Absorption Spectra as Predictors of Algal Biomass and Pigment Content of the Cultured Microalgae Amphidinium carterae, Isochrysis galbana, Nephroselmis sp., and Anabaena sp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Origination of the Microalgae
2.2. Culture Conditions
2.3. Estimation of Culture Density and Absorption Spectra
2.4. Pigment Analyses
2.5. Statistics
3. Results
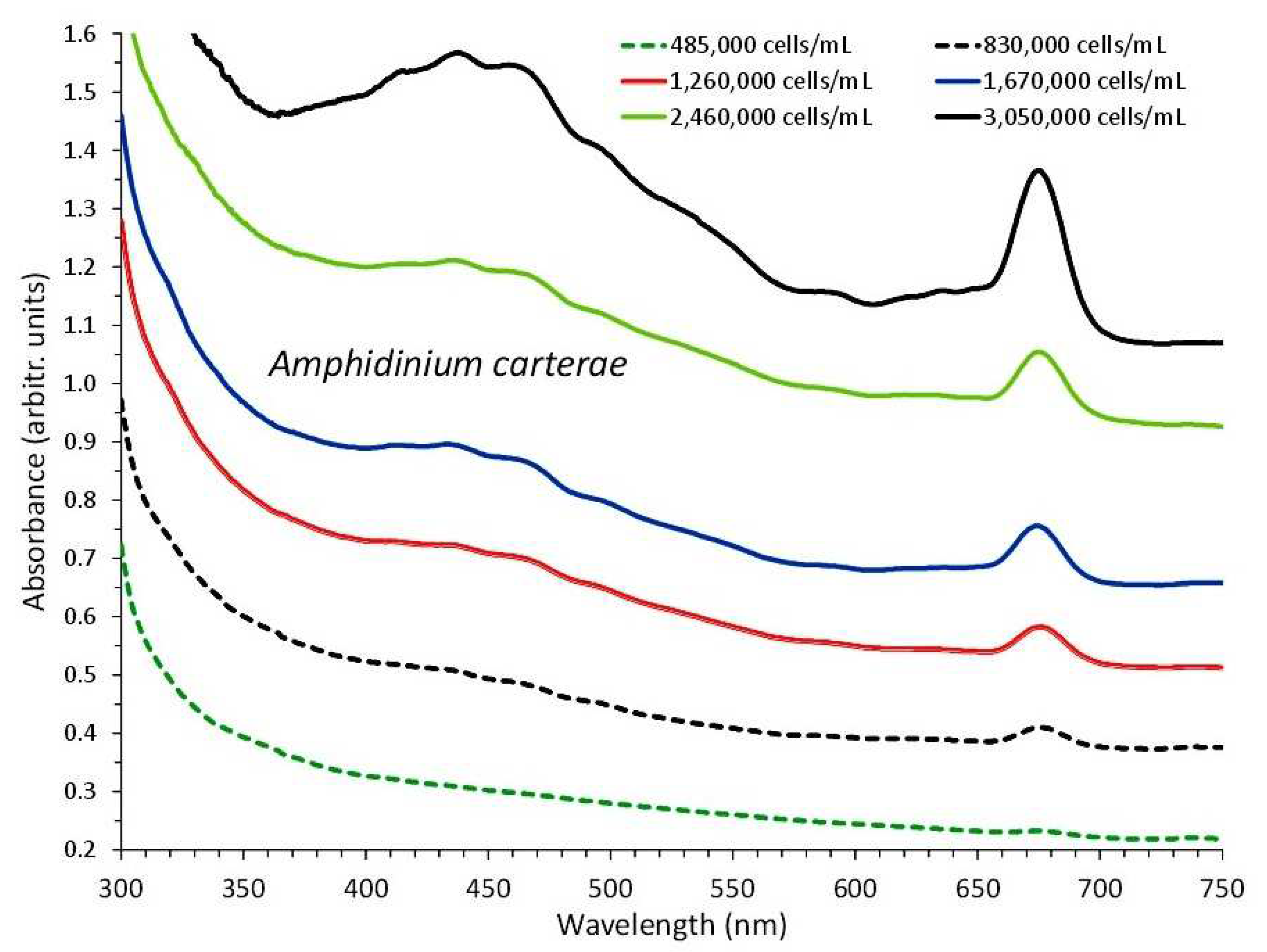
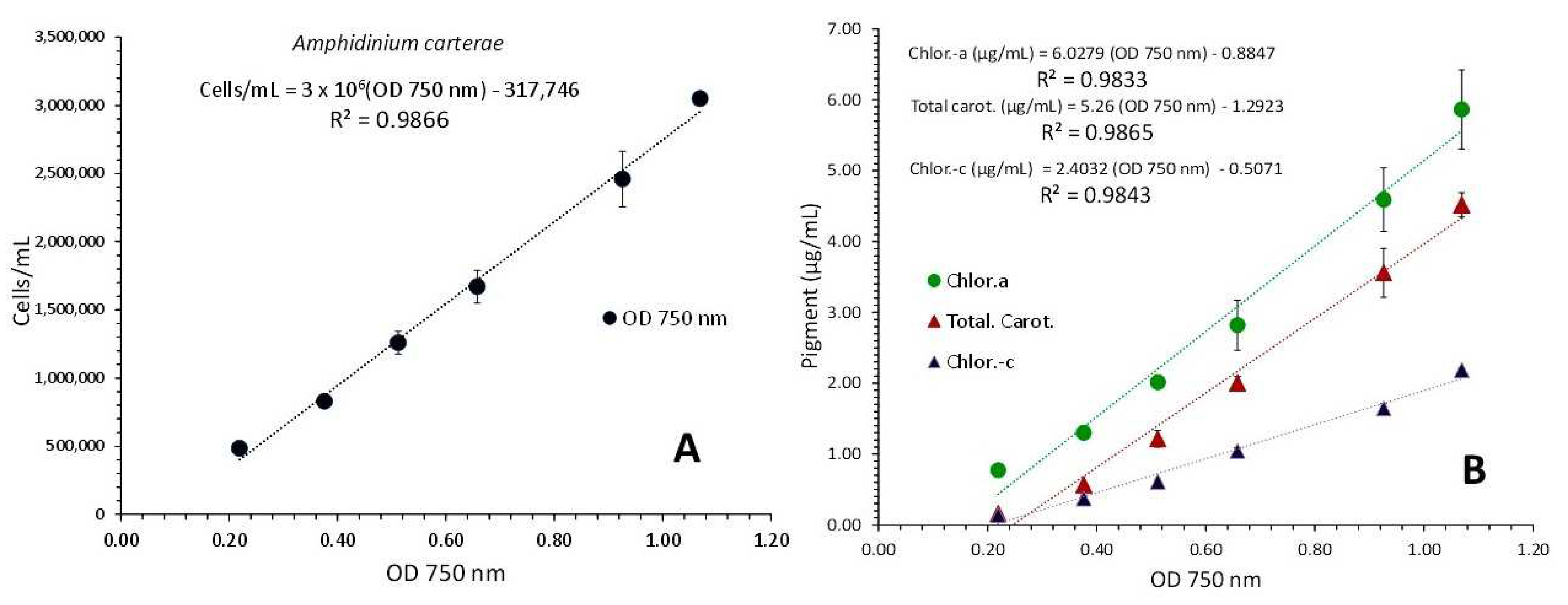
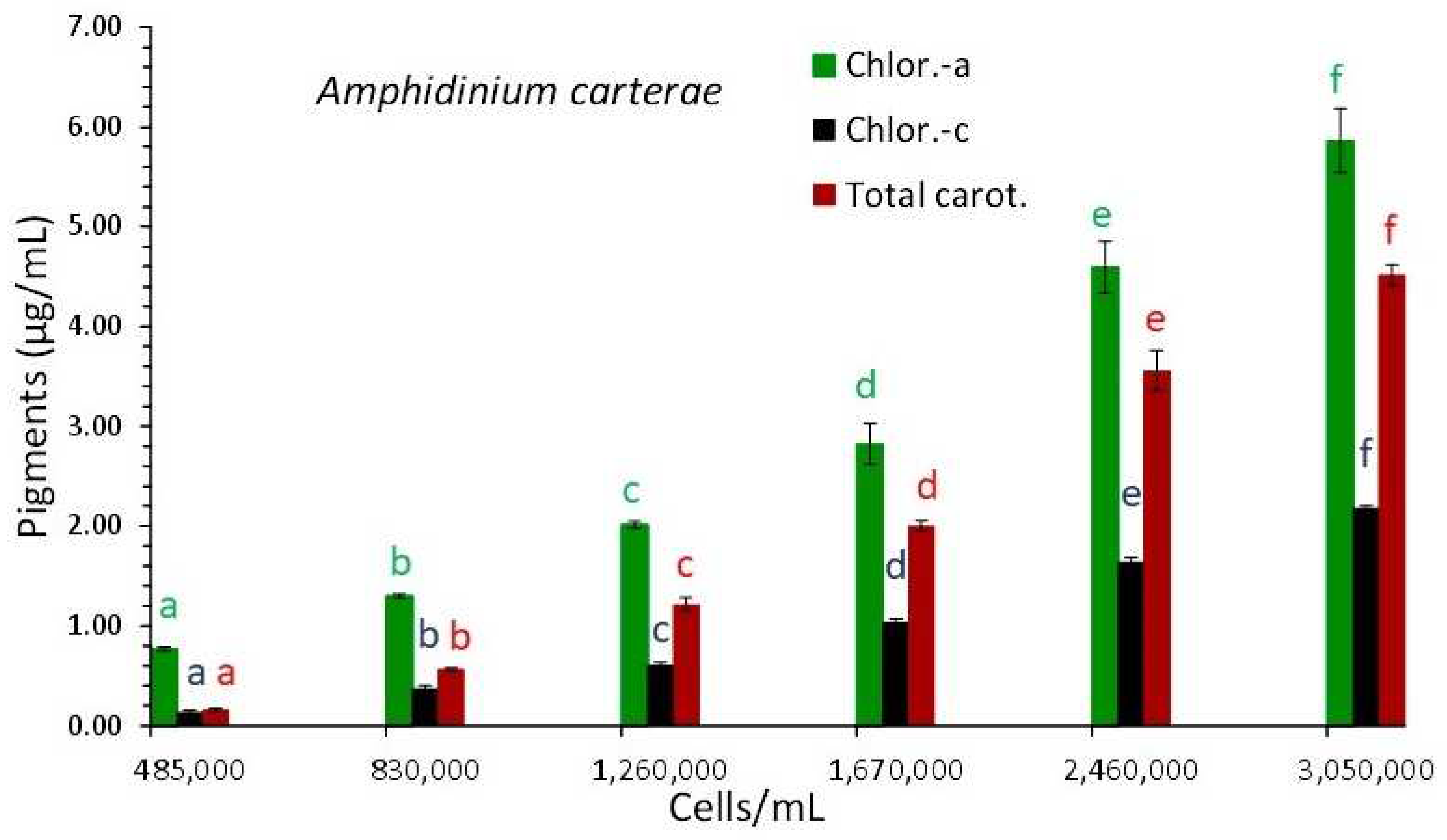
3.1. Amphidinium Carterae
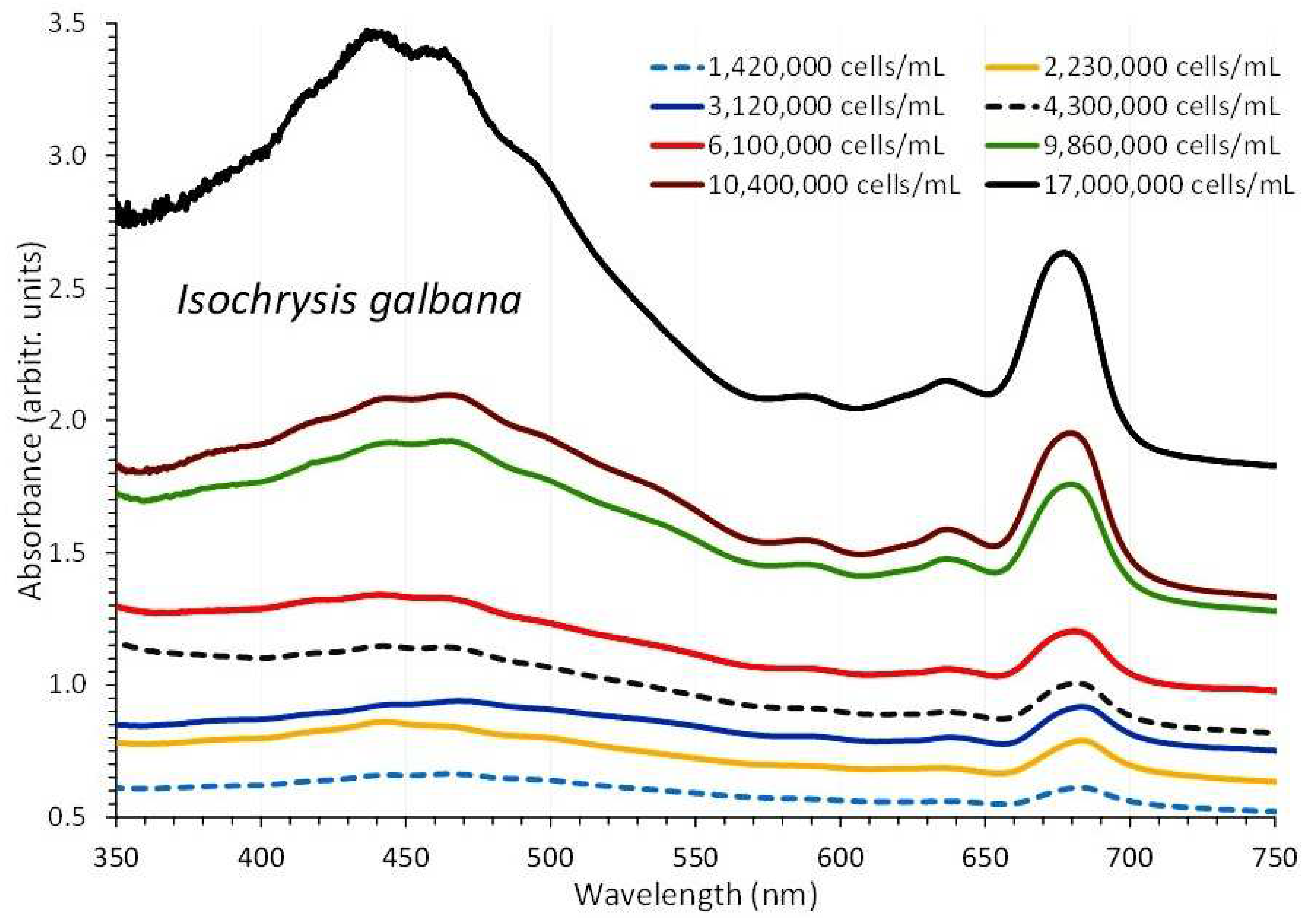
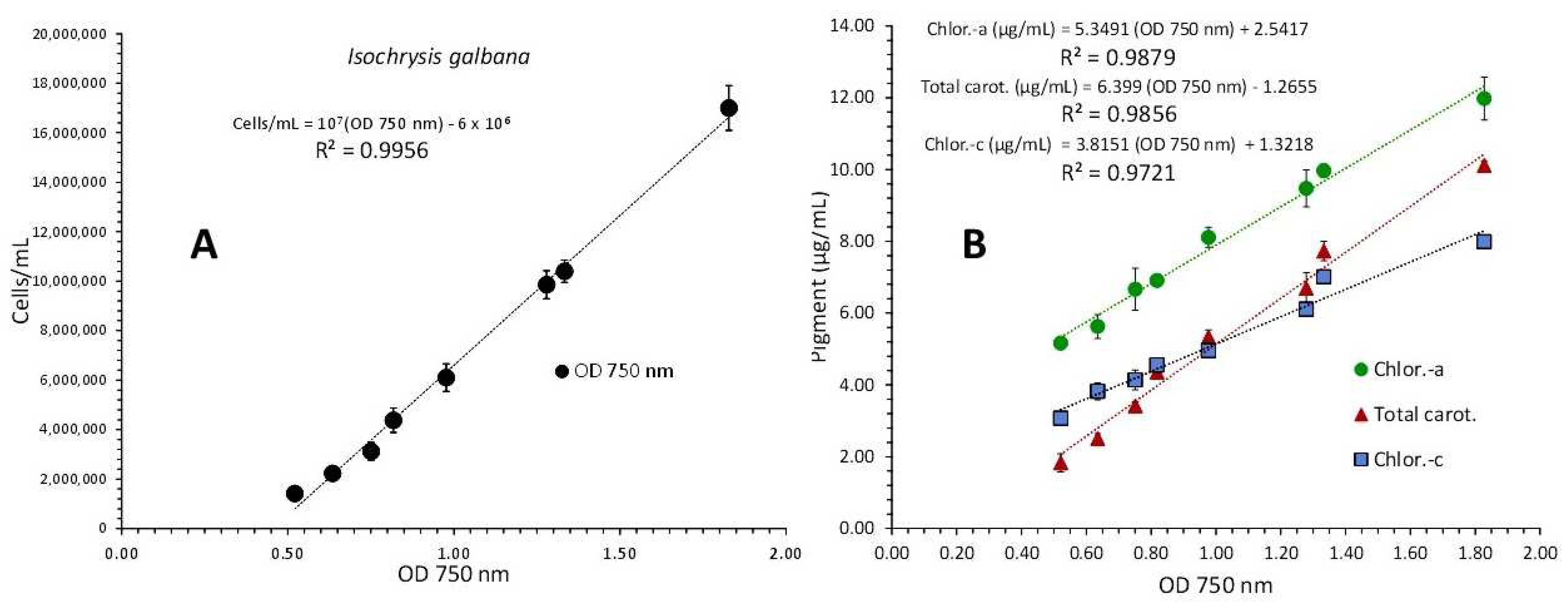
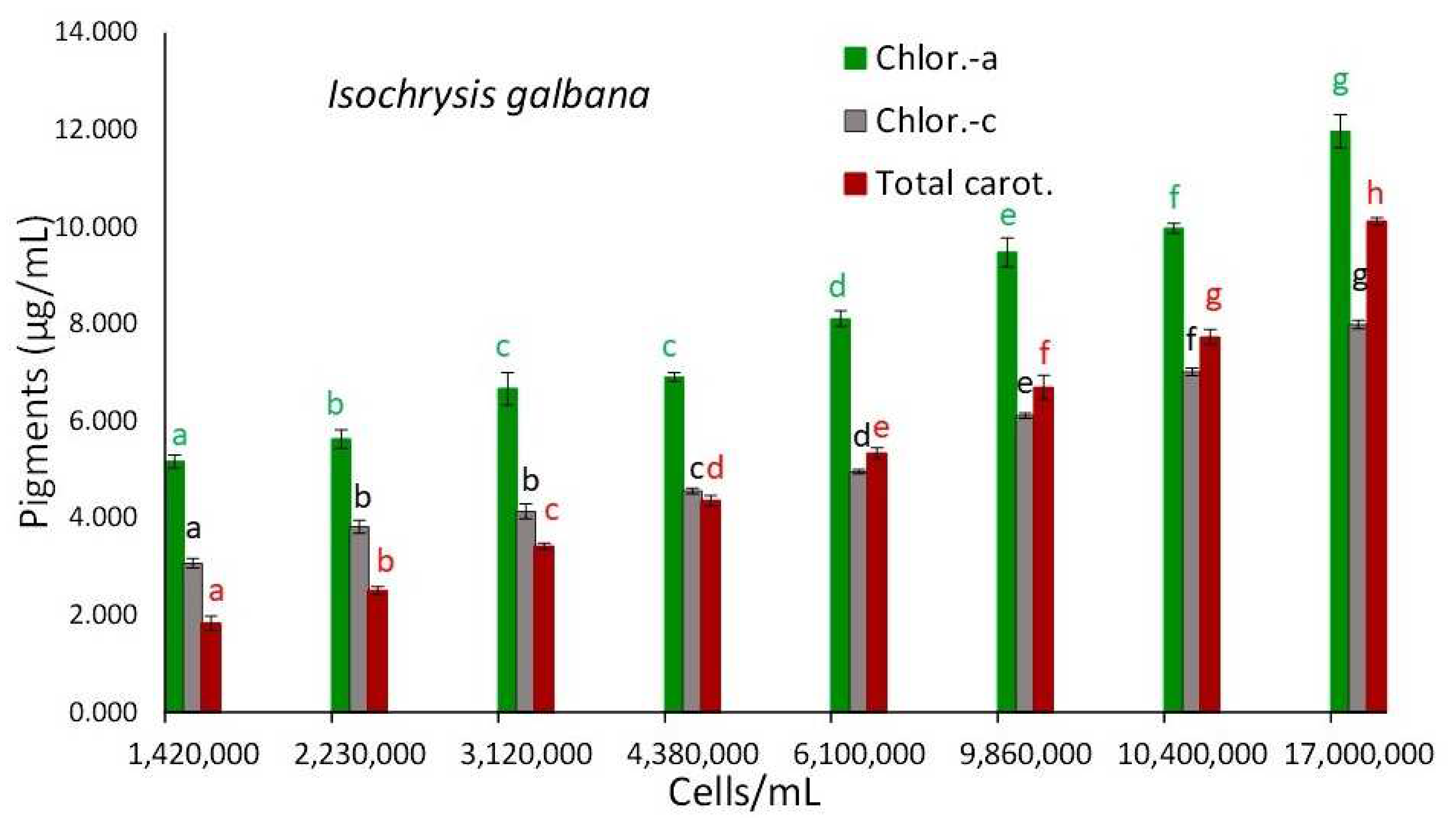
3.2. Isochrysis Galbana
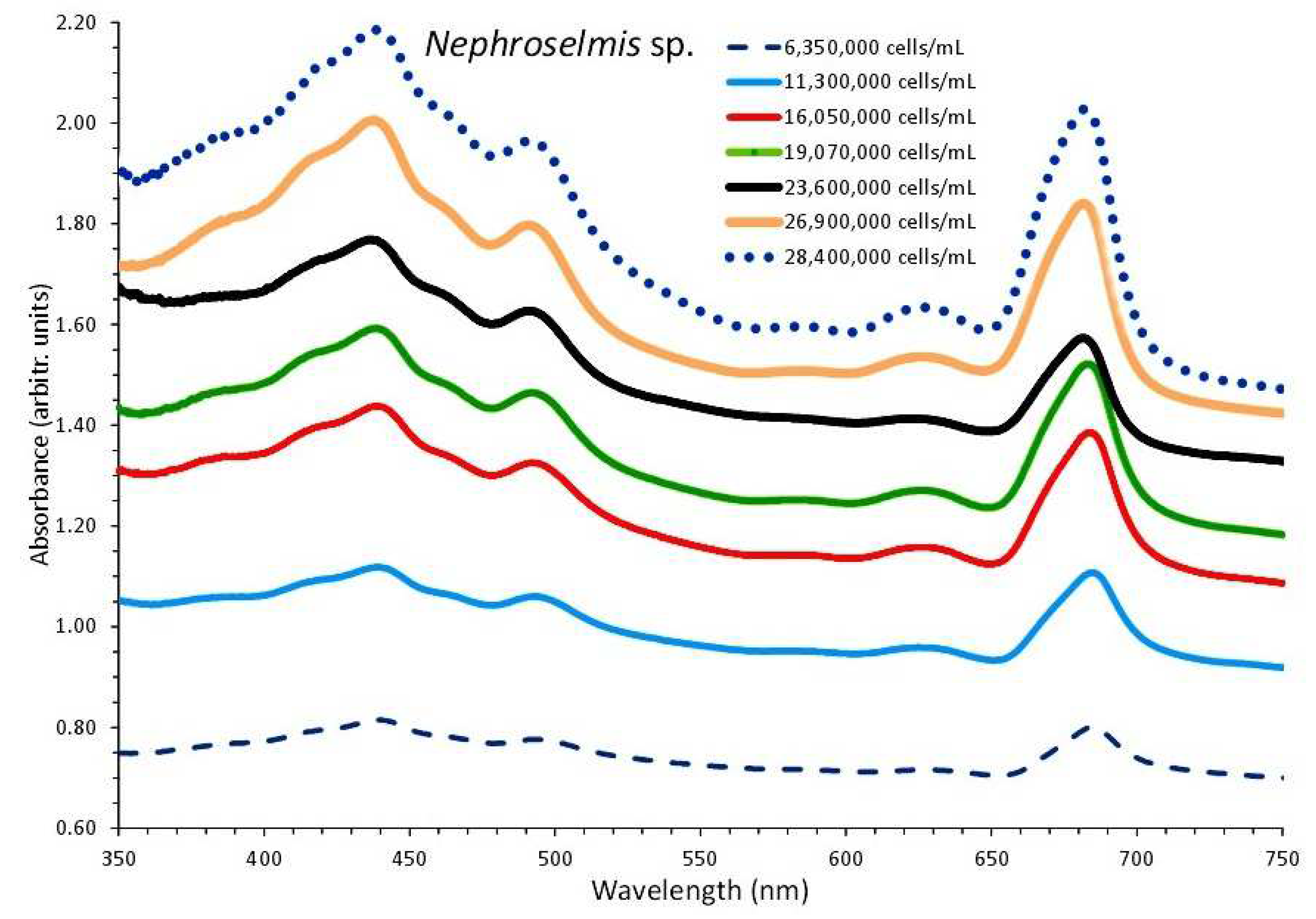
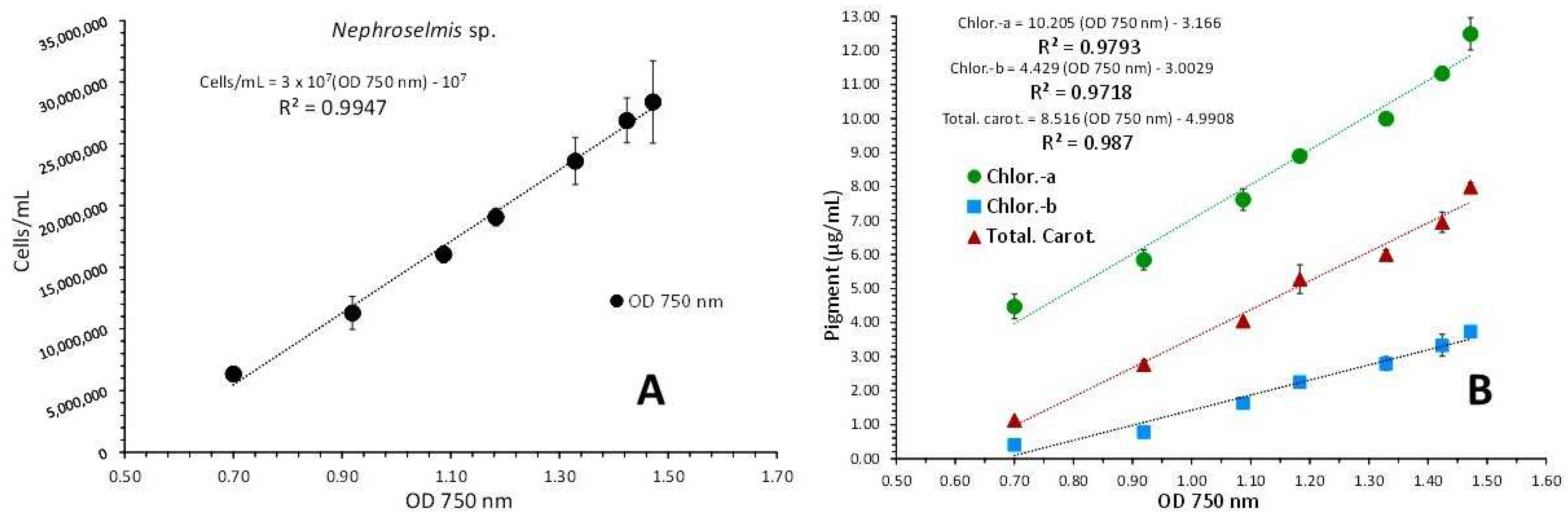
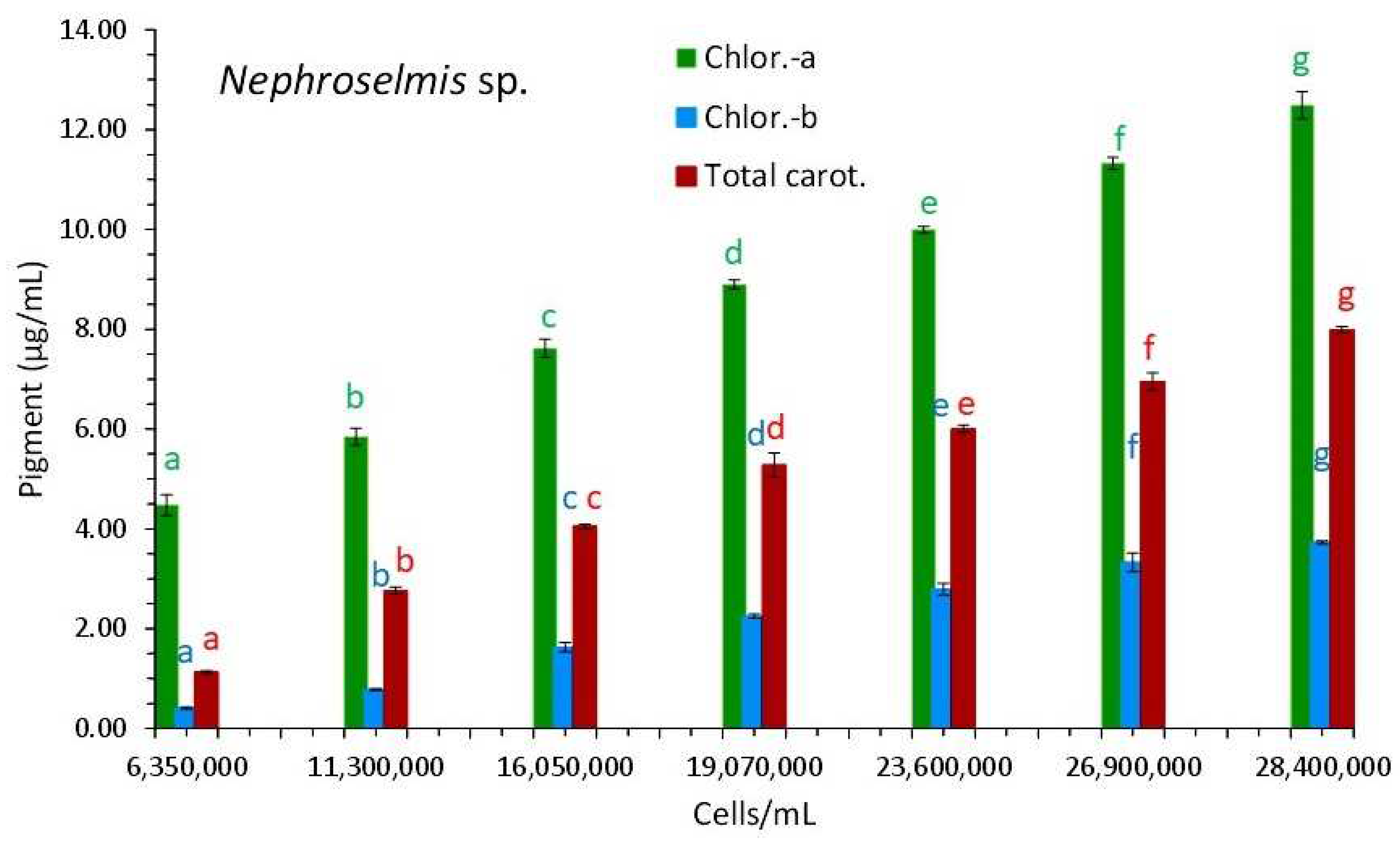
3.3. Nephroselmis sp.
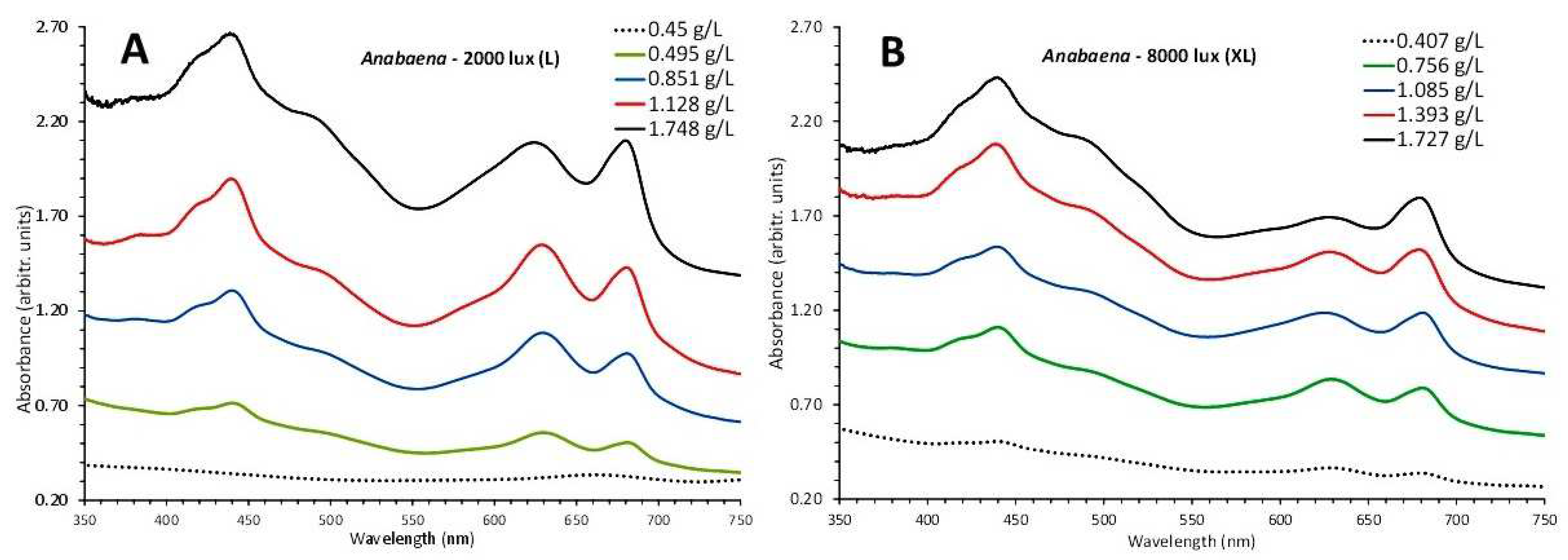
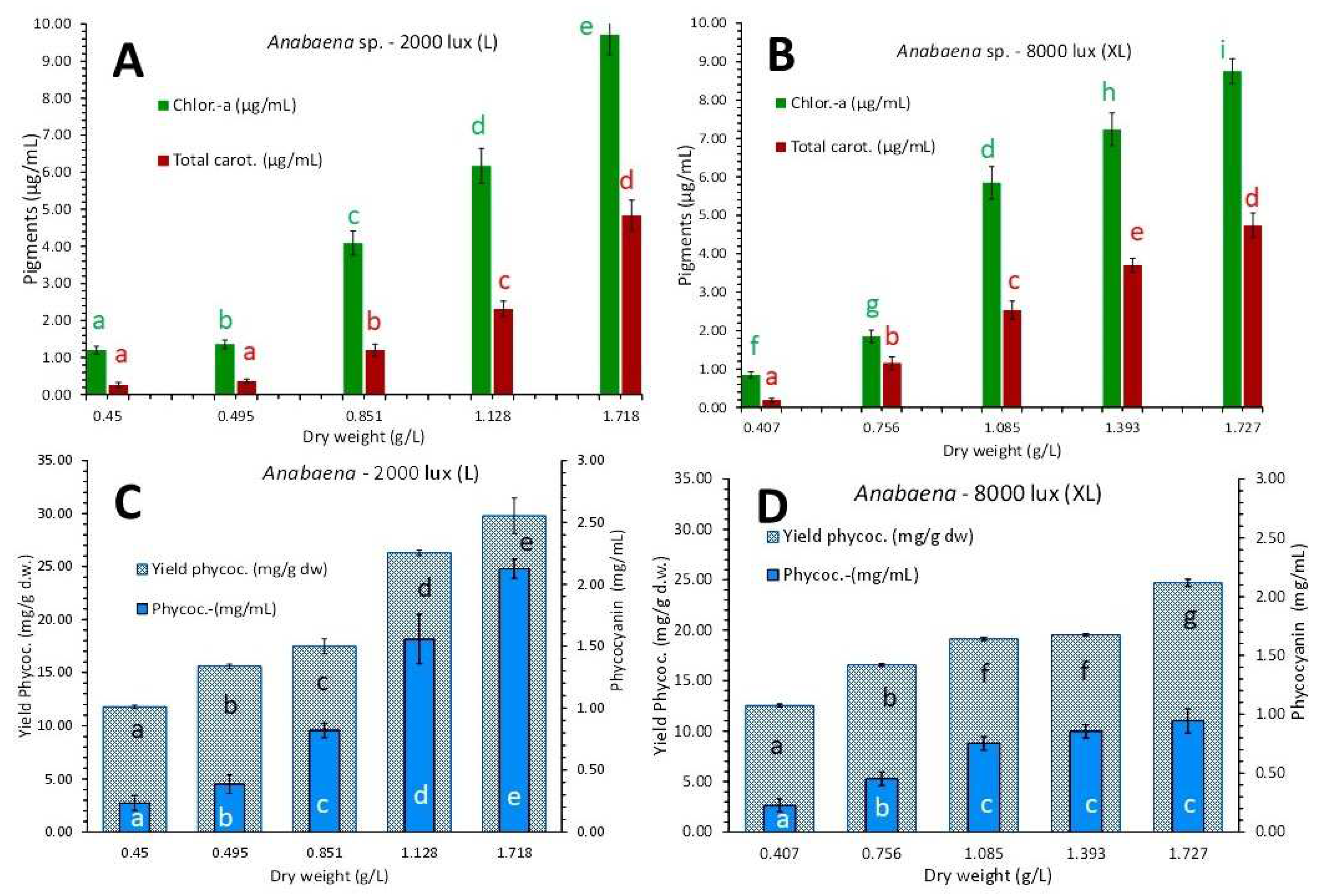
3.4. Anabaena sp.
4. Discussion
- During the duration of the culture of a particular microalge under a more or less predetermined set of culture conditions, a sufficient number of absorption spectra should be recorded.
- On the same day that an absorption spectrum is recorded, the biomass density should be accurately calculated, and the concentrations of different pigments should be painstakingly analyzed. Additionally, each pigment’s peaks and absorbance values at 750 nm are noted.
- For each spectrum that was noted along the culture path, the aforementioned process is repeated. The data are processed after being collected at regular intervals together with information on biomass density and pigment concentrations for a specific culture.
- Using the aforementioned body of data, regression equations are created for the pairs of biomass density and absorbance at 750 nm to determine whether there is a substantial association (i.e., R2 > 0.85). If so, the OD value of 750 nm can be used to predict biomass using the equation. In order to forecast each pigment’s concentration based on the created equations, the identical technique is carried out for the pairs of each pigment concentration and OD at 750 nm.
- The foregoing laborious process is carried out once at the beginning to create the calibration equations for the specific culture. The grower can then utilize the created equations to obtain reasonably accurate estimations for biomass and pigments for subsequent cultures of the same species under almost identical conditions.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chisti, Y. Biodiesel from Microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Ajanovic, A. Renewable Fuels—A Comparative Assessment from Economic, Energetic and Ecological Point-of-View up to 2050 in EU-Countries. Renew. Energy 2013, 60, 733–738. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Chakravarthy, M.; Ranjith Kumar, R.; Yogendran, D.; Yuvaraj, D.; Jayamuthunagai, J.; Praveen Kumar, R.; Palani, S. Aquatic Biomass (Algae) as a Future Feed Stock for Bio-Refineries: A Review on Cultivation, Processing and Products. Renew. Sustain. Energy Rev. 2015, 47, 634–653. [Google Scholar] [CrossRef]
- Guedes, A.C.; Malcata, X.F. Nutritional Value and Uses of Microalgae in Aquaculture. In Aquaculture; Muchlisin, Z.A., Ed.; InTech: London, UK, 2012; pp. 63–66. [Google Scholar]
- Kaparapu, J. Application of Microalgae in Aquaculture. Phykos 2018, 48, 21–26. [Google Scholar]
- Turcihan, G.; Turgay, E.; Yardımcı, R.E.; Eryalçın, K.M. The Effect of Feeding with Different Microalgae on Survival, Growth, and Fatty Acid Composition of Artemia franciscana Metanauplii and on Predominant Bacterial Species of the Rearing Water. Aquac. Int. 2021, 29, 2223–2241. [Google Scholar] [CrossRef]
- Eryalçın, K.M.; Ganuza, E.; Atalah, E.; Hernández Cruz, M.C. Nannochloropsis Gaditana and Crypthecodinium Cohnii, Two Microalgae as Alternative Sources of Essential Fatty Acids in Early Weaning for Gilthead Seabream. Hidrobiológica 2015, 25, 193–203. [Google Scholar]
- Lu, L.; Yang, G.; Zhu, B.; Pan, K. A Comparative Study on Three Quantitating Methods of Microalgal Biomass. Indian J. Geomarine Sci. 2017, 46, 2265–2272. [Google Scholar]
- Liu, J.-Y.; Zeng, L.-H.; Ren, Z.-H. The Application of Spectroscopy Technology in the Monitoring of Microalgae Cells Concentration. Appl. Spectrosc. Rev. 2021, 56, 171–192. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Zeng, L.-H.; Ren, Z.-H. Recent Application of Spectroscopy for the Detection of Microalgae Life Information: A Review. Appl. Spectrosc. Rev. 2020, 55, 26–59. [Google Scholar] [CrossRef]
- Sá, M.; Ferrer-Ledo, N.; Gao, F.; Bertinetto, C.G.; Jansen, J.; Crespo, J.G.; Wijffels, R.H.; Barbosa, M.; Galinha, C.F. Perspectives of Fluorescence Spectroscopy for Online Monitoring in Microalgae Industry. Microb. Biotechnol. 2022, 15, 1824–1838. [Google Scholar] [CrossRef]
- Sandnes, J.M.; Ringstad, T.; Wenner, D.; Heyerdahl, P.H.; Källqvist, T.; Gislerød, H.R. Real-Time Monitoring and Automatic Density Control of Large-Scale Microalgal Cultures Using near Infrared (NIR) Optical Density Sensors. J. Biotechnol. 2006, 122, 209–215. [Google Scholar] [CrossRef]
- White, L.H.; Martin, D.W.; Witt, K.K.; Vogt, F. Impacts of Nutrient Competition on Microalgae Biomass Production. J. Chemom. 2014, 28, 448–461. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Garcin, C.; van Hille, R.P.; Harrison, S.T.L. Interference by Pigment in the Estimation of Microalgal Biomass Concentration by Optical Density. J. Microbiol. Methods 2011, 85, 119–123. [Google Scholar] [CrossRef]
- Ribeiro-Rodrigues, L.H.; Arenzon, A.; Raya-Rodriquez, M.T.; Ferreira Fontouna, N. Algal Density Assessed by Spectrophotometry: A Calibration Curve for the Unicellular Algae Pseudokirchneriella Subcapitata. J. Environ. Chem. Ecotoxicol. 2011, 3, 225–228. [Google Scholar] [CrossRef]
- Santos-Ballardo, D.U.; Rossi, S.; Hernández, V.; Gómez, R.V.; del Carmen Rendón-Unceta, M.; Caro-Corrales, J.; Valdez-Ortiz, A. A Simple Spectrophotometric Method for Biomass Measurement of Important Microalgae Species in Aquaculture. Aquaculture 2015, 448, 87–92. [Google Scholar] [CrossRef]
- Hotos, G.N.; Avramidou, D.; Bekiari, V. Calibration Curves of Culture Density Assessed by Spectrophotometer for Three Microalgae (Nephroselmis sp., Amphidinium carterae and Phormidium sp.). Eur. J. Biol. Biotechnol. 2020, 1, 6. [Google Scholar] [CrossRef]
- Stein, J.R. Dry Weight, Volume and Optical Density. In Handbook of Phycological Methods: Culture Methods and Growth Measurements; Stein, J.R., Ed.; Cambridge University Press: London, UK, 1973; pp. 21–22. [Google Scholar]
- Guillard, R.R.L.; Sieracki, M.S. Counting Cells in Cultures with the Light Microscope. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 239–252. [Google Scholar]
- Hyka, P.; Lickova, S.; Přibyl, P.; Melzoch, K.; Kovar, K. Flow Cytometry for the Development of Biotechnological Processes with Microalgae. Biotechnol. Adv. 2013, 31, 2–16. [Google Scholar] [CrossRef]
- Rottberger, J.; Gruber, A.; Kroth, P.G. Analysing Size Variation during Light-Starvation Response of Nutritionally Diverse Chrysophytes with a Coulter Counter. Arch. Hydrobiol. Suppl. Algol. Stud. 2013, 141, 37–51. [Google Scholar] [CrossRef]
- Marie, D.; Simon, N.; Vaulot, D. Phytoplankton Cell Counting by Flow Cytometry. In Algal Culturing Techniques; Andersen, R.A., Ed.; Academic Press: Cambridge, MA, USA, 2005; pp. 253–267. [Google Scholar]
- Moheimani, N.R.; Borowitzka, M.A.; Isdepsky, A.; Sing, S.F. Standard Methods for Measuring Growth of Algae and Their Composition. In Algae for Biofuels and Energy; Springer: Dordrecht, The Netherlands, 2013; pp. 265–284. [Google Scholar]
- Sarrafzadeh, M.H.; La, H.-J.; Seo, S.-H.; Asgharnejad, H.; Oh, H.-M. Evaluation of Various Techniques for Microalgal Biomass Quantification. J. Biotechnol. 2015, 216, 90–97. [Google Scholar] [CrossRef]
- Almomani, F.A.; Örmeci, B. Monitoring and Measurement of Microalgae Using the First Derivative of Absorbance and Comparison with Chlorophyll Extraction Method. Environ. Monit. Assess. 2018, 190, 90. [Google Scholar] [CrossRef]
- Hotos, G.N.; Avramidou, D.; Samara, A. The Effect of Salinity and Light Intensity on the Batch Cultured Cyanobacteria Anabaena sp. and Cyanothece sp. Hydrobiology 2022, 1, 278–287. [Google Scholar] [CrossRef]
- Hotos, G.N.; Avramidou, D. The Effect of Various Salinities and Light Intensities on the Growth Performance of Five Locally Isolated Microalgae [Amphidinium Carterae, Nephroselmis sp., Tetraselmis sp. (Var. Red Pappas), Asteromonas Gracilis and Dunaliella sp.] in Laboratory Batch Cultures. J. Mar. Sci. Eng. 2021, 9, 1275. [Google Scholar] [CrossRef]
- Hotos, G.; Avramidou, D.; Mastropetros, S.G.; Tsigkou, K.; Kouvara, K.; Makridis, P.; Kornaros, M. Isolation, Identification, and Chemical Composition Analysis of Nine Microalgal and Cyanobacterial Species Isolated in Lagoons of Western Greece. Algal Res. 2023, 69, 102935. [Google Scholar] [CrossRef]
- Hotos, G.N. A Preliminary Survey on the Planktonic Biota in a Hypersaline Pond of Messolonghi Saltworks (W. Greece). Diversity 2021, 13, 270. [Google Scholar] [CrossRef]
- Walne, P.R. Experiments on the Large-Scale Culture of the Larvae of Ostrea Edulis. J. Fish. Invest. Min. Agric. Fish. Lond. Ser. 1966, 2, 25–53. [Google Scholar]
- Hotos, G.N.; Antoniadis, T.I. The Effect of Colored and White Light on Growth and Phycobiliproteins, Chlorophyll and Carotenoids Content of the Marine Cyanobacteria Phormidium sp. and Cyanothece sp. in Batch Cultures. Life 2022, 12, 837. [Google Scholar] [CrossRef]
- Hotos, G.N. Culture Growth of the Cyanobacterium Phormidium sp. in Various Salinity and Light Regimes and Their Influence on Its Phycocyanin and other Pigments Content. J. Mar. Sci. Eng. 2021, 9, 798. [Google Scholar] [CrossRef]
- Ritchie, R.J. Universal Chlorophyll Equations for Estimating Chlorophylls a, b, c, and d and Total Chlorophylls in Natural Assemblages of Photosynthetic Organisms Using Acetone, Methanol, or Ethanol Solvents. Photosynthetica 2008, 46, 115–126. [Google Scholar] [CrossRef]
- Moraes, C.C.; Sala, L.; Cerveira, G.P.; Kalil, S.J. C-Phycocyanin Extraction from Spirulina Platensis Wet Biomass. Braz. J. Chem. Eng. 2011, 28, 45–49. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Kirk, J.T.O. Light Capture by Aquatic Plants. In Light and Photosynthesis in Aquatic Ecosystems; Kirk, J.T.O., Ed.; Cambridge University Press: Cambridge, UK, 1994; pp. 201–218. ISBN 9780511623370. [Google Scholar]
- Borovkov, A.B.; Gudvilovich, I.N.; Avsiyan, A.L. Scale-up of Dunaliella Salina Cultivation: From Strain Selection to Open Ponds. J. Appl. Phycol. 2020, 32, 1545–1558. [Google Scholar] [CrossRef]
- Healey, F.P. Physiological Indicators of Nutrient Deficiency in Algae. Internationale Vereinigung für Theoretische und Angewandte Limnologie: Mitteilungen 1978, 21, 34–41. [Google Scholar] [CrossRef]
- Minhas, A.K.; Hodgson, P.; Barrow, C.J.; Adholeya, A. A Review on the Assessment of Stress Conditions for Simultaneous Production of Microalgal Lipids and Carotenoids. Front. Microbiol. 2016, 7, 546. [Google Scholar] [CrossRef]
- Montero-Lobato, Z.; Vázquez, M.; Navarro, F.; Fuentes, J.; Bermejo, E.; Garbayo, I.; Vílchez, C.; Cuaresma, M. Chemically-Induced Production of Anti-Inflammatory Molecules in Microalgae. Mar. Drugs 2018, 16, 478. [Google Scholar] [CrossRef]
- Valer, R.M.; Glock, L. Quantificação de Algas Clorofíceas Deinteresse Ecotoxicológico a Través Do Método Espectrofotométrico. Acta Limnol. Bras. 1998, 11, 148–156. [Google Scholar]
- Rossi, S.; Fiorillo, I. Biochemical Features of a Protoceratium reticulatum Red Tide in Chipana Bay (Northern Chile) in Summer Conditions. Sci. Mar. 2010, 74, 633–642. [Google Scholar] [CrossRef]
- Ebenezer, V.; Ki, J.-S. Quantification of Toxic Effects of the Herbicide Metolachlor on Marine Microalgae Ditylum brightwellii (Bacillariophyceae), Prorocentrum minimum (Dinophyceae), and Tetraselmis suecica (Chlorophyceae). J. Microbiol. 2013, 51, 136–139. [Google Scholar] [CrossRef]
- Lee, E.; Heng, R.-L.; Pilon, L. Spectral Optical Properties of Selected Photosynthetic Microalgae Producing Biofuels. J. Quant. Spectrosc. Radiat. Transf. 2013, 114, 122–135. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hotos, G.N.; Bekiari, V. Absorption Spectra as Predictors of Algal Biomass and Pigment Content of the Cultured Microalgae Amphidinium carterae, Isochrysis galbana, Nephroselmis sp., and Anabaena sp. Int. J. Plant Biol. 2023, 14, 879-895. https://doi.org/10.3390/ijpb14040065
Hotos GN, Bekiari V. Absorption Spectra as Predictors of Algal Biomass and Pigment Content of the Cultured Microalgae Amphidinium carterae, Isochrysis galbana, Nephroselmis sp., and Anabaena sp. International Journal of Plant Biology. 2023; 14(4):879-895. https://doi.org/10.3390/ijpb14040065
Chicago/Turabian StyleHotos, George N., and Vlasoula Bekiari. 2023. "Absorption Spectra as Predictors of Algal Biomass and Pigment Content of the Cultured Microalgae Amphidinium carterae, Isochrysis galbana, Nephroselmis sp., and Anabaena sp." International Journal of Plant Biology 14, no. 4: 879-895. https://doi.org/10.3390/ijpb14040065
APA StyleHotos, G. N., & Bekiari, V. (2023). Absorption Spectra as Predictors of Algal Biomass and Pigment Content of the Cultured Microalgae Amphidinium carterae, Isochrysis galbana, Nephroselmis sp., and Anabaena sp. International Journal of Plant Biology, 14(4), 879-895. https://doi.org/10.3390/ijpb14040065







