Effect of Non-Native Endophytic Bacteria on Oat (Avena sativa L.) Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Root Vigor Assay
2.2. Greenhouse Study
3. Results
3.1. Effect of Endophytes on Root Development in Oat Seedlings (Root Vigor Assay)
3.2. Effect of Endophytes and Fertilization Level on Root and Shoot Growth of Oat Cultivars in the Greenhouse
3.2.1. Effect of Fertilization Level on Root and Shoot Growth of Oats
3.2.2. Response of Oat Cultivars to Endophyte Inoculation under Full Fertilization Rate
3.2.3. Response of Oat Cultivars to Endophyte Inoculation under Half Fertilization Rate
3.2.4. Comparison of the 10 Oat Cultivars for Their Growth Response Pattern to Endophyte Inoculation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webster, F.H.; Wood, P.J. Oats: Chemistry and Technology, 2nd ed.; AACC International Press: Saint Paul, MN, USA, 2011. [Google Scholar]
- Strychar, R. World Oat Production, Trade, and Usage. In Oats: Chemistry and Technology, 2nd ed.; Webster, F.H., Wood, P.J., Eds.; AACC International Press: St. Paul, MN, USA, 2016. [Google Scholar]
- Martínez-Villaluenga, C.; Peñas, E. Health benefits of oat: Current evidence and molecular mechanisms. Curr. Opin. Food Sci. 2017, 14, 26–31. [Google Scholar] [CrossRef]
- Yan, W.; Fregeau-Reid, J.; Ma, B.; Pageau, D.; Vera, C. Nitrogen fertilizer complements breeding in improving yield and quality of milling oat. Crop Sci. 2017, 57, 3291–3302. [Google Scholar] [CrossRef]
- Rütting, T.; Aronsson, H.; Delin, S. Efficient use of nitrogen in agriculture. Nutr. Cycl. Agroecosyst. 2018, 110, 1–5. [Google Scholar] [CrossRef]
- Wilson, D. Endophyte: The evolution of a term, and clarification of its use and definition. Oikos 1995, 73, 274–276. [Google Scholar] [CrossRef]
- White, J.F.; Kingsley, K.L.; Zhang, Q.; Verma, R.; Obi, N.; Dvinskikh, S.; Elmore, M.T.; Verma, S.K.; Gond, S.K.; Kowalski, K.P. Endophytic microbes and their potential applications in crop management. Pest Manag. Sci. 2019, 75, 2558–2565. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Kandel, S.L.; Joubert, P.M.; Doty, S.L. Bacterial Endophyte Colonization and Distribution within Plants. Microorganisms 2017, 5, 77. [Google Scholar] [CrossRef]
- Irizarry, I.; White, J.F. Bacillus amyloliquefaciens alters gene expression, ROS production and lignin synthesis in cotton seedling roots. J. Appl. Microbiol. 2018, 124, 1589–1603. [Google Scholar] [CrossRef]
- Kafle, A.; Cope, K.; Raths, R.; Krishna, Y.J.; Subramanian, S.; Bucking, H.; Garcia, K. Harnessing Soil Microbes to Improve Plant Phosphate Efficiency in Cropping Systems. Agronomy 2019, 9, 127. [Google Scholar] [CrossRef]
- Walia, A.; Guleria, S.; Chauhan, A.; Mehta, P. Endophytic bacteria: Role in phosphate solubilization. In Endophytes: Crop Productivity and Protection; Springer: Berlin/Heidelberg, Germany, 2017; pp. 61–93. [Google Scholar]
- Bhattacharjee, R.B.; Singh, A.; Mukhopadhyay, S.N. Use of nitrogen-fixing bacteria as biofertiliser for non-legumes: Prospects and challenges. Appl. Microbiol. Biotechnol. 2008, 80, 199–209. [Google Scholar] [CrossRef]
- Boddey, R.M.; de Oliveira, O.C.; Urquiaga, S.; Reis, V.M.; Olivares, F.L.; Baldani, V.L.D.; Döbereiner, J. Biological nitrogen fixation associated with sugar cane and rice: Contributions and prospects for improvement. Plant Soil 1995, 174, 195–209. [Google Scholar] [CrossRef]
- Soares, R.A.; Roesch, L.F.W.; Zanatta, G.; de Oliveira Camargo, F.A.; Passaglia, L.M.P. Occurrence and distribution of nitrogen fixing bacterial community associated with oat (Avena sativa) assessed by molecular and microbiological techniques. Appl. Soil Ecol. 2006, 33, 221–234. [Google Scholar] [CrossRef]
- Venieraki, A.; Dimou, M.; Vezyri, E.; Kefalogianni, I.; Argyris, N.; Liara, G.; Pergalis, P.; Chatzipavlidis, I.; Katinakis, P. Characterization of Nitrogen-Fixing Bacteria Isolated from Field-Grown Barley, Oat, and Wheat. J. Microbiol. 2011, 49, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Bravo, A.; Herrera-Cornelio, L.C.; García-Toscano, D.F.; Kiel-Martínez, A.L.; Guevara-Avendaño, E.; Ramírez-Vázquez, M.; Bautista, Y.P.; Méndez-Bravo, A.; Reverchon, F. Beneficial effects of selected rhizospheric and endophytic bacteria, inoculated individually or in combination, on non-native host plant development. Rhizosphere 2023, 26, 100693. [Google Scholar] [CrossRef]
- ALKahtani, M.D.F.; Fouda, A.; Attia, K.; Al-Otaibi, F.; Eid, A.M.; Ewais, E.; Hijri, M.; St-Arnaud, M.; Hassan, S.; Khan, N.; et al. Isolation and Characterization of Plant Growth Promoting Endophytic Bacteria from Desert Plants and Their Application as Bioinoculants for Sustainable Agriculture. Agronomy 2020, 10, 1325. [Google Scholar] [CrossRef]
- Sapre, S.; Gontia-Mishra, I.; Tiwari, S. Klebsiella sp. confers enhanced tolerance to salinity and plant growth promotion in oat seedlings (Avena sativa). Microbiol. Res. 2018, 206, 25–32. [Google Scholar] [CrossRef]
- Chang, P.; Gerhardt, K.E.; Huang, X.-D.; Yu, X.-M.; Glick, B.R.; Gerwing, P.D.; Greenberg, B.M. Plant growth-promoting bacteria facilitate the growth of barley and oats in salt-impacted soil: Implications for phytoremediation of saline soils. Int. J. Phytoremed. 2014, 16, 1133–1147. [Google Scholar] [CrossRef]
- Peta, V. Utilizing Rhizospheric and Bacterial Endophytes for Use as Potential Bio-fertilizers for Sustainable Agricultural Production. Ph.D. Thesis, South Dakota State University, Brookings, SD, USA, 2020; p. 3913. [Google Scholar]
- Cheplick, G.; Cho, R. Interactive effects of fungal endophyte infection and host genotype on growth and storage in Lolium perenne. New Phytol. 2003, 158, 183–191. [Google Scholar] [CrossRef]
- Iniguez, A.L.; Dong, Y.; Triplett, E.W. Nitrogen fixation in wheat provided by Klebsiella pneumoniae 342. Mol. Plant Microbe Interact. 2004, 17, 1078–1085. [Google Scholar] [CrossRef]
- Neiverth, A.; Delai, S.; Garcia, D.M.; Saatkamp, K.; de Souza, E.M.; de Oliveira Pedrosa, F.; Guimarães, V.F.; Santos, M.F.; da Costa, A.C.T.; Vendruscolo, E.C.G.; et al. Performance of different wheat genotypes inoculated with the plant growth promoting bacterium Herbaspirillum seropedicae. Eur. J. Soil Biol. 2014, 64, 1–5. [Google Scholar] [CrossRef]
- Vargas, L.; de Carvalho, T.L.G.; Ferreira, P.C.G.; Baldani, V.L.D.; Baldani, J.I.; Hemerly, A.S. Early responses of rice (Oryza sativa L.) seedlings to inoculation with beneficial diazotrophic bacteria are dependent on plant and bacterial genotypes. Plant Soil 2012, 356, 127–137. [Google Scholar] [CrossRef]
- Montañez, A.; Abreu, C.; Gill, P.; Hardarson, G.; Sicardi, M. Biological nitrogen fixation in maize (Zea mays L.) by 15N isotope-dilution and identification of associated culturable diazotrophs. Biol. Fert. Soils 2008, 45, 253–263. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research; R Package Version 1.3-5. 2021. Available online: https://www.vps.fmvz.usp.br/CRAN/web/packages/agricolae/vignettes/tutorial.pdf (accessed on 1 June 2022).
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Yanni, Y.G.; Dazzo, F.B.; Zidan, M.I. Beneficial Endophytic Rhizobia as Biofertilizer Inoculants for Rice and the Spatial Ecology of This Bacteria-Plant Association. In Bacteria in Agrobiology: Crop Ecosystems; Springer: Berlin/Heidelberg, Germany, 2011; pp. 265–294. [Google Scholar]
- Ramesh, A.; Sharma, S.K.; Sharma, M.P.; Yadav, N.; Joshi, O.P. Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in Vertisols of central India. Appl. Soil Ecol. 2014, 73, 87–96. [Google Scholar] [CrossRef]
- Midekssa, M.J.; Loscher, C.R.; Schmitz, R.A.; Assefa, F. Characterization of phosphate solubilizing rhizobacteria isolated from lentil growing areas of Ethiopia. Afr. J. Microbiol. Res. 2015, 9, 1637–1648. [Google Scholar]
- Joo, G.-J.; Kim, Y.-M.; Lee, I.-J.; Song, K.-S.; Rhee, I.-K. Growth promotion of red pepper plug seedlings and the production of gibberellins by Bacillus cereus, Bacillus macroides and Bacillus pumilus. Biotechnol. Lett. 2004, 26, 487–491. [Google Scholar] [CrossRef]
- Goswami, D.; Dhandhukia, P.; Patel, P.; Thakker, J.N. Screening of PGPR from saline desert of Kutch: Growth promotion in Arachis hypogea by Bacillus licheniformis A2. Microbiol. Res. 2014, 169, 66–75. [Google Scholar] [CrossRef]
- Mumtaz, M.Z.; Ahmad, M.; Jamil, M.; Hussain, T. Zinc solubilizing Bacillus spp. potential candidates for biofortification in maize. Microbiol. Res. 2017, 202, 51–60. [Google Scholar] [CrossRef]
- Cakmakci, R.; Erat, M.; Erdogan, U.; Donmez, M.F. The influence of plant growth-promoting rhizobacteria on growth and enzyme activities in wheat and spinach plants. J. Plant Nut. Soil Sci. 2007, 170, 288–295. [Google Scholar] [CrossRef]
- Coffman, F.A. Oat History, Identification and Classification; US Department of Agriculture: Washington, DC, USA, 1977; Volume 1516.
- Caffe-Treml, M.; Hall, L.; Bauer, R.; Kleinjan, J.; Hall, N.; Ingemansen, J.A. Registration of oat cultivar ‘Hayden’. J Plant Regist. 2017, 11, 95–99. [Google Scholar] [CrossRef]
- Buckley, H.; Young, C.A.; Charlton, N.D.; Hendricks, W.Q.; Haley, B.; Nagabhyru, P.; Rudgers, J.A. Leaf Endophytes Mediate Fertilizer Effects on Plant Yield and Traits in Northern Oat Grass (Trisetum spicatum). Plant Soil 2019, 434, 425–440. [Google Scholar] [CrossRef]
- Hughes, A.R.; Moore, A.F.; Gehring, C. Plant response to fungal root endophytes varies by host genotype in the foundation species Spartina alterniflora. Am. J. Bot. 2020, 107, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Cheplick, G.P. Costs of fungal endophyte infection in Lolium perenne genotypes from Eurasia and North Africa under extreme resource limitation. Environ. Exp. Bot. 2007, 60, 202–210. [Google Scholar] [CrossRef]
- Brader, G.; Company, S.; Vescio, K.; Mitter, B.; Trognitz, F.; Ma, L.J.; Sessitsch, A. Ecology and genome insights into plant-pathogenic and plant-nonpathogenic endophytes. Annu. Rev. Phytopathol. 2017, 55, 61–83. [Google Scholar] [CrossRef]
- Schultz, C.R.; Brantley, K.M.; Wallace, J.G. The role of genetic variation in Zea mays response to beneficial endophytes. Plant Growth Regul. 2022, 98, 167–177. [Google Scholar] [CrossRef]
- Ravel, C.; Courty, C.; Coudret, A.; Charmet, G. Beneficial Effects of Neotyphodium lolii on the Growth and the Water Status in Perennial Ryegrass Cultivated under Nitrogen Deficiency or Drought Stress. Agronomie 1997, 17, 173–181. [Google Scholar] [CrossRef]
- Khare, E.; Mishra, J.; Arora, N. Multifaceted Interactions between Endophytes and Plant: Developments and Prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef]
- Ren, A.; Wei, M.; Yin, L.; Wu, L.; Zhou, Y.; Li, X.; Gao, Y. Benefits of a fungal endophyte in Leymus chinensis depend more on water than on nutrient availability. Environ. Exp. Bot. 2014, 108, 71–78. [Google Scholar] [CrossRef]
- Ren, A.Z.; Li, X.; Han, R.; Yin, L.J.; Wei, M.Y.; Gao, Y.B. Benefits of a symbiotic association with endophytic fungi are subject to water and nutrient availability in Achnatherum sibiricum. Plant Soil 2011, 346, 363–373. [Google Scholar] [CrossRef]
- Sasaki, K.; Ikeda, S.; Eda, S.; Mitsui, H.; Hanzawa, E.; Kisara, C. Impact of plant genotype and nitrogen level on rice growth response to inoculation with Azospirillum sp. strain B510 under paddy field conditions. Soil Sci. Plant Nutr. 2010, 56, 636–644. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J. Auxin and plant-microbe interactions. Cold Spring Harbor Perspect. Biol. 2011, 3, a001438. [Google Scholar] [CrossRef]
- Morse, L.; Faeth, S.H.; Day, T. Neotyphodium interactions with a wild grass are driven mainly by endophyte haplotype. Funct. Ecol. 2007, 21, 813–822. [Google Scholar] [CrossRef]
- Ul Hassan, T.; Bano, A. Construction of IAA-deficient mutants of Pseudomonas moraviensis and their comparative effects with wild type strains as bio-inoculant on wheat in saline sodic soil. Geomicrobiol. J. 2019, 36, 376–384. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Mace, W.; Qin, J.; Liu, H.; Chen, W.; Ren, A.Z.; Gao, Y.B. Endophyte species influence the biomass production of the native grass Achnatherum sibiricum (L.) Keng under high nitrogen availability. Ecol. Evol. 2016, 6, 8595–8606. [Google Scholar] [CrossRef] [PubMed]
- Saikkonen, K.; Lehtonen, P.; Helander, M.; Koricheva, J.; Faeth, S.H. Model systems in ecology: Dissecting the endophyte–grass literature. Trends Plant Sci. 2006, 11, 428–433. [Google Scholar] [CrossRef]
- Lewis, G.C. Effects of biotic and abiotic stress on the growth of three genotypes of Lolium perenne with and without infection by the fungal endophyte Neotyphodium lolii. Ann. Appl. Biol. 2004, 144, 53–63. [Google Scholar] [CrossRef]
- Thuc, L.V.; Huu, T.N.; Ngoc, T.M.; Hue, N.H.; Quang, L.T.; Xuan, D.T.; Nhan, T.C.; Xuan, L.N.T.; Thu, L.T.M.; Akagi, I.; et al. Effects of nitrogen fertilization and nitrogen fixing endophytic bacteria supplementation on soil fertility, N uptake, growth, and yield of sesame (Sesamum indicum L.) cultivated on alluvial soil in dykes. Appl. Environ. Soil Sci. 2022, 2022, 1972585. [Google Scholar] [CrossRef]
- Cheplick, G.P.; Clay, K.; Marks, S. Interactions between infection by endophytic fungi and nutrient limitation in the grasses Lolium perenne and Festuca arundinacea. New Phytol. 1989, 111, 89–97. [Google Scholar] [CrossRef]
- Rosenblueth, M.; Ormeño-Orrillo, E.; López-López, A.; Rogel, M.A.; Reyes-Hernández, B.J.; Martínez-Romero, J.C.; Reddy, P.M.; Martínez-Romero, E. Nitrogen Fixation in Cereals. Front. Microbiol. 2018, 9, 1794. [Google Scholar] [CrossRef]
- Faeth, S.H.; Fagan, W.F. Fungal endophytes: Common host plant symbionts but uncommon mutualists. Integr. Comp. Biol. 2002, 42, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Vivanco, J.M.; Manter, D.K. Nitrogen fertilizer rate affects root exudation, the rhizosphere microbiome and nitrogen-use-efficiency of maize. Appl. Soil Ecol. 2016, 107, 324–333. [Google Scholar] [CrossRef]
- Knoth, J.L.; Kim, S.; Ettl, G.J.; Doty, S.L. Biological nitrogen fixation and biomass accumulation within poplar clones as a result of inoculations with diazotrophic endophyte consortia. New Phytol. 2014, 201, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.M.; Urquiaga, S.; Döbereiner, J.; Baldani, J.I. The effect of inoculating endophytic N2-fixing bacteria on micropropagated sugarcane plants. Plant Soil 2002, 242, 205–215. [Google Scholar] [CrossRef]
- Govindarajan, M.; Balandreau, J.; Kwon, S.W. Effects of the inoculation of Burkholderia vietnamensis and related endophytic diazotrophic bacteria on grain yield of rice. Microb. Ecol. 2007, 55, 21–37. [Google Scholar] [CrossRef]
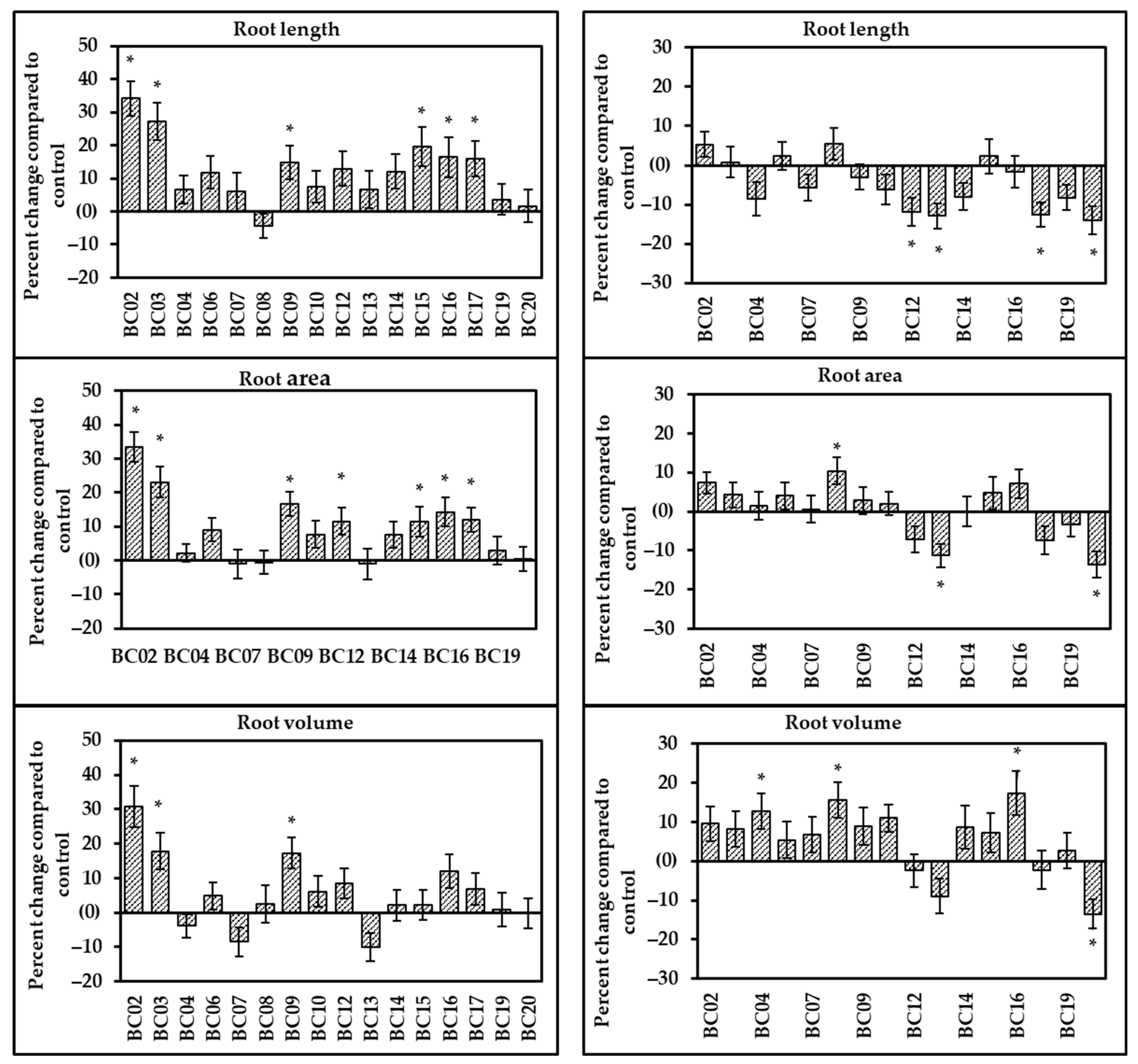

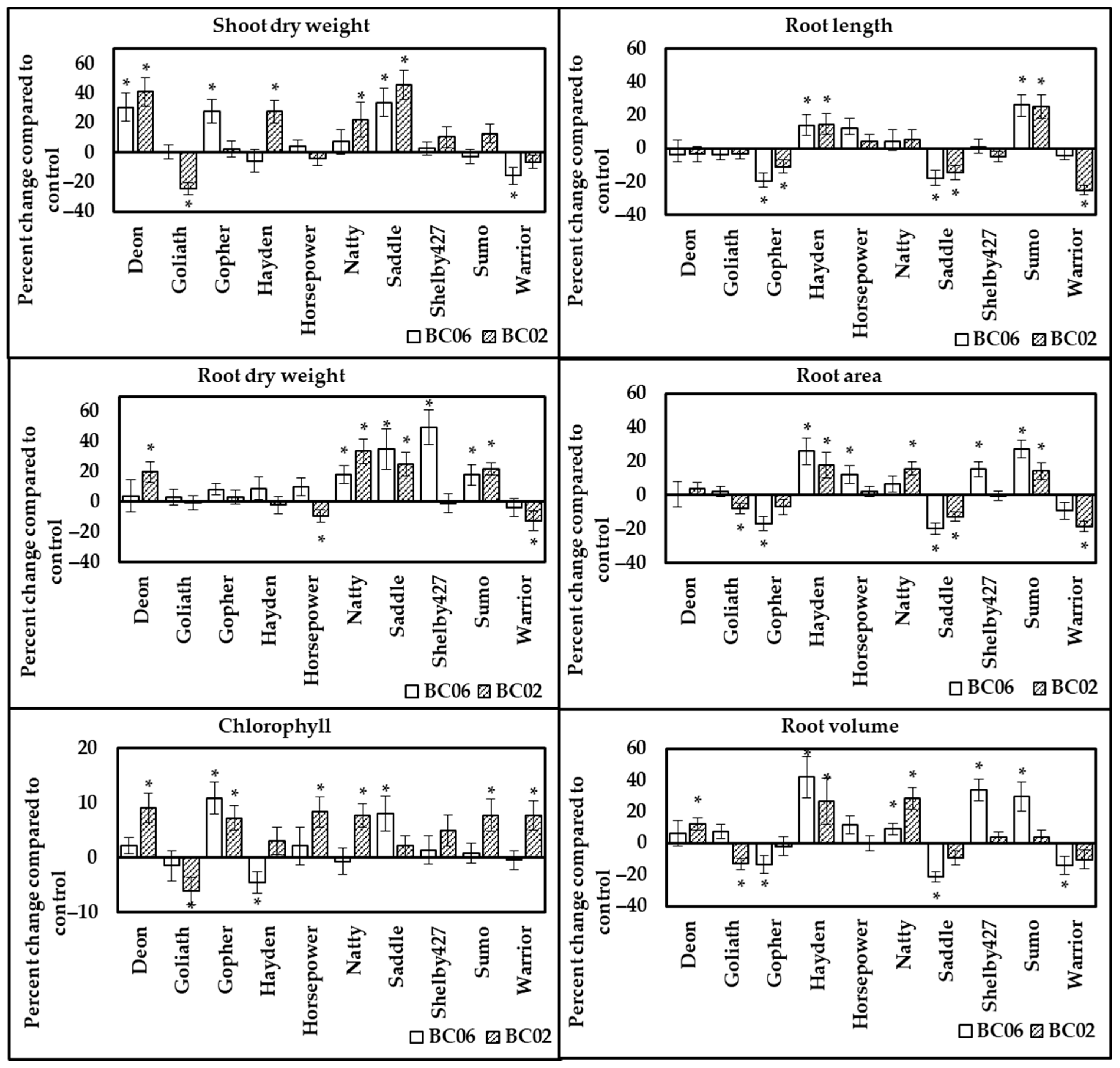
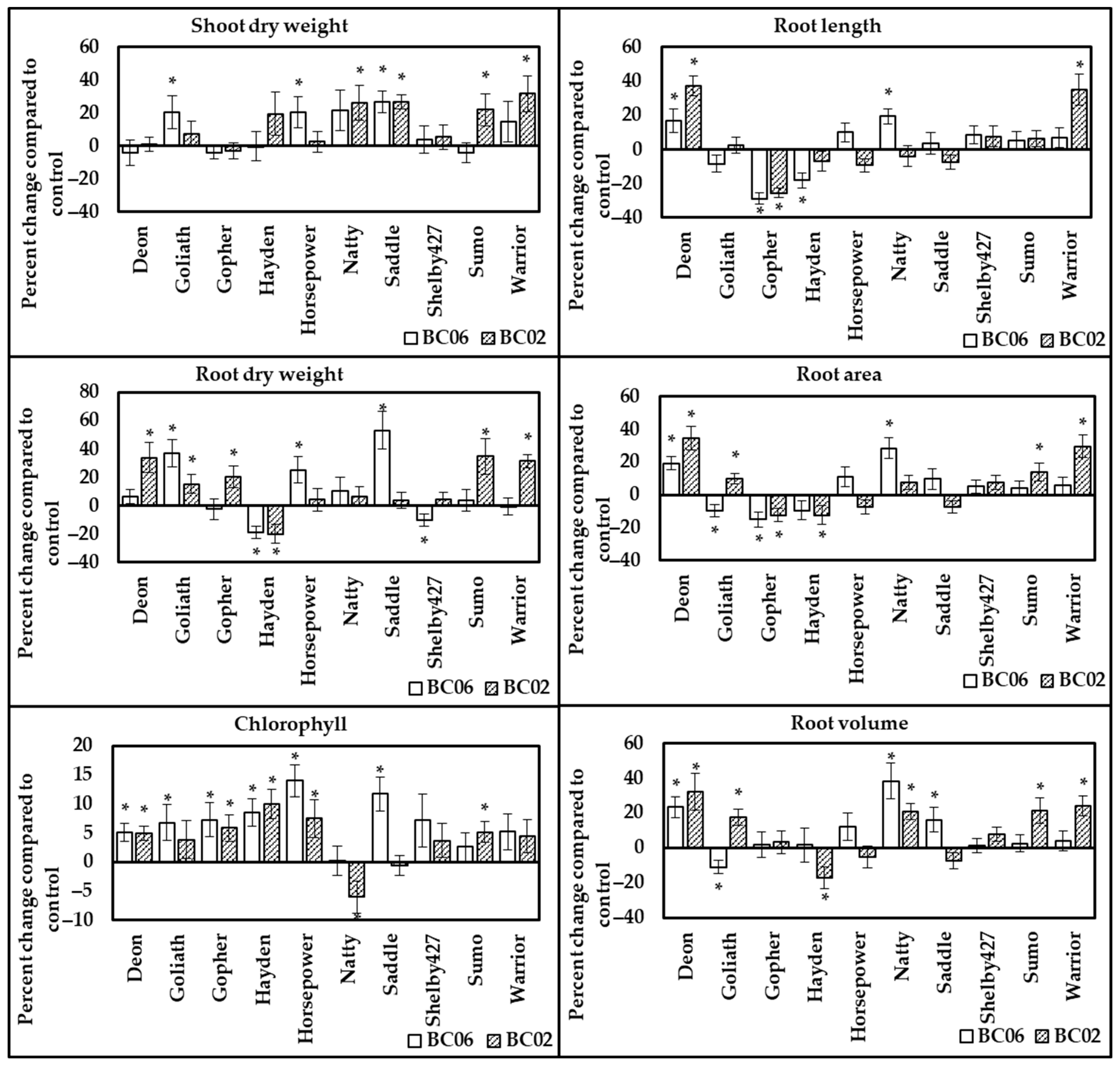
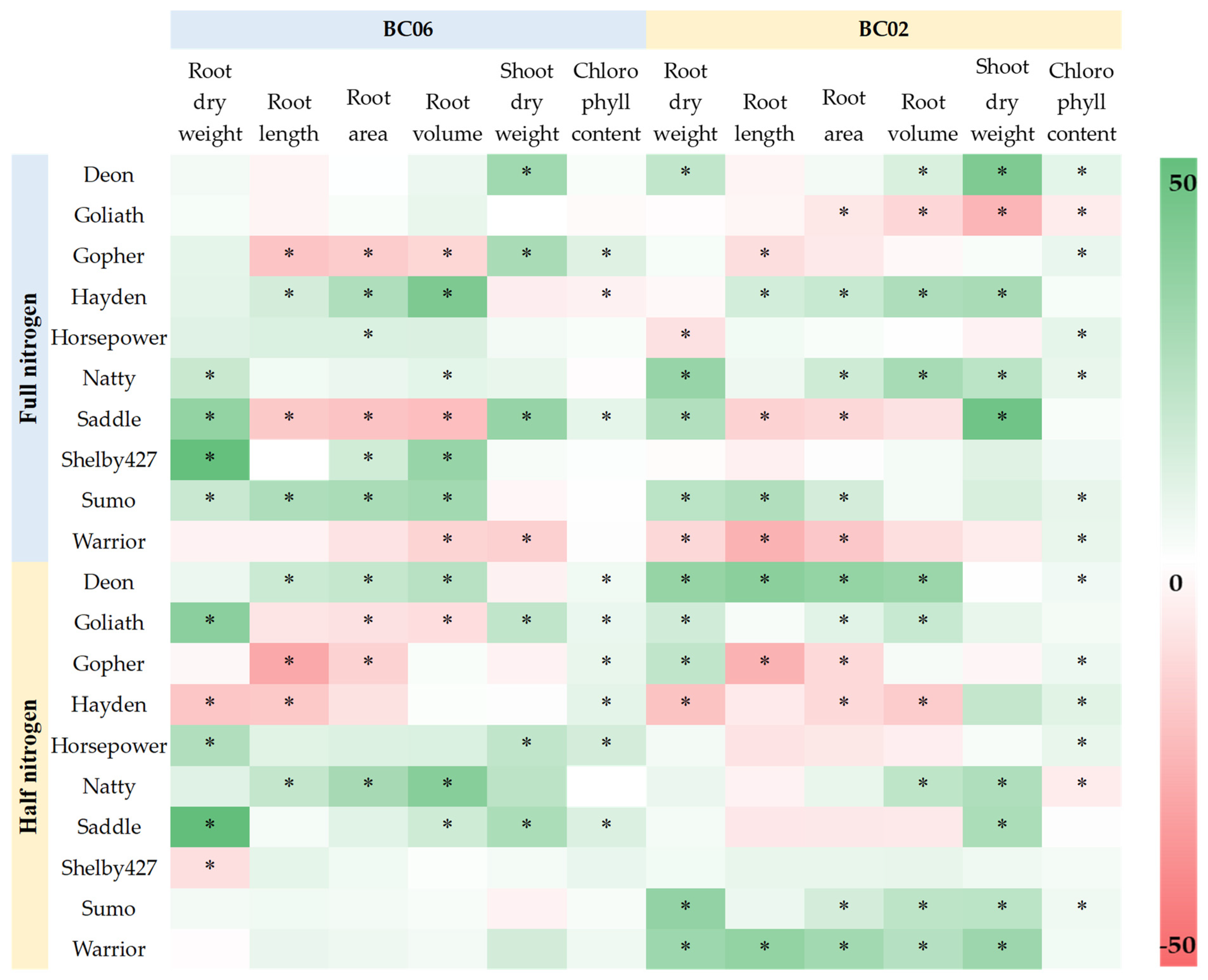
| Bacterial ID | 16S rRNA Taxonomic Identity |
|---|---|
| BC02 | Bacillus licheniformis |
| BC03 | Enterobacter kobei |
| BC04 | Pantoea ananatis |
| BC06 | Enterobacter kobei |
| BC07 | Bacillus pumilus |
| BC08 | Pantoea agglomerans |
| BC09 | Brevibacterium halotolerans |
| BC10 | Bacillus toyonensis |
| BC12 | Bacillus pumilus |
| BC13 | Bacillus pumilus |
| BC14 | Bacillus thuringiensis |
| BC15 | Bacillus cereus |
| BC16 | Bacillus aryabhattai |
| BC17 | Lysinibacillus fusiformis |
| BC19 | Brevibacterium halotolerans |
| BC20 | Pseudomonas spp. |
| Cultivars | Shoot Dry Weight (mg) | Root Dry Weight (mg) | Chlorophyll Content | Root Length (cm) | Root Area (cm2) | Root Volume (cm3) |
|---|---|---|---|---|---|---|
| Deon | 681.4 ± 32.9 b | 188.3 ± 9 de | 55.5 ± 0.9 ab | 407.8 ± 16.7 d | 104.9 ± 3.8 f | 2.2 ± 0.1 de |
| Goliath | 761.1 ± 46.0 ab | 211.4 ± 12.5 cd | 53.9 ± 1.4 abcd | 514.5 ± 21.7 b | 134.9 ± 4.7 b | 2.9 ± 0.2 ab |
| Gopher | 806.9 ± 34.9 a | 222.9 ± 8.5 bc | 50.4 ± 1.2 ef | 649.4 ± 24.9 a | 159.6 ± 5.5 a | 3.2 ± 0.2 a |
| Hayden | 769.8 ± 39.4 ab | 262.7 ± 9.2 a | 55.3 ± 1.1 abc | 486.9 ± 20.7 bc | 129.0 ± 5.2 bc | 2.9 ± 0.2 ab |
| Horsepower | 788.1 ± 32.5 ab | 198.0 ± 9.1 cde | 49.6 ± 1.2 f | 518.4 ± 19.7 b | 125.8 ± 3.2 bcd | 2.5 ± 0.1 cd |
| Natty | 866.9 ± 57.8 a | 243.4 ± 11.9 ab | 52.4 ± 0.9 bcdef | 483.9 ± 18.6 bc | 114.5 ± 4.1 def | 2.2 ± 0.1 de |
| Saddle | 770.6 ± 46.7 ab | 158.1 ± 9.1 f | 52.6 ± 1.1 abcde | 508.5 ± 25.9 bc | 121.5 ± 5.9 cde | 2.7 ± 0.1 cde |
| Shelby427 | 681.6 ± 35.6 b | 177.7 ± 11.8 ef | 51.0 ± 1.2 def | 450.3 ± 20.9 cd | 109.5 ± 3.2 ef | 2.2 ± 0.1 de |
| Sumo | 810.0 ± 33.4 a | 175.5 ± 5.3ef | 55.9 ± 1.0 a | 484.9 ± 24.2 bc | 110.2 ± 2.9ef | 2.1 ± 0.1 e |
| Warrior | 807.7 ± 48.1 a | 201.5 ± 5.1cde | 52.4 ± 1.1 cdef | 466.3 ± 24.2 bcd | 123.7 ± 2.9 bcd | 2.7 ± 0.1 bc |
| Average | 774.4 | 203.9 | 52.9 | 497.1 | 127.2 | 2.5 |
| C.V. (%) | 28.1 | 26.1 | 11.0 | 23.6 | 19.2 | 29.7 |
| Half Fertilization Rate | Full Fertilization Rate | |||||
|---|---|---|---|---|---|---|
| Traits | Mean | Range | Standard Deviation | Mean | Range | Standard Deviation |
| Shoot dry weight (mg) | 736.3 | 163–1437 | 208.6 | 906.6 | 293–1608 | 227.7 |
| Root dry weight (mg) | 200.5 | 68–400 | 57.6 | 231.9 | 97–452 | 61.8 |
| Chlorophyll content | 52.4 | 35.1–69.4 | 5.7 | 56.3 | 34–73.4 | 5.6 |
| Root length (cm) | 469.8 | 178.9–836.2 | 116.5 | 517.7 | 156.2–859.1 | 117.7 |
| Root area (cm2) | 118.3 | 53.2–189.2 | 24.0 | 130.8 | 42.7–217.9 | 25.5 |
| Root volume (cm3) | 2.45 | 1.02–5.38 | 0.72 | 2.72 | 0.93–5.81 | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghimire, K.; Peta, V.; Bücking, H.; Caffe, M. Effect of Non-Native Endophytic Bacteria on Oat (Avena sativa L.) Growth. Int. J. Plant Biol. 2023, 14, 827-844. https://doi.org/10.3390/ijpb14030062
Ghimire K, Peta V, Bücking H, Caffe M. Effect of Non-Native Endophytic Bacteria on Oat (Avena sativa L.) Growth. International Journal of Plant Biology. 2023; 14(3):827-844. https://doi.org/10.3390/ijpb14030062
Chicago/Turabian StyleGhimire, Krishna, Vincent Peta, Heike Bücking, and Melanie Caffe. 2023. "Effect of Non-Native Endophytic Bacteria on Oat (Avena sativa L.) Growth" International Journal of Plant Biology 14, no. 3: 827-844. https://doi.org/10.3390/ijpb14030062
APA StyleGhimire, K., Peta, V., Bücking, H., & Caffe, M. (2023). Effect of Non-Native Endophytic Bacteria on Oat (Avena sativa L.) Growth. International Journal of Plant Biology, 14(3), 827-844. https://doi.org/10.3390/ijpb14030062






