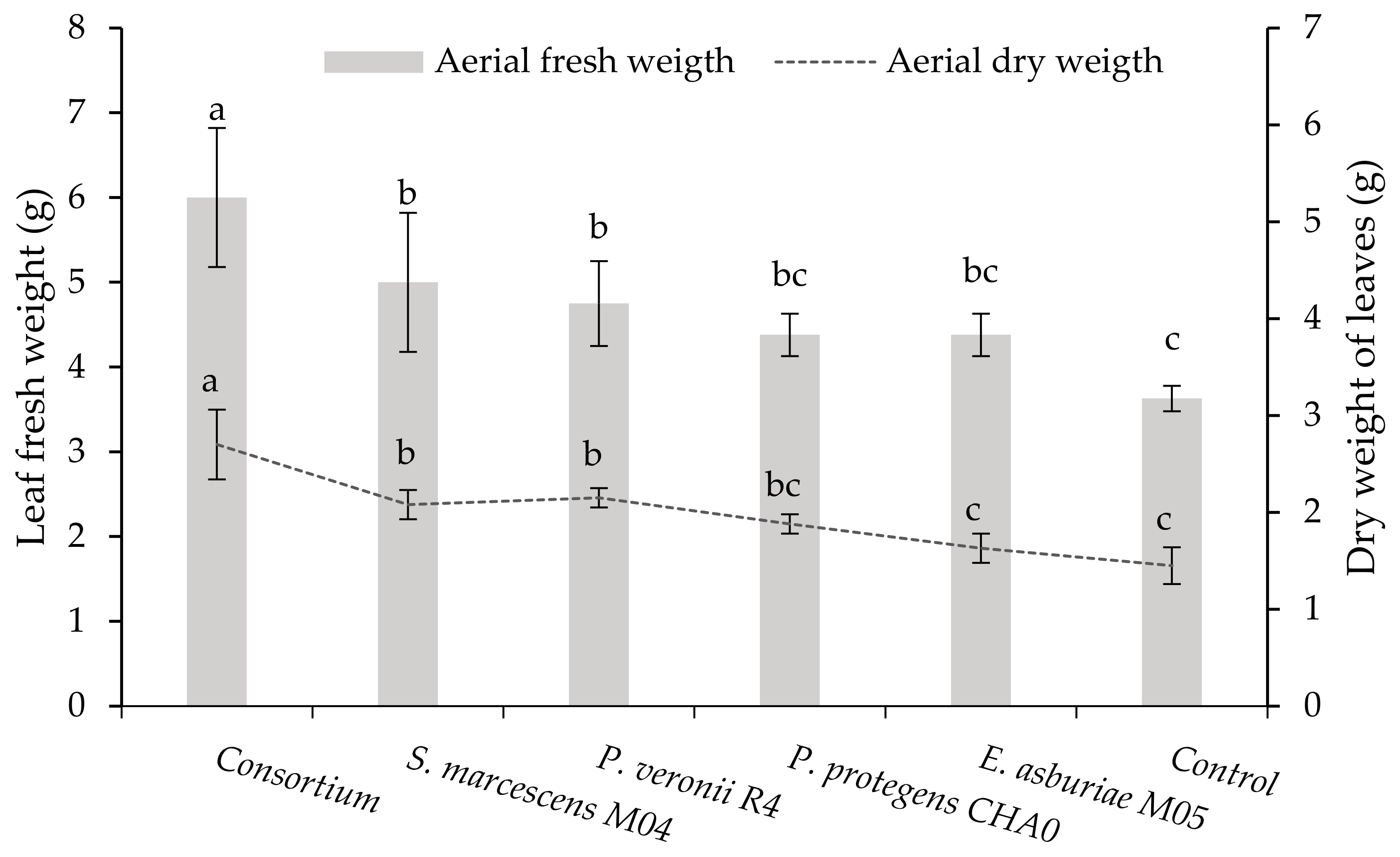
3.3.3. Fresh Weight and Dry Leaf
3.3.4. Content of Colony-Forming Units in Soil (CFU g−1)
4. Discussion
4.1. Aluminum Tolerance in the Germination Process of Two Varieties of Oryza sativa L.
4.2. Degree of Protection of Rhizobacteria with Aluminum Concentrations
4.3. Determination of the Morphological Characteristics of the Oryza sativa L. Plant by the Effect of Rhizobacteria and Aluminum
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Asibi, A.E.; Chai, Q.; Coulter, J.A. Rice Blast: A Disease with Implications for Global Food Security. Agronomy 2019, 9, 451. [Google Scholar] [CrossRef]
- Kumar, A.; Sengar, R.S.; Pathak, R.K.; Singh, A.K. Integrated Approaches to Develop Drought-Tolerant Rice: Demand of Era for Global Food Security. J. Plant Growth Regul. 2023, 42, 96–120. [Google Scholar] [CrossRef]
- Seck, P.A.; Diagne, A.; Mohanty, S.; Wopereis, M.C.S. Crops That Feed the World 7: Rice. Food Secur. 2012, 4, 7–24. [Google Scholar] [CrossRef]
- Mohidem, N.A.; Hashim, N.; Shamsudin, R.; Che Man, H. Rice for Food Security: Revisiting Its Production, Diversity, Rice Milling Process and Nutrient Content. Agriculture 2022, 12, 741. [Google Scholar] [CrossRef]
- Awasthi, J.P.; Saha, B.; Regon, P.; Sahoo, S.; Chowra, U.; Pradhan, A.; Roy, A.; Panda, S.K. Morpho-physiological analysis of tolerance to aluminum toxicity in rice varieties of North East India. PLoS ONE 2017, 12, e0176357. [Google Scholar] [CrossRef]
- Bartholomé, J.; Ospina, J.O.; Sandoval, M.; Espinosa, N.; Arcos, J.; Ospina, Y.; Frouin, J.; Beartschi, C.; Ghneim, T.; Grenier, C. Genomic selection for tolerance to aluminum toxicity in a synthetic population of upland rice. PLoS ONE 2024, 19, e0307009. [Google Scholar] [CrossRef]
- Chakraborty, N.; Das, A.; Pal, S.; Roy, S.; Sil, S.K.; Adak, M.K.; Hasanuzzaman, M. Exploring Aluminum Tolerance Mechanisms in Plants with Reference to Rice and Arabidopsis: A Comprehensive Review of Genetic, Metabolic, and Physiological Adaptations in Acidic Soils. Plants 2024, 13, 1760. [Google Scholar] [CrossRef]
- Hajiboland, R.; Panda, C.K.; Lastochkina, O.; Gavassi, M.A.; Habermann, G.; Pereira, J.F. Aluminum Toxicity in Plants: Present and Future. J. Plant Growth Regul. 2023, 42, 3967–3999. [Google Scholar] [CrossRef]
- Ofoe, R.; Thomas, R.H.; Asiedu, S.K.; Wang-Pruski, G.; Fofana, B.; Abbey, L. Aluminum in Plant: Benefits, Toxicity and Tolerance Mechanisms. Front. Plant Sci. 2023, 13, 1085998. [Google Scholar] [CrossRef]
- Cruz-Macías, W.O.; Rodríguez-Larramendi, L.A.; Salas-Marina, M.Á.; Hernández-García, V.; Campos-Saldaña, R.A.; Chávez-Hernández, M.H.; Gordillo-Curiel, A. Efecto de la materia orgánica y la capacidad de intercambio catiónico en la acidez de suelos cultivados con maíz en dos regiones de Chiapas, México. Terra Latinoam. 2020, 38, 475–480. [Google Scholar] [CrossRef]
- Rahman, R.; Upadhyaya, H. Aluminium Toxicity and Its Tolerance in Plant: A Review. J. Plant Biol. 2021, 64, 101–121. [Google Scholar] [CrossRef]
- Chauhan, D.K.; Yadav, V.; Vaculík, M.; Gassmann, W.; Pike, S.; Arif, N.; Singh, V.P.; Deshmukh, R.; Sahi, S.; Tripathi, D.K. Aluminum Toxicity and Aluminum Stress-Induced Physiological Tolerance Responses in Higher Plants. Crit. Rev. Biotechnol. 2021, 41, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Shuang, C.; Qing, H.; Song, B. Enhanced Technology of Phytoremediation. E3S Web Conf. 2021, 261, 04034. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, S.; Liu, S.; Peng, J.; Zhang, H.; Zhao, Q.; Zheng, L.; Chen, Y.; Shen, Z.; Xu, X.; et al. Enhancing the Phytoremediation of Heavy Metals by Combining Hyperaccumulator and Heavy Metal-Resistant Plant Growth-Promoting Bacteria. Front. Plant Sci. 2022, 13, 912350. [Google Scholar] [CrossRef]
- Nurmustaqimah; Jamilatun, S.; Rahayu, A.; Hakika, D.C.; Muthadin, A.S.; Taufiqurahman, M.A. Heavy Metal Phytoremediation: Plant Hyperaccumulators and Clean Strategies for the Environment. Indones. J. Chem. Eng. 2024, 2, 9–21. [Google Scholar]
- Xiao, X.; Wang, J.L.; Li, J.J.; Li, X.L.; Dai, X.J.; Shen, R.F.; Zhao, X.Q. Distinct Patterns of Rhizosphere Microbiota Associated With Rice Genotypes Differing in Aluminum Tolerance in an Acid Sulfate Soil. Front. Microbiol. 2022, 13, 933722. [Google Scholar] [CrossRef]
- Belimov, A.A.; Shaposhnikov, A.I.; Azarova, T.S.; Yuzikhin, O.S.; Sekste, E.A.; Safronova, V.I.; Tikhonovich, I.A. Aluminum-Immobilizing Rhizobacteria Modulate Root Exudation and Nutrient Uptake and Increase Aluminum Tolerance of Pea Mutant E107 (Brz). Plants 2023, 12, 2334. [Google Scholar] [CrossRef]
- Munyaneza, V.; Zhang, W.; Haider, S.; Xu, F.; Wang, C.; Ding, G. Strategies for Alleviating Aluminum Toxicity in Soils and Plants. Plant Soil. 2024, 504, 167–190. [Google Scholar] [CrossRef]
- Ur Rahman, S.; Han, J.-C.; Ahmad, M.; Ashraf, M.N.; Khaliq, M.A.; Yousaf, M.; Wang, Y.; Yasin, G.; Nawaz, M.F.; Khan, K.A.; et al. Aluminum Phytotoxicity in Acidic Environments: A Comprehensive Review of Plant Tolerance and Adaptation Strategies. Ecotoxicol. Environ. Saf. 2024, 269, 115791. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lee, S.-H.; Ji, H.C.; Kabir, A.H.; Jones, C.S.; Lee, K.-W. Importance of Mineral Nutrition for Mitigating Aluminum Toxicity in Plants on Acidic Soils: Current Status and Opportunities. Int. J. Mol. Sci. 2018, 19, 3073. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Gunsé, B.; Corrales, I.; Barceló, J. A Glance into Aluminum Toxicity and Resistance in Plants. Sci. Total Environ. 2008, 400, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, J.; Gandullo, J.; de la Osa, C.; Feria, A.B.; Echevarría, C.; Monreal, J.A.; García-Mauriño, S. Responses to Aluminum and Cadmium of a RNAi Sorghum Line with Decreased Levels of Phosphoenolpyruvate Carboxylase 3 (PPC3). Environ. Exp. Bot. 2023, 205, 105139. [Google Scholar] [CrossRef]
- Kumari, B.; Mallick, M.A.; Solanki, M.K.; Solanki, A.C.; Hora, A.; Guo, W. Plant Growth Promoting Rhizobacteria (PGPR): Modern Prospects for Sustainable Agriculture. In Plant Health Under Biotic Stress: Volume 2: Microbial Interactions; Ansari, R.A., Mahmood, I., Eds.; Springer: Singapore, 2019; pp. 109–127. ISBN 9789811360404. [Google Scholar]
- Aeron, A.; Khare, E.; Jha, C.K.; Meena, V.S.; Aziz, S.M.A.; Islam, M.T.; Kim, K.; Meena, S.K.; Pattanayak, A.; Rajashekara, H.; et al. Revisiting the Plant Growth-Promoting Rhizobacteria: Lessons from the Past and Objectives for the Future. Arch. Microbiol. 2020, 202, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Panhwar, Q.A.; Naher, U.A.; Radziah, O.; Shamshuddin, J.; Razi, I.M. Eliminating Aluminum Toxicity in an Acid Sulfate Soil for Rice Cultivation Using Plant Growth Promoting Bacteria. Molecules 2015, 20, 3628–3646. [Google Scholar] [CrossRef]
- Velasco-Jiménez, A.; Castellanos-Hernández, O.; Acevedo-Hernández, G.; Aarland, R.C.; Rodríguez-Sahagún, A.; Velasco-Jiménez, A.; Castellanos-Hernández, O.; Acevedo-Hernández, G.; Aarland, R.C.; Rodríguez-Sahagún, A. Bacterias rizosféricas con beneficios potenciales en la agricultura. Terra Latinoam. 2020, 38, 333–345. [Google Scholar] [CrossRef]
- Zerrouk, I.Z.; Rahmoune, B.; Khelifi, L.; Mounir, K.; Baluska, F.; Ludwig-Müller, J. Algerian Sahara PGPR Confers Maize Root Tolerance to Salt and Aluminum Toxicity via ACC Deaminase and IAA. Acta Physiol. Plant 2019, 41, 91. [Google Scholar] [CrossRef]
- Xiao, A.W.; Li, Z.; Li, W.C.; Ye, Z.H. The Effect of Plant Growth-Promoting Rhizobacteria (PGPR) on Arsenic Accumulation and the Growth of Rice Plants (Oryza sativa L.). Chemosphere 2020, 242, 125136. [Google Scholar] [CrossRef]
- Gupta, K.; Dubey, N.K.; Singh, S.P.; Kheni, J.K.; Gupta, S.; Varshney, A. Plant Growth-Promoting Rhizobacteria (PGPR): Current and Future Prospects for Crop Improvement. In Current Trends in Microbial Biotechnology for Sustainable Agriculture; Yadav, A.N., Singh, J., Singh, C., Yadav, N., Eds.; Springer: Singapore, 2021; pp. 203–226. ISBN 9789811569494. [Google Scholar]
- Bhattacharyya, P.N.; Jha, D.K. Plant Growth-Promoting Rhizobacteria (PGPR): Emergence in Agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, N.; Zhang, Z.; Lei, C.; Chen, B.; Qin, G.; Qiu, D.; Lu, T.; Qian, H. Deciphering Microbial Community and Nitrogen Fixation in the Legume Rhizosphere. J. Agric. Food Chem. 2024, 72, 5659–5670. [Google Scholar] [CrossRef]
- Zhiyong, S.; Yaxuan, G.; Yuanyuan, W.; Xiang, Y.; Xu, G.; Zhenhong, L.; Jingping, N.; Jianping, L.; Zhenyu, L. Nitrogen-Fixing Bacteria Promote Growth and Bioactive Components Accumulation of Astragalus Mongholicus by Regulating Plant Metabolism and Rhizosphere Microbiota. BMC Microbiol. 2024, 24, 261. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Z.; Peng, J.; Yang, K.; Zhang, Y.; Chen, Q.-L.; Zhu, Y.-G.; Cui, L. Single-Cell Exploration of Active Phosphate-Solubilizing Bacteria across Diverse Soil Matrices for Sustainable Phosphorus Management. Nat. Food 2024, 5, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rodríguez, J.A.; Reyes-Pérez, J.J.; Adame, L.H.; Llerena-Fuentes, B.L.; Hernandez-Montiel, L.G. Marine Actinomycetes for Biocontrol of Fusarium Solani in Tomato Plants: In Vitro and in Vivo Studies. Not. Bot. Horti Agrobot. Cluj-Napoca 2024, 52, 13562. [Google Scholar] [CrossRef]
- Navarro-Ródenas, A.; Berná, L.M.; Lozano-Carrillo, C.; Andrino, A.; Morte, A. Beneficial Native Bacteria Improve Survival and Mycorrhization of Desert Truffle Mycorrhizal Plants in Nursery Conditions. Mycorrhiza 2016, 26, 769–779. [Google Scholar] [CrossRef]
- Sharifi, R.; Ryu, C.-M. Are Bacterial Volatile Compounds Poisonous Odors to a Fungal Pathogen Botrytis Cinerea, Alarm Signals to Arabidopsis Seedlings for Eliciting Induced Resistance, or Both? Front. Microbiol. 2016, 7, 196. [Google Scholar] [CrossRef]
- Lim, J.-H.; Kim, S.-D. Induction of Drought Stress Resistance by Multi-Functional PGPR Bacillus Licheniformis K11 in Pepper. Plant Pathol. J. 2013, 29, 201–208. [Google Scholar] [CrossRef]
- AbuQamar, S.F.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.-S.M.; Elrys, A.S.; El-Mageed, T.A.A.; Semida, W.M.; Abdelkhalik, A.; Mosa, W.F.A.; Al Kafaas, S.S.; et al. Halotolerant Plant Growth-Promoting Rhizobacteria Improve Soil Fertility and Plant Salinity Tolerance for Sustainable Agriculture—A Review. Plant Stress 2024, 12, 100482. [Google Scholar] [CrossRef]
- Gupta, A.; Meyer, J.M.; Goel, R. Development of Heavy Metal-Resistant Mutants of Phosphate Solubilizing Pseudomonas Sp. NBRI 4014 and Their Characterization. Curr. Microbiol. 2002, 45, 323–327. [Google Scholar] [CrossRef]
- Zhu, D.; Ouyang, L.; Xu, Z.; Zhang, L. Rhizobacteria of Populus Euphratica Promoting Plant Growth Against Heavy Metals. Int. J. Phytoremediat. 2015, 17, 973–980. [Google Scholar] [CrossRef]
- Florez-Márquez, J.D.; Leal-Medina, G.I.; Ardila-Leal, L.D.; Cárdenas-Caro, D.M.; Florez-Márquez, J.D.; Leal-Medina, G.I.; Ardila-Leal, L.D.; Cárdenas-Caro, D.M. Aislamiento y caracterización de rizobacterias asociadas a cultivos de arroz (Oryza sativa L.) del Norte de Santander (Colombia). Agrociencia 2017, 51, 373–391. [Google Scholar]
- Gusain, Y.S.; Sharma, A.K. PGPRs Inoculations Enhances the Grain Yield and Grain Nutrient Content in Four Cultivars of Rice (Oryza sativa L.) under Field Condition. J. Pharmacogn. Phytochem. 2019, 8, 1865–1870. [Google Scholar]
- Ouyabe, M.; Irie, K.; Tanaka, N.; Kikuno, H.; Pachakkil, B.; Shiwachi, H. Response of Upland Rice (Oryza sativa L.) Inoculated with Non-Native Plant Growth-Promoting Bacteria. Agronomy 2020, 10, 903. [Google Scholar] [CrossRef]
- Peñafiel-Jaramillo, M.; Álvarez, A.E.B.; Navarrete, E.D.T.; Martínez, H.F.C.; Prieto-Encalada, H.; Carriel, J.M. Producción de ácido indol-3-acético por Pseudomonas veronii R4 y formación de raíces en hojas de vid “Thompson seedless” in vitro. Cienc. Tecnol. 2016, 9, 31–36. [Google Scholar] [CrossRef]
- Lebuhn, M.; Heulin, T.; Hartmann, A. Production of Auxin and Other Indolic and Phenolic Compounds by Paenibacillus Polymyxa Strains Isolated from Different Proximity to Plant Roots. FEMS Microbiol. Ecol. 1997, 22, 325–334. [Google Scholar] [CrossRef]
- Keswani, C.; Singh, S.P.; Cueto, L.; García-Estrada, C.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Singh, S.P.; Blázquez, M.A.; Sansinenea, E. Auxins of Microbial Origin and Their Use in Agriculture. Appl. Microbiol. Biotechnol. 2020, 104, 8549–8565. [Google Scholar] [CrossRef]
- Farh, M.E.-A.; Kim, Y.-J.; Sukweenadhi, J.; Singh, P.; Yang, D.-C. Aluminium Resistant, Plant Growth Promoting Bacteria Induce Overexpression of Aluminium Stress Related Genes in Arabidopsis thaliana and Increase the Ginseng Tolerance against Aluminium Stress. Microbiol. Res. 2017, 200, 45–52. [Google Scholar] [CrossRef]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor Determination in Soybean Seed by Multiple Criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Scapin, E.; Sarri, D.R.A.; Augusco, M.A.C.; Rodrigues, M.A.M.; Fernandes, R.M.N.; Silva, J.F.M.; Cardoso, C.A.L.; Rambo, M.K.D. Phytochemical Analysis, Toxicity and Evaluation of Antioxidant and Antimicrobial Activities of Leaves of Dipteryx alata Vogel. Braz. J. Biol. 2024, 84, e278004. [Google Scholar] [CrossRef]
- Fernandez, M.; Pereira, P.P.; Agostini, E.; González, P.S. Impact Assessment of Bioaugmented Tannery Effluent Discharge on the Microbiota of Water Bodies. Ecotoxicology 2020, 29, 973–986. [Google Scholar] [CrossRef]
- Espinoza-Vergara, J.; Molina, P.; Walter, M.; Gulppi, M.; Vejar, N.; Melo, F.; Urzua, M.; Muñoz, H.; Zagal, J.H.; Zhou, X.; et al. Effect of pH on the Electrochemical Behavior of Hydrogen Peroxide in the Presence of Pseudomonas Aeruginosa. Front. Bioeng. Biotechnol. 2021, 9, 749057. [Google Scholar] [CrossRef]
- Purwanti, I.F.; Kurniawan, S.B.; Imron, M.F. Potential of Pseudomonas aeruginosa Isolated from Aluminium-Contaminated Site in Aluminium Removal and Recovery from Wastewater. Environ. Technol. Innov. 2019, 15, 100422. [Google Scholar] [CrossRef]
- Hu, B.; Li, Y.; Zhu, S.; Zhang, H.; Jing, Y.; Jiang, D.; He, C.; Zhang, Z. Evaluation of Biohydrogen Yield Potential and Electron Balance in the Photo-Fermentation Process with Different Initial pH from Starch Agricultural Leftover. Bioresour. Technol. 2020, 305, 122900. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, B.; Kotoky, R.; Maheshwari, D.K.; Pandey, P. Rhizoremediation of Cd-Contaminated Soil Using Zea Mays Sturt, with Heavy Metal Resistant Rhizobacteria That Alleviate Cd-Induced Stress in Plant. Environ. Sustain. 2022, 5, 375–387. [Google Scholar] [CrossRef]
- Samad, R.; Rashid, P.; Karmoker, J.L. Anatomical Responses of Rice (Oryza sativa L.) to Aluminium Toxicity. J. Bangladesh Acad. Sci. 2019, 43, 123–131. [Google Scholar] [CrossRef]
- Lan, Y.; Chai, Y.; Xing, C.; Wu, K.; Wang, L.; Cai, M. Nitric Oxide Reduces the Aluminum-Binding Capacity in Rice Root Tips by Regulating the Cell Wall Composition and Enhancing Antioxidant Enzymes. Ecotoxicol. Environ. Saf. 2021, 208, 111499. [Google Scholar] [CrossRef]
- Bhadra, S.; Roy, B. Seedling Vigour as a Potential Indicator for Rapid Screening of Rice (Oryza sativa L.) Genotypes for Aluminium (Al) Toxicity Tolerance. Indian J. Exp. Biol. (IJEB) 2021, 59, 493–499. [Google Scholar] [CrossRef]
- Azura, E.; Shamshuddin, J.; Fauziah, C. Root elongation, root surface area and organic acid by rice seedling under Al 3+ and/or H+ stress. Am. J. Agric. Biol. Sci. 2011, 6, 324–331. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Guo, S.W.; Shinmachi, F.; Sunairi, M.; Noguchi, A.; Hasegawa, I.; Shen, R.F. Aluminium Tolerance in Rice Is Antagonistic with Nitrate Preference and Synergistic with Ammonium Preference. Ann. Bot. 2013, 111, 69–77. [Google Scholar] [CrossRef]
- Chang, S.; Jing-hao, W.; Gao-ling, S.; Lai-qing, L.; Jun-xia, D.; Jian-lin, W.; Qing-sheng, C. Different Aluminum Tolerance among Indica, Japonica and Hybrid Rice Varieties. Rice Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Wei, Y.; Han, R.; Xie, Y.; Jiang, C.; Yu, Y. Recent Advances in Understanding Mechanisms of Plant Tolerance and Response to Aluminum Toxicity. Sustainability 2021, 13, 1782. [Google Scholar] [CrossRef]
- Aizawa, T.; Sato, J.; Saito, S.; Yasuda, T.; Maruyama, Y.; Urai, M. An Extracellular Polysaccharide Is Involved in the Aluminum Tolerance of Pullulanibacillus Sp. CA42, a Newly Isolated Strain from the Chinese Water Chestnut Growing in an Actual Acid Sulfate Soil Area in Vietnam. Front. Microbiol. 2023, 14, 1241244. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Parmar, S.; Vaghela, H.; Dhandhukia, P.; Thakker, J.N. Describing Paenibacillus Mucilaginosus Strain N3 as an Efficient Plant Growth Promoting Rhizobacteria (PGPR). Cogent Food Agric. 2015, 1, 1000714. [Google Scholar] [CrossRef]
- Paredes-Mendoza, M.; Espinosa-Victoria, D. Ácidos orgánicos producidos por rizobacterias que solubilizan fosfato: Una revisión crítica. Terra Latinoam. 2010, 28, 61–70. [Google Scholar]
- Silambarasan, S.; Logeswari, P.; Valentine, A.; Cornejo, P. Role of Curtobacterium herbarum Strain CAH5 on Aluminum Bioaccumulation and Enhancement of Lactuca sativa Growth under Aluminum and Drought Stresses. Ecotoxicol. Environ. Saf. 2019, 183, 109573. [Google Scholar] [CrossRef] [PubMed]
- Serrano, N.F.G.; Moreno, L. Sideróforos De Rizobacterias Y Su Aplicación En La Biorremediación. Cienc. Desarro. 2024, 15, 16996. [Google Scholar] [CrossRef]
- Singh, R.; Beriault, R.; Middaugh, J.; Hamel, R.; Chenier, D.; Appanna, V.D.; Kalyuzhnyi, S. Aluminum-Tolerant Pseudomonas Fluorescens: ROS Toxicity and Enhanced NADPH Production. Extremophiles 2005, 9, 367–373. [Google Scholar] [CrossRef]
- Belimov, A.A.; Shaposhnikov, A.I.; Azarova, T.S.; Syrova, D.S.; Kitaeva, A.B.; Ulyanich, P.S.; Yuzikhin, O.S.; Sekste, E.A.; Safronova, V.I.; Vishnyakova, M.A.; et al. Rhizobacteria Mitigate the Negative Effect of Aluminum on Pea Growth by Immobilizing the Toxicant and Modulating Root Exudation. Plants 2022, 11, 2416. [Google Scholar] [CrossRef]
- Jiang, X.; Li, W.-W.; Han, M.; Chen, G.; Wu, J.; Lai, S.; Fu, Z.; Zhang, S.; Deng, W.-W.; Gao, L.; et al. Aluminum-Tolerant, Growth-Promoting Endophytic Bacteria as Contributors in Promoting Tea Plant Growth and Alleviating Aluminum Stress. Tree Physiol. 2022, 42, 1043–1058. [Google Scholar] [CrossRef]
- Bisht, H.; Kumar, N. Identification and Characterization of Aluminium Tolerant Bacteria Isolated from Soil Contaminated by Electroplating and Automobile Waste. Nat. Environ. Poll. Technol. 2023, 22, 411–416. [Google Scholar] [CrossRef]
- Gyaneshwar, P.; James, E.K.; Reddy, P.M.; Ladha, J.K. Herbaspirillum Colonization Increases Growth and Nitrogen Accumulation in Aluminium-Tolerant Rice Varieties. New Phytol. 2002, 154, 131–145. [Google Scholar] [CrossRef]
- Feld, L.; Nielsen, T.K.; Hansen, L.H.; Aamand, J.; Albers, C.N. Establishment of Bacterial Herbicide Degraders in a Rapid Sand Filter for Bioremediation of Phenoxypropionate-Polluted Groundwater. Appl. Environ. Microbiol. 2016, 82, 878–887. [Google Scholar] [CrossRef]
- Wan, W.; Tan, J.; Wang, Y.; Qin, Y.; He, H.; Wu, H.; Zuo, W.; He, D. Responses of the Rhizosphere Bacterial Community in Acidic Crop Soil to pH: Changes in Diversity, Composition, Interaction, and Function. Sci. Total Environ. 2020, 700, 134418. [Google Scholar] [CrossRef]
- Zhang, N.; Ma, Z.; Li, D.; Ni, H.; Sun, B.; Liang, Y. Soil pH Filters the Association Patterns of Aluminum-Tolerant Microorganisms in Rice Paddies. mSystems 2022, 7, e01022-21. [Google Scholar] [CrossRef]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Allaoui, A.; Biskri, L. Plant Growth Enhancement Using Rhizospheric Halotolerant Phosphate Solubilizing Bacterium Bacillus Licheniformis QA1 and Enterobacter Asburiae QF11 Isolated from Chenopodium Quinoa Willd. Microorganisms 2020, 8, 948. [Google Scholar] [CrossRef]
- Santoyo, G.; Orozco-Mosqueda, M.D.C.; Afridi, M.S.; Mitra, D.; Valencia-Cantero, E.; Macías-Rodríguez, L. Trichoderma and Bacillus Multifunctional Allies for Plant Growth and Health in Saline Soils: Recent Advances and Future Challenges. Front. Microbiol. 2024, 15, 1423980. [Google Scholar] [CrossRef]
- de la Luz Mora, M.; Demanet, R.; Acuña, J.J.; Viscardi, S.; Jorquera, M.; Rengel, Z.; Durán, P. Aluminum-Tolerant Bacteria Improve the Plant Growth and Phosphorus Content in Ryegrass Grown in a Volcanic Soil Amended with Cattle Dung Manure. Appl. Soil. Ecol. 2017, 115, 19–26. [Google Scholar] [CrossRef]
- Ismail, N.I.; Abdullah, S.R.S.; Idris, M.; Kurniawan, S.B.; Effendi Halmi, M.I.; AL Sbani, N.H.; Jehawi, O.H.; Hasan, H.A. Applying Rhizobacteria Consortium for the Enhancement of Scirpus grossus Growth and Phytoaccumulation of Fe and Al in Pilot Constructed Wetlands. J. Environ. Manag. 2020, 267, 110643. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Cornejo, P.; Kannan, V.R. Role of Plant Growth–Promoting Rhizobacterial Consortium in Improving the Vigna Radiata Growth and Alleviation of Aluminum and Drought Stresses. Environ. Sci. Pollut. Res. 2019, 26, 27647–27659. [Google Scholar] [CrossRef]
- Mozumder, A.B.; Chanda, K.; Chorei, R.; Prasad, H.K. An Evaluation of Aluminum Tolerant Pseudomonas Aeruginosa A7 for In Vivo Suppression of Fusarium Wilt of Chickpea Caused by Fusarium oxysporum f. sp. Ciceris and Growth Promotion of Chickpea. Microorganisms 2022, 10, 568. [Google Scholar] [CrossRef]
- Mitra, S.; Pramanik, K.; Sarkar, A.; Ghosh, P.K.; Soren, T.; Maiti, T.K. Bioaccumulation of Cadmium by Enterobacter sp. and Enhancement of Rice Seedling Growth under Cadmium Stress. Ecotoxicol. Environ. Saf. 2018, 156, 183–196. [Google Scholar] [CrossRef]


| Rhizobacteria | Strain | PR | HCN | Prn | DAPG | Siderophores | Indole Acetic Acid |
|---|---|---|---|---|---|---|---|
| Serratia marcescens | M04 | − | − | − | − | + | + |
| Enterobacter asburiae | M05 | − | − | − | − | + | + |
| Pseudomonas veronii | R4 | + | − | + | − | + | + |
| Pseudomonas protegens | CHA0 | + | + | + | + | + | + |
| Rice Variety | Al2(SO4)3 | GP (%) | HL (cm) | RL (cm) | VI | RGI | GI |
|---|---|---|---|---|---|---|---|
| SUPREMA I-1480 | 0 mM | 100 ± 0.00 a | 52.33 ± 0.44 a | 39.33 ± 0.93 a | 655 ± 5.00 a | 1 ± 0.00 a | 1 ± 0.00 a |
| SUPREMA I-1480 | 2 mM | 80 ± 10.00 b | 25.67 ± 1.17 b | 11.50 ± 0.25 b | 149 ± 7.86 b | 0.28 ± 0.02 b | 0.23 ± 0.05 b |
| SUPREMA I-1480 | 4 mM | 80 ± 7.07 b | 26.33 ± 0.93 b | - | 105 ± 7.42 c | - | - |
| SUPREMA I-1480 | 8 mM | 50 ± 8.66 c | 20.67 ± 0.17 b | - | 52 ± 0.83 d | - | - |
| SUPREMA I-1480 | 16 mM | - | - | - | - | - | - |
| INIAP-4M | 0 mM | 100 ± 0.00 a | 47.00 ± 2.65 a | 55.67 ± 2.89 a | 767 ± 4.41 a | 1 ± 0.00 a | 1 ± 0.00 a |
| INIAP-4M | 2 mM | 100 ± 0.00 a | 31.67 ± 0.93 b | 3.90 ± 1.53 b | 178 ± 2.24 b | 0.07 ± 0.04 b | 0.07 ± 0.02 b |
| INIAP-4M | 4 mM | 100 ± 0.00 a | 25.50 ± 0.14 b | 0.60 ± 0.05 c | 129 ± 0.73 c | 0.01 ± 0.06 b | 0.01 ± 0.02 b |
| INIAP-4M | 8 mM | 70 ± 7.07 b | 23.83 ± 0.46 bc | - | 83 ± 3.25 d | - | - |
| INIAP-4M | 16 mM | 60 ± 7.07 c | 15.67 ± 0.33 c | - | 47 ± 2.00 e | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carranza-Patiño, M.S.; Torres-Rodriguez, J.A.; Reyes-Pérez, J.J.; Herrera-Feijoo, R.J.; Cedeño-Moreira, Á.V.; Coello Mieles, A.J.; Macías Holguín, C.J.; Chicaiza-Ortiz, C. The Role of Different Rhizobacteria in Mitigating Aluminum Stress in Rice (Oriza sativa L.). Int. J. Plant Biol. 2024, 15, 1418-1436. https://doi.org/10.3390/ijpb15040098
Carranza-Patiño MS, Torres-Rodriguez JA, Reyes-Pérez JJ, Herrera-Feijoo RJ, Cedeño-Moreira ÁV, Coello Mieles AJ, Macías Holguín CJ, Chicaiza-Ortiz C. The Role of Different Rhizobacteria in Mitigating Aluminum Stress in Rice (Oriza sativa L.). International Journal of Plant Biology. 2024; 15(4):1418-1436. https://doi.org/10.3390/ijpb15040098
Chicago/Turabian StyleCarranza-Patiño, Mercedes Susana, Juan Antonio Torres-Rodriguez, Juan José Reyes-Pérez, Robinson J. Herrera-Feijoo, Ángel Virgilio Cedeño-Moreira, Alejandro Jair Coello Mieles, Cristhian John Macías Holguín, and Cristhian Chicaiza-Ortiz. 2024. "The Role of Different Rhizobacteria in Mitigating Aluminum Stress in Rice (Oriza sativa L.)" International Journal of Plant Biology 15, no. 4: 1418-1436. https://doi.org/10.3390/ijpb15040098
APA StyleCarranza-Patiño, M. S., Torres-Rodriguez, J. A., Reyes-Pérez, J. J., Herrera-Feijoo, R. J., Cedeño-Moreira, Á. V., Coello Mieles, A. J., Macías Holguín, C. J., & Chicaiza-Ortiz, C. (2024). The Role of Different Rhizobacteria in Mitigating Aluminum Stress in Rice (Oriza sativa L.). International Journal of Plant Biology, 15(4), 1418-1436. https://doi.org/10.3390/ijpb15040098










