Abstract
Climate change has prompted agri-food systems to explore new strategies for improving the production of crops in a sustainable manner. This includes green bean, the most important legume in the world for its nutritional value. The use of omeprazole (OMP) and melatonin (MEL) has been proposed as innovative strategy for crop improvement when they are applied as biostimulants. However, although their role in the growth of several species has been studied, the results in photosynthetic efficiency parameters are still scarce. Therefore, the objective of the present study was to evaluate the effect of foliar application of OMP and MEL on biomass, yield, SPAD values, leaf chlorophyll fluorescence (Fv/Fm), photochemical quenching (qP), non-photochemical quenching (NPQ), quantum yield of photosystem II (PhiPSII), and electron transport rate (ETR) in bean plants. Treatments were applied separately at doses of 1, 10, and 100 µM, plus a control without application. The results obtained indicate that OMP and MEL were able to increase biomass; yield; SPAD values; and qP, Fv/Fm, and PhiPSII coefficients. Finally, it is concluded that foliar application of OMP and MEL at a dose of 1 and 10 µM can increase photosynthetic efficiency and decrease photoinhibition, which is reflected in higher biomass accumulation and yield in green bean plants cv. Strike.
1. Introduction
Currently, the global demand for food is increasing at the same rate as the world population [1]. Because of this, technological innovations in agriculture are striving to increase yields of highly important crops in a sustainable manner [2]. The common bean is considered the most important legume in the world due to its high content of protein and minerals such as iron and zinc [3]. However, especially in the last decade and thanks to studies revealing the benefits of bean consumption, its demand has increased significantly [4]. Some of the innovations in agriculture to improve bean yield include genetic improvement, rethinking fertilization, and the use of new-generation fertilizers as well as various biostimulants [5]. However, due to their great potential, the use of biostimulant products has gained great relevance as a tool for crop development improvement and efficient use of inputs.
One of the most innovative strategies that has shown favorable results in the last decade in terms of growth, production and efficient use of nutrients is the use of low weight molecules as biostimulants, such as omeprazole (OMP) and melatonin (MEL) [6,7]. Omeprazole, 5-metoxi-2-[[(4-metoxi3,5-dimetil-2-piridinil)metil]sulfinil]-1H-benzimidazole, is a substituted benzimidazole widely used as an anti-ulcer drug [8]. Melatonin (N-acetil-5-metoxitriptamine) is a multifunctional molecule with physiological effects in various organisms. In humans, it is related to the enhancement of the immune system, circadian rhythms, sleep, mood, body temperature, and appetite [9].
The biological function of both molecules is well identified in humans. However, recent research has shown that micromolar doses of both OMP and MEL in plants improved yield, root structure, nitrogen use efficiency, tolerance to different types of stress, photosynthesis parameters and the expression of genes that regulate these mechanisms [10,11,12]. In the same way, it has been reported that one of the key mechanisms affecting the application of OMP and MEL to increase yield and biomass is related to carbon metabolism [13,14].
Carbon metabolism through the process of photosynthesis is a fundamental process for plants to carry out their routine and primary cellular activities during growth and development [15]. Furthermore, the study of chlorophyll fluorescence parameters as a method of studying photosynthesis is a valuable technique which can serve as an indicator of the efficiency of the thylakoid membranes and the functional changes of the photosynthetic apparatus [16]. There is interaction between this process and key areas of plant function. Therefore, an improvement in photosynthesis would lead to an improvement in crop yields [17].
Although the responses of OMP and MEL in major metabolic processes, such as growth and physiological responses under stress, have been previously studied in other plants, information about the specific function of these molecules related to light harvesting and the efficiency of the photosynthetic machinery in bean plants is limited. Therefore, the present research aims to evaluate the effect of foliar application of OMP and MEL on biomass, yield, SPAD values, leaf chlorophyll fluorescence (Fv/Fm), photochemical quenching (qP), photosystem II quantum yield (PhiPSII), and electron transport rate (ETR) in green bean plants cv. Strike. The results of this study would contribute to the practical use of omeprazole and melatonin in increasing photosynthetic efficiency in bean crops, specifically to help increase yields and meet production demand.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
The experiment was carried out at the Food and Development Research Center, A.C. Delicias unit (CIAD), located in Cd. Delicias, Chihuahua, México during the months of September and October 2022. The experiment was established under shade netting at a mean temperature of 20.2 ± 7.8 °C. Seeds of green bean (Phaseolus vulgaris L.) cv. Strike obtained from Hydro environment® S.A. de C.V. were grown in 13.4 L plastic pots (two plants per pot). The pots had a diameter of 30.5 cm, with a height of 32 cm. The substrate mixture was composed of vermiculite and perlite in a 2:1 (v/v) ratio. During development, plants were irrigated with a standard Hoagland nutrient solution composed of 6 mM NH4NO3, 1.6 mM K2HPO4, 0.3 mM K2SO4, 4 mM CaCl2, 1.4 mM MgSO4, 5 µM Fe-EDDHA, 2 µM MnSO4, 0.25 µM CuSO4, 0.5 µM H3BO3 and 0.3 µM Na2MoO4, at pH 6.0 ± 0.1. A 500 mL nutrient solution was applied every third day, and once the flowering stage (38 days after sowing (DAS)) was initiated, 1 L was applied. The treatments used were applied via foliar application every week from 15 DAS to 43 DAS.
2.2. Experimental Design and Description of Treatments
A completely randomized design (CRD) was used with 7 treatments; the foliar application of omeprazole and melatonin at 3 doses each (1, 10 and 100 µM); and a control without application (Table 1). The doses used were chosen due to the ranges used by different authors, who report that doses above 100 µM can cause toxicity. Each treatment had a total of 6 replicates (pots) with 2 plants each.

Table 1.
Description of treatments applied.
For the preparation of the treatments, commercial tablets were used, considering the molarity of both compounds for the calculation of the concentrations (Table 2). The tablets were macerated in a mortar until a fine powder was obtained. The amount required for the concentration was weighed (Table 1), and the powder was dissolved in distilled water through a magnetic stirring plate at 700 rpm for 30 min (VWR® 7 × 7 CER, Henry Troemmer, LLC, Thorofare, NJ, USA). Once dissolved, the solution was made up to a volume of 1 L, sealed, and sonicated through an aquasonic cleaner for 1 h (VWR® 150D, VWR International, Radnor, PA, USA). Once the treatments were prepared, they were applied via foliar application.

Table 2.
Description of chemical compounds used for treatment preparation.
2.3. Plant Biomass and Yield Measurement
Once the plants reached physiological maturity, at 53 DAS, they were harvested for sampling and separated into their different organs (roots, stems, leaves, and pods). The harvested material was weighted in fresh, washed and dried in a drying oven (Shel Lab Forced Air Laboratory Oven SMO14-108 2, Baltimore, MD, USA) at 70 °C for 48 h. Finally, dry weight was recorded.
Total biomass was evaluated considering all the organs of the plant once the samples were dried. An analytical balance (AND HR-120, San Jose, CA, USA) was used to quantify the weight. Biomass was expressed as grams of dry weight per plant (g·plant−1 DW).
The total fresh weight of pods per plant was measured as yield and expressed as grams of fresh weight per plant (g·plant−1 FW).
2.4. SPAD Values
The quantification of the SPAD (soil–plant analysis development) values was obtained through a Minolta SPAD 502 chlorophyll reader (Konica Minolta Sensing, Inc., Osaka, Japan). The reading was achieved by projecting light through a leaf. Measurement was performed on rib-free parts 32 DAS. The results were expressed as SPAD units [18].
2.5. Measurements of Chlorophyll Fluorescence Parameters
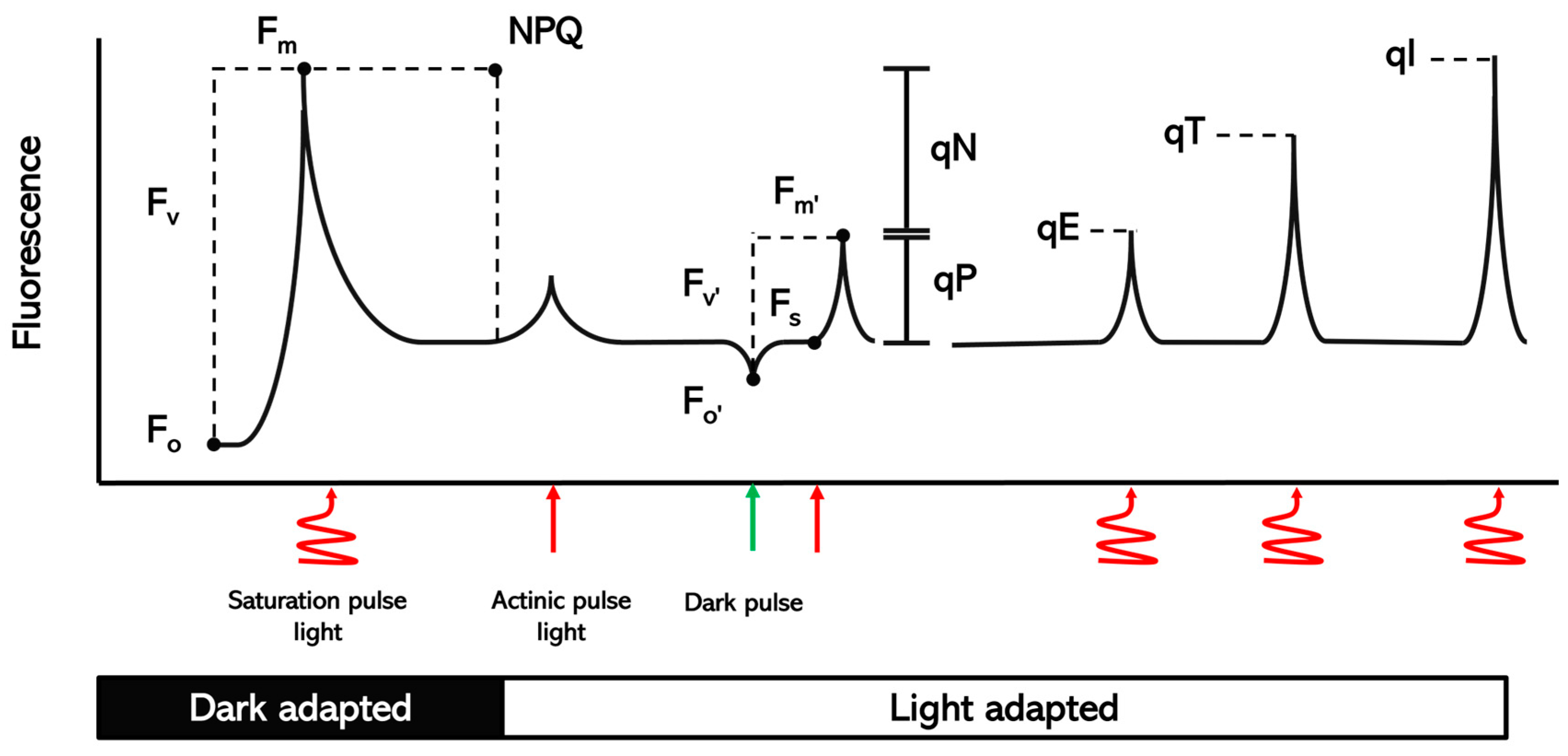
Chlorophyll fluorescence parameters (Table 3) were calculated at 32 DAS to evaluate the transfer, dissipation, and distribution of light in the photosystem of green bean plants grown under different biostimulant molecules, at different doses (Figure 1). Measurement of the variables was performed with a gas exchange system (Li-Cor 6400, Li-Cor, Inc., Lincoln, NE, USA) and an integrated fluorescence camera head (Li-Cor-6400, Li-Cor, Inc., Lincoln, NE, USA). The PPFD (photosynthetic photon flux density) value was 200 µM photons m−2 s−1, and the CO2 concentration was 400 ± 2 µmol·µmol−1 for the reference cell. The vapor pressure deficit of the air vapor pressure in the sample chamber was less than 1.5, and the leaf block temperature was 25 °C.

Table 3.
Description of chlorophyll fluorescence parameters.
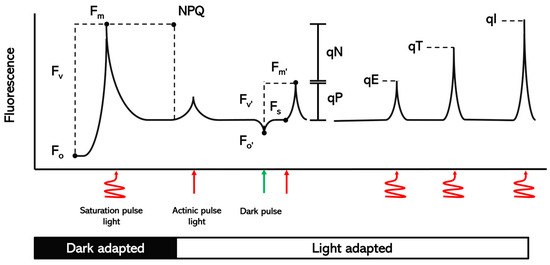
Figure 1.
Schematic of the different parameters of chlorophyll fluorescence measured in dark-adapted and light-adapted plants.
2.6. Statistical Analysis
Once the measurements were taken, the data obtained for each variable were subjected to a Shapiro–Wilk test to check the normal distribution of the data. Additionally, a Bartlett’s test was performed to comorbid the homogeneity of variances. Once these assumptions had been made, a one-way analysis of variance (ANOVA) was performed, with a mean separation test using the LSD method. For all analyses, 6 replicates were used. In addition, a Pearson correlation test was performed between all variables, using the SAS 9.0 statistical package at a significance level (p < 0.05).
3. Results and Discussion
3.1. Total Biomass
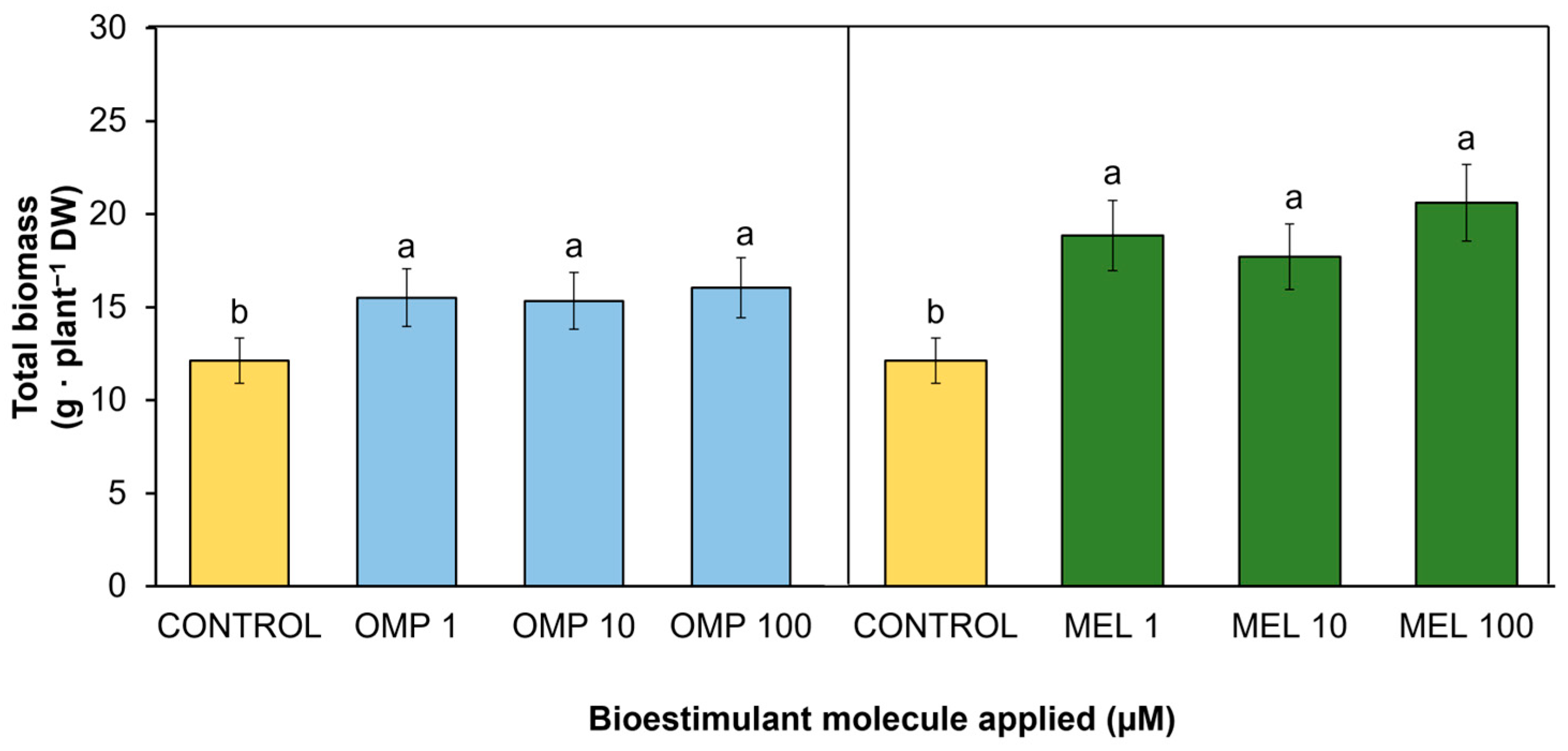
A key variable to determine the effectiveness of the treatments applied is the quantification of accumulated dry biomass [19]. In the present study, significant differences (p < 0.05) were found in total biomass accumulation by effect of OMP and MEL application at different doses (Figure 2 and Figure 3). The treatment that was most favored was MEL 100 with a significant increase of 70% over the control, followed by MEL 1 with a significant increase of 55.4% over the control. However, although the increase in biomass accumulation was higher when the dose was increased from 1 to 100 µM melatonin, there was no significant difference between these treatments (9.3%), so the most efficient treatment was MEL 1 (Figure 3). The data obtained in the present work agree with those obtained by [20], who reported a 71.1% increase in total dry biomass of maize seedlings when a 100 µM dose of melatonin was applied to plants fertilized with an optimum dose of nitrogen. Other authors, such as [21], indicated that maize seedlings treated with 1 µM of melatonin increased their dry biomass by 50.6% under nitrogen-sufficient conditions 28 days after planting compared to untreated plants.

Figure 2.
Effect of foliar application of omeprazole (OMP) and melatonin (MEL) at doses of 1, 10, and 100 µM in green bean plants cv. Strike.
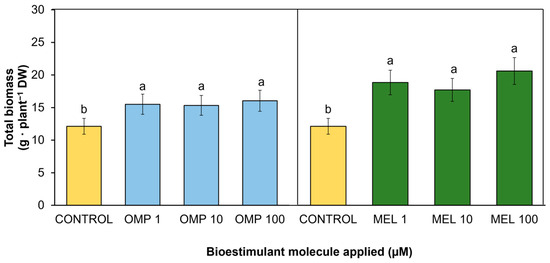
Figure 3.
Effect of foliar application of omeprazole (OMP) and melatonin (MEL) at doses of 1, 10, and 100 µM on total biomass accumulation in green bean plants cv. Strike. Means with equal letters do not differ according to LSD test (p < 0.05).
According to the foliar application of omeprazole, the highest biomass accumulation was recorded in the OMP 100 treatment, with an increase of 32.4% with respect to the control (Figure 3). However, no significant differences were obtained between the different doses of this molecule and the control. Investigations such as the one conducted by [22] obtained significant increases of 14.3%, 21.9%, and 25.7% in dry weight in lettuce plants after adding 10, 50, and 100 µM of omeprazole, respectively.
Models have previously been proposed to explain biomass distribution and relate it to carbon supply from leaf photosynthesis in the aerial part [23]. In addition, it has been indicated that the use of low weight molecules such as OMP and MEL can intervene in growth processes, mainly in photosynthesis, through which the constituent sugars of biomass are obtained [24].
Similarly, the interaction of membrane ATPases and OMP has been described as responsible for the increase in vegetative growth in treated plants. ATPases are regulators of the polar transport of auxin from the source organs (leaves) to the root and fruit. It is possible that this mechanism promotes an increase in the number and size of pods in green bean, ultimately resulting in an increase in yield [25].
Additionally, multiple studies have shown the protective role of MEL in the photosynthetic apparatus of plants of different species, especially under stress conditions [26,27]. One of the processes that is disrupted under stress conditions is photosynthesis. However, the application of MEL favors that this process is carried on continuously through different mechanisms, such as maintaining the integrity of chloroplasts, improving water uptake to boost energy transfer between PSI and PSII, and improving the nitrogen assimilation process, allowing NO3− reduction and subsequently the utilization of carbon skeletons from the glutamine–glutamate pathway generated by the photosynthesis process [13,28]. The result of the enhancement of all these mechanisms may be the possible cause of increased biomass accumulation in bean plants.
3.2. Yield
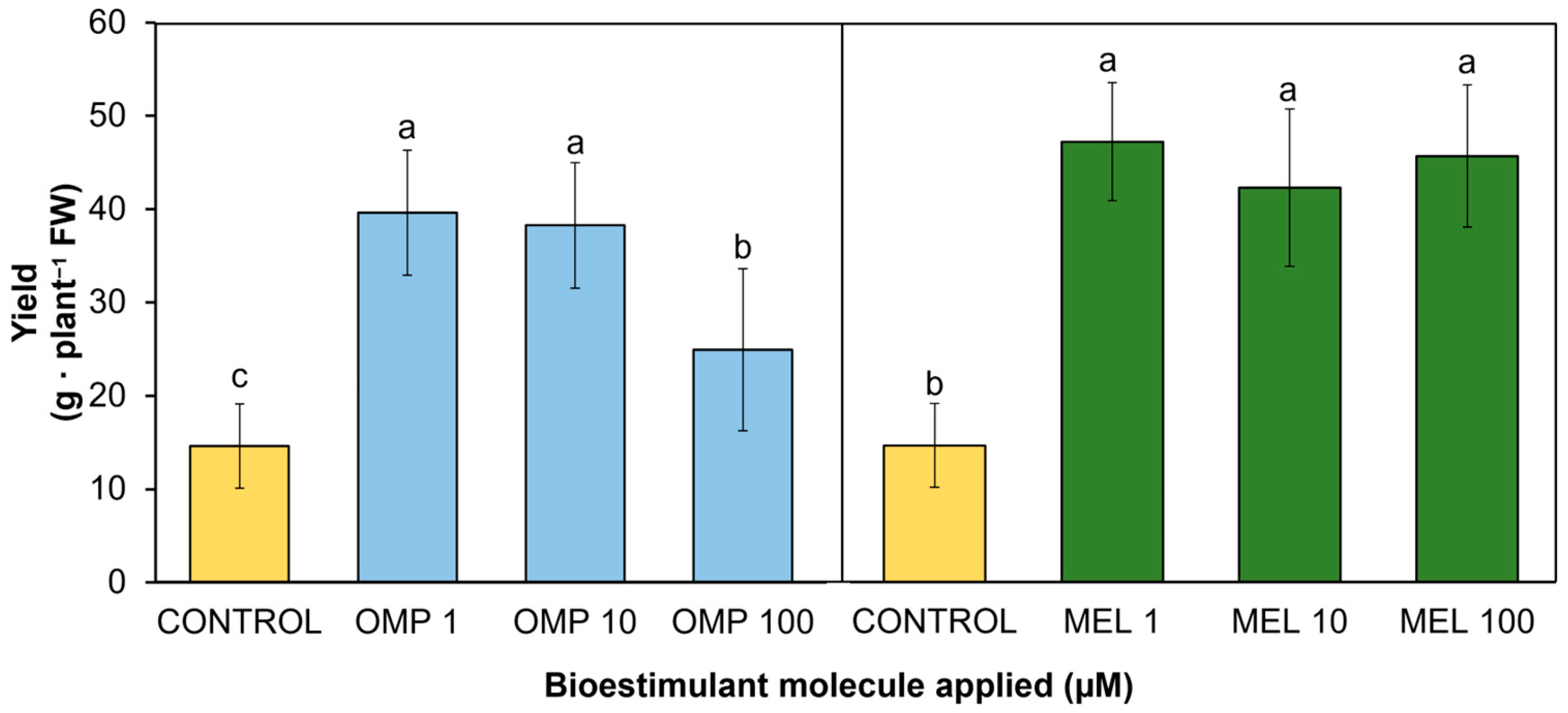
Yield is linked to biomass production and the physiological state of the plants, which makes it an indicator of the functionality of the treatments applied [29]. The data obtained in this research work showed significant differences (p < 0.05) in yield by effect of the application of OMP and MEL at different doses (Figure 4). The treatment that obtained the highest pod production was MEL1, which increased by 222.7% with respect to the control, with no significant difference when increasing the dose 10 and 100 times (Figure 3). The dose of omeprazole that obtained the best result was OMP 1, with a significant increase of 182% with respect to the control (Figure 4). In contrast to the application of melatonin, when the dose of OMP was increased by 10 and 100 times, the yield decreased, which could indicate that this molecule modifies the metabolism of the plant according to the dose, as it is related to the metabolism of endogenous hormones such as auxin. Increasing the concentration to 100 mM may induce an inhibitory response or an imbalance between vegetative growth promotion and fruit production, an effect observed in linseed plants [30].
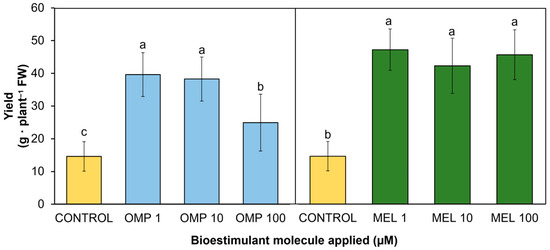
Figure 4.
Effect of foliar application of omeprazole (OMP) and melatonin (MEL) at doses of 1, 10, and 100 µM on yield in green bean plants cv. Strike. Means with equal letters do not differ according to LSD test (p < 0.05).
Similarly, MEL has been linked to the regulation of endogenous growth promoters such as auxin. This regulation has described accelerated growth in the root zone. When root architecture is modified by MEL action, nutrient and water uptake is promoted [31]. Adequate mineral supply and proper water uptake ultimately leads to increased yield and plant capacity to produce biomass. As shown in Figure 3 and Figure 4, plants with the highest biomass accumulation were able to produce the highest amount of yield (r = 0.6859 **). In addition, application at the lowest doses (1 µM) of both OMP and MEL were the most effective in increasing yield—by 1.8 and 2.2 times, respectively—with respect to the control, with a 100-fold lower dose.
3.3. SPAD Values
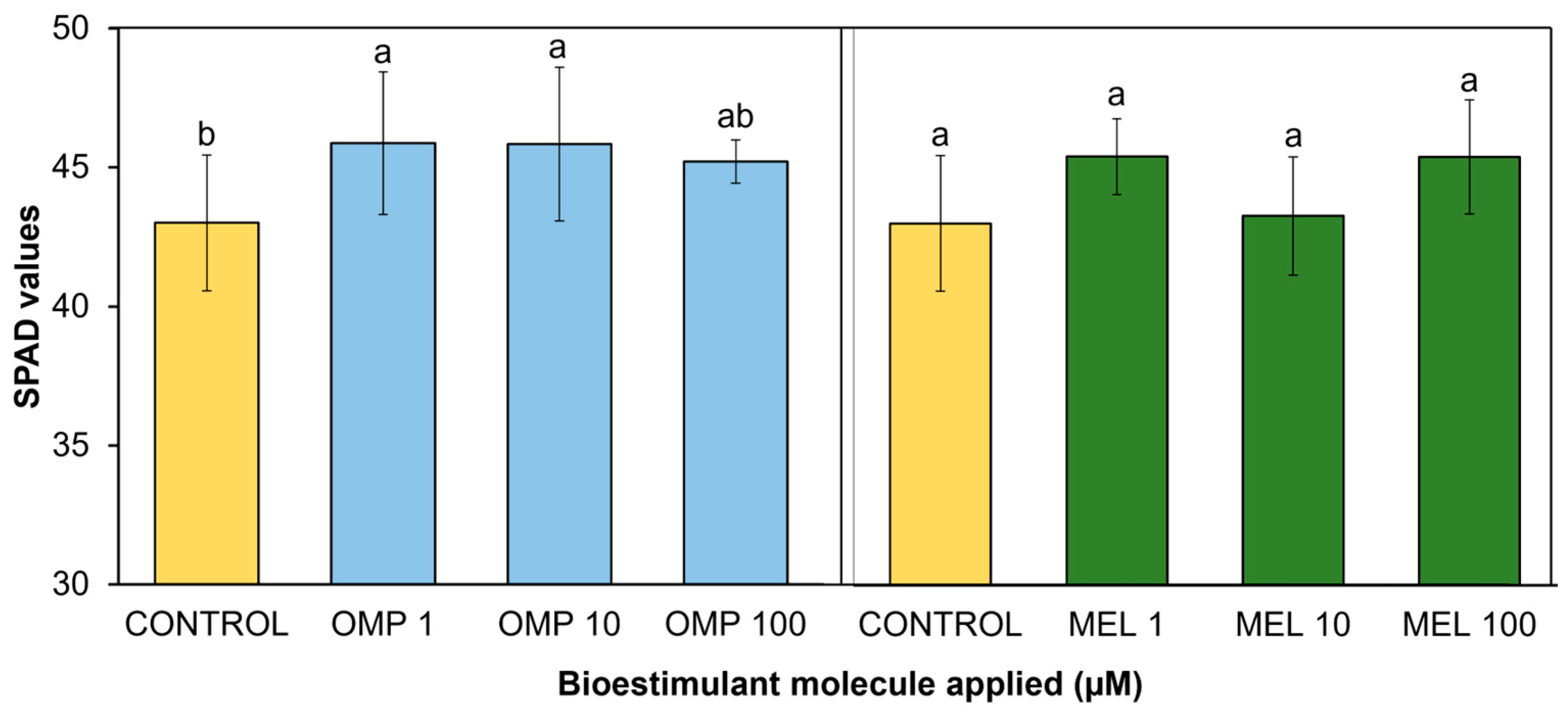
Measurement of pigment concentration through SPAD values can be a good indicator of leaf chlorophyll composition and photosynthetic process as well as plant health status and proper plant functioning [32]. In the present study, significant differences (p < 0.05) were found in photosynthetic activity expressed through SPAD units via the effects of OMP and MEL application at different doses (Figure 5). The most favored treatment, and therefore that with the highest photosynthetic activity, was OMP 1, with a significant increase over the control of 9.3%, even higher than the photosynthetic activity recorded when the foliar dose of OMP was increased to 10 and 100 µM, making it the most efficient dose. Regarding the application of melatonin, the treatment that showed the highest photosynthetic activity was MEL10, with a significant increase of 6.5% over the control, being significantly equal to the doses of 1 and 100 µM.
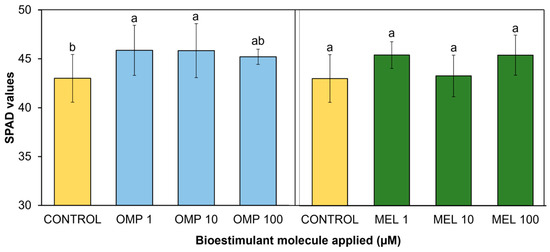
Figure 5.
Effect of foliar application of omeprazole (OMP) and melatonin (MEL) at doses of 1, 10, and 100 µM on SPAD values in green bean plants cv. Strike. Means with equal letters do not differ according to LSD test (p < 0.05).
The data obtained agree with those reported by [22], who found significant increases of 5.9% in basil cultivation under greenhouse conditions after the application of omeprazole at a dose of 10 µM. Similar results are reported by [33], who observed an increase in photosynthetic activity with exogenous addition of melatonin in maize seedlings.
The role of OMP in promoting the photosynthetic process is mainly related to the enhancement of nitrate and potassium uptake due to the regulation of ATPases and to the protection of the photosynthetic apparatus and regulation that these nutrients provide to the transpiration process [10]. In addition, a protective role against harmful ions, such as chlorine, has been described, facilitating their compartmentalization in vacuoles and excluding them from active sites during photosynthesis. Thus, with adequate nitrate uptake, leaves avoid the senescence state, allowing chloroplasts to maintain integrity and photosynthetic activity to be favored [34].
As for the role of MEL, it is believed that one of the mechanisms driving the photosynthetic process is ionic balance in the cytosol, especially in the vegetative growth stage, by increasing the uptake of potassium and calcium ions [35]. Additionally, according to [36], the presence of MEL affects the expression of the genes responsible for photosynthetic pigment biosynthesis. More importantly, it has been reported to down-regulate the production of chlorophyllase, the enzyme responsible for chlorophyll degradation [37]. Finally, the improvement in photosynthetic activity of the treatments compared to the control is due to different protective mechanisms that OMP and MEL molecules trigger inside the plant, leading to an improvement in the efficiency of light utilization for transformation into energy.
3.4. Chlorophyll Fluorescence Parameters
3.4.1. Chlorophyll Fluorescence
Quantification of chlorophyll fluorescence is one of the most important parameters when considering photosynthetic efficiency. The energy absorbed by photosynthetic pigments is sent to the reaction centers of photosystems I (PSI) and II (PSII) for transformation and remission as fluorescence, used as photochemical energy for photosynthesis or dissipated as heat [38].
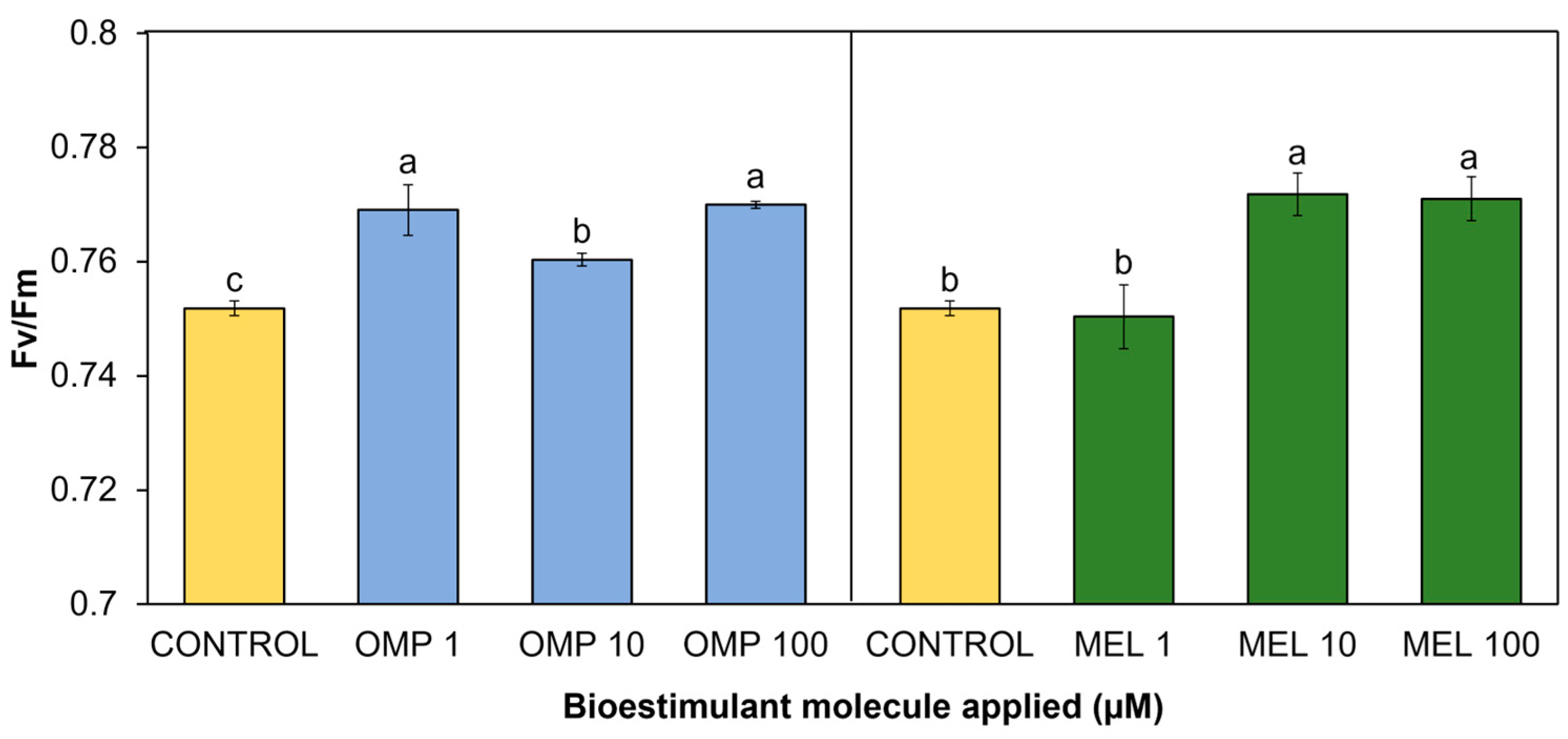
In the present study, significant differences (p < 0.05) were found in chlorophyll fluorescence as a result of the application of OMP and MEL at different doses (Figure 6). All applied treatments showed increases with respect to the control, except for MEL 1. The treatment that expressed the highest fluorescence was MEL 10, with a significant increase over the control of 2.6%; however, it did not show a significant difference against MEL 100, whose increase was 2.5% over the control (Figure 6).
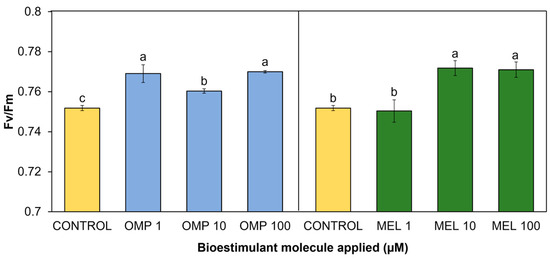
Figure 6.
Effect of foliar application of omeprazole (OMP) and melatonin (MEL) at doses of 1, 10, and 100 µM on chlorophyll fluorescence (Fv/Fm) in green bean plants cv. Strike. Means with equal letters do not differ according to LSD test (p < 0.05).
The omeprazole application that obtained the best fluorescence values was MEL 100, with a significant increase of 2.4% with respect to the control. However, the MEL 1 treatment obtained a similar increase of 2.2% compared to the control (Figure 6).
The range of fluorescence values for bean plants from non-stress plants is between 0.7 and 0.8 [39]. As indicated by numerous authors, a decrease in fluorescence expressed as (Fv/Fm) is indicative of a decrease in PSII [40]. The results found in this study agree with those reported by [41], who found increases in fluorescence as well as an increase in photosynthesis driven by the production of protective molecules such as catalase, peroxidase, and superoxide dismutase in tomato plants treated with melatonin via foliar at 50 and 100 µM. Other authors have described that the increase in PSII efficiency related to the application of melatonin is due to a decrease in PSII electron flow [42]. This decrease in flux allows the captured energy to be used to drive energetic reactions for photosynthesis, and less energy is dissipated by other pathways.
Another mechanism described for the increase in photosynthesis efficiency caused by melatonin is protection against reactive oxygen species. When the energy stored in chlorophyll is not dissipated by any of the possible pathways, the excited state of chlorophyll (Chl*) can react with molecular oxygen to form singlet oxygen (O2*), which is highly reactive and can degrade cellular components [43]. Thanks to the high stability of the central ring of the molecule and its two side chains, once a functional group interacts with ROS to stabilize them, melatonin retains its stability and its ability to scavenge free radicals [44].
3.4.2. Photochemical and Non-Photochemical Quenching
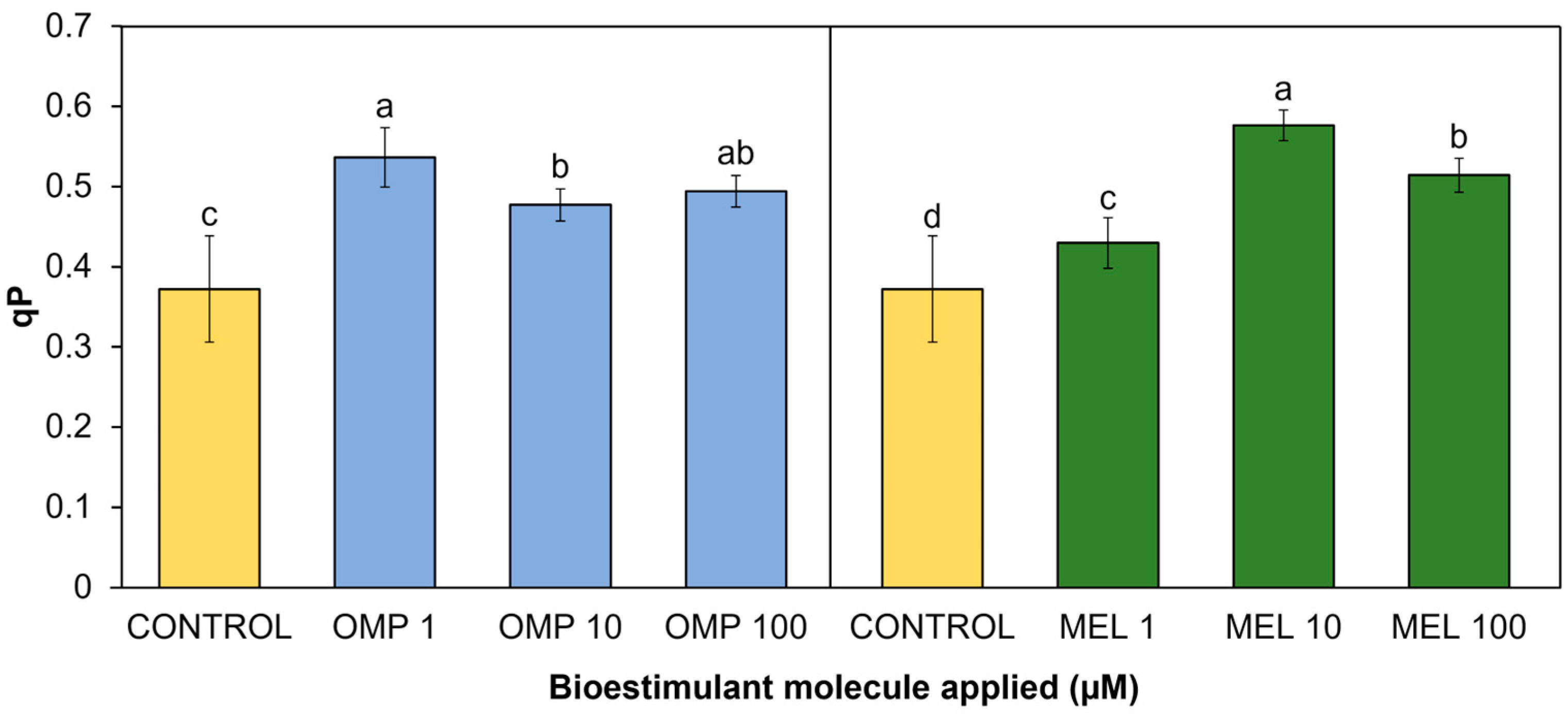
Photochemical quenching (qP) refers to the set of processes of photosynthetic electron consumption in an excited state through metabolic sinks, i.e., a different pathway from non-photosynthetic quenching (NPQ) processes, which returns electrons in excited state to their normal state through their remission by heat [45]. In the present research work, significant differences (p < 0.05) were found in the photochemical quenching coefficient due to the effect of applying OMP and MEL at different doses (Figure 7). The treatment with the highest rate was MEL 10, with a significant increase of 54.8% compared to the control, followed by the OMP 1 treatment, with an increase of 15.5% compared to the control (Figure 7).
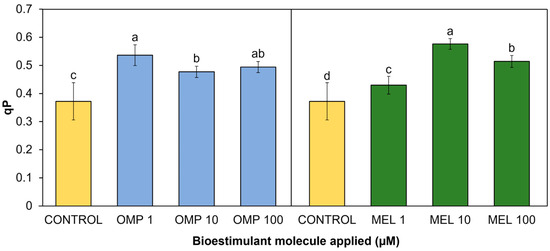
Figure 7.
Effect of foliar application of omeprazole (OMP) and melatonin (MEL) at doses of 1, 10, and 100 µM on photochemical quenching (qP) in green bean plants cv. Strike. Means with equal letters do not differ according to LSD test (p < 0.05).
The trend observed in the decrease in yield as the dose of omeprazole increased (Figure 5) is repeated in this variable (r = 0.4821), so it is possible that the lower dose is the one that gives the plant the greatest capacity to transfer the “extra” energy to sinks, avoiding photoinhibition. The enhancement of photosynthetic quenching in omeprazole-treated plants has already been reported. Authors explain that there is a group of genes related to the integral maintenance of PSII and protein synthesis of the thylakoids that are overexpressed in the presence of omeprazole as well as photosynthetic genes that protect against ROS, such as SlPSII and SlFTSH. One of the enzymes that are biosynthesized with the application of OMP is catalase, which is related to H2O2 scavenging [46].
Similarly, a higher qP value is observed in MEL 10 than in MEL 1—both increased with respect to the control—and their difference in yield is only 9%, indicating that the use of melatonin at low doses (between 1 to 10 µM) may confer the ability to protect the PSII reaction centers. Finally, this indicates that both treatments at low doses obtained the highest operational efficiency by being able to achieve the highest electron transport rate (r = 0.9554) to possible acceptors, avoiding photoinhibition by excess energy [47].
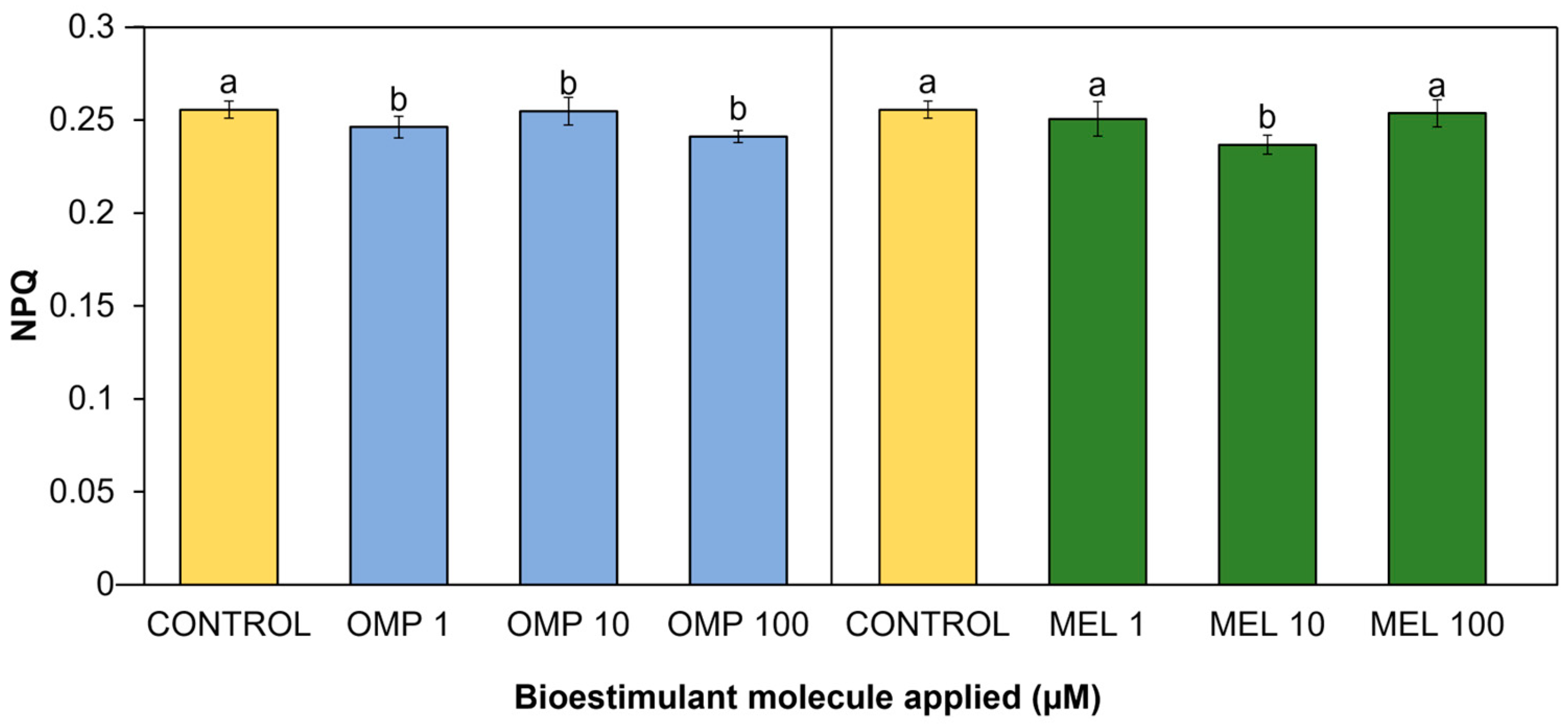
Through the thermal dissipation contained in PSI and PSII, the plant avoids inactivation of the photosynthesis process or damage caused by excess energy. This thermal dissipation is known as non-photosynthetic quenching (NPQ), and its quantification can be an important index to measure the health of the photosynthetic system in plants [48]. Significant differences (p < 0.05) in the present work were found in the non-photochemical quenching coefficient due to the effect of applying OMP and MEL at different doses (Figure 8). The treatment with the lowest value was MEL 10, with a significant decrease of 7.4% with respect to the control. On the omeprazole side, the lowest index was obtained in the OMP 100 treatment, with a significant decrease with respect to the control of 5.6% (Figure 8).
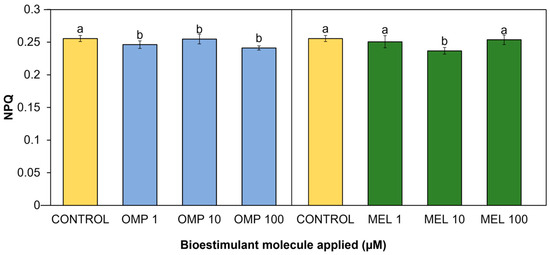
Figure 8.
Effect of foliar application of omeprazole (OMP) and melatonin (MEL) at doses of 1, 10, and 100 µM on non-photochemical quenching (NPQ) in green bean plants cv. Strike. Means with equal letters do not differ according to LSD test (p < 0.05).
Although an increase in NPQ has been reported as a protective mechanism against photoinhibition, it may also indicate a loss of energy that can be used for photosynthesis, leading to a decrease in growth and production [49]. As demonstrated above, the increase in photochemical quenching (qP) caused by the application of omeprazole and melatonin may induce an improvement in the conversion of energy used for photosynthesis but in turn a decrease in the NPQ index because the protection mechanism against excess light is not as necessary in plants with high photosynthetic efficiency. The increase in NPQ found in the control treatment may be due to the fact that plants without OMP and MEL-driven production of antioxidant molecules require the activation of alternative mechanisms, such as the induction of the xanthophyll cycle and carotenoid biosynthesis [50]. However, these mechanisms, being energy-dependent, may have an energetic cost to the plant, which will ultimately lead to reductions in growth and yield [51].
3.4.3. Electron Transport Quantum Yield of PSII and Electron Transport Rate
To complement the photochemical quenching results, an important indicator can be quantified: the electron transport quantum yield of PSII (PhiPSII) [52,53,54]. This measurement allows an estimation of the overall process of photosynthesis, as it quantifies the linear transport of electrons and thus allows estimating the photosynthetic capacity of the plant as well as a bio-indicator of growth and development [55].
In the present study, significant differences (p < 0.05) were found in the coefficients of PhiPSII and ETR by the effect of OMP and MEL application at different doses (Table 4). With respect to PhiPSII, the highest yield was recorded in the MEL 10 treatment, with a significant increase of 71.4% over the control, followed by the OMP 1 treatment, with a significant increase of 52.8% over the control (Table 4). These are similar to those obtained by [56], who reported a greater increase in PhiPSII in tomato seedlings to which exogenous melatonin was foliar applied compared to solution application and the control. It is possible that the increased electron transport quantum yield of PSII is related to a higher amount of open PSII centers, which maintain their activity due to the protective role of melatonin as a redox regulator and promoter of D1 protein synthesis, the central subunit of PSII [57].

Table 4.
Effect of foliar application of omeprazole (OMP) and melatonin (MEL) at doses of 1, 10, and 100 µM on quantum yield of PSII (PhiPSII) and electron transport rate (ETR) in green bean plants cv. Strike. Means with equal letters do not differ according to LSD test (p < 0.05).
Authors such as [58] have reported an increase in electron transport rate caused by higher photochemical quenching. The results of the present experiment showed the treatment with higher qP (Figure 6) as the most favored treatment in terms of PhiPSII (Table 4). Similarly, the treatment with lower qP and higher NPQ recorded the lowest PhiPSII.
The improvement in electron transport and PSII quantum yield could indicate a positive effect of melatonin and omeprazole on the reaction centers. In addition, these molecules could enhance quinone activation in PSII and PSI, which is primarily responsible for electron uptake and transfer between photosystems [59].
When quinones (QA) are in the oxidized state, PSII reaction centers are open and drained (Fo), ready to initiate light harvesting, which would enhance both electron reception and electron transport of photosystems (Figure 8) [60]. Finally, the role of melatonin in the antioxidant protection of the photosynthetic apparatus has been highlighted. Possibly, the reduction of ROS stress was reduced in the treatments to which MEL was applied. Photoinhibition and blockage of electron transport was lower in the applied treatments, thus improving the capacity of photosynthetic pigments to transform energy used for photosynthesis [61].
In general, application at lower doses stimulated growth, expressed as a higher biomass recorded in treatments MEL 1 and OMP 1 (Figure 2). Similarly, greater leaf greenness expressed in SPAD units was found in the OMP 1 treatment (Figure 4). The treatments that benefited photosynthetic efficiency parameters the most were generally MEL 10 and OMP 1 (Figure 5, Figure 6 and Figure 7, Table 5). Finally, this resulted in higher fruit yield in treatments MEL 1 and OMP 1. As indicated by numerous authors, bioactive agents, such as salicylic acid, brassinosteroids, calcium ions, polyamine, abscisic acid, and now melatonin and omeprazole, achieve remarkable effects on plant metabolism after a millimolar dose [62]. In addition, other neurotransmitters isolated in humans and plants are being investigated more thoroughly, as their beneficial effects when applied to plants have been recorded [63]. Finally, it should be noted that further studies on the use of low weight molecules in agriculture are required to elucidate the mechanisms of action at the physiological, biochemical, and molecular levels that are involved in the processes of photosynthesis, nitrogen assimilation, carbon metabolism, and crop growth.

Table 5.
Pearson correlation analysis for 8 green bean cv. Strike variables.
4. Conclusions
In general, foliar application of omeprazole and melatonin, both at 1 µM doses, were the treatments that most benefited biomass accumulation and yield in green bean plants cv. Strike. Application of omeprazole at 1µM increased SPAD units and obtained the best results in fluorescence and photochemical quenching. On the other hand, the application of melatonin at 10 µM obtained the highest PhiPSII coefficient. These results could indicate that the use of both omeprazole and melatonin at doses of 1 µM applied foliarly in green bean plants can increase photosynthetic efficiency and decrease photoinhibition, which is reflected in higher biomass accumulation and yield. These results suggest the ability of low-weight molecules such as omeprazole and melatonin to improve important metabolic processes in crops and their potential to be incorporated into agricultural biostimulant products for sustainable production.
Author Contributions
To carry out the present study, E.S. and C.A.R.-E. designed the study; S.P.-Á., C.A.R.-E. and E.S. conducted the experiment and collected the samples; C.C.-M. conducted the statistical analysis; and C.A.R.-E., E.S., S.P.-Á., M.A.F.-C., L.C.N.-M. and C.C.-M. contributed to the writing, revision, and editing of the article. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
This work was supported by the National Science and Technology Council (CONACYT, México) under the project of the Call to Address National Problems: Project #1529 “Biofortification of basic agricultural crops representing the key to combat malnutrition and ensure food security in Mexico”. We are grateful to the National Science and Technology Council (CONACYT, México) for the financial support provided through the national program of scholarships for postgraduate studies under CVU No. 1011744. We also thank the Food and Development Research Center, A.C. (CIAD) Delicias unit; the Faculty of Agrotechnological Sciences; and the Autonomous University of Chihuahua (UACH) for providing the facilities and laboratory equipment necessary to carry out the study.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bajželj, B.; Richards, K.S.; Allwood, J.M.; Smith, P.; Dennis, J.S.; Curmi, E.; Gilligan, C.A. Importance of food-demand management for climate mitigation. Nat. Clim. Change 2014, 4, 924–929. [Google Scholar] [CrossRef]
- Simkin, A.J.; López-Calcagno, P.E.; Raines, C.A. Feeding the world: Improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 2019, 70, 1119–1140. [Google Scholar] [CrossRef] [PubMed]
- Karavidas, I.; Ntatsi, G.; Vougeleka, V.; Karkanis, A.; Ntanasi, T.; Saitanis, C.; Agathokleous, D.; Ropokis, A.; Sabatino, L.; Tran, F.; et al. Agronomic practices to increase the yield and quality of common bean (Phaseolus vulgaris L.): A systematic review. Agronomy 2022, 12, 271. [Google Scholar] [CrossRef]
- Celmeli, T.; Sari, H.; Canci, H.; Sari, D.; Adak, A.; Eker, T.; Toker, C. The nutritional content of common bean (Phaseolus vulgaris L.) landraces in comparison to modern varieties. Agronomy 2018, 8, 166. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin in plants–diversity of levels and multiplicity of functions. Front. Plant Sci. 2016, 7, 198. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Dell’Aversana, E.; Ruggiero, A.; Cirillo, V.; Gibon, Y.; Woodrow, P.; Maggio, A.; Carillo, P. Omeprazole treatment enhances nitrogen use efficiency through increased nitrogen uptake and assimilation in corn. Front. Plant Sci. 2019, 10, 1507. [Google Scholar] [CrossRef]
- Shah, N.; Gossman, W. Omeprazole; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef]
- Du, P.; Yin, B.; Zhou, S.; Li, Z.; Zhang, X.; Cao, Y.; Han, R.; Shi, C.; Liang, B.; Xu, J. Melatonin and dopamine mediate the regulation of nitrogen uptake and metabolism at low ammonium levels in Malus hupehensis. Plant Physiol. Biochem. 2022, 171, 182–190. [Google Scholar] [CrossRef]
- Cirillo, V.; Van Oosten, M.J.; Izzo, M.; Maggio, A. Omeprazole treatment elicits contrasting responses to salt stress in two basil genotypes. Ann. Appl. Biol. 2019, 174, 329–338. [Google Scholar] [CrossRef]
- Zhao, Q.; Shen, W.; Gu, Y.; Hu, J.; Ma, Y.; Zhang, X.; Du, J.; Zhang, Y. Exogenous melatonin mitigates saline-alkali stress by decreasing DNA oxidative damage and enhancing photosynthetic carbon metabolism in soybean (Glycine max [L.] Merr.) leaves. Physiol. Plant. 2023, 175, e13983. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; El-Abedin, T.K.Z. Omeprazole alleviates water stress in peppermint and modulates the expression of menthol biosynthesis genes. Plant Physiol. Biochem. 2019, 139, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Erdal, S. Melatonin promotes plant growth by maintaining integration and coordination between carbon and nitrogen metabolisms. Plant Cell Rep. 2019, 38, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Lu, N.; Xu, H.; Maruo, T.; Guo, S. Root zone cooling and exogenous spermidine root-pretreatment promoting Lactuca sativa L. growth and photosynthesis in the high-temperature season. Front. Plant Sci. 2016, 7, 368. [Google Scholar] [CrossRef]
- Yamori, W.; Kondo, E.; Sugiura, D.; Terashima, I.; Suzuki, Y.; Makino, A. Enhanced leaf photosynthesis as a target to increase grain yield: Insights from transgenic rice lines with variable Rieske FeS protein content in the cytochrome b6/f complex. Plant Cell Environ. 2016, 39, 80–87. [Google Scholar] [CrossRef]
- Wang, L.Y.; Liu, J.L.; Wang, W.X.; Sun, Y. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 2016, 54, 19–27. [Google Scholar] [CrossRef]
- Shrestha, S.; Brueck, H.; Asch, F. Chlorophyll index, photochemical reflectance index and chlorophyll fluorescence measurements of rice leaves supplied with different N levels. J. Photochem. Photobiol. B Biol. 2012, 113, 7–13. [Google Scholar] [CrossRef]
- Sánchez, E.; Ruiz, J.M.; Romero, L. Compuestos nitrogenados indicadores de estrés en respuesta a las dosis tóxicas y deficientes de Nitrógeno en frijol ejotero. Nova Sci. 2016, 8, 228–244. [Google Scholar] [CrossRef]
- Ahmad, S.; Wang, G.Y.; Muhammad, I.; Chi, Y.X.; Zeeshan, M.; Nasar, J.; Zhou, X.B. Interactive effects of melatonin and nitrogen improve drought tolerance of maize seedlings by regulating growth and physiochemical attributes. Antioxidants 2022, 11, 359. [Google Scholar] [CrossRef]
- Qiao, Y.; Yin, L.; Wang, B.; Ke, Q.; Deng, X.; Wang, S. Melatonin promotes plant growth by increasing nitrogen uptake and assimilation under nitrogen deficient condition in winter wheat. Plant Physiol. Biochem. 2019, 139, 342–349. [Google Scholar] [CrossRef]
- Carillo, P.; Raimondi, G.; Kyriacou, M.C.; Pannico, A.; El-Nakhel, C.; Colla, G.; De Pascale, S.; Cirillo, V.; Rouphael, Y. Morpho-physiological and homeostatic adaptive responses triggered by omeprazole enhance lettuce tolerance to salt stress. Sci. Hortic. 2019, 249, 22–30. [Google Scholar] [CrossRef]
- Turner, M.F.; Heuberger, A.L.; Kirkwood, J.S.; Collins, C.C.; Wolfrum, E.J.; Broeckling, C.D.; Prenni, J.E.; Jahn, C.E. Non-targeted metabolomics in diverse sorghum breeding lines indicates primary and secondary metabolite profiles are associated with plant biomass accumulation and photosynthesis. Front. Plant Sci. 2016, 7, 953. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Ni, Z.; Xia, H.; Xie, Y.; Lv, X.; Wang, J.; Ling, L.; Deng, Q.; Luo, X. Exogenous melatonin promotes biomass accumulation and photosynthesis of kiwifruit seedlings under drought stress. Sci. Hortic. 2019, 246, 34–43. [Google Scholar] [CrossRef]
- Li, L.; Gallei, M.; Friml, J. Bending to auxin: Fast acid growth for tropisms. Trends Plant Sci. 2021, 27, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S.; Guo, S.; Sun, J.; Shu, S.; Wang, Y.; El-Yazied, A.A.; Alabdallah, N.M.; Hikal, M.; Mostafa, H.M.; Ibrahim, F.M.; et al. Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol. Biochem. 2021, 167, 309–320. [Google Scholar] [CrossRef]
- Zhang, R.; Yue, Z.; Chen, X.; Wang, Y.; Zhou, Y.; Xu, W.; Huang, R. Foliar applications of urea and melatonin to alleviate water-logging stress on photosynthesis and antioxidant metabolism in sorghum seedlings. Plant Growth Regul. 2021, 97, 429–438. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, J.; Li, W.; Ding, Y.; Zhong, Q.; Xu, X.; Wei, H.; Li, G. Exogenous melatonin alleviates salt stress by improv-ing leaf photosynthesis in rice seedlings. Plant Physiol. Biochem. 2021, 163, 367–375. [Google Scholar] [CrossRef]
- Salcido-Martínez, A.; Sanchez, E.; Licon-Trillo, L.P.; Perez-Alvarez, S.; Palacio-Márquez, A.; Amaya-Olivas, N.I.; Preciado-Rangel, P. Impact of the foliar application of magnesium nanofertilizer on physiological and biochemical parameters and yield in green beans. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 2167. [Google Scholar] [CrossRef]
- Rastogi, A.; Siddiqui, A.; Mishra, B.K.; Srivastava, M.; Pandey, R.; Misra, P.; Singh, M.; Shukla, S. Effect of auxin and gibberellic acid on growth and yield components of linseed (Linum usitatissimum L.). Crop. Breed. Appl. Biotechnol. 2013, 13, 136–143. [Google Scholar] [CrossRef]
- Sukumar, P.; Maloney, G.S.; Muday, G.K. Localized induction of the ATP-binding cassette B19 auxin transporter enhances adventitious root formation in Arabidopsis. Plant Physiol. 2013, 162, 1392–1405. [Google Scholar] [CrossRef]
- Jiang, C.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Maruo, T. A correlation analysis on chlorophyll content and SPAD value in tomato leaves. Hort. Res. 2017, 71, 37–42. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Alsubaie, Q.D.; Ali, H.M. Melatonin and gibberellic acid promote growth and chlorophyll biosynthesis by regulating antioxidant and methylglyoxal detoxification system in tomato seedlings under salinity. J. Plant Growth Regul. 2020, 39, 1488–1502. [Google Scholar] [CrossRef]
- Soltabayeva, A.; Srivastava, S.; Kurmanbayeva, A.; Bekturova, A.; Fluhr, R.; Sagi, M. Early senescence in older leaves of low nitrate-grown Atxdh1 uncovers a role for purine catabolism in N supply. Plant Physiol. 2018, 178, 1027–1044. [Google Scholar] [CrossRef]
- Hussain, S.; Khalid, M.F.; Hussain, M.; Ali, M.A.; Nawaz, A.; Zakir, I.; Fatima, Z.; Ahmad, S. Role of micronutrients in salt stress tolerance to plants. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Singapore, 2018; pp. 363–376. [Google Scholar] [CrossRef]
- Mir, A.R.; Siddiqui, H.; Alam, P.; Hayat, S. Melatonin modulates photosynthesis, redox status, and elemental composition to promote growth of Brassica juncea—A dose-dependent effect. Protoplasm 2020, 257, 1685–1700. [Google Scholar] [CrossRef] [PubMed]
- Weeda, S.; Zhang, N.; Zhao, X.; Ndip, G.; Guo, Y.; Buck, G.A.; Fu, C.; Ren, S. Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS ONE 2014, 9, e93462. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Wang, Y.; Chi, Y.; Zhou, L.; Chen, J.; Zhou, W.; Song, J.; Zhao, N.; Ding, J. Drought stress strengthens the link between chlorophyll fluorescence parameters and photosynthetic traits. PeerJ 2020, 8, e10046. [Google Scholar] [CrossRef]
- Sánchez-Reinoso, A.D.; Ligarreto-Moreno, G.A.; Restrepo-Díaz, H. Physiological and biochemical expressions of a determinated growth common bean genotype (Phaseolus vulgaris L.) to water deficit stress periods. J. Anim. Plant Sci. 2018, 28, 119–127. [Google Scholar]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef]
- Khan, T.A.; Saleem, M.; Fariduddin, Q. Melatonin influences stomatal behavior, root morphology, cell viability, photosynthetic responses, fruit yield, and fruit quality of tomato plants exposed to salt stress. J. Plant Growth Regul. 2022, 42, 2408–2432. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Yang, S.J.; Chen, Y.Y. Effects of melatonin on photosynthetic performance and antioxidants in melon during cold and recovery. Biol. Plant. 2017, 61, 571–578. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a potent and inducible endogenous antioxidant: Synthesis and metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, G.C.; Govindjee. The Non-Photochemical Quenching of the Electronically Excited State of Chlorophyll a in Plants: Definitions, Timelines, Viewpoints, Open Questions. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Demmig-Adams, B., Garab, G., Adams, W., III, Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 1–44. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Silletti, S.; Guida, G.; Cirillo, V.; Di Stasio, E.; Carillo, P.; Woodrow, P.; Maggio, A.; Raimondi, G. A benzimidazole proton pump inhibitor increases growth and tolerance to salt stress in tomato. Front. Plant Sci. 2017, 8, 1220. [Google Scholar] [CrossRef]
- Tyystjärvi, E. Photoinhibition of photosystem II. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 300, pp. 243–303. [Google Scholar] [CrossRef]
- Murchie, E.H.; Ruban, A.V. Dynamic non-photochemical quenching in plants: From molecular mechanism to productivity. Plant J. 2020, 101, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Berteotti, S.; Ballottari, M.; Bassi, R. Increased biomass productivity in green algae by tuning non-photochemical quenching. Sci. Rep. 2016, 6, 21339. [Google Scholar] [CrossRef] [PubMed]
- Quaas, T.; Berteotti, S.; Ballottari, M.; Flieger, K.; Bassi, R.; Wilhelm, C.; Goss, R. Non-photochemical quenching and xanthophyll cycle activities in six green algal species suggest mechanistic differences in the process of excess energy dissipation. J. Plant Physiol. 2015, 172, 92–103. [Google Scholar] [CrossRef]
- Kromdijk, J.; Walter, J. Relaxing Non-Photochemical Quenching (NPQ) to Improve Photosynthesis in Crops; Burleigh Dodds Science Publishing: Cambridge, UK, 2023; pp. 1–19. [Google Scholar] [CrossRef]
- Janka, E.; Körner, O.; Rosenqvist, E.; Ottosen, C.O. Using the quantum yields of photosystem II and the rate of net photosynthesis to monitor high irradiance and temperature stress in chrysanthemum (Dendranthema grandiflora). Plant Physiol. Biochem. 2015, 90, 14–22. [Google Scholar] [CrossRef]
- Olvera-González, E.; Alaniz-Lumbreras, D.; Ivanov-Tsonchev, R.; Villa-Hernández, J.; de la Rosa-Vargas, I.; López-Cruz, I.; Silos-Espino, H.; Lara-Herrera, A. Chlorophyll fluorescence emission of tomato plants as a response to pulsed light based LEDs. Plant Growth Regul. 2013, 69, 117–123. [Google Scholar] [CrossRef]
- Saito, A.; Shinjo, S.; Ito, D.; Doi, Y.; Sato, A.; Wakabayashi, Y.; Honda, J.; Arai, Y.; Maeda, T.; Ohyama, T.; et al. Enhancement of photosynthetic iron-use efficiency is an important trait of Hordeum vulgare for adaptation of photosystems to iron deficiency. Plants 2021, 10, 234. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.; Liang, D.; Ma, F. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2013, 54, 292–302. [Google Scholar] [CrossRef]
- Yang, X.L.; Xu, H.; Li, D.; Gao, X.; Li, T.L.; Wang, R. Effect of melatonin priming on photosynthetic capacity of tomato leaves under low-temperature stress. Photosynthetica 2018, 56, 884–892. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, H.; Cao, K.; Hu, L.; Du, T.; Baluška, F.; Zou, Z. Beneficial roles of melatonin on redox regulation of photosynthetic electron transport and synthesis of D1 protein in tomato seedlings under salt stress. Front. Plant Sci. 2016, 7, 1823. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.E.A.; Rodrigues, F.A.; Moreira, W.R.; DaMatta, F.M. Leaf gas exchange and chlorophyll a fluorescence in wheat plants supplied with silicon and infected with Pyricularia oryzae. Phytopathology 2014, 104, 143–149. [Google Scholar] [CrossRef]
- Pereira, Y.C.; Rodrigues, W.S.; Lima, E.J.A.; Santos, L.R.; Silva, M.H.L.; Lobato, A.K.S. Brassinosteroids increase electron transport and photosynthesis in soybean plants under water deficit. Photosynthetica 2019, 57, 181–191. [Google Scholar] [CrossRef]
- Lu, X.F.; Zhang, H.; Lyu, S.S.; Du, G.D.; Wang, X.Q.; Wu, C.H.; Lyu, D.G. Effects of exogenous phenolic acids on photosystem functions and photosynthetic electron transport rate in strawberry leaves. Photosynthetica 2018, 56, 616–622. [Google Scholar] [CrossRef]
- Wang, D.Y.; Wang, J.; Shi, S.H.; Huang, L.X.; Zhu, M.; Li, F.H. Exogenous melatonin ameliorates salinity-induced oxidative stress and improves photosynthetic capacity in sweet corn seedlings. Photosynthetica 2021, 59, 327–336. [Google Scholar] [CrossRef]
- Borges, A.A.; Jiménez-Arias, D.; Expósito-Rodríguez, M.; Sandalio, L.M.; Pérez, J.A. Priming crops against biotic and abiotic stresses: MSB as a tool for studying mechanisms. Front. Plant Sci. 2014, 5, 642. [Google Scholar] [CrossRef]
- Erland, L.A.; Saxena, P.K. Melatonin and serotonin in plant morphogenesis and development. In Neurotransmitters in Plants: Perspectives and Applications; Ramakrishna, A., Roshchina, V.V., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 57–66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).