The Response of Botrytis cinerea to Fire in a Coast Redwood Forest
Abstract
1. Introduction
2. Materials and Methods
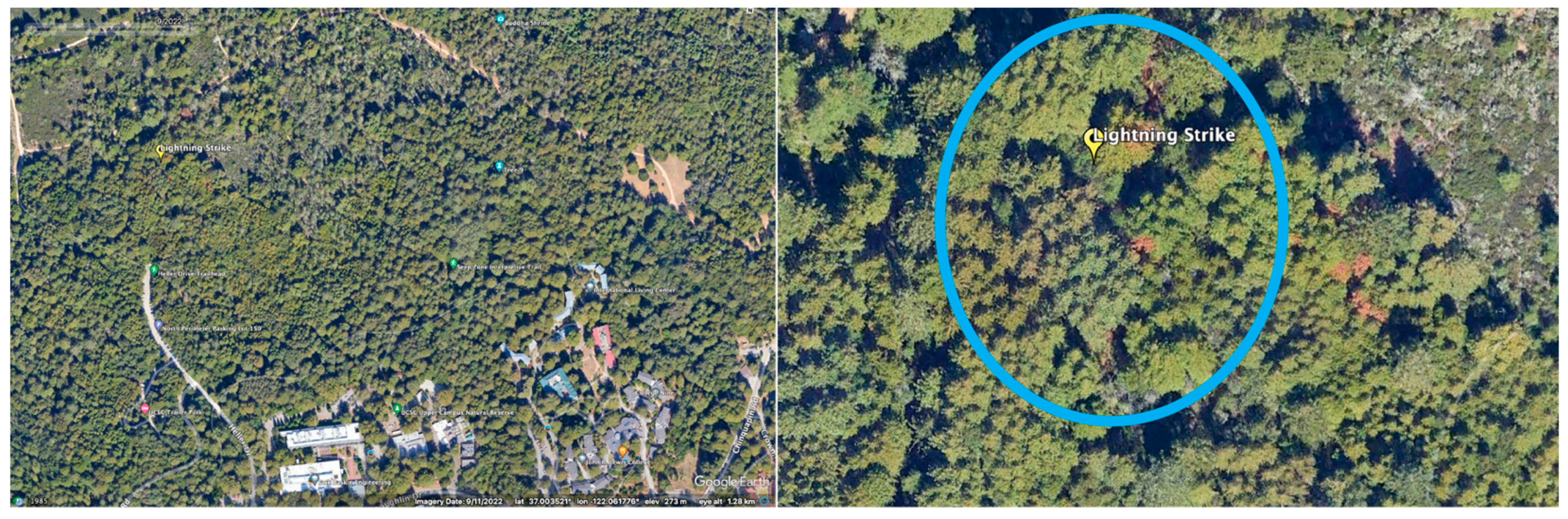
2.1. Study Site
2.2. Media
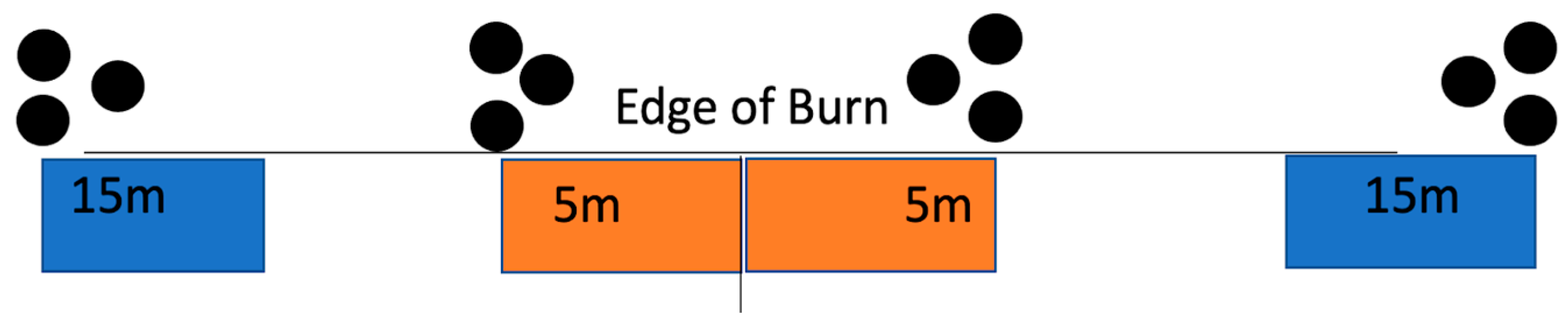
2.3. Spore Trapping and Counting
2.4. Seed Germination and Seedling Growth
2.5. Statistical Analysis
3. Results
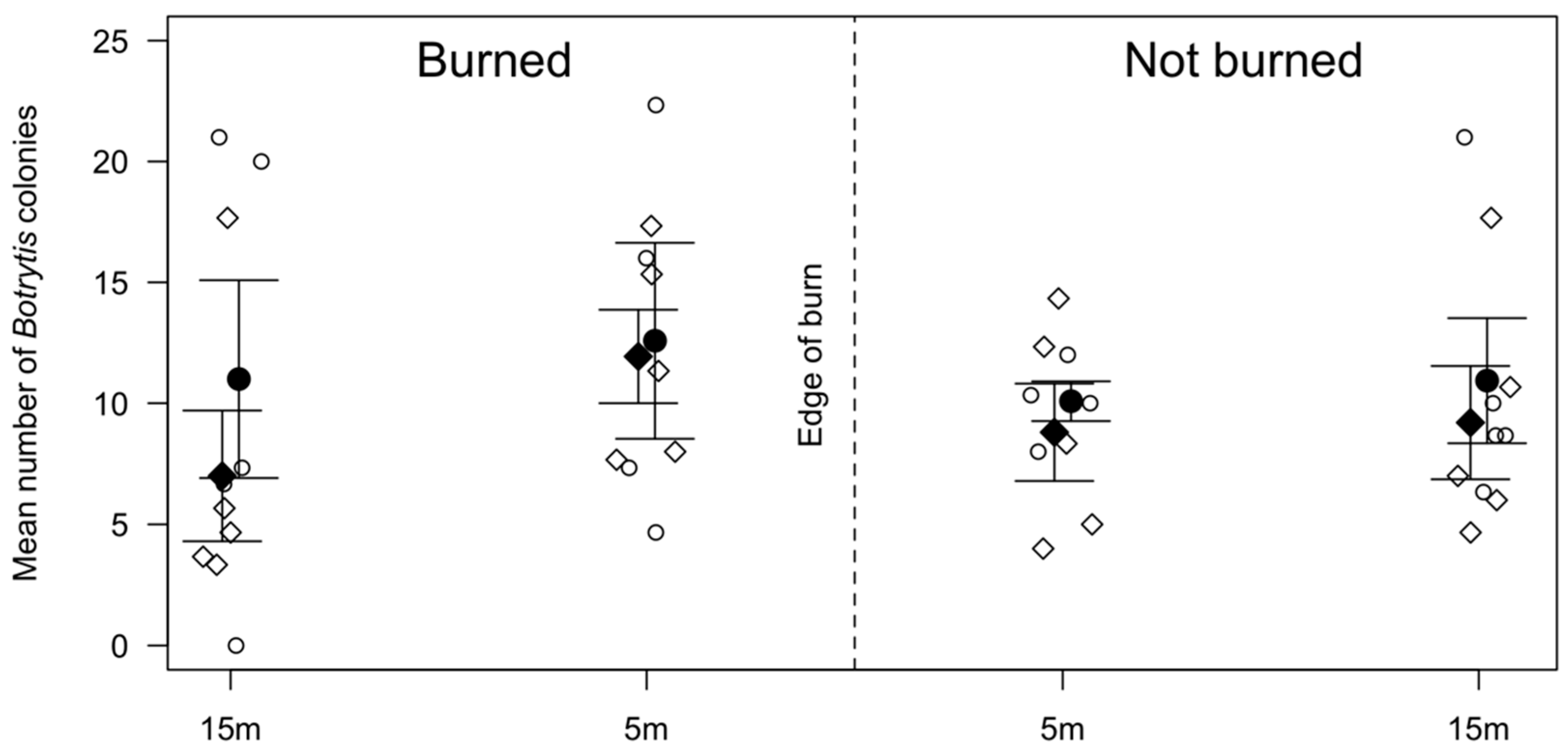
3.1. Spore Trapping
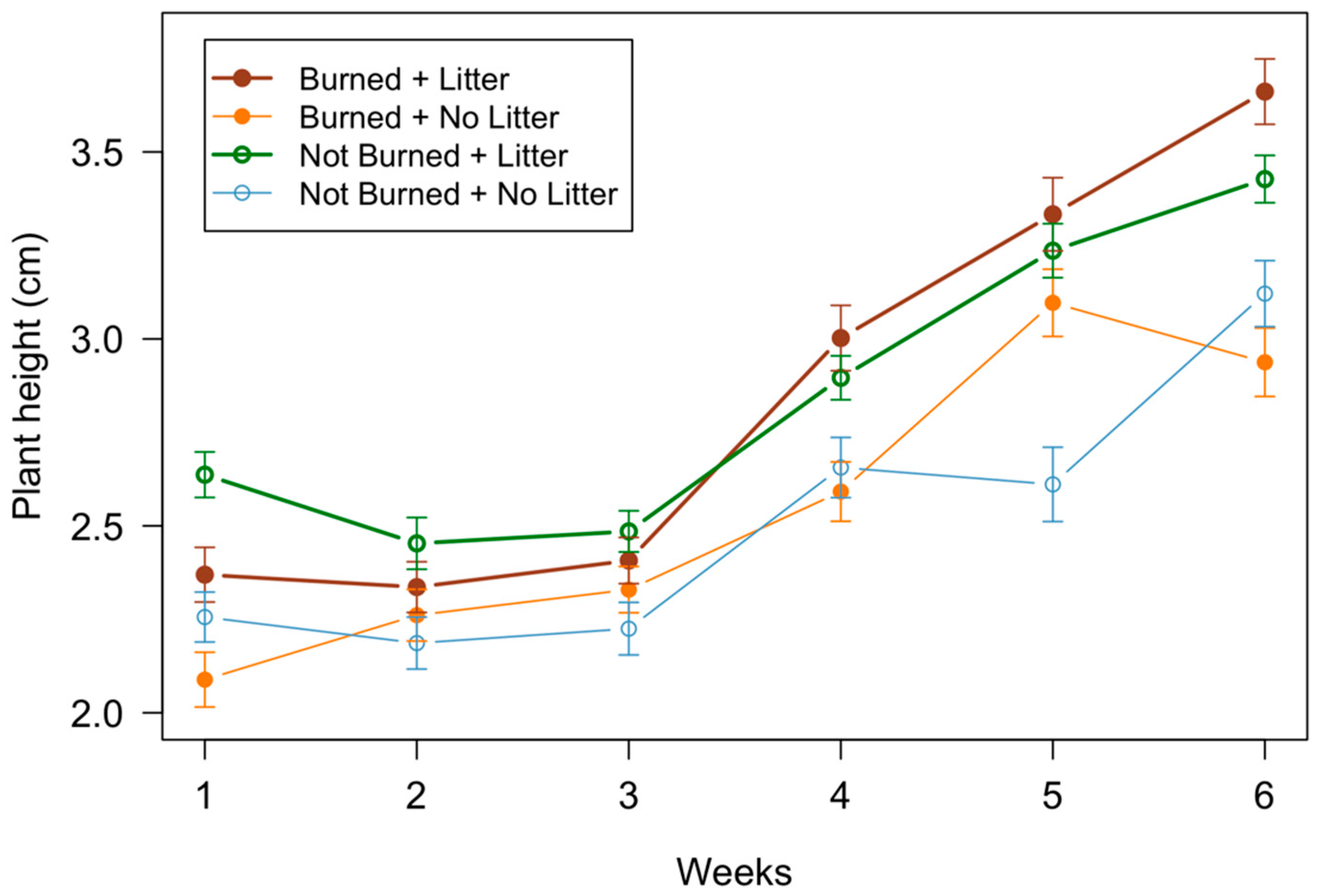
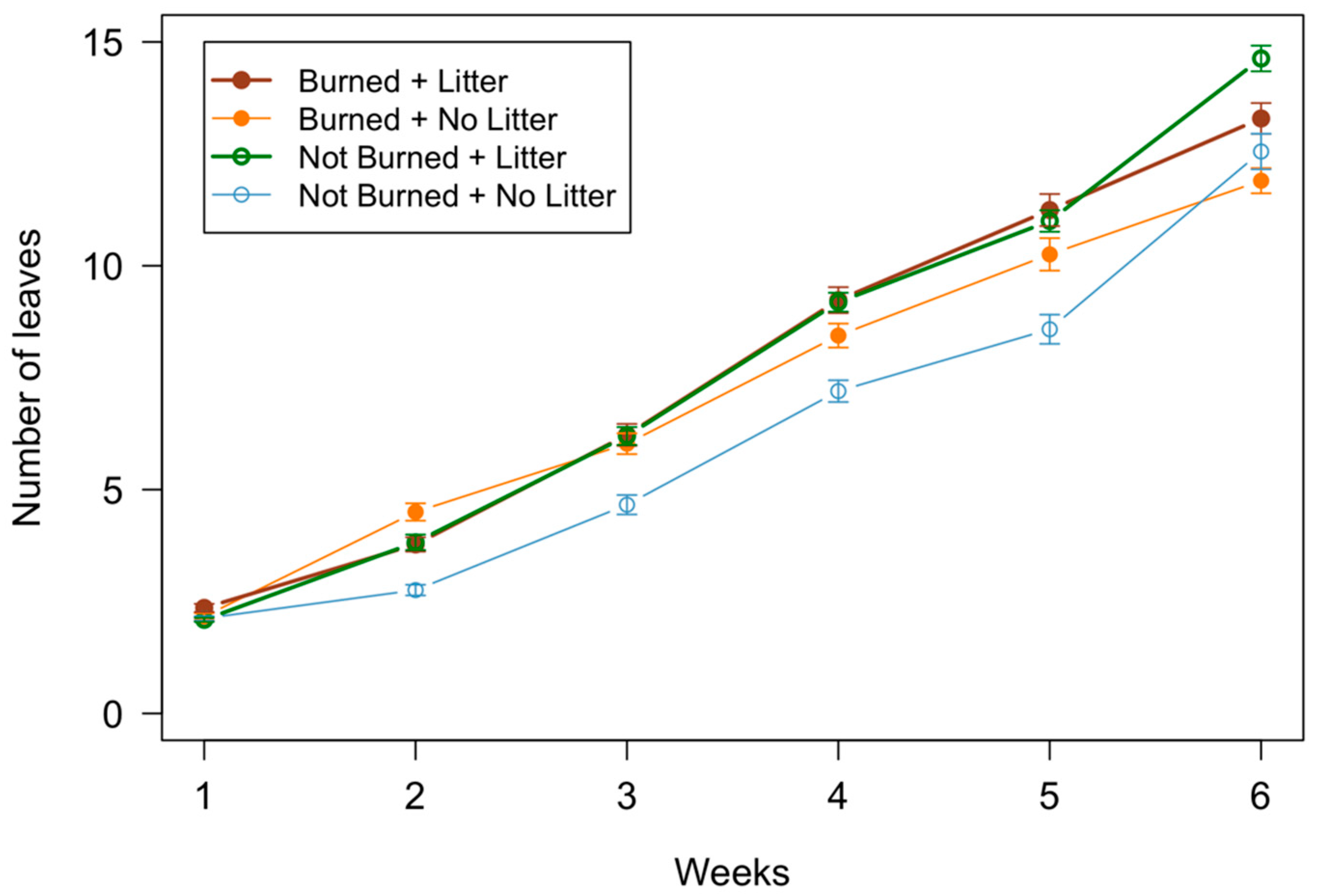
3.2. Seedling Measurements
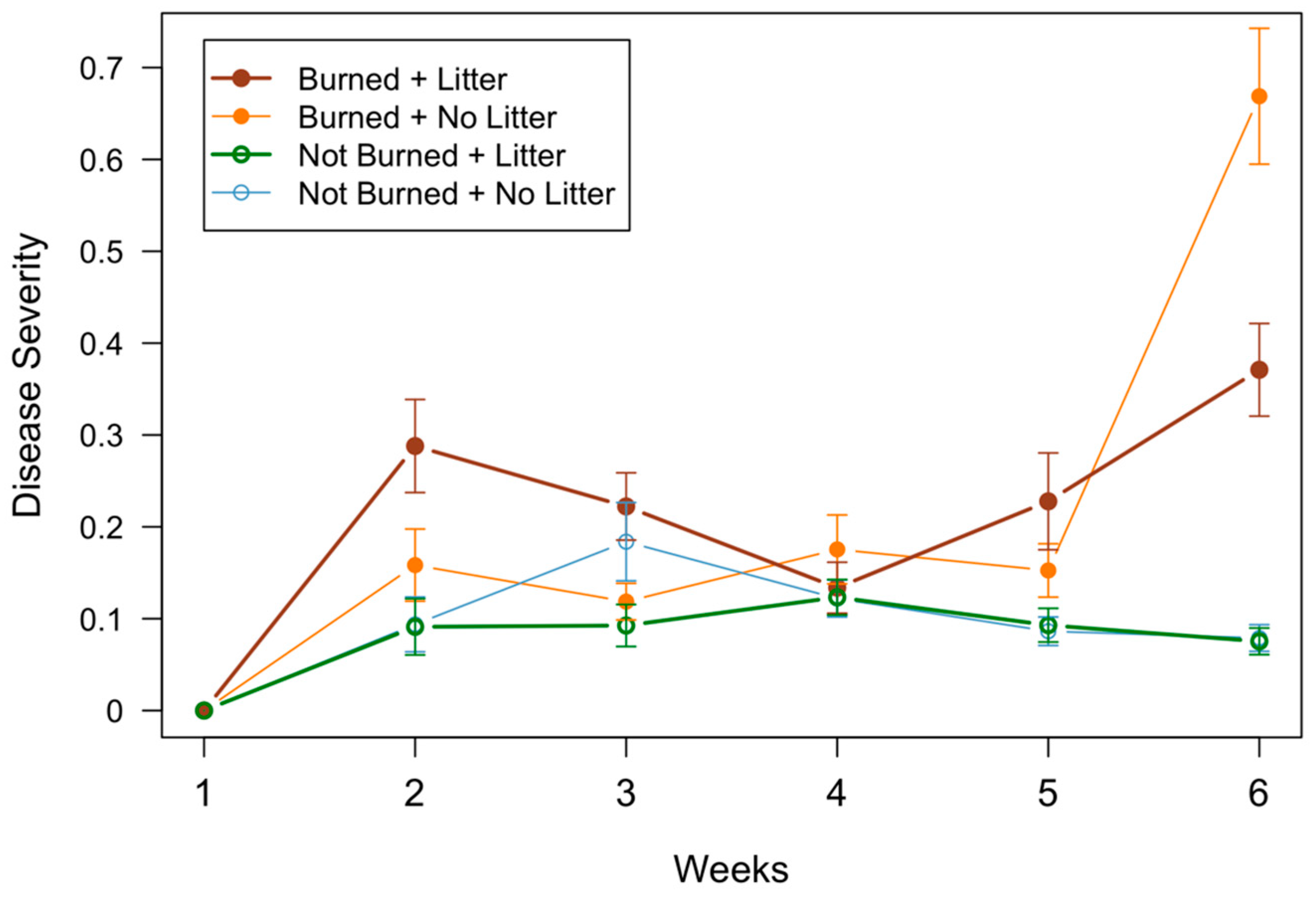
3.3. Disease Development
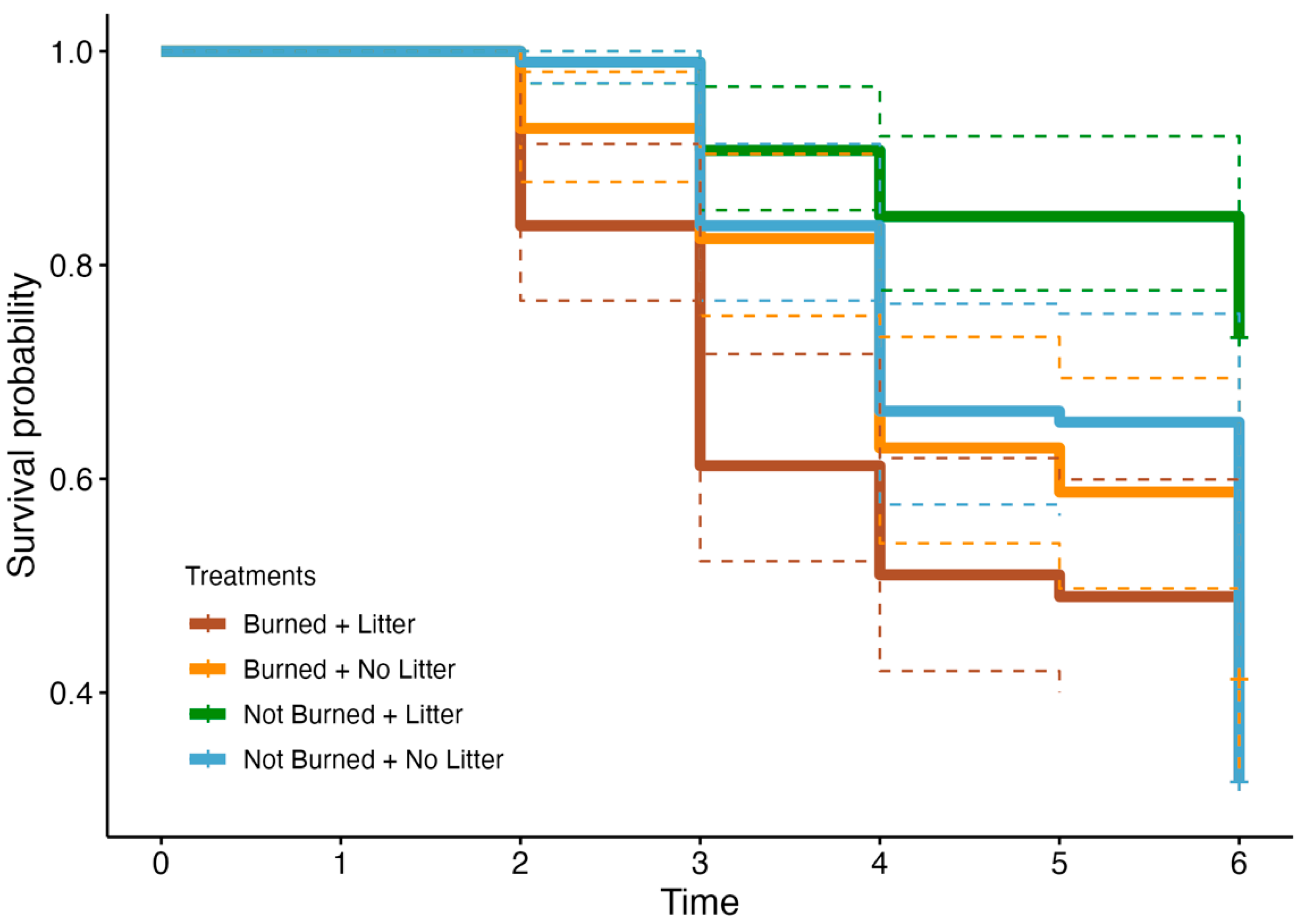
3.4. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haase, D.L.; Taylor, M. Gray Mold. In Forest Nursery Pests; Cram, M.M., Frank, M.S., Mallams, K.M., Eds.; US Department of Agriculture, Forest Service: Washington, DC, USA, 2012; pp. 121–122. [Google Scholar]
- Hua, L.; Yong, C.; Zhanquan, Z.; Boqiang, L.; Guozheng, Q.; Shiping, T. Pathogenic mechanisms and control strategies of Botrytis cinerea causing post-harvest decay in fruits and vegetables. Food Qual. Saf. 2018, 2, 111–119. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E. Botrytis cinerea (Gray Mold). In Postharvest Decay: Control Strategies; Bautista-Baños, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Nakajima, M.; Akutsu, K. Virulence factors of Botrytis cinerea. J. Gen. Plant Pathol. 2014, 80, 15–23. [Google Scholar] [CrossRef]
- Van Kan, J.A.; Shaw, M.W.; Grant-Downton, R.T. Botrytis species: Relentless necrotrophic thugs or endophytes gone rogue? Mol. Plant Pathol. 2014, 15, 957–961. [Google Scholar] [CrossRef]
- Mittal, R.; Singh, P.; Wang, B. Botrytis: A hazard to reforestation: A literature review. Eur. J. For. Pathol. 1987, 17, 369–384. [Google Scholar] [CrossRef]
- Hepting, G.H. Diseases of Forest and Shade Trees of the United States; US Department of Agriculture, Forest Service: Washington, DC, USA, 1971.
- Baker, K.F. Observations on some Botrytis diseases in California. Plant Dis. Rep. 1946, 30, 145–155. [Google Scholar]
- Smith, R.E. Botrytis and Sclerotinia: Their relation to certain plant diseases and to each other. Bot. Gaz. 1900, 29, 369–407. [Google Scholar] [CrossRef]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J.A. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef]
- Parmeter, J.R., Jr. Effects of fire on pathogens. In Proceedings of the Symposium on Environmental Consequences of Fire and Fuel Management in Mediterranean Ecosystems, Palo Alto, California, USDA Forest Service. General Technical Report WO-3, Washington, DC, USA, 1–5 August 1977; pp. 58–64. [Google Scholar]
- Lorimer, C.G.; Porter, D.J.; Madej, M.A.; Stuart, J.D.; Veirs, S.D., Jr.; Norman, S.P.; O’Hara, K.L.; Libby, W.J. Presettlement and modern disturbance regimes in coast redwood forests: Implications for the conservation of old-growth stands. For. Ecol. Manag. 2009, 258, 1038–1054. [Google Scholar] [CrossRef]
- Parker, T.J.; Clancy, K.M.; Mathiasen, R.L. Interactions among fire, insects and pathogens in coniferous forests of the interior western United States and Canada. Agric. For. Entomol. 2006, 8, 167–189. [Google Scholar] [CrossRef]
- Edwards, S.G.; Seddon, B. Selective media for the specific isolation and enumeration of Botrytis cinerea conidia. Lett. Appl. Microbiol. 2001, 32, 63–66. [Google Scholar] [CrossRef]
- Moore, R.A.; Bomar, C.; Kobziar, L.N.; Christner, B.C. Wildland fire as an atmospheric source of viable microbial aerosols and biological ice nucleating particles. ISME J. 2021, 15, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Kobziar, L.N.; Pingree, M.R.; Larson, H.; Dreaden, T.J.; Green, S.; Smith, J.A. Pyroaerobiology: The aerosolization and transport of viable microbial life by wildland fire. Ecosphere 2018, 9, e02507. [Google Scholar] [CrossRef]
- Kobziar, L.N.; Thompson, G.R., III. Wildfire smoke, a potential infectious agent. Science 2020, 370, 1408–1410. [Google Scholar] [CrossRef] [PubMed]
- Kobziar, L.N.; Pingree, M.R.; Watts, A.C.; Nelson, K.N.; Dreaden, T.J.; Ridout, M. Accessing the life in smoke: A new application of unmanned aircraft systems (UAS) to sample wildland fire bioaerosol emissions and their environment. Fire 2019, 2, 56. [Google Scholar] [CrossRef]
- Camacho, I.; Góis, A.; Camacho, R.; Nóbrega, V.; Fernandez. The impact of urban and forest fires on the airborne fungal spore aerobiology. Aerobiologia 2018, 34, 585–592. [Google Scholar] [CrossRef]
- Cairney, J.W.; Bastias, B.A. Influences of fire on forest soil fungal communities. Can. J. For. Res. 2007, 37, 207–215. [Google Scholar] [CrossRef]
- Widden, P.; Parkinson, D. The effects of a forest fire on soil microfungi. Soil Biol. Biochem. 1975, 7, 125–138. [Google Scholar] [CrossRef]
- Holden, S.R.; Rogers, B.M.; Treseder, K.K.; Randerson, J.T. Fire severity influences the response of soil microbes to a boreal forest fire. Environ. Res. Lett. 2016, 11, 035004. [Google Scholar] [CrossRef]
- Sun, H.; Santalahti, M.; Pumpanen, J.; Köster, K.; Berninger, F.; Raffaello, T.; Jumpponen, A.; Asiegbu, F.O.; Heinonsalo, J. Fungal community shifts in structure and function across a boreal forest fire chronosequence. Appl. Environ. Microbiol. 2015, 81, 7869–7880. [Google Scholar] [CrossRef]
- Harrison, J.G.; Forister, M.L.; Parchman, T.L.; Koch, G.W. Vertical stratification of the foliar fungal community in the world’s tallest trees. Am. J. Bot. 2016, 103, 2087–2095. [Google Scholar] [CrossRef]
- Espinosa-Garcia, F.J.; Langenheim, J.H. The endophytic fungal community in leaves of a coastal redwood population: Diversity and spatial patterns. New Phytol. 1990, 116, 89–98. [Google Scholar] [CrossRef]
- Ramage, B.S.; O’Hara, K.; Caldwell, B. The role of fire in the competitive dynamics of coast redwood forests. Ecosphere 2010, 1, 1–18. [Google Scholar] [CrossRef]
- Enright, D.J.; Frangioso, K.M.; Isobe, K.; Rizzo, D.M.; Glassman, S.I. Mega-fire in redwood tanoak forest reduces bacterial and fungal richness and selects for pyrophilous taxa that are phylogenetically conserved. Mol. Ecol. 2022, 31, 2475–2493. [Google Scholar] [CrossRef] [PubMed]
| Rank | % Plant Affected | Associated Symptoms |
|---|---|---|
| 0.00 | 0% | None |
| 0.25 | 1–10% | Leaf yellowing appears on tips of leaves |
| 0.50 | 10–20% | Leaf yellowing appears on the whole leaf |
| 1.00 | 20–50% | Leaf yellowing on one or more whole leaves, leaves start to drop |
| 1.25 | 50–70% | One or more leaves has turned white, top half of plant fallen over |
| 1.50 | 70–90% | One or more leaves white and yellow, top half of the plant fallen over |
| 2.00 | 90–100% | Plant has no color, plant completely fallen down, and is dead |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas, D.S.; Gilbert, G.S. The Response of Botrytis cinerea to Fire in a Coast Redwood Forest. Int. J. Plant Biol. 2024, 15, 94-101. https://doi.org/10.3390/ijpb15010008
Rojas DS, Gilbert GS. The Response of Botrytis cinerea to Fire in a Coast Redwood Forest. International Journal of Plant Biology. 2024; 15(1):94-101. https://doi.org/10.3390/ijpb15010008
Chicago/Turabian StyleRojas, Damiana S., and Gregory S. Gilbert. 2024. "The Response of Botrytis cinerea to Fire in a Coast Redwood Forest" International Journal of Plant Biology 15, no. 1: 94-101. https://doi.org/10.3390/ijpb15010008
APA StyleRojas, D. S., & Gilbert, G. S. (2024). The Response of Botrytis cinerea to Fire in a Coast Redwood Forest. International Journal of Plant Biology, 15(1), 94-101. https://doi.org/10.3390/ijpb15010008






