Influence of Fruit Load and Water Deficit on Olive Fruit Phenolic Profiling and Yield
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Orchard and Experimental Design
2.2. Field Measurements and Fruit Harvest
2.3. Maturation Index and Fruit Yield
2.4. Sample Preparation and Extraction for Metabolomic Analysis
2.5. Phenolic Profiling Using Untargeted Metabolomics
2.6. Statistical Analysis
3. Results
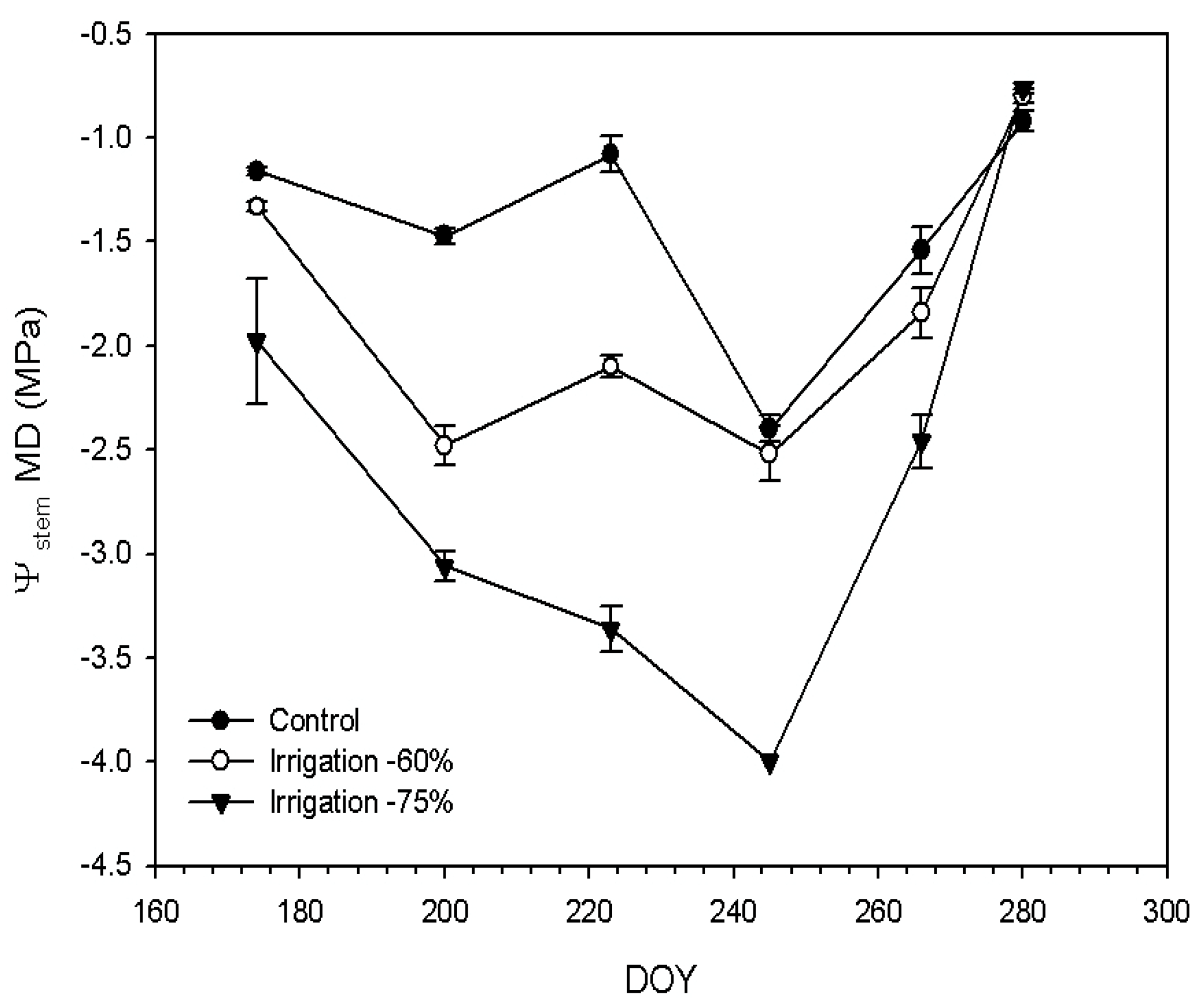
3.1. Fruit Load and Deficit Irrigation Effect on Oil Accumulation, Water Potential, and Olive Growth Dynamics
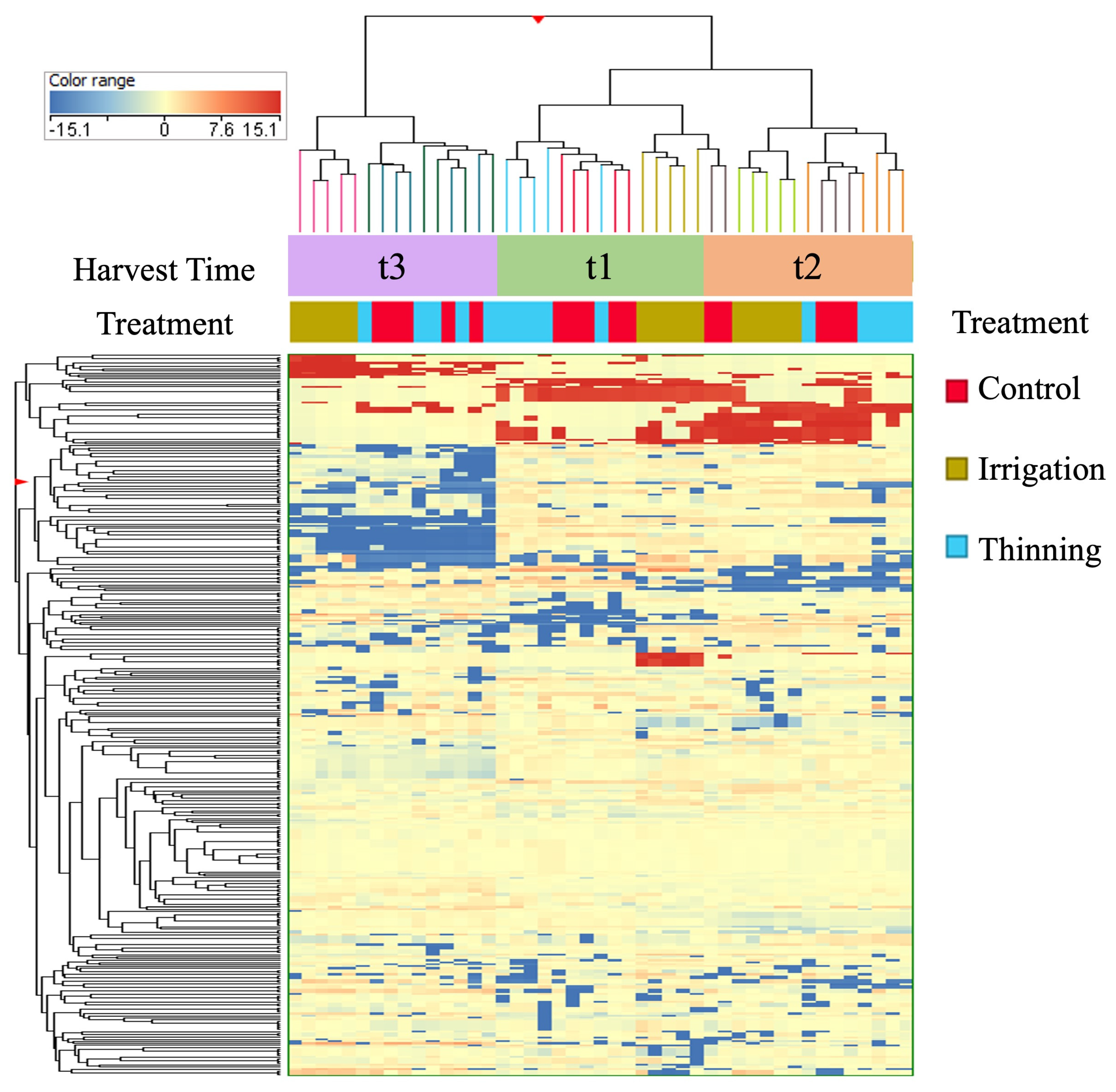
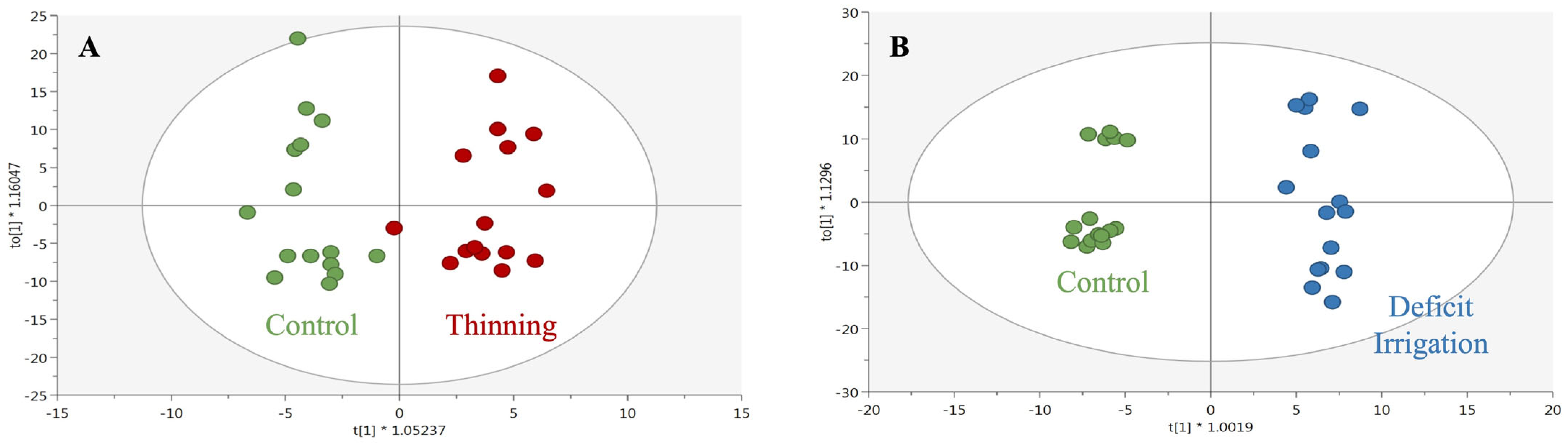
3.2. Fruit Load and Deficit Irrigation Effect on the Phenolic Profile of Olive Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Y.; Zhao, J.; Xin, Q.; Yuan, R.; Miao, Y.; Yang, M.; Mo, H.; Chen, K.; Cong, W. Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases. Food Sci. Hum. Wellness 2024, 13, 529–540. [Google Scholar] [CrossRef]
- EC—European Commission, Expert Group for Agricultural Markets—Arable crops and olive oil. Market Situation in the Olive Oil and Table Olive Sectors. 2023. Available online: https://agriculture.ec.europa.eu/system/files/2023-12/market-situation-olive-oil-table-olives_en.pdf (accessed on 2 January 2024).
- D’Adamo, I.; Falcone, P.M.; Gastaldi, M.; Morone, P. A social analysis of the olive oil sector: The role of family business. Resources 2019, 8, 151. [Google Scholar] [CrossRef]
- Guarino, F.; Falcone, G.; Stillitano, T.; de Luca, A.I.; Gulisano, G.; Mistretta, M.; Strano, A. Life cycle assessment of olive oil: A case study in southern Italy. J. Environ. Manag. 2019, 238, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, L.; Farolfi, C.; Capri, E. Sustainability certification, a new path of value creation in the olive oil sector: The Italian case study. Foods 2021, 10, 501. [Google Scholar] [CrossRef] [PubMed]
- Stillitano, T.; Falcone, G.; Nicolò, B.F.; di Girolamo, C.; Gulisano, G.; de Luca, A.I. Technical efficiency assessment of intensive and traditional olive farms in Southern Italy. Agris Line Pap. Econ. Inform. 2019, 11, 81–93. [Google Scholar] [CrossRef]
- D’Adamo, I.; Falcone, P.M.; Gastaldi, M. Price analysis of extra virgin olive oil. Br. Food J. 2019, 121, 1899–1911. [Google Scholar] [CrossRef]
- Lombardo, L.; Farolfi, C.; Tombesi, S.; Novelli, E.; Capri, E. Development of a sustainability technical guide for the Italian olive oil supply chain. Sci. Total Environ. 2022, 820, 153332. [Google Scholar] [CrossRef]
- Orlandi, F.; Rojo, J.; Picornell, A.; Oteros, J.; Pérez-Badia, R.; Fornaciari, M. Impact of climate change on olive crop production in Italy. Atmosphere 2020, 11, 595. [Google Scholar] [CrossRef]
- International Olive Council. International Olive Oil Production Costs Study: Results, Conclusions and Recommendations. 2015. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/INTERNATIONAL-OLIVE-OIL-PRODUCTION-COSTS-STUDY-.pdf (accessed on 25 January 2023).
- Criscuolo, N.; Guarino, F.; Angelini, C.; Castiglione, S.; Caruso, T.; Cicatelli, A. High biodiversity arises from the analyses of morphometric, biochemical and genetic data in ancient olive trees of South of Italy. Plants 2019, 8, 297. [Google Scholar] [CrossRef]
- Roselli, L.; Clodoveo, M.L.; Corbo, F.; de Gennaro, B. Are health claims a useful tool to segment the category of extra-virgin olive oil? Threats and opportunities for the Italian olive oil supply chain. Trends Food Sci. Technol. 2017, 68, 176–181. [Google Scholar] [CrossRef]
- Ferrara, R.M.; Bruno, M.R.; Campi, P.; Camposeo, S.; de Carolis, G.; Gaeta, L.; Martinelli, N.; Mastrorilli, M.; Modugno, A.F.; Mongelli, T.; et al. Water use of a super high-density olive orchard submitted to regulated deficit irrigation in Mediterranean environment over three contrasted years. Irrig. Sci. 2024, 42, 57–73. [Google Scholar] [CrossRef]
- Guerrero-Casado, J.; Carpio, A.J.; Tortosa, F.S.; Villanueva, A.J. Environmental challenges of intensive woody crops: The case of super high-density olive groves. Sci. Total Environ. 2021, 798, 149212. [Google Scholar] [CrossRef] [PubMed]
- Trentacoste, E.R.; Puertas, C.M.; Sadras, V.O. Effect of irrigation and tree density on vegetative growth, oil yield and water use efficiency in young olive orchard under arid conditions in Mendoza, Argentina. Irrig. Sci. 2015, 33, 429–440. [Google Scholar] [CrossRef]
- Navas-Lopez, J.F.; León, L.; Trentacoste, E.R.; de la Rosa, R. Multi-environment evaluation of oil accumulation pattern parameters in olive. Plant Physiol. Biochem. 2019, 139, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Gucci, R.; Lodolini, E.; Rapoport, H.F. Productivity of olive trees with different water status and crop load. J. Hortic. Sci. Biotechnol. 2007, 82, 648–656. [Google Scholar] [CrossRef]
- Trentacoste, E.R.; Puertas, C.M.; Sadras, V.O. Effect of fruit load on oil yield components and dynamics of fruit growth and oil accumulation in olive (Olea europaea L.). Eur. J. Agron. 2010, 32, 249–254. [Google Scholar] [CrossRef]
- Gucci, R.; Caruso, G.; Gennai, C.; Esposto, S.; Urbani, S.; Servili, M. Fruit growth, yield and oil quality changes induced by deficit irrigation at different stages of olive fruit development. Agric. Water Manag. 2019, 212, 88–98. [Google Scholar] [CrossRef]
- Siakou, M.; Bruggeman, A.; Eliades, M.; Zoumides, C.; Djuma, H.; Kyriacou, M.C.; Emmanouilidou, M.G.; Spyros, A.; Manolopoulou, E.; Moriana, A. Effects of deficit irrigation on ‘Koroneiki’ olive tree growth, physiology and olive oil quality at different harvest dates. Agric. Water Manag. 2021, 258, 107200. [Google Scholar] [CrossRef]
- Fernández, F.J.; Ladux, J.L.; Hammami, S.B.M.; Rapoport, H.F.; Searles, P.S. Fruit, mesocarp, and endocarp responses to crop load and to different estimates of source: Sink ratio in olive (cv. Arauco) at final harvest. Sci. Hortic. 2018, 234, 49–57. [Google Scholar] [CrossRef]
- Kokkotos, E.; Zotos, A.; Patakas, A. The Ecophysiological Response of Olive Trees under Different Fruit Loads. Life 2024, 14, 128. [Google Scholar] [CrossRef]
- Ivancic, T.; Jakopic, J.; Veberic, R.; Vesel, V.; Hudina, M. Effect of Ripening on the Phenolic and Sugar Contents in the Meso- and Epicarp of Olive Fruits (Olea europaea L.) Cultivar ‘Leccino’. Agriculture 2022, 12, 1347. [Google Scholar] [CrossRef]
- Rao, G.; Liu, X.; Zha, W.; Wu, W.; Zhang, J. Metabolomics reveals variation and correlation among different tissues of olive (Olea europaea L.). Biol. Open 2017, 6, 1317–1323. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Figueiredo, C.; Santos, C.; Silva, A.M.S. Phenolic and lipophilic metabolite adjustments in Olea europaea (olive) trees during drought stress and recovery. Phytochemistry 2021, 185, 112695. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL-cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13 of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033. [Google Scholar] [CrossRef]
- Jerman Klen, T.; Mozetič Vodopivec, B. The fate of olive fruit phenols during commercial olive oil processing: Traditional press versus continuous two- and three-phase centrifuge. LWT Food Sci. Technol. 2012, 49, 267–274. [Google Scholar] [CrossRef]
- Talhaoui, N.; Gómez-Caravaca, A.M.; León, L.; de la Rosa, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. From olive fruits to olive Oil: Phenolic compound transfer in six different olive cultivars grown under the same agronomical conditions. Int. J. Mol. Sci. 2016, 17, 337. [Google Scholar] [CrossRef]
- Ortega-García, F.; Peragón, J. Phenylalanine ammonia-lyase, polyphenol oxidase, and phenol concentration in fruits of Olea europaea L. cv. picual, verdial, arbequina, and frantoio during ripening. J. Agric. Food Chem. 2009, 57, 10331–10340. [Google Scholar] [CrossRef]
- Servili, M.; Sordini, B.; Esposto, S.; Taticchi, A.; Urbani, S.; Sebastiani, L. Metabolomics of Olive Fruit: A Focus on the Secondary In The Olive Tree Genome, Compendium of Plant Genomes; Rugini, E., Baldoni, L., Muleo, R., Sebastiani, L., Eds.; Springer: Cham, Switzerland, 2016; pp. 123–139. [Google Scholar] [CrossRef]
- Pierantozzi, P.; Torres, M.; Tivani, M.; Contreras, C.; Gentili, L.; Mastio, V.; Parera, C.; Maestri, D. Yield and chemical components from the constitutive parts of olive (cv. Genovesa) fruits are barely affected by spring deficit irrigation. J. Food Compos. Anal. 2021, 102, 104072. [Google Scholar] [CrossRef]
- Angilè, F.; Vivaldi, G.A.; Girelli, C.R.; del Coco, L.; Caponio, G.; Lopriore, G.; Fanizzi, F.P.; Camposeo, S. Treated Unconventional Waters Combined with Different Irrigation Strategies Affect 1 H NMR Metabolic Profile of a Monovarietal Extra Virgin Olive Oil. Sustainability 2022, 14, 1592. [Google Scholar] [CrossRef]
- Faghim, J.; Ben Mohamed, M.; Bagues, M.; Guasmi, F.; Triki, T.; Nagaz, K. Irrigation effects on phenolic profile and extra virgin olive oil quality of “‘Chemlali’” variety grown in South Tunisia. S. Afr. J. Bot. 2021, 141, 322–329. [Google Scholar] [CrossRef]
- García-Garví, J.M.; Sánchez-Bravo, P.; Hernández, F.; Sendra, E.; Corell, M.; Moriana, A.; Burgos-Hernández, A.; Carbonell-Barrachina, Á.A. Effect of Regulated Deficit Irrigation on the Quality of ‘Arbequina’ Extra Virgin Olive Oil Produced on a Super-High-Intensive Orchard. Agronomy 2022, 12, 1892. [Google Scholar] [CrossRef]
- Sastre, B.; Arbonés, A.; Pérez-Jiménez, M.Á.; Pascual, M.; Benito, A.; de Lorenzo, C.; Villar, J.M.; Bonet, L.J.; Paz, S.; Santos, Á.; et al. Influence of Regulated Deficit Irrigation on Arbequina’s Crop Yield and EVOOs Quality and Sensory Profile. Agronomy 2022, 13, 31. [Google Scholar] [CrossRef]
- Fernandes-Silva, A.; Oliveira, M.; APaço, T.; Ferreira, I. Deficit Irrigation in Mediterranean Fruit Trees and Grapevines: Water Stress Indicators and Crop Responses. In Irrigation in Agroecosystems; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Martinelli, F.; Basile, B.; Morelli, G.; d’Andria, R.; Tonutti, P. Effects of irrigation on fruit ripening behavior and metabolic changes in olive. Sci. Hortic. 2012, 144, 201–207. [Google Scholar] [CrossRef]
- Martinelli, F.; Remorini, D.; Saia, S.; Massai, R.; Tonutti, P. Metabolic profiling of ripe olive fruit in response to moderate water stress. Sci. Hortic. 2013, 159, 52–58. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration—Guidelines for Computing Crop Water Requirements—FAO Irrigation and Drainage Paper 56; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 1998. [Google Scholar]
- McCutchan, H.; Shackel, K.A. Stem-water potential as a sensitive indicator of water stress in prune trees (Prunus domestica L. cv. French). J. Am. Soc. Hortic. Sci. 1992, 117, 607–611. [Google Scholar] [CrossRef]
- International Olive Council. Guide for the Determination of the Characteristics of Oil-Olives. 2011. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-OH-Doc.-1-2011-Eng.pdf (accessed on 15 March 2023).
- García-Pérez, P.; Rocchetti, G.; Giuberti, G.; Lucchini, F.; Lucini, L. Phenolic acids, lignans, and low-molecular-weight phenolics exhibit the highest in vitro cellular bioavailability in different digested and faecal-fermented phenolics-rich plant extracts. Food Chem. 2023, 412, 135549. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. J. Biol. Databases Curation 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Salek, R.M.; Steinbeck, C.; Viant, M.R.; Goodacre, R.; Dunn, W.B. The role of reporting standards for metabolite annotation and identification in metabolomic studies. GigaScience 2013, 2, 1–3. [Google Scholar] [CrossRef]
- Falchi, R.; Bonghi, C.; Drincovich, M.F.; Famiani, F.; Lara, M.V.; Walker, R.P.; Vizzotto, G. Sugar Metabolism in Stone Fruit: Source-Sink Relationships and Environmental and Agronomical Effects. Front. Plant Sci. 2020, 11, 573982. [Google Scholar] [CrossRef]
- Lavee, S.; Wodner, M. The effect of yield, harvest time and fruit size on the oil content in fruits of irrigated olive trees (Olea europaea), cvs. Barnea and Manzanillo. Sci. Hortic. 2004, 99, 267–277. [Google Scholar] [CrossRef]
- Gonçalves, A.; Silva, E.; Brito, C.; Martins, S.; Pinto, L.; Dinis, L.T.; Luzio, A.; Martins-Gomes, C.; Fernandes-Silva, A.; Ribeiro, C.; et al. Olive tree physiology and chemical composition of fruits are modulated by different deficit irrigation strategies. J. Sci. Food Agric. 2020, 100, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Petridis, A.; Therios, I.; Samouris, G.; Koundouras, S.; Giannakoula, A. Effect of water deficit on leaf phenolic composition, gas exchange, oxidative damage and antioxidant activity of four Greek olive (Olea europaea L.) cultivars. Plant Physiol. Biochem. 2012, 60, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hueso, A.; Trentacoste, E.R.; Junquera, P.; Gómez-Miguel, V.; Gómez-del-Campo, M. Differences in stem water potential during oil synthesis determine fruit characteristics and production but not vegetative growth or return bloom in an olive hedgerow orchard (cv. Arbequina). Agric. Water Manag. 2019, 223, 105589. [Google Scholar] [CrossRef]
- Marra, F.P.; Marino, G.; Marchese, A.; Caruso, T. Effects of different irrigation regimes on a super-high-density olive grove cv. “Arbequina”: Vegetative growth, productivity and polyphenol content of the oil. Irrig. Sci. 2016, 34, 313–325. [Google Scholar] [CrossRef]
- Dag, A.M.; Kerem, Z.; Yogev, N.; Zipori, I.; Lavee, S.; Ben-David, E. Influence of time of harvest and maturity index on olive oil yield and quality. Sci. Hortic. 2011, 127, 358–366. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Kurtural, S.K. Source–Sink manipulations have major implications for grapevine berry and wine flavonoids and aromas that go beyond the changes in berry sugar accumulation. Food Res. Int. 2023, 169, 112826. [Google Scholar] [CrossRef]
- Nasini, L.; Proietti, P. Olive harvesting. In The Extra-Virgin Olive Oil Handbook; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 87–105. [Google Scholar] [CrossRef]
- Karagiannis, E.; Michailidis, M.; Skodra, C.; Stamatakis, G.; Dasenaki, M.; Ganopoulos, I.; Samiotaki, M.; Thomaidis, N.S.; Molassiotis, A.; Tanou, G. Proteo-metabolomic journey across olive drupe development and maturation. Food Chem. 2021, 363, 130339. [Google Scholar] [CrossRef]
- Mechri, B.; Tekaya, M.; Hammami, M.; Chehab, H. Effects of drought stress on phenolic accumulation in greenhouse-grown olive trees (Olea europaea). Biochem. Syst. Ecol. 2020, 92, 104112. [Google Scholar] [CrossRef]
- Buendía, B.; Allende, A.; Nicolás, E.; Alarcón, J.J.; Gil, M.I. Effect of regulated deficit irrigation and crop load on the antioxidant compounds of peaches. J. Agric. Food Chem. 2008, 56, 3601–3608. [Google Scholar] [CrossRef]
- Andreotti, C.; Ravaglia, D.; Costa, G. Effects of Fruit Load and Reflective Mulch on Phenolic Compounds Accumulation in Nectarine Fruit. Eur. J. Hort. Sci. 2010, 75, 53. [Google Scholar]
- Fotiadou, R.; Lefas, D.; Vougiouklaki, D.; Tsakni, A.; Houhoula, D.; Stamatis, H. Enzymatic Modification of Pomace Olive Oil with Natural Antioxidants: Effect on Oxidative Stability. Biomolecules 2023, 13, 1034. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, N.; El Jaziri, M.; Baucher, M.; Behr, M. UGT72, a major glycosyltransferase family for flavonoid and monolignol homeostasis in plants. Biology 2022, 11, 441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, S.; Yang, J.; Kang, C.; Huang, L.; Guo, L. Glycosylation of plant secondary metabolites: Regulating from chaos to harmony. Environ. Exp. Bot. 2022, 194, 104703. [Google Scholar] [CrossRef]
- Macoy, D.M.; Kim, W.Y.; Lee, S.Y.; Kim, M.G. Biosynthesis, physiology, and functions of hydroxycinnamic acid amides in plants. Plant Biotechnol. Rep. 2015, 9, 269–278. [Google Scholar] [CrossRef]
- Hoffmann, L.; Besseau, S.; Geoffroy, P.; Ritztenthaler, C.; Meyer, D.; Lapierre, C.; Pollet, B.; Legrand, M. Silencing of hydroxycinnamoyl-coenzyme A shikimate/quinate hydroxycinnamoyltransferase affects phenylpropanoid biosynthesis. Plant Cell 2004, 16, 1446–1465. [Google Scholar] [CrossRef]
- Alagna, F.; Mariotti, R.; Panara, F.; Caporali, S.; Urbani, S.; Veneziani, G.; Esposto, S.; Taticchi, A.; Rosati, A.; Rao, R.; et al. Olive phenolic compounds: Metabolic and transcriptional profiling during fruit development. BMC Plant Biol. 2012, 12, 162. [Google Scholar] [CrossRef]
- Machado, M.; Felizardo, C.; Fernandes-Silva, A.A.; Nunes, F.M.; Barros, A. Polyphenolic compounds, antioxidant activity and l-phenylalanine ammonia-lyase activity during ripening of olive cv. “Cobrançosa” under different irrigation regimes. Food Res. Int. 2013, 51, 412–421. [Google Scholar] [CrossRef]
- Cirilli, M.; Caruso, G.; Gennai, C.; Urbani, S.; Frioni, E.; Ruzzi, M.; Servili, M.; Gucci, R.; Poerio, E.; Muleo, R. The role of polyphenoloxidase, peroxidase, and β-glucosidase in phenolics accumulation in Olea europaea L. fruits under different water regimes. Front. Plant Sci. 2017, 8, 717. [Google Scholar] [CrossRef]

| Treatments | Sampling Dates | |||||||
|---|---|---|---|---|---|---|---|---|
| 06/23 | 07/19 | 08/11 | 09/02 | 09/23 | 10/07 | 10/25 | 11/15 | |
| Control | 0.0% ± 0.00 | 1.4% ± 0.22 | 4.8% ± 0.61 | 11.8% ± 0.43 | 12.7% ± 0.80 | 14.7% ± 1.02 | 19.3% ± 0.40 | 22.3% ± 0.29 |
| Thinning −33% | 0.0% ± 0.00 | 1.3% ± 0.12 | 5.8% ± 0.33 | 12.6% ± 0.38 | 12.6% ± 0.59 | 15.7% ± 0.61 | 20.3% ± 0.87 | 23.6% ± 1.13 |
| Thinning −50% | 0.0% ± 0.00 | 1.2% ± 0.25 | 6.1% ± 0.47 | 11.9% ± 0.27 | 12.8% ± 0.72 | 14.8% ± 0.27 | 19.6% ± 0.84 | 23.8% ± 0.72 |
| Thinning −66% | 0.0% ± 0.00 | 1.0% ± 0.26 | 6.3% ± 0.29 | 12.5% ± 0.38 | 12.6% ± 0.71 | 15.0% ± 0.76 | 19.2% ± 1.47 | 21.6% ± 0.96 |
| Factor (p-value) | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Control | 0.0% ± 0.00 | 1.4% ± 0.22 | 4.8% ± 0.61 | 11.8% ± 0.43 | 12.7% ± 0.80 a | 14.7% ± 1.02 | 19.3% ± 0.40 a | 22.3% ± 0.29 a |
| Irrigation −60% | 0.0% ± 0.00 | 1.1% ± 0.38 | 6.6% ± 0.72 | 9.6% ± 1.31 | 12.7% ± 0.38 a | 13.6% ± 0.71 | 19.3% ± 0.62 a | 21.2% ± 0.31 a |
| Irrigation −75% | 0.0% ± 0.00 | 1.8% ± 0.16 | 6.9% ± 0.56 | 9.4% ± 0.21 | 9.5% ± 0.61 b | 13.1% ± 1.17 | 14.2% ± 0.71 b | 19.3% ± 0.78 b |
| Factor (p-value) | n.s. | n.s. | n.s. | n.s. | 0.004 | n.s. | <0.001 | 0.003 |
| Sampling Date | Treatments | Fresh Weight (g) | Dry Weight (g) | Pulp–Stone Ratio | Maturity Index |
|---|---|---|---|---|---|
| 2 September | Control | 1.11 ± 0.06 | 0.55 ± 0.03 | 1.46 ± 0.03 | 2.1 ± 0.07 |
| Thinning −66% | 1.39 ± 0.05 | 0.71 ± 0.03 | 1.07 ± 0.11 | 2.9 ± 0.04 | |
| p-value | 0.006 | 0.005 | 0.011 | <0.001 | |
| Control | 1.11 ± 0.06 | 0.55 ± 0.03 | 1.46 ± 0.03 | 2.1 ± 0.07 | |
| Irrigation −75% | 0.63 ± 0.05 | 0.39 ± 0.03 | 1.01 ± 0.07 | 4.0 ± 0.07 | |
| p-value | <0.001 | 0.004 | <0.001 | <0.001 | |
| 23 September | Control | 1.40 ± 0.09 | 0.69 ± 0.05 | 1.80 ± 0.05 | 3.20 ± 0.09 |
| Thinning −66% | 1.50 ± 0.08 | 0.74 ± 0.05 | 1.93 ± 0.11 | 4.50 ± 0.06 | |
| p-value | n.s. | n.s. | n.s. | <0.001 | |
| Control | 1.40 ± 0.09 | 0.69 ± 0.05 | 1.80 ± 0.05 | 3.20 ± 0.09 | |
| Irrigation −75% | 0.90 ± 0.03 | 0.44 ± 0.01 | 1.65 ± 0.12 | 6.00 ± 0.11 | |
| p-value | <0.001 | 0.001 | n.s. | <0.001 | |
| 15 November | Control | 2.46 ± 0.08 | 1.10 ± 0.03 | 3.04 ± 0.08 | 6.94 ± 0.02 |
| Thinning −66% | 2.85 ± 0.15 | 1.41 ± 0.12 | 3.47 ± 0.20 | 7.00 ± 0.01 | |
| p-value | n.s. | 0.038 | n.s. | 0.043 | |
| Control | 2.46 ± 0.08 | 1.10 ± 0.03 | 3.04 ± 0.08 | 6.94 ± 0.02 | |
| Irrigation −75% | 1.89 ± 0.09 | 0.73 ± 0.01 | 1.78 ± 0.05 | 6.98 ± 0.03 | |
| p-value | 0.001 | <0.001 | <0.001 | n.s. | |
| Sampling Date | Treatments | Canopy Volume (m3/tree) | Production (kg/plant) | ||
| 15 November | Control | 2.69 ± 0.04 | 1.13 ± 0.15 | ||
| Thinning −66% | 3.15 ± 0.01 | 0.52 ± 0.09 | |||
| p-value | <0.001 | 0.009 | |||
| Control | 2.69 ± 0.04 | 1.13 ± 0.15 | |||
| Irrigation −75% | 1.97 ± 0.01 | 0.34 ± 0.08 | |||
| p-value | <0.001 | 0.003 | |||
| Phenolic Class | Phenolic Subclass | Compound Name | VIP Score 1 | LogFC 2 |
|---|---|---|---|---|
| Flavonoids | Flavones | Apigenin 6,8-di-C-glucoside | 2.31 ± 1.54 | −0.32 |
| Chalcones | Butein | 1.68 ± 0.79 | 1.12 | |
| Flavanones | 6-Prenylnaringenin | 1.65 ± 1.82 | −0.38 | |
| Flavones | Luteolin 7-O-glucuronide | 1.63 ± 1.93 | 0.34 | |
| Anthocyanins | Malvidin 3-O-(6″-p-coumaroyl-glucoside) | 1.62 ± 1.20 | 4.00 | |
| Isoflavonoids | Glycitin | 1.59 ± 2.67 | 0.68 | |
| Flavones | Isorhoifolin | 1.55 ± 0.84 | −0.28 | |
| Flavonols | Spinacetin 3-O-(2″-p-coumaroylglucosyl) (1–6)-[apiosyl(1–2)]-glucoside | 1.44 ± 1.95 | −0.75 | |
| Flavones | 5,6-Dihydroxy-7,8,3′,4′-tetramethoxyflavone | 1.42 ± 0.87 | 1.11 | |
| Anthocyanins | Cyanidin 3-O-rutinoside | 1.41 ± 1.32 | −0.21 | |
| Flavonols | Spinacetin 3-O-glucosyl-(1–6)-[apiosyl(1–2)]-glucoside | 1.37 ± 1.38 | −4.00 | |
| Flavanols | Theaflavin | 1.35 ± 0.81 | −0.63 | |
| Flavanols | (+)-Catechin | 1.35 ± 1.49 | 0.13 | |
| Lignans | Lignans | Conidendrin | 1.50 ± 1.12 | 0.36 |
| Lignans | Secoisolariciresinol | 1.41 ± 1.34 | −0.52 | |
| LMW and others | Hydroxycinnamaldehydes | Ferulaldehyde | 1.64 ± 1.07 | 0.27 |
| Alkylphenols | 3-Methylcatechol | 1.41 ± 1.08 | 0.38 | |
| Hydroxyphenylpropenes | Acetyl eugenol | 1.35 ± 1.36 | −0.32 | |
| Tyrosols | p-HPEA-EDA | 1.34 ± 1.04 | 0.28 | |
| Tyrosols | p-HPEA-AC | 1.32 ± 1.84 | −0.71 | |
| Other polyphenols | Coumestrol | 1.30 ± 1.49 | −4.00 | |
| Phenolic acids | Hydroxycinnamic acids | Sinapine | 1.92 ± 2.32 | 1.35 |
| Hydroxybenzoic acids | Ellagic acid | 1.58 ± 1.15 | 0.80 | |
| Hydroxycinnamic acids | 3,4-Dicaffeoylquinic acid | 1.50 ± 1.54 | 1.18 | |
| Hydroxycinnamic acids | p-Coumaric acid ethyl ester | 1.43 ± 1.22 | −0.15 | |
| Hydroxycinnamic acids | 1,2-Diferuloylgentiobiose | 1.38 ± 1.10 | −0.28 | |
| Stilbenes | Stilbenes | d-Viniferin | 1.76 ± 1.17 | 0.80 |
| Phenolic Class | Phenolic Subclass | Compound Name | VIP Score 1 | LogFC 2 |
|---|---|---|---|---|
| Flavonoids | Dihydrochalcones | Phloretin 2′-O-xylosyl-glucoside | 1.60 ± 0.54 | 1.65 |
| Flavanones | Naringenin 7-O-glucoside | 1.58 ± 0.51 | 0.48 | |
| Anthocyanins | Petunidin 3-O-rhamnoside | 1.41 ± 0.38 | 0.27 | |
| Flavones | Apigenin 6,8-di-C-glucoside | 1.40 ± 0.26 | 0.07 | |
| Flavonols | Myricetin 3-O-rhamnoside | 1.39 ± 0.57 | 0.15 | |
| Lignans | Lignans | Cyclolariciresinol | 1.63 ± 0.35 | 1.38 |
| Lignans | Secoisolariciresinol | 1.56 ± 0.41 | 0.20 | |
| Lignans | Sesamol | 1.42 ± 0.67 | 1.09 | |
| LMW and others | Other polyphenols | Pyrogallol | 1.54 ± 0.63 | 0.72 |
| Curcuminoids | Curcumin | 1.53 ± 0.73 | 0.55 | |
| Tyrosols | Ligstroside | 1.52 ± 0.33 | 1.42 | |
| Hydroxycoumarins | Scopoletin | 1.47 ± 0.56 | 0.75 | |
| Other polyphenols | 3,4-Dihydroxyphenylglycol | 1.38 ± 0.63 | 0.48 | |
| Phenolic acids | Hydroxycinnamic acids | Cinnamic acid | 1.66 ± 0.74 | 0.94 |
| Hydroxycinnamic acids | Sinapine | 1.65 ± 0.76 | 2.33 | |
| Hydroxycinnamic acids | Caffeoyl aspartic acid | 1.60 ± 0.50 | 0.79 | |
| Hydroxycinnamic acids | 3-Sinapoylquinic acid | 1.59 ± 0.42 | 1.25 | |
| Hydroxycinnamic acids | p-Coumaric acid | 1.44 ± 1.01 | 1.92 | |
| Hydroxycinnamic acids | 1-Sinapoyl-2-feruloylgentiobiose | 1.38 ± 0.52 | 0.68 | |
| Hydroxycinnamic acids | 1,2-Diferuloylgentiobiose | 1.37 ± 1.09 | 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farolfi, C.; Tombesi, S.; Lucini, L.; Capri, E.; García-Pérez, P. Influence of Fruit Load and Water Deficit on Olive Fruit Phenolic Profiling and Yield. Int. J. Plant Biol. 2024, 15, 895-913. https://doi.org/10.3390/ijpb15030064
Farolfi C, Tombesi S, Lucini L, Capri E, García-Pérez P. Influence of Fruit Load and Water Deficit on Olive Fruit Phenolic Profiling and Yield. International Journal of Plant Biology. 2024; 15(3):895-913. https://doi.org/10.3390/ijpb15030064
Chicago/Turabian StyleFarolfi, Camilla, Sergio Tombesi, Luigi Lucini, Ettore Capri, and Pascual García-Pérez. 2024. "Influence of Fruit Load and Water Deficit on Olive Fruit Phenolic Profiling and Yield" International Journal of Plant Biology 15, no. 3: 895-913. https://doi.org/10.3390/ijpb15030064
APA StyleFarolfi, C., Tombesi, S., Lucini, L., Capri, E., & García-Pérez, P. (2024). Influence of Fruit Load and Water Deficit on Olive Fruit Phenolic Profiling and Yield. International Journal of Plant Biology, 15(3), 895-913. https://doi.org/10.3390/ijpb15030064









