Abstract
Using agricultural gypsum promotes nutrient descent and generates a less aggressive environment for roots, favoring their development and better use of water and nutrients. However, studies on apple trees are scarce, and there is no official recommendation for gypsum application in the soils of southern Brazil. This work aimed to evaluate the potential use of agricultural gypsum to increase soil fertility and apple tree productivity. The research was carried out in the municipality of Ipê, RS, in an apple orchard cv. Condessa planted fifteen years ago and with acidity in the soil layer of 20–40 cm. The design of the experiment was consisted of randomized blocks, containing five treatments and four replications, with 80 m2 each. The treatments consisted of five doses of agricultural gypsum, applied at the beginning of the productive cycle. The study evaluated soil chemical parameters at 0–20 cm and 20–40 cm, chlorophyll content, and crop productivity. The results showed an increase in the sulfur content up to the gypsum dose of 6.0 t∙ha−1 and a reduction in the magnesium content in the soil at both evaluated depths. Agricultural gypsum application did not significantly influence the other chemical parameters evaluated. Chlorophyll content in apple leaves was reduced, and there was an increase in productivity with the increase in the dose of agricultural gypsum. Therefore, gypsum can potentially improve soil fertility and increase apple tree productivity.
1. Introduction
World production of apples in 2020 was 86.4 million tons, with China being the largest producer and exporter of this fruit, with 40.5 million tons and 1.1 million tons, respectively [1]. In 2020, Brazil produced 983,000 t, cultivated in 32,468 ha, corresponding to an average productivity of 30.28 t∙ha−1 [2]. According to the Associação Gaúcha dos Produtores de Maçã (AGAPOMI) [3], apple production in Rio Grande do Sul in the 2020/2021 season was 440,300 t, cultivated in 14,195 ha, of which the municipality of Ipê represented 4000 t, coming from 447 ha.
The apple tree adapts well to different soil conditions, with pH being the main factor that can limit the production of this fruit species [4]. In the predominant regions of apple tree cultivation, the soils are naturally acidic, with pH values below 5.0 [5]. However, the highest production and fruit quality are obtained from a pH range of 5.6–6.8 [6], and the recommendation for the southern region of Brazil is to raise the soil pH to 6.5 through liming [7].
Most tropical soils of basaltic origin are acidic [8] and, in general, have low concentrations of the base cations calcium (Ca2+) and magnesium (Mg2+), in addition to toxic concentrations of aluminum (Al3+) and manganese (Mn2+) [9]. This acidity can occur because of several factors that increase the concentration of hydrogen ions (H+) and decrease the pH of the soil, such as the weathering of minerals, leaching of bases by rainfall, ammonia fertilization, root extrusion of hydrogen by plants, and the action of microorganisms in the soil, especially in organic matter [8,9,10,11,12].
Most problems related to low pH values encompass the presence of Al3+ in the soil solution and deleterious effects on the microbiota, reducing or halting the biochemical processes of microorganisms [9]. At pH values greater than 5.5, aluminum precipitates as oxides and hydroxides. However, with a decrease in pH, there is the dissolution of the precipitates, which bind to soil colloids, remaining available for absorption by the plant in low concentrations [13].
The most sensitive root region to aluminum is the root apex, specifically in the transition zone between the meristem and the cell elongation zone, where a greater interaction with the element occurs due to the anatomy of the cells [13]. Under the toxic effect of Al3+, root cells express protein kinases of the type Wall-associated protein kinase 1 (WAK1), which is responsible for the perception and transmission of external stimuli such as wounds and pathogen attacks [13,14]. In addition, plants also synthesize callose in adverse situations. Callose is a polysaccharide that disrupts communication between cells and the transport of solutes [13,15]. In addition, the basipetal transport of auxin may be interrupted at the root apex, which ceases elongation in the cell elongation zone. However, a set of variables such as the pH, the ionic strength of the solution, the presence of organic compounds, and the concentration of divalent cations influence the interaction of aluminum with the root, which may cause greater or lesser toxicity to the plants [13].
The absorption of nutrients by plants is linked to their concentration in the soil solution and, indirectly, to the reserve in the solid phase [16]. Most solution and soil colloids in acidic soils are filled with H+ and Al3+ ions; however, the presence and content of Al depends on the soil type and the minerals that compose the clay fraction [17]. To eliminate the problems generated by soil acidity, it is necessary to correct the soil with a material capable of neutralizing these ions and providing nutrients [9]. The primary input is limestone, consisting mainly of calcium and magnesium carbonate [18].
In an aqueous medium, the carbonate ion (CO32−) promotes water hydrolysis, generating carbonic acid (H2CO3). Carbonic acid is an unstable compound that decomposes into water (H2O) and carbon dioxide (CO2), effectively removing acidity (H+) from the soil [19]. The reduction in acidity (increase in pH) leads to the precipitation of aluminum in the form of aluminum hydroxide (Al(OH)3), consequently making it unavailable for absorption by plant roots [9,10].
One of the greatest benefits of liming is aluminum precipitation and base substitution (Ca2+ and Mg2+), increasing the effective cation exchange capacity (CECeff) and base saturation of the soil [12]. Furthermore, at pH values between 6.0 and 6.5, there is greater availability of most of the nutrients needed by plants [9,10].
However, for the acidity corrector to be effective, it must be in contact with the soil due to its low solubility [20], with its action restricted to a few centimeters below the incorporated depth [21]. Fruit trees generally have an active root system at a depth of up to 40 cm. Thus, the recommendation is that liming reaches at least this depth [22]. This deep incorporation, however, is generally difficult, mainly due to inadequate implements or high cost [9].
An alternative to soften the effects of acidity in the soil along the profile is the use of calcium sulfate dihydrate (CaSO4∙2H2O), commonly called ‘agricultural gypsum’. This material can be obtained naturally in sedimentary basins, then called gypsum, with great importance in civil construction, explored mainly in the northeast region of Brazil [23]. However, most of the used agricultural gypsum is of industrial origin [24], resulting from the action of sulfuric acid on phosphate rocks to obtain phosphoric acid and/or concentrated phosphate fertilizers [23].
Agricultural gypsum has a water solubility of 2.4 g∙L−1, 172 times more soluble than calcium carbonate [23]. It mainly comprises sulfur and calcium, with smaller proportions of phosphorus, fluorine, and magnesium. CaSO4 cannot hydrolyze water, unlike CaCO3. However, it can increase calcium levels and change aluminum saturation in the subsurface [9].
When applied to the soil, agricultural gypsum solubilizes. It dissociates into calcium (Ca2+) and sulfate (SO42−) ions, which can originate neutral compounds of great mobility in the profile, called ionic pairs, formed from the sulfate bonding with calcium, magnesium, or potassium [9]. As soil depth increases, a natural decrease in pH occurs, with a favorable environment for sulfate adsorption [25], which leads to the dissociation of previously formed ionic pairs. This provides an accumulation of base cations, especially calcium, which interacts with the exchange complex, promoting the displacement of adsorbed aluminum [9].
After the application of agricultural gypsum in corrected soil in the surface layer, an increase in calcium, magnesium, and sulfur contents, and a decrease in aluminum saturation in the subsurface layer can be observed. This improves the soil’s chemical characteristics and fertility [23]. This increase in fertility promotes an increase in crop production, a fact observed by several authors in pastures [26], corn [27,28,29,30], and wheat [31]. In addition, increased productivity may be associated with increased plant root characteristics [32], indicating that the productive response may be related to greater soil exploration. On the other hand, in some studies, no effect of gypsum application on crop productivity was observed [33,34]. Furthermore, gypsum application should be avoided in soils with low CEC and low subsurface acidity [35].
In apple trees, the annual application of agricultural gypsum for eight consecutive years at 0, 1.0, 2.0, and 3.0 t∙ha−1 only caused changes in magnesium leaching from the surface layer and increased calcium levels along the profile [36]. Danner et al. [37], comparing the application of different calcium sources in apple trees during two cycles, observed that, in those treated with agricultural gypsum, there was a significant difference in the calcium contents in the subsurface layer compared to the control in the first year, an effect that extended into the following year for the surface layer. In addition, the same authors found a significant difference in the calcium content in the leaves during the two cycles and in the fruits only in the second evaluated productive cycle.
Gypsum application recommendations exist for the Cerrado region and the Brazilian state of Minas Gerais. However, these recommendations are not suitable for all soils in Brazil [38], and studies on apple trees are scarce. Considering that using agricultural gypsum may enhance soil fertility, the present work aimed to evaluate the potential use of increasing doses of agricultural gypsum to promote soil fertility and, consequently, apple tree productivity in the soils in southern Brazil.
2. Materials and Methods
The experiment was carried out during the 2021/2022 season in an apple orchard cv. ‘Condessa’ with M9 rootstock, aged 15 years. The area was in the municipality of Ipê, RS, 3.0 km from the urban area, at the geographical coordinates 28°48′ S and 51°17′ W, and with an altitude of approximately 770 m above sea level.
Soil analysis followed the methods proposed by Tedesco et al. [39]. Clay content was determined by the densimeter method. Organic matter was assessed by oxidation with a sulfochromic solution followed by colorimetry. Ca, Mg, Mn, and Al were extracted with KCl 1 mol∙L−1; Al and H + Al were determined by titration; and Ca, Mg, and Mn were determined by atomic absorption spectroscopy (AAS). S was extracted with 500 mg·L−1 Ca(H2PO4)2, followed by turbidimetry with BaCl2. B was extracted with hot water and assessed by colorimetry with azomethine-H. K, P, Cu, and Zn were extracted with Mehlich-1 solution; K was determined by flame photometry; Cu and Zn by AAS; and P by the molybdenum blue method. CEC at pH 7.0 was calculated by the summation of cations (K, Ca, and Mg), and base saturation was calculated based on the percentage of CEC composed of the cations other than Al.
Soil fertility parameters for the layers of 0–20 cm and 20–40 cm, as well as their interpretation, are compiled in Table 1.

Table 1.
Compilation of results referring to soil fertility parameters of the soil used in the experiment and their interpretation in the layers of 0–20 cm and 20–40 cm depth.
The experimental design consisted of randomized blocks, consisting of five treatments with four replications each, allocated in four blocks arranged in two planting rows, totaling 20 plots (Figure 1).
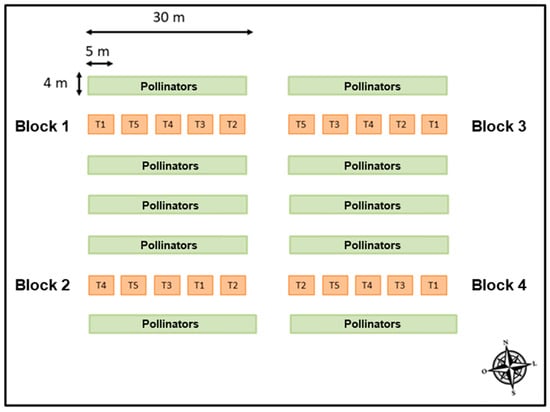
Figure 1.
Sketch of the experimental design carried out in the study. Ipê, RS, 2021.
The treatments consisted of five doses of agricultural gypsum, corresponding to T1: zero (control), T2: 2.0 t∙ha−1, T3: 4.0 t∙ha−1, T4: 6.0 t∙ha−1, and T5: 8.0 t∙ha−1, applied on the soil surface on 1 July 2021, corresponding to the flowering period of the crop. Each plot consisted of 80 m2, containing five plants, with an application range covering the center of the two adjacent rows. Of the five plants contained in each plot, only the three central ones were evaluated.
The agricultural gypsum was supplied by Calcários Mocellin (Vacaria, Brazil) and had 17.7 wt.% S, 30.9 wt.% CaO, 0.2 wt.% F, and 0.7 wt.% P2O5 [41]. All cultural practices, such as phytosanitary treatments, thinning, and fertilization, were carried out according to the standards adopted by the producer and his technical assistance.
During the experiment, soil chemical attributes were evaluated in the depth layers of 0–20 cm and 20–40 cm. The parameters of chlorophyll A, B, and total chlorophyll contents and crop productivity were also assessed.
Soil sampling was carried out with a soil collection kit from the company Saci (Vacaria, Brazil), composed of a 25 mm diameter and 600 mm length drill and two pots intended for collection in the two depths. This kit was coupled to a screwdriver, with ten perforations per plot, so that, in each one, the surface layer (0–20 cm) was first collected and, later, with the change of the pot, the collection of the subsurface layer (20–40 cm) was carried out. All samples were collected on the same side of the planting line, at 50 cm from the tree trunks, and limited to the projection of the crown of the three central plants. The collections were carried out at the end of February 2022, approximately six months after the gypsum treatments were applied.
The chlorophyll contents were measured using a clorofiLOG® device (Falker, Brazil). The measurements were carried out twice, on 15 November 2021 and 15 January 2022 (post-harvest). On both sides, six leaves per plant were evaluated in the lower, middle, and upper portions, totaling 18 leaves per plot.
Productivity was evaluated on 10 January 2022 and all the fruits in each plot’s three central plants were collected. Then, the fruits were weighed using a commercial scale with a capacity of 40 kg.
The obtained results were analyzed for homoscedasticity (Levene’s test) and normality of residuals (Shapiro–Wilk test), followed by an analysis of variance (ANOVA) at a 5 % error probability (p ≤ 0.05). In the case of statistical significance, the means of the treatments were compared by regression analysis at a 5% error probability (p ≤ 0.05). The statistical analyses were conducted using the AgroEstat® software (São Paulo, Brazil).
3. Results
The results obtained in the chemical analyses of the soil did not demonstrate a significant interaction between the use of agricultural gypsum and the evaluated depth. Furthermore, no significant difference was observed when considering the gypsum doses applied. On the other hand, significant effects occurred relative to the soil depth, as can be seen in Table 2.

Table 2.
Soil fertility parameters with different agricultural gypsum dosages in the 0–40 cm layer. Ipê, RS, 2022.
There was no statistical difference between the different treatments for pH, organic matter (OM), H + Al, Ca, K, P, base saturation, and aluminum saturation. However, as expected, all parameters were influenced by the assessed depth.
The contents of all micronutrients evaluated (Cu, Zn, B, and Mn) were not significantly affected by the application of agricultural gypsum, as seen in Table 3.

Table 3.
Soil micronutrient contents as a function of different agricultural gypsum dosages in zero to 40 cm depth. Ipê, RS, 2022.
Although agricultural gypsum has traces of micronutrients in its composition, it was not possible, in this study, to notice significant results with the dosages used, probably due to the low concentration and normal variability of the contents of these elements in the soil.
The results of S content in the soil showed an increase in the levels with the increase in the applied agricultural gypsum dose (Figure 2).
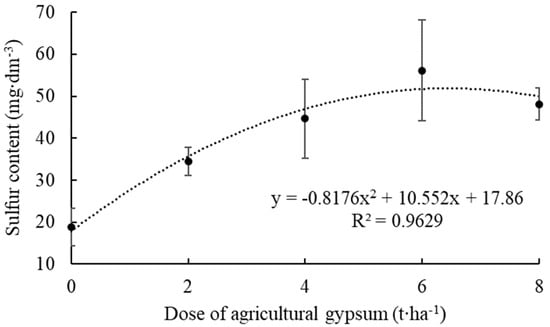
Figure 2.
Sulfur content in the soil as a function of applying agricultural gypsum in an apple orchard, considering the average content of the layer from zero to 40 cm. Ipê, RS, 2022.
Quadratic regression was adjusted for the content of this nutrient, and the dose of 6.0 t∙ha−1 showed the highest S content, corresponding to 56.1 mg∙dm−3. The control, in turn, presented an average S content of 18.9 mg∙dm−3, which represented about three times less sulfur, S, compared to the dose of 6.0 t∙ha−1 agricultural gypsum.
For the Mg contents in the soil, a linear regression (Figure 3) was adjusted to the observed values, making it possible to notice a tendency of decline in the contents, which, in control (zero), was 2.35 cmolc∙dm−3. At the highest agricultural gypsum dosage (8.0 t∙ha−1), the Mg content decreased to 1.43 cmolc∙dm−3.
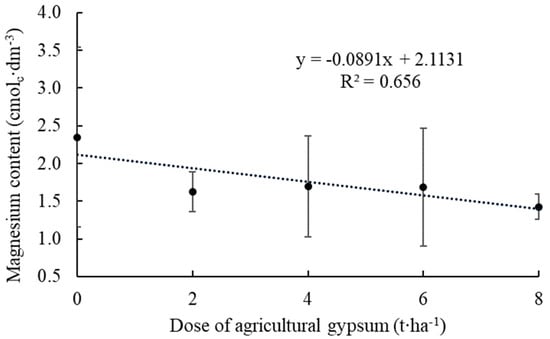
Figure 3.
Magnesium content in the soil as a function of applying agricultural gypsum in an apple orchard, considering the average content of the layer from zero to 40 cm. Ipê, RS, 2022.
No significant differences regarding the total chlorophyll leaf indices were verified for the first evaluation in November 2021. However, a significant linear relationship was found in the data referring to the measurements from January 2022, with a tendency observed for a decline in values as the dosage of agricultural gypsum increased (Figure 4).
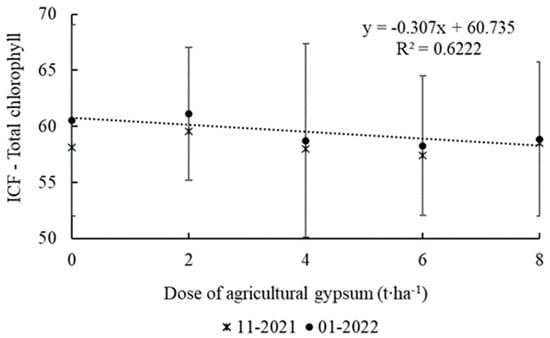
Figure 4.
Index of leaf chlorophyll (ICF) to total chlorophyll in apple leaves as a function of applying agricultural gypsum in two evaluation periods. Ipê, RS, 2022.
The productivity evaluation verified a significant effect by applying agricultural gypsum. There was a trend of increased productivity with increasing gypsum dose, with an ascending linear regression adjustment for the data obtained (Figure 5).
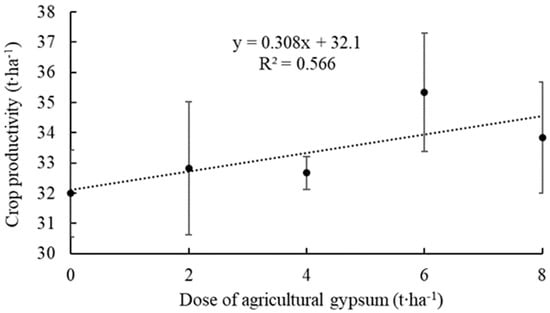
Figure 5.
Productivity of an apple orchard as a function of applying increasing doses of agricultural gypsum. Ipê, RS, 2022.
The control (no gypsum application) showed a productivity of 32.0 t∙ha−1. In contrast, the agricultural gypsum dosage of 6.0 t∙ha−1 in the experiment showed the highest productivity (35.3 t∙ha−1), corresponding to a productivity increase of 10.3% relative to the control.
4. Discussion
The effects of soil chemical parameters observed in this study were only significant regarding the sampling depth. A higher fertility in the superficial layer (zero to 20 cm) can be assigned to liming and fertilization. These operations promote the greater availability of nutrients in the upper layer, unlike the subsurface, which does not receive soil correction and maintains high concentrations of H+ and Al3+ [42]. Likewise, Michalovicz et al. [43] did not observe significant differences for H + Al in all layers, and Pauletti et al. [44] did not observe the effects of gypsum application on soil pH. This occurred because agricultural gypsum cannot hydrolyze water and does not significantly alter soil acidity [9].
The result for K contents in this study, which showed no statistical difference, is probably related to the surface 40 cm of soil evaluated per sample, which may have diluted the leaching effect of potassium from the upper layer. Rampim et al. [45], six months after gypsum application, noticed a reduction in the levels of K in the 10–20 cm layer and no reduction in the 0–10 cm and 20–40 cm layers. The evaluation was performed for every 10 cm of soil, with a difference detected only in the 10–20 cm layer. Given this, the same effect may have occurred in this experiment, but as the evaluation was performed every 20 cm, the potassium leaching effect was possibly not noticed.
The results for Ca in this study did not show significant differences, contrary to what is widely verified in other studies [29,43,44,45,46,47,48]. It is possible that there was a greater leaching of the element, transporting it to the layer below the 40 cm that was evaluated in the study.
For P, one of the components of agricultural gypsum, the results agreed with those of Caires et al. [46] and Michalovicz et al. [43], in which no effect was detected in any evaluated layer, and with Caires et al. [47], who observed differences only in the most superficial 5 cm of the soil. This behavior results from the low mobility of P and its greater retention in soil clay [42], which can dilute its effect in samples from 0–20 cm.
Base saturation results were like those observed by Caires et al. [46], with no significant effect on soil evaluated down to a depth of 60 cm. Pauletti et al. [44] only found a difference in aluminum saturation in the soil layer deeper than 60 cm, which was not evaluated in the present study.
On the other hand, the trend towards an increase in the S content and a decrease in the magnesium content of the soil has also been observed by other authors. Foloni et al. [48] tested the effects of gypsum on corn dry matter and soil attributes in a greenhouse, noting an increase in the S content of the soil from a dose of 2.0 t∙ha−1. Testing gypsum doses in maize, Amaral et al. [29] observed a similar result, corresponding to the increase in S content, showing that, with the rise in gypsum doses, there is an increase in the levels of this element in the soil. This is because S is one of the components of agricultural gypsum and is highly mobile in the soil compared to other elements such as phosphorus [9].
Caires et al. [49], evaluating the effects of limestone and gypsum on soil fertility parameters and soybean production without tillage, observed Mg leaching when agricultural gypsum was applied. In addition, the authors found a decrease in Mg foliar levels in the second crop from the area two years after the installation of the experiment, which, according to them, harmed soybean productivity. Caires et al. [47], evaluating the effect of gypsum and limestone in soybean production with no tillage, observed that there was Mg leaching to the deeper layers eight months after the application of agricultural gypsum, with the effect persisting 32 months after application. Similarly, there was a reduction in the foliar Mg levels in soybeans; however, productivity was not influenced by the application of agricultural gypsum.
Pauletti et al. [44], evaluating the long-term effects of agricultural gypsum, pointed to Mg leaching at 36 months after application. However, the effect was pronounced when assessing the levels in the soil 72 months after application. The same authors also noted that a dose of 12 t∙ha−1 was equivalent to the lowest Mg levels in the soil, indicating a tendency for Mg leaching from agricultural gypsum. Nava et al. [36], testing annual applications of 0, 1.0 t∙ha−1, 2.0 t∙ha−1, and 3.0 t∙ha−1 of agricultural gypsum on apple trees for eight years, observed that there was a decrease in Mg content only up to a depth of 20 cm, remaining adsorbed in the deeper layers.
Mg leaching along the soil profile may be related to the competition that Ca exerts on adsorption in CEC. Ca is the element with the highest preference for the exchange site when both elements are compared [24]. Once displaced from the CEC, Mg can form the, neutral, MgSO4 ion pair which is capable of percolating through the soil profile and accumulating in the subsurface [9], which may have been potentiated by rainfall in the initial period after application, which was 270 mm in September.
No studies were found that related the decrease in the total chlorophyll content with the increase in the dosage of agricultural gypsum. However, this reduction is probably due to the decline in Mg levels in the soil and the increase in the Ca/Mg ratio, resulting from the leaching of the element from the 0 to 40 cm layer. In the study by Salvador et al. [50], in which different Ca/Mg ratios in the soil were tested, the authors found that higher Ca/Mg ratios diminish foliar Mg content. It is important to highlight that Mg is a structural constituent of the chlorophyll molecule [51], and its root absorption can be affected by the presence of other cations, such as Ca [52]. Furthermore, the absorption of Mg and chlorophyll biosynthesis may be affected by nutritional imbalances or other factors. The addition of agricultural gypsum in this experiment, despite not demonstrating a significant effect on Ca levels, increased the Ca/Mg ratio. Considering the gypsum doses applied, the Ca/Mg ratios ranged between 3.6 in the control, 5.4 at 2.0 t∙ha−1, 5.2 at 4.0 t∙ha−1, 4.8 at 6.0 t∙ha−1, and 7.1 in the treatment with 8.0 t∙ha−1, an overall increase in the Ca/Mg ratio of approximately 97%. As observed by Webster [53], higher Ca/Mg ratios may decrease chlorophyll contents with no effects that enhance plant productivity.
Research demonstrating the effects of using agricultural gypsum on productivity is mainly focused on grains. Zandoná et al. [28] evaluated, during the same agricultural year, the application of six doses of agricultural gypsum in soybeans and corn and found that, in both cultures, there was an increase in productivity up to the application of 2.0 t∙ha−1 of gypsum. Michalovicz et al. [42] also observed increased corn and barley productivity by applying gypsum in the same year the evaluated crops were sown. Amaral et al. [29] verified a linear increase in corn productivity by applying up to 4.0 t∙ha−1 of agricultural gypsum.
The increase in productivity observed in the literature could be related to the increase in fertility in the layer below 40 cm, which was not evaluated in this work. Mg leaching associated with the non-occurrence of significant results in Ca contents in the evaluated layer suggests that these nutrients were probably transported to deeper layers than those assessed in the study (depth of up to 40 cm), supposedly due to the intense rainfall (270 mm) verified in the 30 days following the application of the gypsum. It is hypothesized that, with the increase in the concentration of cations by depth, there was a greater root growth of the crop, which was crucial to the rise in production in a season in which a water deficit was verified in the final third of fruit growth, as rainfall equivalent to 65 mm was recorded in October, 80 mm in November and 50 mm in December, totaling 195 mm for the quarter. However, the soil should be analyzed at deeper layers (below 40 cm) to assess such a hypothesis properly.
5. Conclusions
The application of agricultural gypsum increased the S content and decreased the Mg content in the soil at the evaluated depths (0–40 cm), according to the applied dose. Post-harvest chlorophyll levels in apple tree leaves decreased with increasing gypsum dosages. Regarding the productivity of the crop, the application of agricultural gypsum at 6.0 t∙ha−1 caused a 10% increase in apple tree productivity relative to the control. Thus, agricultural gypsum can potentially improve the soil’s chemical conditions in the subsurface, positively affecting fruit trees such as the apple tree. However, further research is needed under different soil types and climatic conditions to establish a suitable gypsum dose for application to this crop.
Author Contributions
Conceptualization, F.M.C.S. and E.D.C.; Methodology, W.P.S., T.D.M., G.F.P. and E.D.C. Data collection, F.M.C.S.; Data analysis and interpretation, F.M.C.S., W.P.S., C.C., T.D.M., G.F.P. and E.D.C.; Writing and editing, F.M.C.S., W.P.S., C.C., T.D.M., G.F.P. and E.D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data produced in the study are shown in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Food and Agriculture Organization of the United Nations (FAO). Faostat. 2021. Available online: http://www.fao.org/faostat/en/#data (accessed on 12 April 2023).
- Instituto Brasileiro de Geografia e Estatística (IBGE). Sistema IBGE de Recuperação Automática (SIDRA): Produção Agrícola Municipal. 2021. Available online: https://sidra.ibge.gov.br/tabela/1613 (accessed on 25 May 2023).
- Associação Gaúcha de Produtores de Maçã (AGAPOMI). 2021. Available online: http://agapomi.com.br/ (accessed on 12 April 2023).
- Sezerino, A.A. Sistema de Produção para a Cultura da Macieira em Santa Catarina; Epagri: Florianópolis, Brazil, 2018. [Google Scholar]
- Freire, C.J.S.; Camelatto, D.; Flores-Cantillano, R.F.; Kovaleski, A.; Fortes, J.F. A Cultura da Maçã; Embrapa—SPI: Brasília, Brazil, 1994. [Google Scholar]
- Raese, T.J. Effect of fertilizers on soil pH and performance of apple and pear trees. ii. grown in different soils in the orchard. Comm. Soil Sci. Plant Anal. 1994, 25, 9–10. [Google Scholar] [CrossRef]
- Comissão de Química e Fertilidade do Solo–RS/SC (CQFS). Manual de Calagem e Adubação para os Estados do Rio Grande do Sul e Santa Catarina, 11th ed.; Sociedade Brasileira de Ciência do Solo–Núcleo Regional Sul: Porto Alegre, Brazil, 2016. [Google Scholar]
- Veloso, C.A.C.; Botelho, S.M.; Rodrigues, J.E.L.F.; Silva, A.R. Correção da acidez do solo. In Recomendações de Calagem e Adubação para o Estado do Pará, 2nd ed.; Brasil, E.C., Cravo, M.S., Viégas, I.J.M., Eds.; Embrapa: Brasília, Brazil, 2020; pp. 121–132. [Google Scholar]
- Sousa, D.M.G.; Miranda, L.N.; Oliveira, S.A. Acidez do solo e sua correção. In Fertilidade do solo, 1st ed.; Novais, R.F., Alvarez, V.H., Barros, N.F., Fontes, R.L., Cantarutti, R.B., Neves, J.C.L., Eds.; Sociedade Brasileira de Ciência do Solo: Viçosa, Brazil, 2007; pp. 205–274. [Google Scholar]
- Lopes, A.S. Manual Internacional de Fertilidade do solo, 2nd ed.; Potafos: Piracicaba, Brazil, 1998. [Google Scholar]
- Sousa, R.A.; Silva, T.R.B. Acidificação de um latossolo vermelho distroférrico em função da aplicação de nitrogênio oriundo de uréia, sulfato de amônio e sulfammo. Cult. Saber 2009, 2, 78–83. [Google Scholar]
- Raij, B.V. Fertilidade do solo e Manejo de Nutrientes; IPNI: Piracicaba, Brazil, 2011. [Google Scholar]
- Rossiello, R.O.P.; Netto, J.J. Toxidez de alumínio em plantas: Novos enfoques para um velho problema. In Nutrição Mineral de Plantas; Fernandes, M.F., Ed.; Sociedade Brasileira de Ciência do Solo: Viçosa, Brazil, 2006; pp. 375–418. [Google Scholar]
- Sivaguru, M.; Ezaki, B.; He, Z.H.; Tong, H.; Osawa, H.; Baluska, F.; Volkmann, D.; Matsumoto, H. Aluminum-induced gene expression and protein localization of a cell wall-associated receptor kinase in Arabidopsis. Plant Physiol. 2003, 132, 2256–2266. [Google Scholar] [CrossRef] [PubMed]
- Sivaguru, M.; Fujiwara, T.; Samaj, J.; Baluska, F.; Yang, Z.; Osawa, H.; Maeda, T.; Mori, T.; Volkmann, D.; Matsumoto, H. Aluminum-Induced 1→3-β-d-Glucan Inhibits Cell-to-Cell Trafficking of Molecules through Plasmodesmata. A New Mechanism of Aluminum Toxicity in Plants. Plant Physiol. 2000, 124, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Novais, R.F.; Mello, J.W. Relação solo-planta. In Fertilidade do solo; Novais, R.F., Alvarez, V.H., Barros, N.F., Fontes, R.L., Cantarutti, R.B., Neves, J.C.L., Eds.; Sociedade Brasileira de Ciência do Solo: Viçosa, Brazil, 2007; pp. 133–204. [Google Scholar]
- Ronquim, C.C. Conceitos de Fertilidade do solo e Manejo Adequado para as Regiões Tropicais, 2nd ed.; Embrapa Territorial: Campinas, Brazil, 2020. [Google Scholar]
- Primavesi, A.C.; Primavesi, O. Características de Corretivos Agrícolas; Embrapa Pecuária Sudeste: São Carlos, Brazil, 2004. [Google Scholar]
- Wiethölter, S. Calagem no Brasil; Embrapa Trigo: Passo Fundo, Brazil, 2000. [Google Scholar]
- Weirich Neto, P.H.; Caires, E.F.; Justino, A.; Dias, J. Correção da acidez do solo em função de modos de incorporação de calcário. Ciênc. Rural 2000, 30, 257–261. [Google Scholar] [CrossRef]
- Freiria, A.C.; Mantovani, J.R.; Ferreira, M.E.; Cruz, M.C.P.; Yagi, R. Alterações em atributos químicos do solo pela aplicação de calcário na superfície ou incorporado na superfície ou incorporado. Acta Sci. Agron. 2008, 30, 285–291. [Google Scholar]
- Fachinello, J.C.; Nachtigal, J.C.; Kersten, E. Fruticultura: Fundamentos e Práticas; Embrapa Clima Temperado: Pelotas, Brazil, 2008. [Google Scholar]
- Brasil, E.C.; Lima, E.V.; Cravo, M.S. Uso de gesso na agricultura. In Recomendações de Calagem e Adubação Para o Estado do Pará, 2nd ed.; Brasil, E.C., Cravo, M.S., Viégas, I.J.M., Eds.; Embrapa: Brasília, Brazil, 2020; pp. 133–146. [Google Scholar]
- Raij, B.V. Gesso na Agricultura; Instituto Agronômico: Campinas, Brazil, 2008. [Google Scholar]
- Alvarez, V.H.; Roscoe, R.; Kurihara, C.H.; Pereira, N.F. Enxofre. In Fertilidade do solo; Novais, R.F., Alvarez, V.H., Barros, N.F., Fontes, R.L., Cantarutti, R.B., Neves, J.C.L., Eds.; Sociedade Brasileira de Ciência do Solo: Viçosa, Brazil, 2007; pp. 595–644. [Google Scholar]
- Custódio, D.P.; Oliveira, I.P.; Costa, I.A.P.; Faria, C.D. Avaliação do gesso no desenvolvimento e produção do capim-tanzânia. Ciênc. Anim. Bras. 2005, 6, 27–34. [Google Scholar]
- Caires, E.F.; Fonseca, A.F.; Mendes, J.; Chueiri, W.A.; Madruga, E.F. Produção de milho, trigo e soja em função das alterações das características químicas do solo pela aplicação de calcário e gesso na superfície, em sistema de plantio direto. Rev. Bras. Ciênc. Solo 1999, 23, 315–327. [Google Scholar] [CrossRef]
- Zandoná, R.R.; Beutler, A.N.; Burg, G.M.; Barreto, C.F.; Schmidt, M.R. Gesso e calcário aumentam a produtividade e amenizam o efeito do déficit hídrico em milho e soja. Pesq. Agropec. Trop. 2015, 45, 128–137. [Google Scholar] [CrossRef]
- Amaral, L.A.; Ascari, J.P.; Duarte, W.M.; Mendes, I.R.N.; Santos, E.S.; Julio, O.L.L. Efeito de doses de gesso agrícola na cultura do milho e alterações químicas no solo. Rev. Agrar. 2017, 10, 31–41. [Google Scholar] [CrossRef]
- Conte, E.D.; Amaral, L.O.; Macedo, C.K.B.; Dal Magro, T.; Marchioretto, L.R.; Schenkel, V.O.; Scopel, E.S. Rates of Agricultural Gypsum in Soil Under No-tillage System with Surface Lime in the Southern of Brazil. J. Agric. Sci. 2018, 10, 544–552. [Google Scholar] [CrossRef][Green Version]
- Caires, E.F.; Feldhaus, I.C.; Barth, G.; Garbuio, F.J. Lime and gypsum application on the wheat crop. Sci. Agric. 2002, 59, 357–364. [Google Scholar] [CrossRef]
- Sousa, D.M.G.; Vilela, L.; Lobato, E.; Soares, W.V. Uso de Gesso, Calcário e Adubos para Pastagens no Cerrado; Embrapa Cerrados: Planaltina, Brazil, 2001. [Google Scholar]
- Costa, B.P.; Duarte Júnior, J.B.; Costa, A.C.T.; Lana, M.C. Uso do calcário e do gesso agrícola em duas épocas de implantação do Coffea arabica L. Rev. Bras. Agropec. Sust. 2020, 10, 241–247. [Google Scholar]
- Raut, Y.Y.; Shedekar, V.S.; Islam, K.R.; Gonzalez, J.M.; Watts, D.B.; Dick, W.A.; Flanagan, D.C.; Fausey, N.R.; Batte, M.T.; Reeder, R.C.; et al. Soybean yield response to gypsum soil amendment, cover crop, and rotation. Agric. Environ. Lett. 2020, 5, e20020. [Google Scholar] [CrossRef]
- Alves, L.A.; Tiecher, T.L.; Flores, J.P.M.; Filippi, D.; Gatiboni, L.C.; Bayer, C.; Pias, O.H.C.; Marquez, A.A.; Bordignon, V.; Goulart, R.Z.; et al. Soil chemical properties and crop response to gypsum and limestone on a coarse-textured Ultisol under no-till in the Brazilian Pampa biome. Geoderma. Reg. 2021, 25, e00372. [Google Scholar] [CrossRef]
- Nava, G.; Ernani, P.R.; Sá, A.A.; Pereira, A.J. Soil composition and nutritional status of apple as affected by long-term application of gypsum. Rev. Bras. Ciên. Solo 2012, 36, 215–222. [Google Scholar] [CrossRef]
- Danner, M.A.; Scariotti, S.; Ciadin, I.; Penso, G.A.; Cassol, L.C. Calcium sources applied to soil can replace leaf application in ‘Fuji’ apple tree. Pesq. Agropec. Trop 2015, 45, 266–273. [Google Scholar] [CrossRef]
- Tiecher, T.; Pias, O.H.C.; Bayer, C.; Martins, A.P.; Denardin, L.G.O.; Anghinoni, I. Crop Response to Gypsum Application to Subtropical Soils Under No-Till in Brazil: A Systematic Review. Rev. Bras. Ciênc. Solo 2018, 42, e0170025. [Google Scholar] [CrossRef]
- Tedesco, M.J.; Gianello, C.; Bissani, C.A.; Bonhen, H.; Wolkweiss, S.J. Análise de solo, Plantas e Outros Materiais, 2nd ed.; Departamento de Solos da Universidade Federal do Rio Grande do Sul: Porto Alegre, Brazil, 1995. [Google Scholar]
- Prezotti, L.C.; Guarçoni, A. Guia de Interpretação de Análise de solo e Foliar; Incaper: Vitória, Brazil, 2013. [Google Scholar]
- Dias, L.E. Uso de gesso Como Insumo Agrícola; Embrapa: Brasília, Brazil, 1992. [Google Scholar]
- Novais, R.F. Fertilidade do solo, 1st ed.; Alvarez, V.H., Barros, N.F., Fontes, R.L., Cantarutti, R.B., Neves, J.C.L., Eds.; Sociedade Brasileira de Ciência do Solo: Viçosa, Brazil, 2007. [Google Scholar]
- Michalovicz, L.; Müller, M.M.L.; Foloni, J.S.S.; Kawakami, J.; Nascimento, R.; Kramer, L.F.M. Soil fertility, nutrition and yield of maize and barley with gypsum application on soil surface in no-till. Rev. Bras. Ciênc. Solo 2014, 38, 1496–1505. [Google Scholar] [CrossRef]
- Pauletti, V.; Pierri, L.; Ranzan, T.; Barth, G.; Motta, A.C.V. Efeitos em longo prazo da aplicação de gesso e calcário no sistema de plantio direto. Rev. Bras. Ciênc. Solo 2014, 38, 495–505. [Google Scholar] [CrossRef]
- Rampim, L.; Lana, M.C.; Frandaloso, J.F.; Fontaniva, S. Atributos químicos de solo e resposta do trigo e da soja ao gesso em sistema semeadura direta. Rev. Bras. Ciênc. Solo 2011, 35, 1687–1698. [Google Scholar] [CrossRef]
- Caires, E.F.; Fonseca, A.F.; Feldhaous, I.C.; Blum, J. Crescimento radicular e nutrição da soja cultivada no sistema plantio direto em resposta ao calcário e gesso na superfície. Rev. Bras. Ciênc. Solo 2001, 25, 1029–1040. [Google Scholar] [CrossRef]
- Caires, E.F.; Blum, J.; Barth, G. Alterações químicas do solo e resposta da soja ao calcário e gesso aplicados na implantação do sistema plantio direto. Rev. Bras. Ciênc. Solo 2003, 27, 275–286. [Google Scholar] [CrossRef]
- Foloni, J.S.S.; Santos, D.H.; Creste, J.E.; Câmara, M. Produção de matéria seca do milho e fertilidade do solo em função da gessagem em excesso. Colloq. Agrar. 2008, 4, 42–51. [Google Scholar] [CrossRef]
- Caires, E.F.; Chueiri, W.A.; Madruga, E.F.; Figueiredo, A. Alterações de características químicas do solo e resposta da soja ao calcário e gesso aplicados na superfície em sistema de cultivo sem preparo do solo. Rev. Bras. Ciênc. Solo 1998, 22, 27–34. [Google Scholar] [CrossRef]
- Salvador, J.T.; Carvalho, T.C.; Lucchesi, L.A.C. Relações cálcio e magnésio presentes no solo e teores foliares de macronutrientes. Rev. Acad. Ciênc. Agrár. Amb. 2011, 9, 27–32. [Google Scholar] [CrossRef][Green Version]
- Kerbauy, G.B. Fisiologia Vegetal; Guanabara Koogan: Rio de Janeiro, Brazil, 2004. [Google Scholar]
- Vitti, G.C.; Lima, E.; Cicarone, F. Cálcio, magnésio e enxofre. In Nutrição Mineral de Plantas; Fernandes, M.F., Ed.; Sociedade Brasileira de Ciência do Solo: Viçosa, Brazil, 2006; pp. 299–325. [Google Scholar]
- Webster, D.H. Effect of varied Ca/Mg ratios on Ca and Mg concentrations in leaf samples of two apple cultivars. Can. J. Plant Sci. 1985, 65, 959–968. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
