Abstract
Minicutting has been used for several woody species, presenting greater efficiency than propagation by conventional cutting due to its high yield of propagative material and higher percentage of rooting in minicuttings. This work was carried out to evaluate the efficiency of minicutting techniques for the ‘Bluegem’ blueberry and find the best concentration of IBA for rooting the minicuttings. The minigarden was installed in pots using micropropagated plants. Four collections of minicuttings were carried out between 27 October 2020 and 12 April 2021, and received the following treatments: 0; 2000; 4000; 6000; and 8000 mg L−1 of IBA. The minicutting was carried out in a mist chamber and evaluated after 60 days. The yield of minicuttings increased from the first to the third collection, decreasing in the fourth collection when the plants began to enter dormancy. The rooting of the minicuttings was influenced by the collection time and the application of IBA. Increasing IBA concentration increased the percentage of rooted minicuttings and root dry mass to concentrations close to 5000 mg L−1 on almost all collection dates. High concentrations of IBA reduced the percentage of sprouted minicuttings and leaf retention and increased mortality. It is recommended for the ‘Bluegem’ blueberry minicutting to apply 5000 mg L−1 of IBA.
1. Introduction
The demand for blueberry seedlings has increased worldwide due to the expansion of its cultivation area. In the period from 2012 to 2022, the global cultivated area of blueberries increased by 78.5%, from 97,439 ha to 173,924 ha [1]. Considering an average planting density of 2222 plants per hectare, more than 169 million seedlings were planted during this period. Therefore, nurseries need to adopt more efficient techniques for rapid and large-quantity propagation of blueberries.
Cutting is the most used technique for blueberry propagation, but several factors affect the formation of adventitious roots, such as the genetic potential of each cultivar [2,3], the collection time and the type of cutting [4,5], and the use of auxins [6]. Woody cuttings are considered difficult to root, with better results obtained with green cuttings collected during the blueberry bushes most intense vegetative growth period, which generally occurs in summer [4,5]. Another way of producing seedlings is micropropagation, which is highly efficient, as has already been achieved with blueberries [7]. However, there are few nurseries that have a tissue culture laboratory to carry out this technique. Therefore, it is necessary to develop more efficient vegetative propagation techniques for the rapid production of blueberry seedlings.
Minicutting is a technique that produces good results for species that are difficult to root. This technique has some advantages for cuttings, such as greater phytosanitary control of the parent plants, a smaller area for the formation of the clonal minigarden concerning stock plants maintained in the field, greater production of minicuttings per unit area, and greater efficiency in the propagation of species. difficult to root [8,9,10].
Minicutting was initially used on a commercial scale for eucalyptus (Eucalyptus spp.) [11,12,13] and showed good results for other forest species, such as Piptocarpha angustifolia [14], Handroanthus heptaphyllus [15], Acacia mearsii [16], and Aniba rosaeodora [10]. It has also been used with good results for fruit species, such as Psidium guineense, Psidium cattleyanum, and Psidium guajava [17], Eugenia uniflora [18], and blackberry [19].
Blueberry propagation has already been carried out using minicutting, demonstrating the viability of the technique. However, it was observed that the efficiency of the technique is affected by the rooting capacity of the cultivars [3], by the time of year when the minicutting is carried out [20,21], by the substrate [22], by the type of minicuttings [23], and by the size of the minicuttings [24]. The application of IBA also influences root formation, and there is no consensus on the most effective concentration, with studies found using 1000 mg L−1 [24], 2000 mg L−1 [23], and 3000 mg L−1 [22].
Considering the uncertainty regarding the ideal concentration for blueberry minicutting and the variation in response about the time of year for collecting minicuttings, this work was carried out to define a minicutting protocol for the ‘Bluegem’ blueberry.
2. Materials and Methods
The ‘Bluegem’ blueberry stock plants used in this experiment were micropropagated following a typical protocol [7]. After acclimatization, the plants were grown in pots in a greenhouse. The experiment was conducted from September 2021 to February 2022, in Curitiba, PR, Brazil.
The substrate used was a mixture of Mecplant® (MecPlant, Telêmaco Borba, PR, Brazil) commercial substrate and vermiculite, in a 2:1 ratio, and for initial fertilization, slow-release fertilizer was applied (Basacote® Plus 3M 16-8-12 plus micronutrients, Compo Expert GmbH, Munster, Germany) at a concentration of 3 g L−1 of substrate.
The minigarden was installed in 5 L pots, with one plant per pot. The pots were placed on a bench occupying a space of 1.7 m2. The minigarden was watered according to water needs and received fertilization with 3 g of Basacote® per pot after each collection of cuttings. The plants were pruned, leaving the branches approximately 15 cm long on 28 August 2020 (Figure 1A).

Figure 1.
‘Bluegem’ blueberry clonal minigarden: (A) Minigarden after pruning on 28 August 2020; (B) Minigarden before collecting branches on 27 October 2020; (C) Minigarden after collecting branches on 27 October 2020; (D) Minigarden after collecting the branches on 21 December 2020.
Four collections were carried out on 27 October 2020 (early spring) (Figure 1C), 21 December 2020 (first day of summer) (Figure 1D), 15 February 2021 (mid-summer), and 12 April 2021 (early autumn), with an interval of 55 days between the first and second collection and 56 days between the rest. In each collection, the number (Figure 2A) and length (Figure 2B) of shoots produced by the clonal minigarden were evaluated.

Figure 2.
Preparation of ‘Bluegem’ blueberry minicuttings: (A) Blueberry branches immediately after collection; (B) Branch is about 50 cm long; (C) Branch segmented into 8 cm long minicuttings; (D) Minicuttings prepared with a pair of leaves cut in half; (E) Treatment of the base of minicuttings in IBA solution; (F) Minicuttings after installation of the experiment, in tubes with commercial substrate.
For each collection, an experiment was set up with a completely randomized design, containing 5 treatments, 4 replications, and 10 minicuttings per plot. The treatments included 0, 2000, 4000, 6000, and 8000 mg L−1 of IBA (Neon®, 99,45%, Neon Reagentes Analíticos, Suzano, SP, Brazil). The IBA treatment was carried out by immersing the base of the cuttings for 10 s in the IBA solution diluted in 50% ethanol (Figure 2E). The minicuttings were standardized to be 8 cm long (Figure 2C), containing two buds in the apical part and two leaves cut in half (Figure 2D).
The cuttings were planted in 140 cm3 plastic tubes containing Agrofior F6® (Agrofior Produção de Mudas, Colombo, PR, Brazil) commercial substrate, whose composition was made up of pine bark, Sphagnum peat, and carbonized rice husk, pH 6.0, electrical conductivity of 0.7 mS cm−1, and dry density of 288 kg m−3. The cuttings were kept in a mist chamber (Figure 2F). The watering frequency was 30 s every 30 min during the period from 8 a.m. to 7 p.m. and 30 s every 3 h during the period from 7 p.m. to 8 a.m.
The temperature was not determined inside the greenhouse. The maximum, average, and minimum temperatures that occurred during the experiment were obtained from the Meteorological Station of the National Institute of Meteorology (INMET), located in Curitiba-PR, Brazil.
Assessments were carried out 60 days after the installation of each collection. The number of minicuttings with dead, sprouted, or callus roots that maintained their leaves and the dry mass of roots per minicutting were evaluated. Rooted minicuttings were defined as those possessing at least one root measuring longer than 1 mm. Leaf retention was calculated by the percentage of minicuttings that had leaves left during their preparation. The data were analyzed by an analysis of variance, and the means were subjected to regression analysis using the SISVAR® program (Version 5.8) [25].
3. Results and Discussion
The clonal minigarden showed a high survival rate of the ministumps, with no death of mother plants occurring during the experiment. The minigarden yield increased from the first to the second collection and decreased in the fourth collection (Table 1). In the first collection, 71.2 branches m−2 were obtained; in the second, 206.2 branches m−2; in the third, 243.6 branches m−2; and the fourth, 83.6 branches m−2. The length of the branches did not vary greatly, remaining between 18.9 and 20.4 cm. The increase in the number of shoots per plant from the first to the second collection was from 4.7 to 14.6, which is justified by the pruning carried out and the increase in the number of buds on the mother plant. But this number dropped to 5.5 in the fourth collection.

Table 1.
Number of shoots emitted per plant, average length of shoots, number and total length of shoots formed per m2 in a clonal minigarden of ‘Bluegem’ blueberry micropropagated during four collections.
The reduction in the number of shoots per plant in the fourth collection carried out in April occurred due to the arrival of autumn. This was also observed in other species, where collections carried out in autumn and winter showed a lower yield of minicuttings compared to the warmer seasons of the year [18,19,26]. Thus, the ministumps showed a reduction in vegetative growth activity after the third collection, and after the fourth collection, no more shoot growth would allow new collections. The blueberry is a temperate species that has a dormant period during the coldest months of the year. In the region where this research was carried out, under field conditions, the Bluegem cultivar enters dormancy in April [27].
By 22 February 2020, there had been a noticeable decline in average temperatures, settling at 18.5 °C up to the fourth collection date. This marked a decrease from the 20.5 °C average recorded between the second and third collections. The maximum temperatures that occurred during the collections were 25.7 °C in the first collection, 26.9 °C in the second collection, and 26.5 °C in the third collection. In the fourth collection, the maximum temperature was much lower, just 21.8 °C (Figure 3). Following the fourth collection, the plants commenced their dormancy phase.
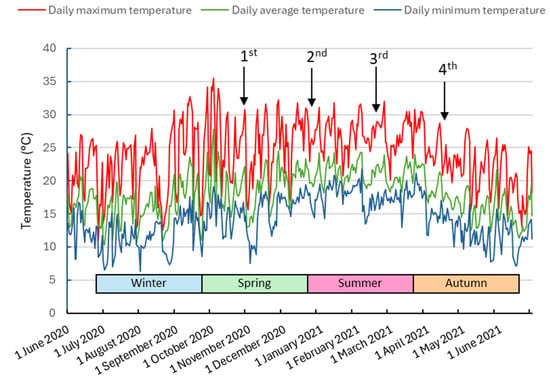
Figure 3.
Maximum, average, and minimum daily temperature for the period from 1 June 2020 to 30 June 2021 in Curitiba-PR. Arrows indicate the dates of the 1st, 2nd, 3rd, and 4th collection of minicuttings.
The increase in the number of shoots emitted per plant resulted in a proportional increase in the total length of shoots formed per square meter, allowing the obtaining of 168, 525, 579, and 197 minicuttings per m2 in the first, second, third, and fourth collections, respectively (Table 1). In a blueberry minigarden of the cultivar Climax, the highest number of minicuttings was obtained in the third collection carried out in February, with 182.9 minicuttings per square meter [20]. The collection in February also resulted in the largest number of minicuttings (579 m−2) in this work for the ‘Bluegem’, but with a much higher yield, probably because in this study, pots with a capacity of 5 L were used, while in the work with the cultivar Climax, there were 1 L pots, and also due to the interval between collections, which was around 30 days in the work with ‘Climax’ and 60 days in this work with ‘Bluegem’.
In the minicutting with the ‘Xingu’ blackberry there was also an increase in the minigarden’s yield up until the collection conducted in January, which reached 365 minicuttings m−2, with a reduction in the collection carried out in March, with the production of 219 minicuttings m−2 [19]. In the minigarden with the seminal origin from pitangueira (Eugenia uniflora), installed in 240 cm3 tubes, eight collections were carried out over one year, with an average of 616 minicuttings obtained per collection [18]. In the minigarden with the seminal origin of araçazeiro (Psidium guineense and Psidium cattleyanum) and guava tree (Psidium guajava), grown in 280 cm3 tubes, it was possible to carry out seven collections of minicuttings in 397 days. There was an increase in the number of shoots emitted by ministumps up to 166 days after the first pruning, with the production of 3.99, 4.83, and 3.62 shoots per ministump, with an average length of 5.88, 4.56, and 11.51 cm, respectively [17].
The percentage of rooted minicuttings was influenced by the application of IBA at all collection times, but in a different way. In the first three collections, the increase in IBA concentration promoted an increase in the rooting percentage up to estimated concentrations of 4500 mg L−1, 6000 mg L−1, and 5000 mg L−1, for the first, second, and third collections, respectively. In the fourth collection, the response to the application of IBA was linear, with the highest percentage of rooting (72%) being obtained with a concentration of 8000 mg L−1 (Figure 4a). This probably happened because in the fourth collection, carried out in April, the plants were already entering dormancy [27], which is a period in which the concentration of growth hormones tends to decrease, requiring a greater amount of exogenous auxin for the formation of adventitious roots. In the propagation by minicutting of the same cultivar, carried out in February with minicuttings with a whole leaf, treated with 2000 mg L−1 of IBA, and evaluated after 90 days, 95% rooting was obtained [3]. In another experiment with the Powderblue cultivar, it was observed that the rooting percentage was 55% when the evaluation was carried out 70 days after installation and 84% after 110 days [22]. This may have been one of the reasons for the lower percentage of rooting found in the present study, which was evaluated after 60 days. However, adventitious formation in blueberry cuttings is influenced by many factors, including the nutritional and physiological condition of the mother plant [20], collection time [4,5], basal lesions in the cuttings [21], and substrate used for rooting the cuttings [23].
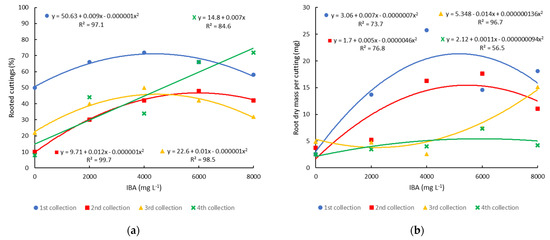
Figure 4.
Propagation of blueberry cultivar Bluegem by minicutting on four collection dates (1st collection—27 October 2020; 2nd collection—21 December 2020; 3rd collection—12 February 2021; and 4th collection—12 April 2021) and treated with different concentrations of IBA: (a) percentage of rooted minicuttings; and (b) dry mass of roots formed per minicutting.
In semi-hardwood cuttings of the Bluegem cultivar, using 15 cm long cuttings with two leaves cut in half and treated with IBA, up to a concentration of 2000 mg L−1, average rooting percentages of 47.5%, 57.1%, and 27.4% were obtained in the collections carried out on 15th October, 15th January, and 15th April, respectively [4]. These values were lower than those obtained in this study, with the minicutting yield of the clonal minigarden being much higher in terms of the number of minicuttings produced per square meter.
Root formation in minicuttings, measured by root dry mass, showed a similar response, with an increase in dry mass up to estimated concentrations of 5000 mg L−1, 5434 mg L−1, and 6111 mg L−1, for the first, second, and fourth collection, respectively. In the third collection, the highest dry mass of roots was obtained with a concentration of 8000 mg L−1 (Figure 4b). The highest root dry mass values found in this study with the Bluegem cultivar were between 15 and 25 mg, which are close to those found with the Powderblue cultivar, whose minicutting was carried out in March with the application of 3000 mg L−1 of IBA in the form of talc and liquid and on three different substrates. Root dry mass was higher in the pine bark substrate (27 mg) than in vermiculite (11.8 mg) [22].
The sprouting of minicuttings was influenced by the application of IBA only in the third and fourth collections, where higher concentrations of IBA reduced sprouting. In the fourth collection, there was the lowest percentage of minicuttings sprouted due to the plants beginning to enter dormancy, which was further reduced with the increase in IBA concentration, reaching just 2% sprouting with the application of 8000 mg L−1. In the first and second collections, there was no significant effect of IBA, and the average sprouting percentage was 52.8% and 21.2%, respectively (Figure 5a).
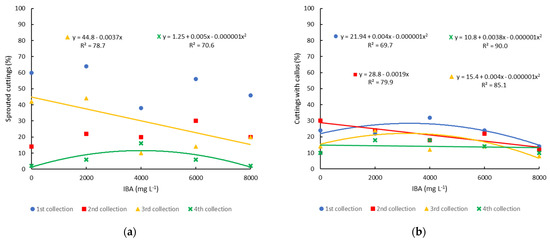
Figure 5.
Propagation of blueberry cultivar Bluegem by minicutting on four collection dates (1st collection—27 October 2020; 2nd collection—21 December 2020; 3rd collection—12 February 2021; 4th collection—12 April 2021) and treated with different concentrations of IBA: (a) percentage of minicuttings sprouted; and (b) percentage of minicuttings with callus.
Callus formation occurred in all collection periods similarly, with values between 10.8 and 28.8% (Figure 5b). The formation of callus occurred independently of the rooting of the cuttings, with cuttings being found with only callus and no roots (Figure 6F), with callus and roots (Figure 6G), and only roots (Figure 6H). Callus formation in this cultivar was also low in semi-hardwood cuttings, with the highest percentage of cuttings with calluses (13%) found in spring (15 October) [4].
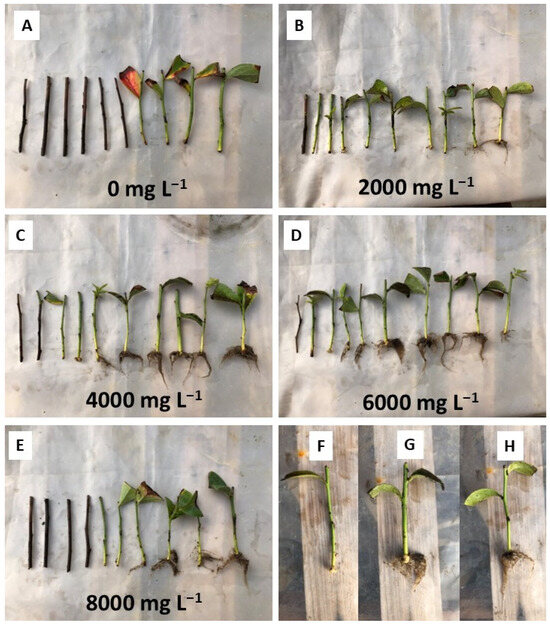
Figure 6.
Appearance of the minicuttings 60 days after the installation of the experiment on December 21, 2020: (A) Control treatment without application of IBA; (B) Treatment with 2000 mg L−1 of IBA; (C) Treatment with 4000 mg L−1 of IBA; (D) Treatment with 6000 mg L−1 of IBA; (E) Treatment with 8000 mg L−1 of IBA; (F) Minicutting with callus and no roots; (G) Minicutting with callus and roots; (H) Minicutting with roots and no callus.
The percentage of dead cuttings was very low in the first and fourth collections, with values between 0 and 5%. In the second and third collections, mortality was higher and increased with the increase in IBA concentration, reaching values close to 45% with the application of 8000 mg L−1 of IBA (Figure 7a). The death of the cuttings must be related to the higher temperatures that occurred during the summer period when these collection periods were installed. Leaf retention was highest in the fourth (88.4%) and first collections (58%) (Figure 7b). In the second and third collections, there was lower leaf retention, which must also be associated with higher temperatures during the cutting period on these dates. Increasing IBA concentration reduced the percentage of retained leaves, demonstrating that higher concentrations had a toxic effect on blueberry cuttings. In the propagation by minicutting of the Climax cultivar in Brazil, high values of leaf retention (97.5%) were observed, even in the summer months (December, January, and February) [20], indicating that the environmental conditions at the minicutting site were maintained properly to avoid dehydration and leaf fall.
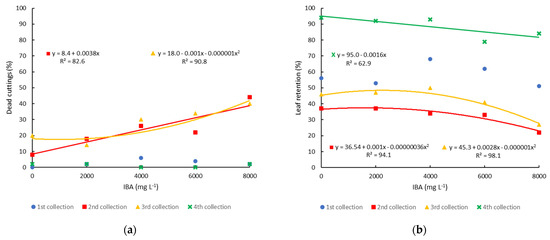
Figure 7.
Propagation of blueberry cultivar Bluegem by minicutting on four collection dates (1st collection—27 October 2020; 2nd collection—21 December 2020; 3rd collection—12 February 2021; and 4th collection—12 April 2021) and treated with different concentrations of IBA: (a) percentage of dead minicuttings; and (b) percentage of leaf retention.
4. Conclusions
Minicutting is an efficient technique for the rapid and large-scale propagation of the ‘Bluegem’ blueberry. The application of 5000 mg L−1 of IBA is recommended to increase the percentage of rooting and root formation in minicuttings.
Author Contributions
Conceptualization, L.A.B.; formal analysis, J.R.P. and A.C.C.; investigation, J.R.P. and A.C.C.; resources, L.A.B. and R.A.A.; writing—original draft preparation, L.A.B.; writing—review and editing, R.A.A.; supervision, L.A.B.; funding acquisition, L.A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) grant number 307.705/2021-8 to L.A.B. and financed, in part, by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—Finance Code 001, granting scholarships to J.R.P. and A.C.C.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAOSTAT. Food and Agriculture Organization of the United Nations. Crops and livestock products. Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 19 April 2024).
- Peña, M.L.P.; Gubert, C.; Tagliani, M.C.; Bueno, P.M.C.; Biasi, L.A. Concentrações e formas de aplicação do ácido indolbutírico na propagação por estaquia dos mirtileiros cvs. Flórida e Clímax. Semin. Ciênc. Agrár. 2012, 33, 57–64. [Google Scholar]
- Giacobbo, C.L.; Fischer, D.L.O.; Fischer, C.; Fischer, L.O.; Galina, J.; Damis, R. Rooting of semi-hardwood blueberry mini-cuttings from different cultivars from the highbush and rabbiteye groups. Sci. Electron. Arch. 2023, 16, 9–14. [Google Scholar]
- Marangon, M.A.; Biasi, L.A. Estaquia de mirtileiros nas estações do ano com ácido indolbutírico e aquecimento do substrato. Pesqui. Agropecu. Bras. 2013, 48, 25–32. [Google Scholar]
- Higuchi, M.T.; Ribeiro, L.T.M.; Aguiar, A.C.; Zeffa, D.M.; Roberto, S.R.; Koyama, R. Methods of application of indolebutyric acid and basal lesion on ‘Woodard’ blueberry cuttings in different seasons. Rev. Bras. Frutic. 2021, 43, e-022. [Google Scholar]
- An, H.; Meng, J.; Xu, F.; Jiang, S.; Wang, X.; Shi, C.; Zhou, B.; Luo, J.; Zhang, X. Rooting ability of hardwood cuttings in highbush blueberry (Vaccinium corymbosum L.) using different indole-butyric acid concentrations. HortScience 2019, 54, 194–199. [Google Scholar]
- Schuchovski, C.; Biasi, L.A. Micropropagation of Vaccinium virgatum ‘Delite’: A rabbiteye cultivar adapted to mild winters. Plant Biosyst. 2022, 156, 1117–1128. [Google Scholar]
- Xavier, A.; Wendling, I.; Silva, R.L. Silvicultura Clonal: Princípios e Técnicas; Ed UFV: Viçosa, Brazil, 2009. [Google Scholar]
- Dias, P.C.; Oliveira, L.S.; Xavier, A.; Wendling, I. Estaquia e miniestaquia de espécies florestais lenhosas do Brasil. Pesq. Florest. Bras. 2012, 32, 453–462. [Google Scholar]
- Menezes, A.; Sampaio, P.T.B.; Blind, A.D. Propagação de pau-rosa (Aniba rosaeodora Ducke) por estacas e miniestacas. Nucleus 2018, 15, 515–521. [Google Scholar]
- Wendling, I.; Xavier, A.; Gomes, J.M.; Pires, I.E.; Andrade, H.B. Propagação clonal de híbridos de Eucalyptus spp. por mini-estaquia. Rev. Árvore 2000, 24, 181–186. [Google Scholar]
- Xavier, A.; Andrade, H.B.; Oliveira, M.L.; Wendling, I. Desempenho do enraizamento de micro-estacas e mini-estacas de clones híbridos de Eucalyptus grandis. Rev. Árvore 2001, 25, 403–411. [Google Scholar]
- Brondani, G.E.; Baccarin, F.J.B.; Bergonci, T.; Gonçalves, A.N.; Almeida, M. Miniestaquia de Eucalyptus benthamii: Efeito do genótipo, AIB, zinco, boro e coletas de brotações. Cerne 2014, 20, 147–156. [Google Scholar]
- Ferriani, A.P.; Zuffellato-Ribas, K.C.; Helm, C.V.; Boza, A.; Wendling, I.; Koehler, H.S. Produção de brotações e enraizamento de miniestacas de Piptocarpha angustifolia. Pesq. Florest. Bras. 2011, 31, 257–264. [Google Scholar]
- Oliveira, T.P.F.; Barroso, D.G.; Lamônica, K.R.; Carvalho, G.C.M.W. Aplicação de AIB e tipo de miniestacas na produção de mudas de Handroanthus heptaphyllus Mattos. Ciênc. Florest. 2016, 26, 313–320. [Google Scholar]
- Engel, M.L.; Higa, A.R.; Alcantara, G.B.; Flôres Junior, P.C.; Soares, I.D. Enraizamento de miniestacas de diferentes clones de Acacia mearnsii De Wildeman com aplicação de AIB. Rev. Espac. 2017, 38, 8. [Google Scholar]
- Altoé, J.A.; Marinho, C.S.; Terra, M.I.C.; Barroso, D.G. Propagação de araçazeiro e goiabeira via miniestaquia de material juvenil. Bragantia 2011, 70, 312–318. [Google Scholar]
- Peña, M.L.P.; Zanette, F.; Biasi, L.A. Época de coleta e ácido indolbutírico no enraizamento de miniestacas de pitangueira. Semin. Ciênc. Agrár. 2015, 36, 3055–3068. [Google Scholar]
- Joaquini, F.A.; Biasi, L.A.; Tofanelli, M.B.D. Propagação clonal rápida de amoreira preta ‘Xingu’ por miniestaquia. Res. Soc. Dev. 2021, 10, e15910111239. [Google Scholar]
- Stuepp, C.A.; Amaral, B.A.D.; Ayub, R.A.; Fragoso, R.D.O. Pruning management of mini-stumps for mass propagation of blueberry. Pesqui. Agropecu. Bras. 2021, 56, e02486. [Google Scholar]
- Koyama, R.; Hussain, I.; Shahab, M.; Ahmed, S.; Assis, A.M.; Zeffa, D.M.; Antunes, L.D.C.; Roberto, S.R. Indole butyric acid application methods in ‘Brite Blue’ blueberry cuttings collected in different seasons. Rev. Bras. Ciênc. Agrár. 2019, 14, e6542. [Google Scholar]
- Colombo, R.C.; Carvalho, D.U.; Cruz, M.A.; Roberto, S.R. Blueberry propagation by minicuttings in response to substrates and indolebutyric acid application methods. J. Agric. Sci. 2018, 10, 450–458. [Google Scholar]
- Pelizza, T.R.; Damiani, C.R.; Rufato, A.R.; Souza, A.L.K.; Ribeiro, M.F.; Schuch, M.W. Microestaquia em mirtileiro com diferentes porções do ramo e substratos. Bragantia 2011, 70, 319–324. [Google Scholar]
- Koyama, R.; Assis, A.M.; Borges, W.F.S.; Yamamoto, L.Y.; Colombo, R.C.; Zeffa, D.M.; Barros, L.G.; Barreira, B.; Hussain, I.; Shahab, M.; et al. Multiplication of blueberry mini-cuttings in different growth media. Agron. Sci. Biotechnol. 2018, 4, 28–35. [Google Scholar]
- Ferreira, D.F. Sisvar: Um sistema computacional de análise estatística. Ciênc. Agrotec. 2011, 35, 1039–1042. [Google Scholar]
- Ferreira, B.G.A.; Zuffellato-Ribas, K.C.; Wendling, I.; Koehler, H.S.; Nogueira, A.C. Miniestaquia de Sapium glandulatum (Vell.) Pax com o uso de ácido indol butírico e ácido naftaleno acético. Ciênc. Florest. 2010, 20, 19–31. [Google Scholar]
- Schuchovski, C.; Biasi, L.A. Dormancy of floral buds of Rabbiteye blueberry in a mild winter climate. Braz. Arch. Biol. Technol. 2021, 64, e21190755. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).