Abstract
Elephant grass (Pennisetum purpureum) exhibits high biomass production, is readily accepted by animals, and demonstrates good adaptation to the various soil types. Consequently, the utilization of P. purpureum shows promise in amending surface and subsurface soil acidity, thereby contributing to increased crop yield while enhancing water and nutrient utilization efficiency. In this study, the effects of corrective processes involving limestone and plastering on the root anatomy of P. purpureum were investigated. Roots subjected to different treatments were fixed, dehydrated, and embedded in historesin. Subsequently, samples were sectioned in a microtome, stained with toluidine blue for the conventional analysis, or submitted to the histochemical test. The qualitative and quantitative anatomical analyses were conducted to evaluate the impact of liming and plastering on the root structure of P. purpureum. The results showed that liming led to an increase in both the number and diameter of vessel elements, while plastering reduced these parameters compared to the acidic soils. Additionally, liming induced the formation of suberized endodermal cell walls. These findings highlight the significance of effective soil management to obtain P. purpureum plants with a well-developed vascular system, thereby promoting optimal plant performance in agricultural crops.
1. Introduction
Agriculture plays an important role in the Brazilian economy. According to data from the Center for Advanced Studies in Applied Economics (Cepea—Esalq/USP) and the Confederation of Agriculture and Livestock in Brazil (CNA), the Gross Domestic Product (GPD) of Brazilian agribusiness grew by 35.9% in 2023 compared to 2022, reaching USD 517 billion. Animal feed has a major role in animal production, mainly in terms of viable pastures and forages for animal nutrition. In this context, elephant grass (Pennisetum purpureum Schum) emerges as a highly promising option for biomass production, displaying superior forage quality, vigor, and resilience. While it is primarily used in grazing, Pennisetum purpureum can also be effectively used in ensiling and as feedstock [1].
Therefore, considering the benefits of P. purpureum, improving soil conditions is essential for boosting crop yields. This requires comprehensive analyses to assess soil fertility, incorporating data on acidity and nutrient availability. Liming practices are essential to obtain high yields by improving soil conditions, including raising pH levels and decreasing exchangeable aluminum (Al) content, while increasing levels of available calcium (Ca) and magnesium (Mg), and enhancing phosphorus (P) availability for plants [2]. Typically, liming is one of the most used techniques by farmers when they implement their crops, which, in most situations, provides economic benefits that vary according to the species and soil type [3]. The effects of liming are due to the increase in pH, the contents of Ca and Mg, and in the neutralization of exchangeable toxic Al [4]. Liming also provides improvements in the absorption of N and K in barley [5].
Calcium plays a vital role in maintaining the structural and functional integrity of membranes and cell walls. In the event of Ca deficiency, cell membranes become permeable, resulting in the leakage of cytoplasmatic contents. This compromises cellular compartmentalization and disrupts Ca binding with pectin in the cell wall. Calcium also contributes to the formation of water-insoluble compounds, such as pectate, in the middle lamella of the cell wall, leading to limited redistribution or immobility in the phloem [6].
In addition to liming, plastering involves the application of Ca and sulfur (S) to improve the soil subsurface environment (20–40 cm) [7]. This procedure also promotes root growth by increasing the availability of nutrients at deeper soil layers.
In an unfavorable chemical environment, the plant may experience reduced root growth and hindered nutrient absorption [8]. Thus, understanding the roles of nutrients in plant physiology is essential for optimizing crop yield and continually assessing the effectiveness of applied crop management practices [9].
However, excess copper (Cu) can cause damage to the root system, leading to anatomical and morphological alterations that compromise nutrient absorption and hinder plant growth [10]. A study [10] analyzed the influence of liming on the development of young vine plants exposed to excessive Cu levels in the soil. The researchers observed that liming resulted in a reduction of Cu concentration, increased absorption of essential nutrients by plants, prevented changes in root anatomy, and contributed to the growth of the species in areas under high Cu concentrations [8]. Through various analyses, the authors reported significant differences in the root structure between samples without Cu addition (a, c, and e) and those with Cu and limestone addition (f). They observed that in the experiment using Cu and limestone, the root apex exhibited division zones, expansion, and differentiation.
Sulfur is a non-metallic element commonly found in the form of the sulphate (SO42) anion in the soil, which is also the form absorbed by plants. As sulphate is highly soluble and can bound to Ca2+, Mg2+, and K+, liming can contribute to the leaching of sulphate by increasing the soil negative charge, leading to a higher accumulation of nutrients in the subsoil layers [2]. Another benefit of plastering is the reduction of Al toxicity and the promotion of root growth by increasing the availability of nutrients and water at deeper soil layers [5]. With the application of liming and plastering, Ca becomes available to the plant, both on the surface and at depth, which is important due to its function in root growth and development and its absorption that occurs in the root cap [11].
Soil nutrients are absorbed by plant roots through the rhizosphere, which constitutes the region of interaction between the plant and the soil [12]. Consequently, enhancing this interaction greatly influences the effective use of nutrients by plants, thereby affecting crop yield and sustainability [13]. It is, therefore, essential to understand how alterations in the root system parameters affect plant growth, mainly elephant grass (Pennisetum purpureum Schum), a grass species of economic significance.
Optimizing root zone management to synchronize root growth and soil nutrient supply are ubiquitous characteristics for improving cropping systems [14]. The implementation of corrective measures, specifically liming and plastering, can impact the root anatomy of elephant grass plants. Enhanced development of the root system and vascular tissues promotes plant growth and biomass production. In this study, we investigated the effects of liming and plastering on the root anatomy of P. purpureum, focusing on their implications for water and nutrient use, as well as their impact on plant growth and crop yield.
2. Materials and Methods
2.1. Experimental Site and Material Used
Seedlings of elephant grass (Pennisetum purpureum cv. Capiaçu) sourced from the Agrostological Field of the Faculty of Animal Science and Food Engineering at the University of São Paulo (FZEA—USP) in Pirassununga, São Paulo, Brazil, were sectioned in the median region. Subsequently, the billets were transplanted into trays filled with substrate to initiate the sprouting process. After a 30-day period, plantlets exhibiting more homogeneous growth were transplanted into pots allocated to different treatments: pots without gypsum or limestone (control), pots with lime, pots with gypsum, and pots with a combination of gypsum and limestone. The experiment was conducted using a randomized design in a greenhouse.
2.2. Soil Preparation
Initially, soil samples were collected from the FZEA-USP campus, and then subsequently analyzed in the Soil Laboratory of the same institution to determine the fertility level and the nutritional status.
The soil used in the experiment was sandy and exhibited low fertility, classified as Quartzarenic Neosol [15]. Liming, plastering, and fertilization with each nutrient were established based on the findings of the initial soil analysis (Table 1).

Table 1.
Results of the soil chemical analysis in the experiment. pH—Hydrogen potential; P—phosphorus; S—sulfur; K—potassium; Ca—calcium; Mg—magnesium; Al—aluminum; H + Al—hydrogen + aluminum; OM—organic matter; TC—total carbon; SB—sum of bases; CEC—cation exchange capacity; V—base saturation; m—aluminum saturation; B—boron; Cu—copper; Mn—manganese; Zn—zinc.
The experiment was performed in the Animal Science and Food Engineering College (FZEA) of the University of São Paulo (USP) in Pirassununga (Sao Paulo State/Brazil). The treatments were carried out in pots, comprising the control (T1), liming alone (T2), plastering alone (T3), and with a combination of liming and plastering (T4). The lime utilized in the experiment was conducted with PRNT 125%, CaO 43%, MgO 9%, and PN 96%. The gypsum CaSO4 + 2H2O used contained Ca = 16%, S = 13%. Each treatment was replicated 5 times, totaling 20 pots. The soil was sieved and approximately 6.5 kg of soil was placed in each pot. Subsequently, limestone was applied, followed by a 30-day incubation period. Afterward, gypsum was applied to the respective treatments without requiring any waiting time. The plants were kept under the temperature averaged at ±30 °C, irrigated daily (2 mm, 3 times per day), under a 10 h photoperiod.
The interpretation of the soil test results is crucial for identifying deficient soil nutrients and determining the necessary rates to fulfill plant requirements [15]. This process involves interpreting the results of the soil chemical and physical tests, which classify the nutrient levels as very low, low, medium, high, or very high, as well as a physical characterization based on the clay content [16].
The liming requirement for forage was determined to be 1.8 tons/ha, equivalent to 6.0 g of limestone per pot, with a Total Neutralizing Relative Power (TNRP) of the limestone used set at 125 [12]. The gypsum requirement was calculated based on the soil clay content (30% clay), requiring 1.5 ton/ha, which corresponds to 5 g of gypsum per pot. Fertilization with nitrogen (N), phosphorus pentoxide (P2O5), and potassium oxide (K2O) was carried out at rates of 40, 70, and 40 kg/ha, respectively. Monoammonium phosphate (MAP) was used as a source of both N and P, applied at a dosage of 0.7 g per pot, while potassium chloride (KCl; 60% K2O) served as the K source, applied at a dosage of 0.3 g per pot. Elephant grass seedlings were initially planted in plastic trays filled with washed sand and were irrigated until emergence. Following an assessment of uniformity, one plant per plot was selected and transplanted, applying fertilizers and the respective treatments accordingly.
2.3. Light Microscopy
Samples of P. purpureum elephant grass roots from various treatments were collected 45 days after the experimental implementation. The samples were sectioned at the apical part and 3 cm above the apical portion of the adventious root, and then immersed in Karnovsk’s fixative, consisting of 10 mL of 25% glutaraldehyde, 10 mL of 20% paraformaldehyde, 25 mL of buffer (0.2 M sodium cacodylate pH 7.2), and 1 mL of calcium chloride (0.1 M) [17]. The samples were subjected to vacuum pump treatment for improved fixation while still in the fixative. Subsequently, the samples were dehydrated using an increasing series of ethyl alcohol concentrations (30%, 50%, 70%, 90%, and 100%) and infiltrated in Tecknovit® resin following the manufacturer’s instructions. The blocks were then sectioned at a thickness of 2–7 μm using a Leica RM 2265 rotary microtome. The sections were mounted on glass slides, stained with 0.05% Toluidine Blue [18] for 7 min, and analyzed on a Zeiss microscope Axionscope. Additionally, the root samples were submitted to the histochemical tests: Sudan Black B dye was used to analyze lipophilic compounds [19], and autofluorescence of elephant grass cell walls was examined using a 365 nm excitation filter and a 420 nm long-pass emission filter.
2.4. Statistical Analysis
To assess the effects of limestone and gypsum addition on the anatomy of P. purpureum roots, all quantitative data, including the number of vessel elements, vascular cylinder diameter, vessel element diameter, root diameter, and cortex width, obtained from at least four independent biological replicates for each variable, underwent mean comparison testing using the one-way analysis of variance followed by the Tukey test, with a 95% confidence interval (p < 0.05). The normality of the data distribution was assessed using the Shapiro–Wilk normality test (p > 0.05), confirming that all variables were drawn from a normally distributed population. Besides, relative change values were calculated for each parameter as a function of the control. Data analysis was performed using Prism software (version 9.2.0, GraphPad Software, Boston, MA, USA).
3. Results
3.1. Characteristics of Cross-Sections of Elephant Grass Roots Obtained by Microscopy
Analysis with Ultraviolet Light
In this study, we analyzed cross-sections of P. purpureum elephant grass roots, focusing on the fundamental and vascular tissues under ultraviolet (UV) light (Figure 1). The cortex predominantly exhibited aerenchyma, with no noticeable differences in the aerenchyma between treatments. In contrast, significant variations were noted in the vascular tissues.
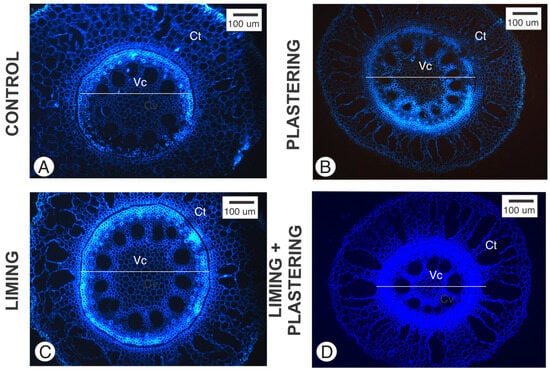
Figure 1.
Cross-section of Pennisetum purpurem adventious root analyzed under ultraviolet light (Ex. 365 nm; Em: 420 nm). (A) Control, (B) plastering, (C) liming, and (D) liming + plastering. Ct—Cortex; Vc—Vascular cylinder.
Hence, this method became essential for our analysis, complementing the conventional method with Toluidine Blue (Figure 1). Its efficiency in quickly conducting analyses facilitated the process of capturing images for quantitative studies (Figure 1 and Figure 2; Table 2).
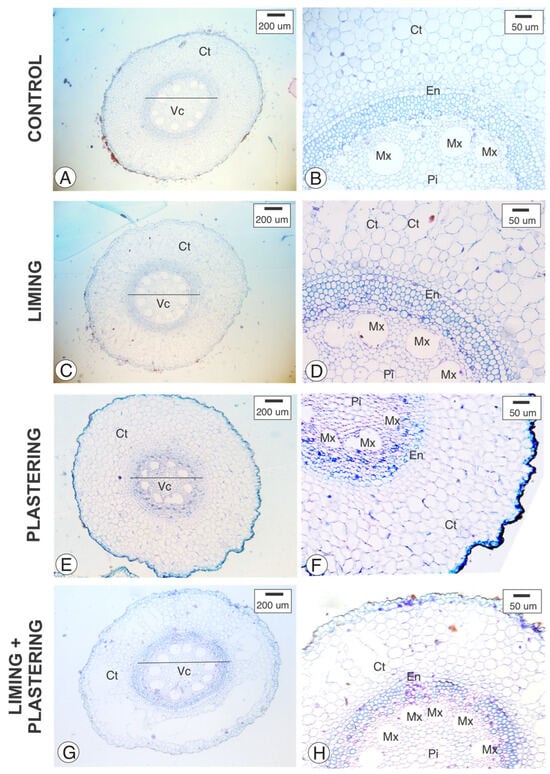
Figure 2.
Cross-section of Pennisetum purpurem adventious root analyzed under staining with Toluidine Blue. (A,B) Control, (C,D) liming, (E,F) plastering, (G,H) liming + plastering. Ct—Cortex; En—Endodermis. Pi—Pith; Mx—Metaxylem; Vc—Vascular cylinder.

Table 2.
Number of vessel elements (NVE), diameter of vessel elements (VE), root (RD), vascular cylinder (VCD), and thickness of the cortex layer (CW) exhibited by plants subjected to liming (L), plastering (P), or both liming and plastering (L + P). The data represent the mean ± standard error of measurements of at least four independent biological replications. Different letters indicate significant statistical differences between the treatments according to a one-way analysis of variance (ANOVA) followed by the Turkey’s post hoc test, at a 95% confidence interval (p < 0.05).
From the images, measurements were taken of the vessel elements, diameter of the vascular cylinder, vessel element diameter, root diameter, and cortex width. The analysis revealed significant statistical differences in the number of vessel elements between the liming treatment and the control plants, as well as between the liming and plastering treatments. Additionally, differences were observed in the diameter of the vascular cylinder between liming and plastering treatments (Figure 2 and Table 2).
Although liming and gypsum plastering are practices known to increase the root depth in other crops, their effect on elephant grass roots without the addition of other nutrients has not been previously reported. Upon conducting the quantitative analysis in this study, it was observed that liming promoted an increase in the number of vessel elements and in the diameter of the vascular cylinder (Figure 2) compared to the other treatments evaluated.
However, no significant statistical differences were observed in the vessel element diameter of the vessel element, root diameter, and cortex width (Figure 3 and Table 2). A significant reduction in root and vessel elements’ diameters was evident in plants subjected to plastering, compared to the other treatments.
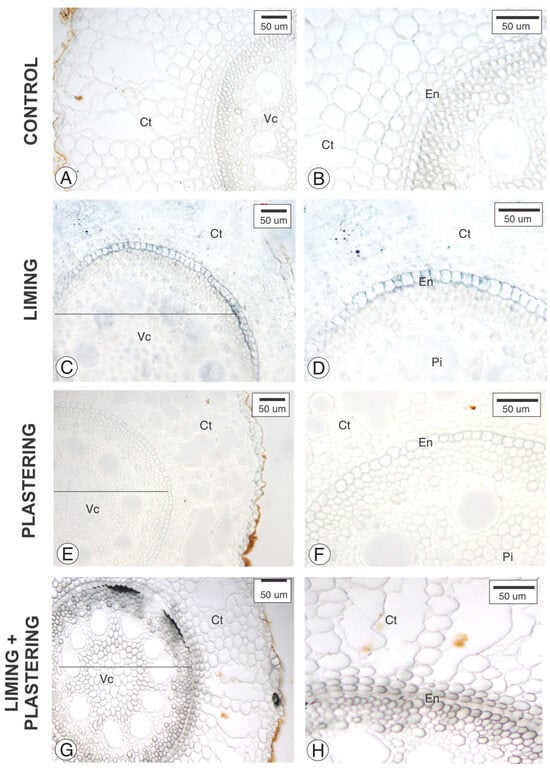
Figure 3.
Cross-section of Pennisetum purpurem adventious root analyzed under staining with Sudan Black B. (A,B) Control, (C,D) liming, (E,F) plastering, (G,H) liming + plastering. Note the suberin (black cell walls) next to the walls of the endodermis (En); Ct—Cortex; Pi—Pith; Vc—Vascular cylinder.
The greater suberization in endodermal cells facilitates the selective transport of substances across the plasma membrane. Possibly, liming (Figure 3) altered the structure of elephant grass roots, resulting in ticker walls that exhibited a strong reaction to the Sudan Black B dye, which is sensitive to suberin.
4. Discussion
The agricultural practices of liming and plastering have been utilized since 1925 [20]; however, few studies have explored the anatomical changes induced by these correction and conditioning methods on the root system of elephant grass (Pennisetum purpureum Schum). Nevertheless, some studies have documented the influence of liming on the development of root systems. There are reports indicating that liming enhances nutrient uptake by plants and prevents alterations in root anatomy, such as changes in the division zones and expansion and differentiation of the apex root, while also to contributing to the growth of aerial parts [10].
The use of autofluorescence in plant tissues is a valuable method to identify various chemical compounds [19]. Grasses, in particular, contain significant levels of ferulic acid in their cell walls [21], which are rich in phenolic compounds that emit blue light under ultraviolet illumination. In addition, lignified cell walls, such as those found in xylem vessel elements, also emit blue light (Figure 1).
Conversely, researchers found that corn plants grown in soil contaminated with varying doses of cadmium (Cd) exhibited an increase in the diameter of elements when subjected to the liming process compared to samples without liming [22]. These findings were attributed due to the negative correlation between the Cd content—a toxic element to plants—and the observed changes in diameter. Moreover, salinity and biofertilizers can also modulate the metaxylem diameter of Amaranthus tricolor stem [23].
Liming demonstrated the capacity to modulate the number of vessel elements of elephant grass roots. The vessel elements and the vascular cylinder are responsible for transporting water and minerals from the roots to the leaves and to other parts of the plant, as well as for forming lateral roots. Therefore, the increase in the number of vessel elements and in the diameter of the vascular cylinder is believed to provide elephant grass with improved distribution of nutrients, water, and increased plant height. Furthermore, changes in vascularization are directly linked to plant survival in adverse conditions, potentially benefiting the plant during water scarcity. Variations in vessel diameter have adaptive significance, as species originating from arid environments typically possess narrower vessels. The quantitative analyses carried out in this work revealed that plastering reduced vessel diameter values (Figure 3) compared to the other treatments, potentially enhancing the capacity of P. purpureum to withstand water stress conditions [24]. Similar results, of a decrease in vessel element diameter, were observed in Paspalum dilatam and Zea mays cv Saracura under water stress [25,26]. Narrower vessels improve the capacity of restraining embolism and may retard the effect of drought in plants [25]. One possible explanation is that this reduction facilitated an increase in the root system width and consequently increased the contact surface. However, to verify this hypothesis, quantification of the height of the roots, stems, and leaves of elephant grass would be necessary, which was not performed in this study due to the destructive nature of our analyses.
In addition to the anatomical and biometric analyses, histochemical tests were also conducted to detect lipophilic compounds using the Sudan IV dye (Figure 3). Through this method, the presence of suberin was identified in the cell walls of the endodermis, particularly evident in the liming treatment (Figure 4). Thicker walls displaying an intense reaction to the Sudan IV dye were observed in the liming treatment compared to the control, as well as the plastering and liming + plastering treatments.
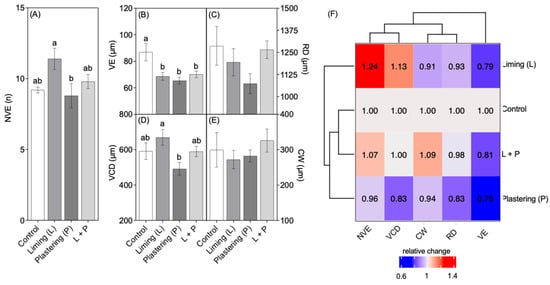
Figure 4.
Number of vessel elements (NVE) (A), diameter of vessel elements (VE) (B), root (RD) (C), vascular cylinder (VCD) (D), and thickness of the cortex layer (CW) (E) exhibited by plants subjected to liming (L), plastering (P), or both liming and plastering (L + P) treatments (A–E). The data represent the mean ± standard error of measurements of at least four independent biological replications. Different letters indicate significant statistical differences between the treatments according to a one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test, at a 95% confidence interval (p < 0.05). Heat map of the relative change of all measured variables compared to the control group (F).
The endodermis, in the innermost layer of the cortex, plays a crucial role in ensuring ionic selectivity to the vascular cylinder [27]. The cell walls of the endodermis are thickened with suberin or lignin, depending on their maturation stage. In the roots of Pennisetum purpureum subjected to liming, greater suberization was observed compared to the other treatments. It is important to highlight that liming promotes nutrient uptake in the environment; thus, the increased availability of nutrients requires an enhanced ion selection capacity for the vascular cylinder. Therefore, an endodermis with more suberized cell walls enhances the function of this tissue [28].
5. Conclusions
In the present study, we analyzed the effects of liming and plastering on the roots of elephant grass (Pennisetum purpureum). The findings showed that liming increased the number of vessel elements and the diameter of the vascular cylinder. Conversely, plastering did not change these parameters, comparable to the control treatment. Therefore, while a combination of liming and plastering is recommended to improve the plant vascular system, the application of liming alone effectively modulates the development of the vascular system and the endodermis.
Author Contributions
Conceptualization, S.P.S. and J.P.R.M.; Methodology, S.P.S., G.S.M. and J.P.R.M. Formal Analysis: S.P.S., G.S.M., F.d.F.d.S.D. and J.P.R.M.; Data curation, G.S.M. and J.P.R.M.; Writing—original draft preparation: S.P.S. and J.P.R.M. Writing—review and editing: S.P.S., G.S.M., F.d.F.d.S.D. and J.P.R.M.; Supervision, J.P.R.M.; Funding addition: J.P.R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by São Paulo Research Foundation [grant numbers 2023/09543-9, 2020/07721-9]. SPS was funded by Institutional Scientific Initiation Scholarship Program, National Council for Scientific and Technological Development, Process Number 2897-2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pereira, A.V.; Ledo, F.D.S.; Morens, M.J.F.; Leite, J.L.B.; Brighenti, A.M.; Martins, C.E.; Machado, J.C. BRS Capiaçu: High-yielding elephant grass cultivar for silage production. Embrapa Dairy Cattle 2016. [Google Scholar]
- Pandey, P.; Srivastava, R.K.; Dubey, R.S. Salicylic acid alleviates aluminum toxicity in rice seedlings better than magnesium and calcium by reducing aluminum uptake, suppressing oxidative damage and increasing antioxidative defense. Ecotoxicology 2013, 22, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Pottker, D.; Ben, J.R. Calagem para uma rotação de culturas no sistema plantio direto. Rev. Bras. Ciênc. Solo 1998, 22, 75–684. [Google Scholar] [CrossRef]
- Ernani, P.R.; Ribeiro, M.S.; Bayer, C. Modificações químicas em solos ácidos ocasionadas pelo método de aplicação de corretivos da acidez e de gesso agrícola. Sci. Agric. 2001, 58, 825–831. [Google Scholar] [CrossRef]
- Caires, E.F.; Feldhaus, I.C.; Blum, J. Crescimento radicular e nutrição da cevada em função da calagem e aplicação de gesso. Bragantia 2001, 60, 213–223. [Google Scholar] [CrossRef]
- Malavolta, E. Manual of Mineral Nutrition of Plants; Editora Agronômica Ceres: São Paulo, Brazil, 2006; 638p. [Google Scholar]
- Souza, D.M.G.; Lobato, E.; Rein, T.A. Use of Agricultural Gypsum in Cerrado Soils; Technical circular, 32; Embrapa; CPAC: Planaltina, Brazil, 1995; 20p. [Google Scholar]
- Van Raij, B.; Cantarella, H.; Quaggio, J.A.; Furlani, A.M.C. (Eds.) Fertilization and Liming Recommendations for the State of São Paulo, 2nd ed.; Technical Bulletin, 100; Instituto Agronomic: Campinas, Brazil, 1996; pp. 56–59. [Google Scholar]
- Goulding, K.; Jarvis, S.; Whitmore, A. Optimizing nutrient management for farm systems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, V.G.; Rosa, D.J.; Corredor Prado, J.P.; Borghezan, M.; Bastos de Melo, G.W.; Fonsêca de Sousa Soares, C.R.; Comin, J.J.; Simão, D.G.; Brunetto, G. Reduction of copper phytotoxicity by liming: A study of the root anatomy of young vines (Vitis labrusca L.). Plant Physiol. Biochem. 2015, 96, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E. Fisiologia Vegetal, 4th ed.; Artmed: Porto Alegre, Brazil, 2009; 819p. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier: London, UK, 2012; 643p. [Google Scholar]
- Zhang, F.; Shen, J.; Zhang, J.; Zuo, Y.; Li, L.; Chen, X. Chapter One-Rhizosphere Processes and Management for Improving Nutrient Use Efficiency and Crop Productivity: Implications for China. Adv. Agron. 2010, 107, 1–32. [Google Scholar] [CrossRef]
- Shen, J.; Li, C.; Mi, G.; Li, L.; Yuan, L.; Jiang, R.; Zhang, F. Maximizing root/rhizosphere efficiency to improve crop productivity and nutrient use efficiency in intensive agriculture of China. J. Exp. Bot. 2013, 64, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- EMBRAPA—Brazilian Agricultural Research Company. Brazilian Soil Classification System/Humberto Gonçalves dos Santos… [et al.], 3rd ed.; Embrapa: Brasília, Brazil, 2013; 353p, Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1094001/brazilian-soil-classification-system (accessed on 20 May 2024).
- Cantarella, H.; Quaggio, J.A.; Mattos, D., Jr.; Boaretto, R.M.; van Raij, B. Bulletin 100: Fertilization and Liming Recommendations for the State of São Paulo; Cantarella, H., Quaggio, J.A., Mattos, D., Jr., Boaretto, R.M., van Raij, B., Eds.; Agronomic Institute of Campinas: Campinas, Brazil, 2022; 489p. [Google Scholar]
- Karnovsky, M.J. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 1965, 27, 137–138. [Google Scholar]
- Sakai, W.S. Simple method for differential staining of paraffin embedded plant material using toluidine blue O. Stain Technol. 1973, 48, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.P.R.; Soares, M.K.M. Manual of Techniques Applied to Plant Histopathology; Ed. FEALQ: Piracicaba, Brazil, 2021; 140p. [Google Scholar]
- Wietholter, S. Liming in Brazil; Embrapa Trigo: Passo Fundo, Brazil, 2000; 104p. [Google Scholar]
- Albersheim, P.; Darvill, A.; Roberts, K.; Sederoff, R.; Staehelin, A. Plant Cell Walls. From Chemistry to Biology; Garland Science: New York, NY, USA, 2010; 430p. [Google Scholar]
- Farid, M.; Shakoor, M.B.; Ehsan, S.; Ali, S.; Zubair, M.; Hanif, M.S. Morphological and Physiological Responses of Plants to Cadmium Toxicity: A Review. Pedosphere 2017, 27, 421–438. [Google Scholar] [CrossRef]
- Riesty, O.K.; Siswanti, D.W. Effect of biofertilizer on growth and metaxylem diameter of Amaranthus tricolor L. in salinity stress condition. Biogenes. J. Ilm. Biol. 2021, 9, 178–188. [Google Scholar] [CrossRef]
- Carlquist, S. Comparative Wood Anatomy; Springer: New York, NY, USA, 1988; 450p. [Google Scholar]
- Vasellati, V.; Oesterheld, M.; Medan, D.; Loreti, J. Effects of flooding and drought on the anatomy of Paspalum dilatatum. Ann. Bot. 2001, 88, 355–360. [Google Scholar] [CrossRef]
- Souza, T.C.; Castro, E.M.; Pereira, F.J.; Parentoni, S.N.; Magalhăes, P.C. Morpho-anatomical characterization of root in recurrent selection cycles for flood tolerance of maize (Zea mays L.). Plant Soil Environ. 2009, 55, 504–510. [Google Scholar] [CrossRef]
- Appezzato da Glória, B.; Carmello Guerreiro, S.M. Vegetal Anatomy, 4th ed.; Universidade Federal de Viçosa: Viçosa, Brazil, 2022; 422p. [Google Scholar]
- Doblas, V.G.; Smakowska-Luzan, E.; Fujita, S.; Alassimone, J.; Barberon, M.; Madalinski, M.; Belkhadir, Y.; Geldner, N. Root diffusion barrier control by a vasculature-derived peptide binding to the SGN3 receptor. Science 2017, 355, 280–284. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).