Habitat Characteristics of Camellia quephongensis and Adaptation Mechanisms in Que Phong District, North-Central Vietnam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
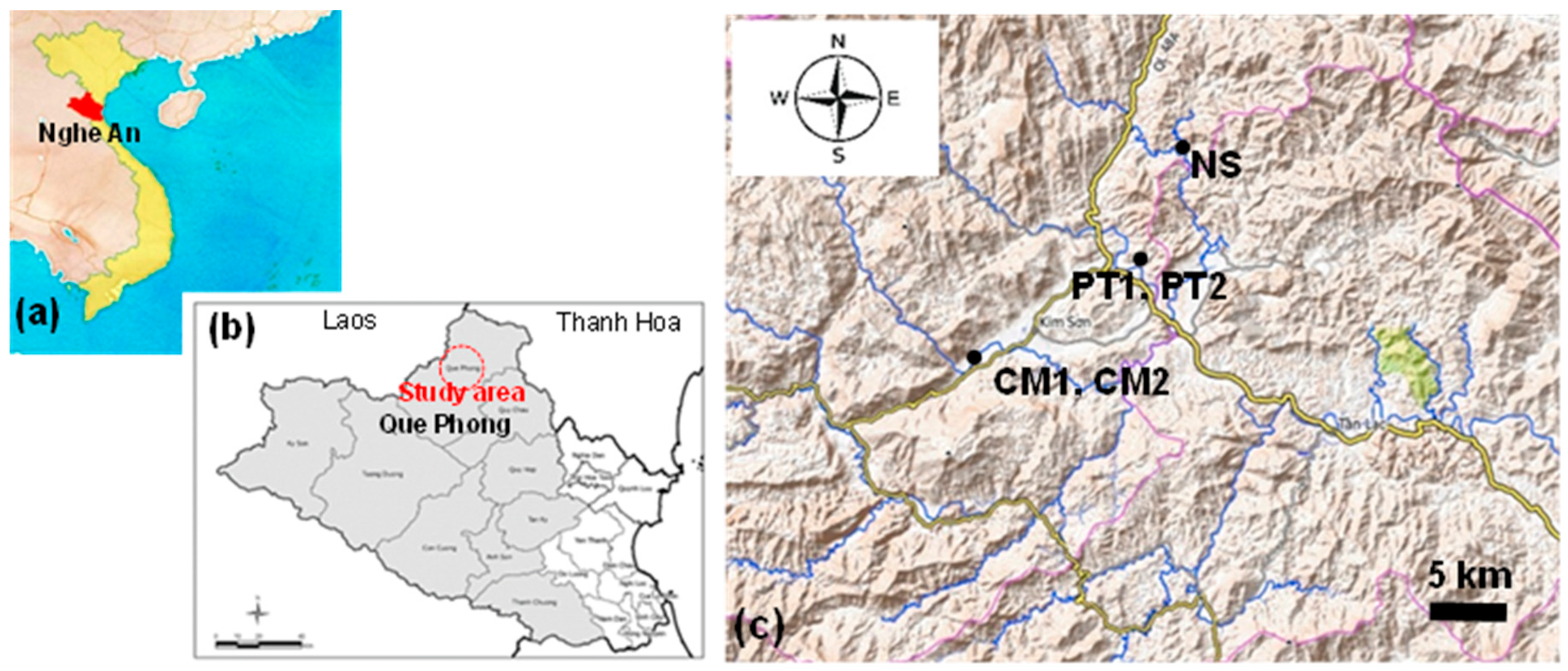
2.1.1. Study Area and Sites
2.1.2. Climate
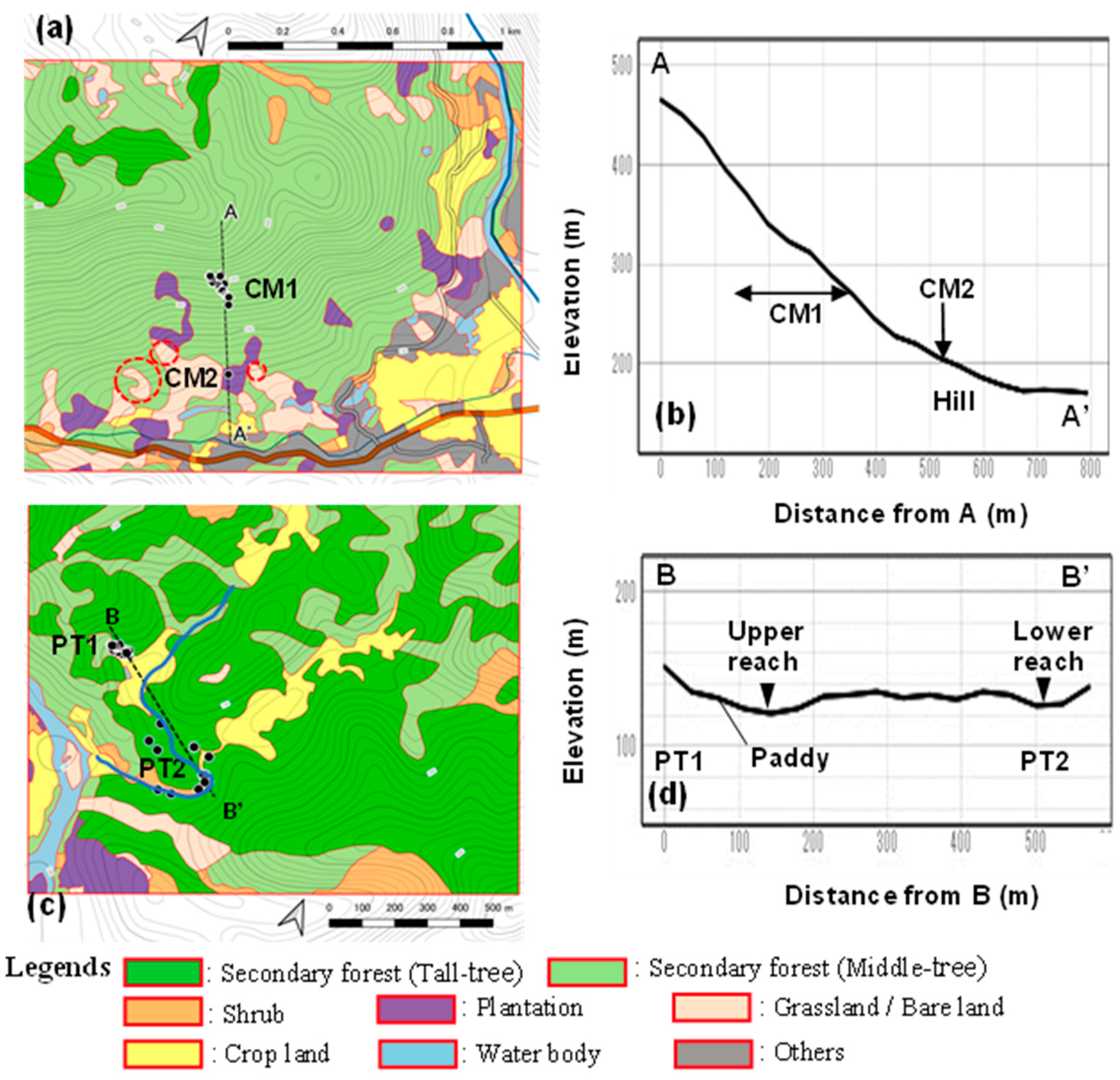
2.1.3. Vegetation and Topography
2.2. Methods
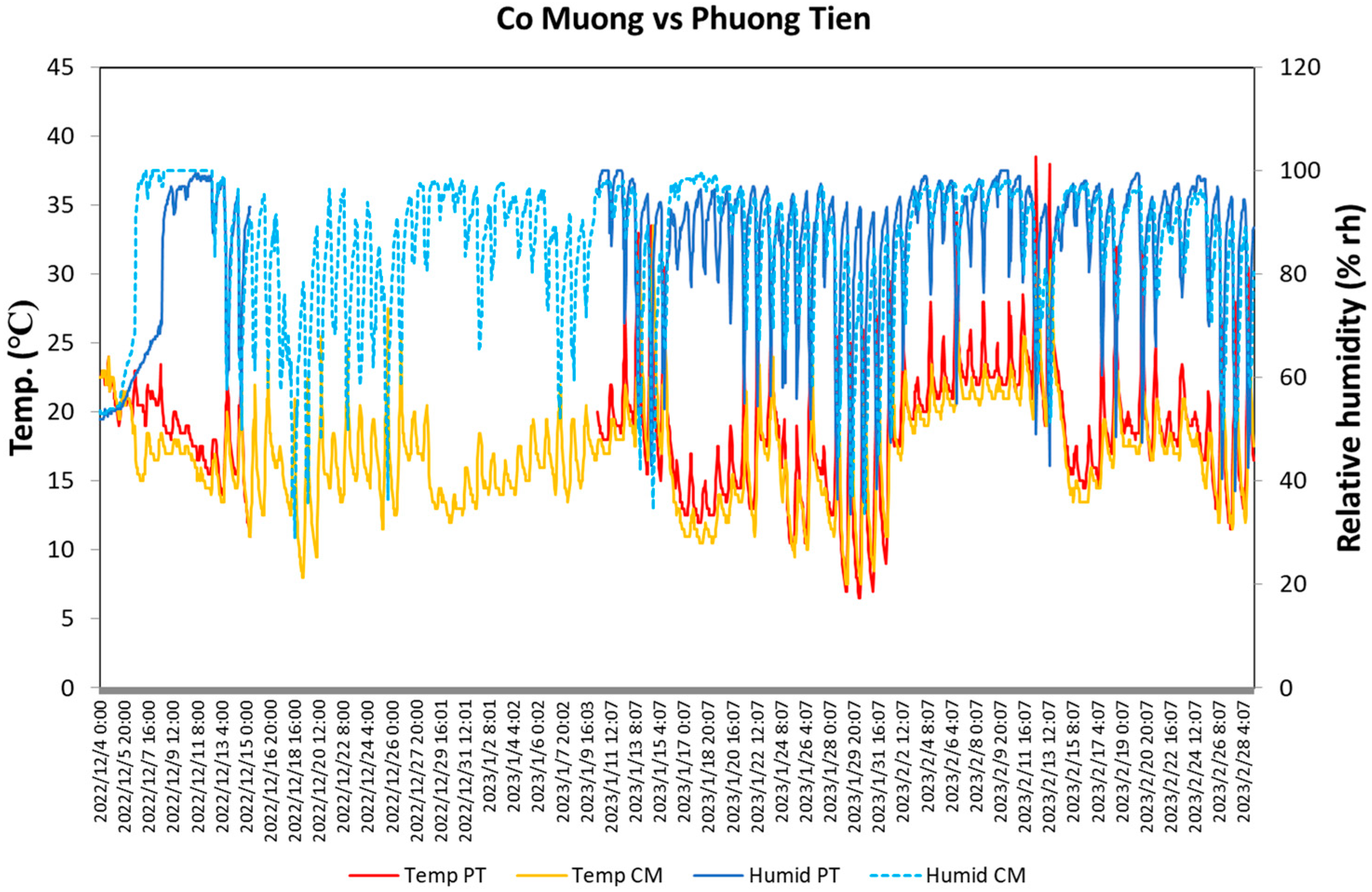
2.2.1. Temperature and Humidity
2.2.2. Population Size and Floristic Composition of the Habitats
2.2.3. Landscape Pattern Analysis
2.2.4. Age Distribution Patterns
2.2.5. Multi-Stemming
3. Results
3.1. Micro-Climate
3.2. Population Size and Floristic Composition of the Habitats
3.3. Habitat Characteristics in the Landscape Perspective
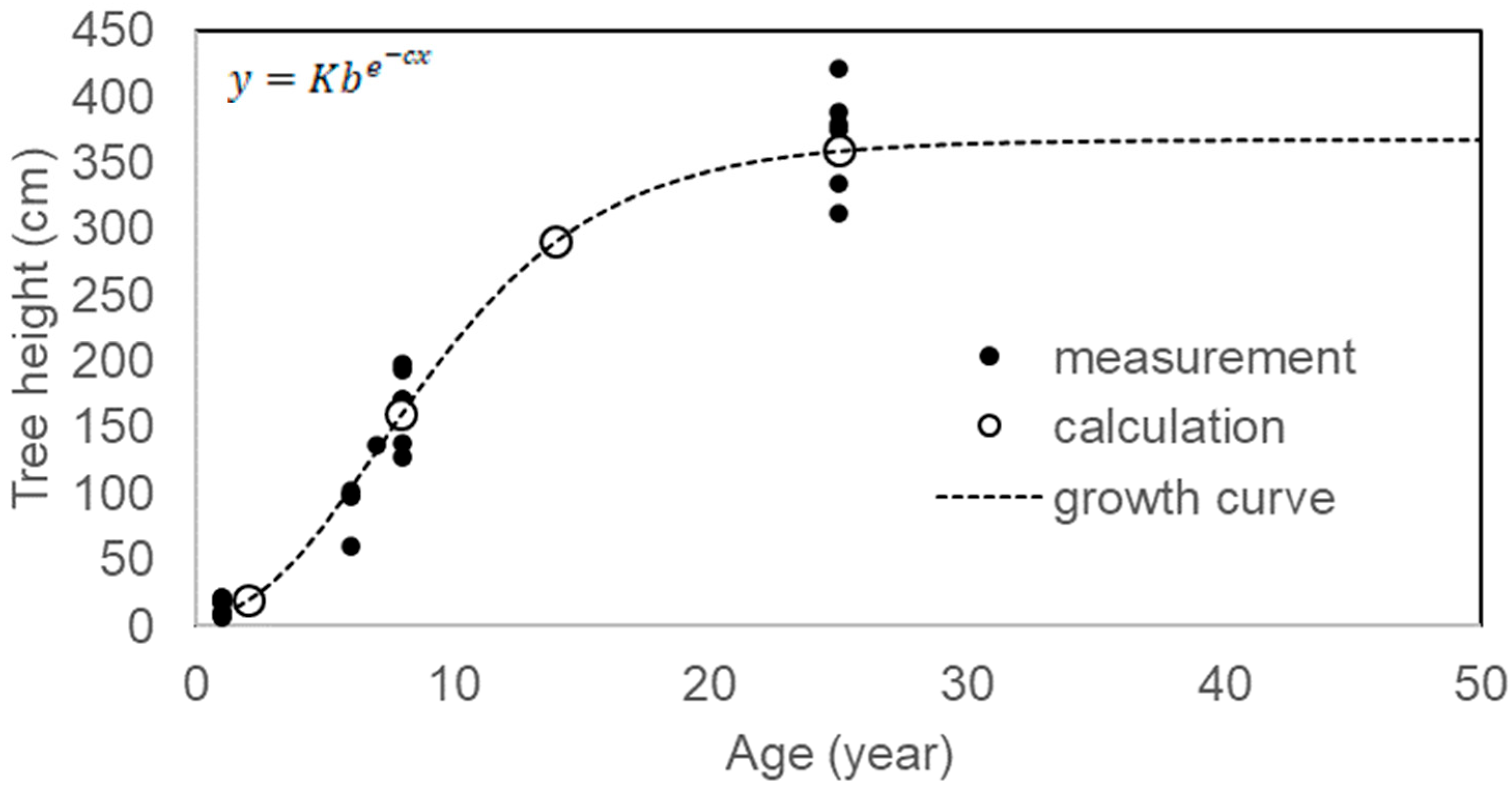
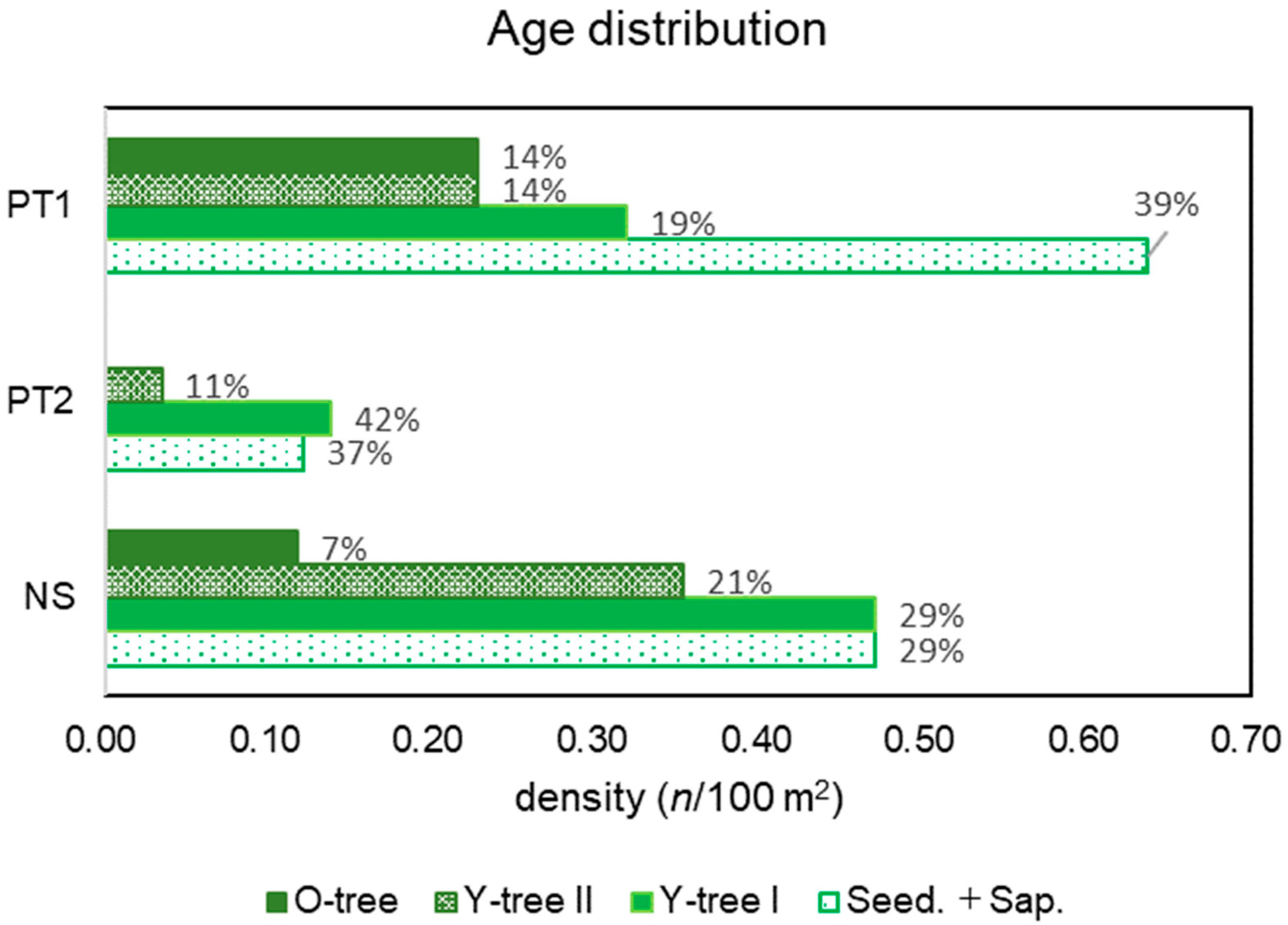
3.4. Age Distribution Patterns
3.5. Multi-Stemming and Growth
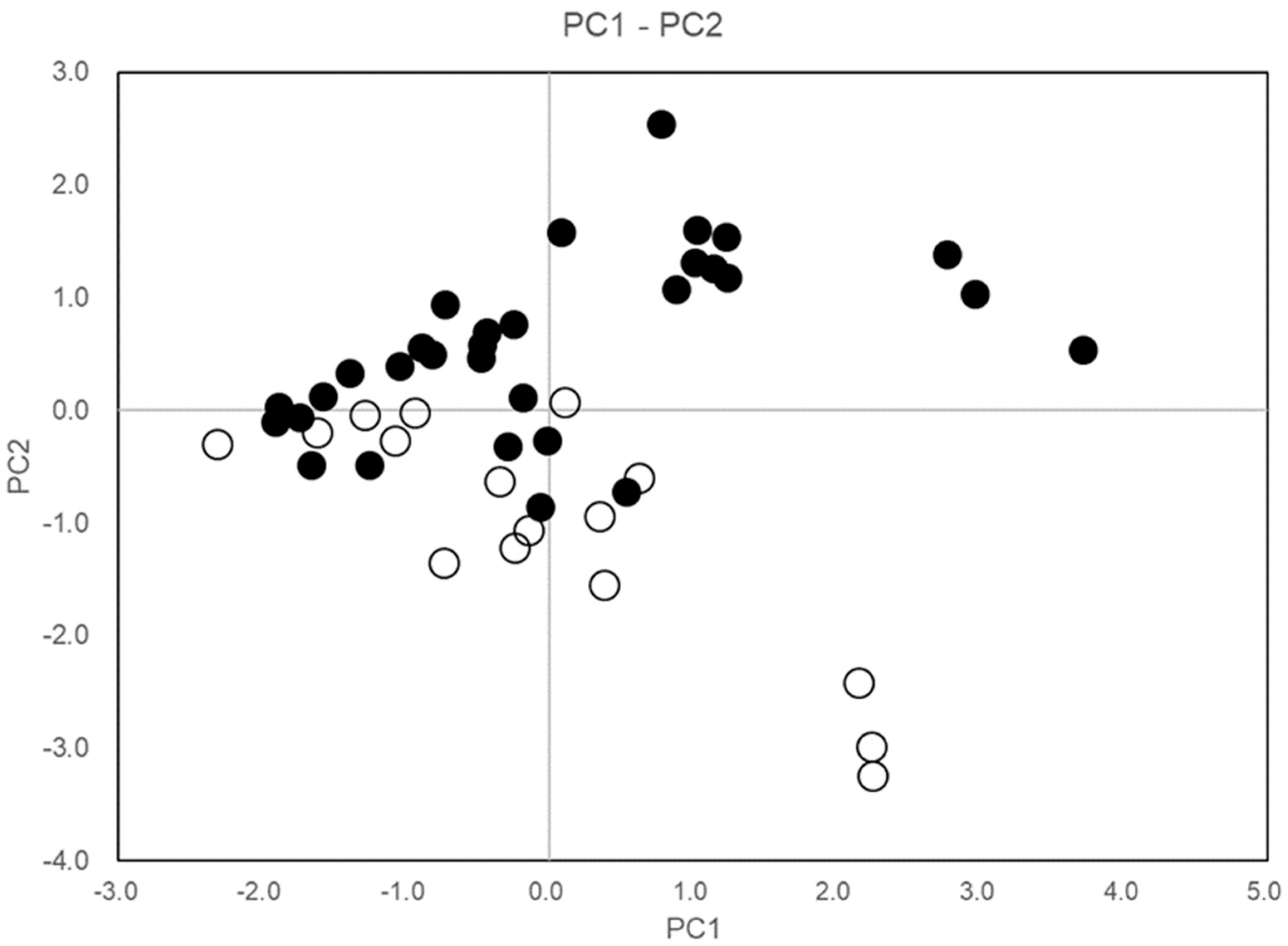
3.6. Variability between Patches
4. Discussion
4.1. Vulnerability of the Habitats
4.2. Adaptation Mechanisms
4.2.1. Growth Pattern Perspective
4.2.2. Population Structure Perspective
4.3. Recommended Measures for Conservation
4.3.1. Zonation
4.3.2. Control of Succession
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le, N.N.H.; Luong, D.V.; Do, D.N. Additional conditions for effective publication of Camellia quephongensis and Camellia hamyenensis. Int. Camellia J. 2021, 53, 99–107. [Google Scholar]
- Tran, M.D.; Nguyen, T.T.; Hoang, S.T.; Dang, T.V.; Phung, T.D.; Nguyen, T.V.; Dao, D.T.; Mai, L.T.; Vu, L.T.; Nguyen, T.H.; et al. Golden Camellias: A Review. Arch. Curr. Res. Int. 2019, 16, 1–8. [Google Scholar]
- Le, N.N.H.; Luong, D.V.; Nguyen, C.V.; Pham, T.D.T.; Luu, T.T.; Pham, T.V. An updated checklist of Theaceae and a new species of Polyspora from Vietnam. Taiwania 2020, 65, 216–227. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Tran, N.; Uematsu, C.; Katayama, H.; Luong, D.V.; Hoang, S.T.; Nguyen, K.D.; Nguyen, H.V.; Thai, T.C. Two new species of Camellia (Theaceae) from Vietnam. Korean J. Plant Taxon. 2018, 48, 115–122. [Google Scholar] [CrossRef]
- Do, D.N.; Luong, D.V.; Nguyen, C.D.; Hoang, S.T.; Le, H.T.; Han, J.E.; Park, H. A new yellow Camellia (Theaceae) from central Vietnam. Korean J. Plant Taxon. 2019, 49, 90–95. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Luong, D.V.; Le, H.T.; Tran, T.Q.; Do, D.N.; Ly, S.N. Camellia puhoatensis (Sect. Archecamellia—Theaceae), a new species from Vietnam. PhytoKeys 2020, 153, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Do, D.N.; Luong, D.V.; Le, H.T.; Nguyen, H.D.; Nguyen, N.T.; Ly, S.N. Camellia ngheanensis (Sect. Chrythanta: Theaceae), a new species from Central Vietnam. Phytotaxa 2020, 452, 209–216. [Google Scholar] [CrossRef]
- Ohmiya, A. Review Diversity of carotenoid composition of flower petals. Jpn. Agri. Res. Q. 2011, 45, 163–172. [Google Scholar] [CrossRef]
- Nguyen, H.V.T.; Pham, B.C.; Cam, I.T.; Doan, P.L.; Le, T.T.; Tran, T.Q.; Pham, L.Q. Flavonoids isolated from the flowers of Camellia chrysantha. Vietnam J. Sci. Technol. 2019, 57, 287–293. [Google Scholar] [CrossRef]
- Diep, T.T. Yellow camellias: A review of chemical constituents and biological activities. Dalat Univ. J. Sci. 2022, 12, 117–144. [Google Scholar]
- Tanikawa, N.; Kashiwabara, T.; Hokura, A.; Abe, T.; Shibata, M.; Nakayama, M. A Peculiar Yellow Flower Coloration of Camellia Using Aluminum-flavonoid Interaction. J. Jpn. Soc. Hort. Sci. 2008, 77, 402–407. [Google Scholar] [CrossRef]
- Dao, D.T.; Mai, L.T.; Tran, M.D.; Dang, T.V.; Ly, T.H.T.; Nguyen, T.V.; Phung, T.D.; Nguyen, T.P.T.; Ninh, K.V.; Dang, H.H.T.; et al. Cutting size and position affect rooting efficiency of Camellia impressinervis: A golden camellia. Plant Cell Biotechnol. Mol. Biol. 2019, 20, 179–187. [Google Scholar]
- Tran, D.V.; Tran, M.D.; Dao, D.T.; Mai, L.T.; Nguyen, T.T.; Dang, T.V.; Ly, T.H.T.; Nguyen, T.V.; Phung, T.D.; Nguyen, T.P.T.; et al. Effect of exogenous hormone and rooting medium on cutting propagation of golden Camellia (Camellia impressinervis). J. Appl. Hortic. 2020, 22, 159–163. [Google Scholar] [CrossRef]
- Chai, S.; Trang, J.; Mallik, A.; Shi1, Y.; Zou, R.; Li, J.; Wei, X. Eco-physiological basis of shade adaptation of Camellia nitidissima, a rare and endangered forest understory plant of Southeast Asia. BioMed Central Biol. 2018, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Nishikawa, H.; Tanabe, R.; Tran, D.Q. Golden camellia as a driver of forest regeneration and conservation: A case study of value-chain forestry with Camellia quephongensis in Que Phong, Nghe An, North-Central Vietnam. Forests 2023, 14, 1087. [Google Scholar] [CrossRef]
- Zhu, X.; Tang, J.; Qin, H.; Bai, K.; Chen, Z.; Zou, R.; Liu, S.; Yang, Q.; Wei, X.; Chai, S. Contrasting adaptation mechanisms of golden camellia species to different soil habitats revealed by Nutrient Characteristics. Agronomy 2022, 12, 1511. [Google Scholar] [CrossRef]
- Xu, Z.; Ren, H.; Wei, X.; Ouyang, K.; Li, D.; Guo, Y.; Wen, S.; Long, J.; Wang, J.; Hui, D. Distribution and conservation status of Camellia longzhouensis (Theaceae), a critically endangered plant species endemic to southern China. Glob. Ecol. Conserv. 2021, 27. [Google Scholar] [CrossRef]
- Inoue, K.; Fukushi, M.; Le, T.V.; Tsuruoka, H.; Kasahara, S.; Nimelan, V. Distribution of gamma radiation dose rate related with natural radionuclides in all of Vietnam and radiological risk assessment of the built-up environment. Sci. Rep. 2020, 10, 12428. [Google Scholar] [CrossRef]
- Trinh, H.D.; Luu, T.C.; Nguyen, A.T.; Tran, A.V.; Phan, G.H.; Takahashi, N.; Saddsy, B.L. Paleogene granite magmatism in the north of the Truong Son belt and implication for crustal evolution. Vietnam J. Earth Sci. 2021, 43, 444–464. [Google Scholar] [CrossRef]
- Que Phong District People’s Committee: Location, Natural Conditions. Available online: https://nghean.gov.vn (accessed on 30 April 2023).
- Stuppy, W.; van Welzen, P.C.; Klinratana, P.; Posa, M.C.T. Revision of the genera Aleurites, Reutealis and Vernicia (Euphorbiaceae). Blumea Biodivers. Evol. Biogeogr. Plants 1999, 44, 73–98. [Google Scholar]
- EL-Juhany, L.I. Evaluation of some wood quality measures of eight-year-old Melia azedarach trees. Turk. J. Agric. For. 2011, 35, 165–171. [Google Scholar] [CrossRef]
- Fritsch, P.W.; Kelly, L.M.; Wang, Y.; Almeda, F.; Kriebel, R. Revised infrafamilial classification of Symplocaceae based on phylogenetic data from DNA sequences and morphology. Taxon 2008, 57, 823–852. [Google Scholar] [CrossRef]
- Yang, X.; Yan, H.; Li, B.; Han, Y.; Song, B. Spatial distribution patterns of Symplocos congeners in a subtropical evergreen broad-leaf forest of southern China. J. For. Res. 2018, 29, 773–784. [Google Scholar] [CrossRef]
- Nguyen, N.L.; Do, D.S.; Nguyen, X.Q.; Tran, V.L.; Ngo, D.Q.; Tran, V.C.; Nguyen, D.K.; Lai, V.C.; Do, H.T.; Ngo, T.G.; et al. Final Report on Forest Ecological Stratification in Vietnam; UN-REDD Programme: Hanoi, Vietnam, 2011; p. 130. [Google Scholar]
- Camarero, J.J.; Colangelo, M.; Rodríguez-Gonzalez, P.M.; Sanchez-Miranda, Á.; Sanchez-Salguero, R.; Campelo, F.; Rita, A.; Ripullone, F. Wood anatomy and tree growth covary in riparian ash forests along climatic and ecological gradients. Dendrochronologia 2021, 70, 125891. [Google Scholar] [CrossRef]
- Berthelot, J.S.; Saint-Laurent, D.; Gervais-Beaulac, V.; Savoie, D. Assessing the Effects of Periodic Flooding on the Population Structure and Recruitment Rates of Riparian Tree Forests. Water 2014, 6, 2614–2633. [Google Scholar] [CrossRef]
- Greet, J.; Fischer, S.; Walsh, C.J.; Sammonds, M.J.; Catford, J.A. Restored river-floodplain connectivity promotes riparian tree maintenance and recruitment. For. Ecol. Manag. 2022, 506, 119952. [Google Scholar] [CrossRef]
- Fischer, S.; Greet, J.; Walsh, C.J.; Catford, J.A. Flood disturbance affects morphology and reproduction of woody riparian plants. Sci. Rep. 2021, 11, 16477. [Google Scholar] [CrossRef]
- Tanentzap, A.J.; Edward, P.; Mountford, E.P.; Cooke, A.S.; Coomes, D.A. The more stems the merrier: Advantages of multi-stemmed architecture for the demography of understorey trees in a temperate broadleaf woodland. J. Ecol. 2012, 100, 171–183. [Google Scholar] [CrossRef]
- Kavsnica, I.; Matula, R.; Rejzek, M.; Ewers, R.M.; Riutta, T.; Turner, E.C.; Nilus, R.; Svatek, M. Multi-stemming enhances tree survival and growth in Borneo’s logged forests. For. Ecol. Manag. 2023, 544, 121140. [Google Scholar] [CrossRef]
- Fahey, T.J.; Sherman, R.E.; Tanner, E.V.J. Tropical montane cloud forest: Environmental drivers of vegetation structure and ecosystem function. J. Trop. Ecol. 2015, 32. [Google Scholar] [CrossRef]
- Dias, P.C. Sources and sinks in population biology. Tree 1996, 11, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Matsuki, R.; Ueno, S.; Nashimoto, M.; Hasegawa, M. Dispersal of Camellia japonica seeds by Apodemus speciosus revealed by maternity analysis of plants and behavioral observation of animal vectors. Ecol. Res. 2006, 21, 732–740. [Google Scholar] [CrossRef]


| Highest | Lowest | |
|---|---|---|
| Temperature (°C) | ||
| CM | 21.2 ± 0.6 | 15.2 ± 0.5 |
| PT | 24.7 ± 0.7 | 15.9 ± 0.5 |
| Relative humidity (%rh) | ||
| CM | 94.0 ± 1.0 | 74.1 ± 2.5 |
| PT | 94.1 ± 1.2 | 65.9 ± 2.3 |
| Local Population | PT | CM | NS | ||||
|---|---|---|---|---|---|---|---|
| Riparian Type | Mountain Slope Type | Riparian Type | |||||
| Patch | PT2 | PT1 | CM1 | CM2 | − | ||
| Habitat Land morphology | stream bank | valley slope | mountain slope | hill slope | valley slope | ||
| Anthropogenic impacts | − | regenerated forest for 10–20 years | regenerated forest for 25 years | transplanted population | |||
| Population size Distributed area (m × m) | 580 × 10 | 220 × 10 | 275 × 10 | − | 85 × 10 | ||
| Population density (n/100 m2) | 0.3 | 1.4 | 1.1 | − | 1.4 | ||
| Stand characteristics Stand type | riparian forest | secondary forest (evergreen broadleaved forest) | plantation | bamboo forest | |||
| Elevation (m) | 130 | 140 | 330 | 300 | 200 | 160 | |
| Slope | NNE—SSW | SE | SE | SE | SW | ||
| Gradient (°) | 35 | 43 | 15 | 30 | 15 | 42 | 43 |
| Canopy openness (%) | 20.2 | 36.5 | 22.7 | 18.8 | 27.6 | 28.0 | 28.3 |
| Canopy height (m) | 16 | 22 | 20 | 21 | 20 | 12 | 14 |
| Tree density (n/100 m2) | 3.0 | 2.7 | 3.3 | 2.3 | 4.5 | 7.1 | 7.6 |
| Dominant species | |||||||
| Acacia mangium | − | − | − | − | − | ++ | ++ |
| Anodendron sp. | − | − | − | (++) | − | − | − |
| Cinnamomum loureiroi | − | − | − | − | ++ | − | − |
| Dendrocalamus sp. | − | − | − | − | − | ++ | ++ |
| Fraxinus griffithii | ++ | − | − | − | − | − | − |
| Livistona sp. | − | (++) | − | − | − | − | − |
| Melia azedarach | − | ++ | ++ | ++ | + | − | − |
| Quercus sp. | − | ++ | − | − | − | − | − |
| − | (++) | − | − | − | − | − | |
| Symplocos sp. | − | − | (++) | − | − | − | − |
| Vernicia montana | − | ++ | ++ | ++ | + | − | − |
| PCs | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| Eigenvalue | 1.934 | 1.357 | 0.526 | 0.183 |
| Cumulative % | 48.35 | 82.28 | 95.42 | 100.00 |
| Variables | ||||
| TH | 0.6556 | 0.2287 | −0.0894 | −0.7140 |
| CW | 0.5626 | −0.3565 | −0.5756 | 0.4744 |
| NOS | 0.3216 | −0.6288 | 0.7079 | 0.0052 |
| SD | 0.3876 | 0.6521 | 0.3994 | 0.5148 |
| Stand | Land Morphology | Dominant in Canopy Layer | Vulnerability |
|---|---|---|---|
| Natural population | |||
| CM | Mountain slope | Pioneer forest (evergreen broadleaved forest) | |
| 15°–30° (20° on average) | Vernicia montana Melia azedarach | Slope failure occurred | |
| PT1 | Valley slope | Pioneer forest (evergreen broadleaved forest) | |
| 43° | Vernicia monata Melia azedarach Quercus sp. | Soil erosion occurred, slope failure possibly occurs. | |
| PT2 | Stream bank | Riparian forest | |
| 35° | Fraxinus griffithii | Flooded | |
| Anthropogenic population | |||
| CM2 | Hill slope | Plantation | |
| 15° | Cinnamomun loureiroi Vernicia montana * Melia azedarach * | Stable | |
| NS | Valley slope | Half planted | |
| 42°–43° | Bamboo Acacia mangium | Occupied by bush-like shrubs | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.T.; Takahashi, K.; Nishikawa, H.; Tanabe, R.; Nguyen, T.T.T.; Nguyen, V.H.T.; Nguyen, T.M.T.; Bui, H.V. Habitat Characteristics of Camellia quephongensis and Adaptation Mechanisms in Que Phong District, North-Central Vietnam. Int. J. Plant Biol. 2023, 14, 959-973. https://doi.org/10.3390/ijpb14040070
Tran TT, Takahashi K, Nishikawa H, Tanabe R, Nguyen TTT, Nguyen VHT, Nguyen TMT, Bui HV. Habitat Characteristics of Camellia quephongensis and Adaptation Mechanisms in Que Phong District, North-Central Vietnam. International Journal of Plant Biology. 2023; 14(4):959-973. https://doi.org/10.3390/ijpb14040070
Chicago/Turabian StyleTran, Tuyen Thi, Kazuya Takahashi, Hiroaki Nishikawa, Reiko Tanabe, Trang Thanh Thi Nguyen, Viet Ha Thi Nguyen, Thanh Mai Thi Nguyen, and Hien Van Bui. 2023. "Habitat Characteristics of Camellia quephongensis and Adaptation Mechanisms in Que Phong District, North-Central Vietnam" International Journal of Plant Biology 14, no. 4: 959-973. https://doi.org/10.3390/ijpb14040070
APA StyleTran, T. T., Takahashi, K., Nishikawa, H., Tanabe, R., Nguyen, T. T. T., Nguyen, V. H. T., Nguyen, T. M. T., & Bui, H. V. (2023). Habitat Characteristics of Camellia quephongensis and Adaptation Mechanisms in Que Phong District, North-Central Vietnam. International Journal of Plant Biology, 14(4), 959-973. https://doi.org/10.3390/ijpb14040070





