Effects of Biochar on Drought Tolerance of Pinus banksiana Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drought Onset Experiment
2.1.1. Biochar
2.1.2. Water Treatment
2.2. Water Amount Experiment
2.2.1. Biochar
2.2.2. Water Amount Treatment
2.3. Data Collection
2.4. Data Analysis
3. Results
3.1. Drought Onset Experiment
3.2. Water Amount Experiment
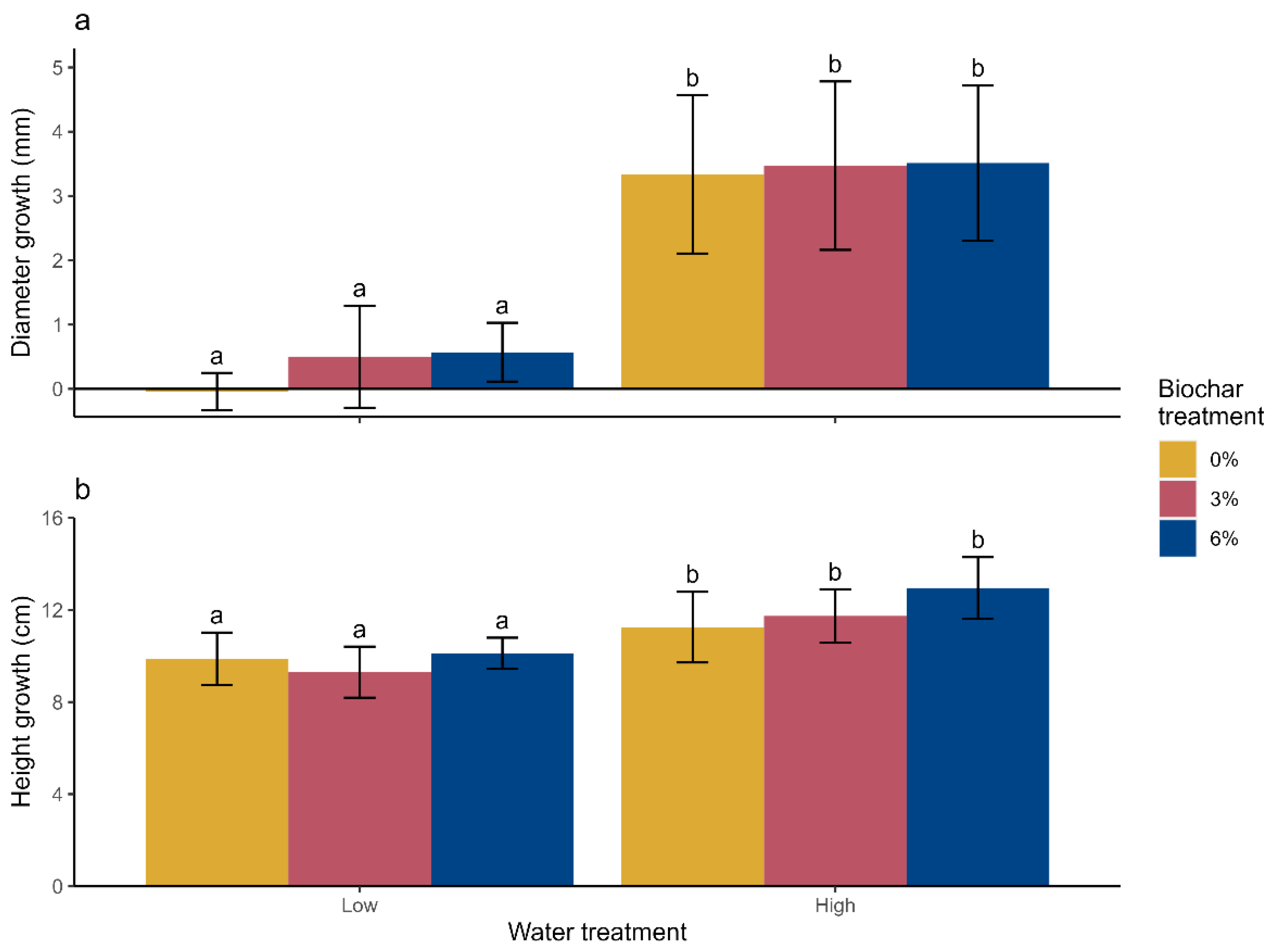
3.2.1. Height and Diameter Growth
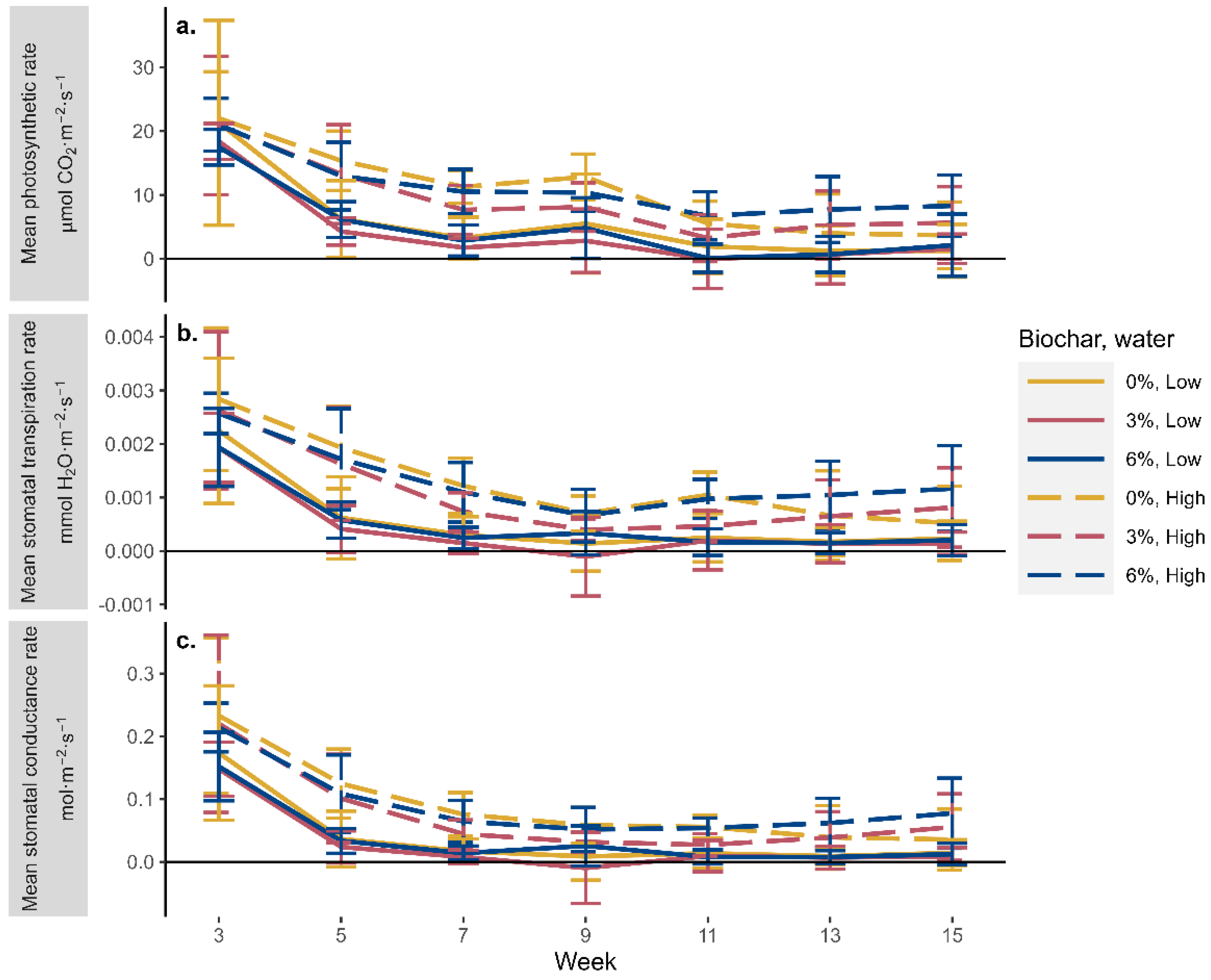
3.2.2. Physiology Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Easterling, D.R.; Arnold, J.R.; Knutson, T.; Kunkel, K.E.; LeGrande, A.N.; Leung, L.R.; Vose, R.S.; Waliser, D.E.; Wehner, M.F. Precipitation Change in the United States. In Climate Science Special Report: Fourth National Climate Assessment, Volume I; Wuebbles, D.J., Fahey, D.W., Hibbard, K.A., Dokken, D.J., Stewart, B.C., Maycock, T.K., Eds.; U.S. Global Change Research Program: Washington, DC, USA, 2017; pp. 207–230. [Google Scholar]
- Vose, J.M.; Clark, J.S.; Luce, C.H.; Patel-Weynand, T. Effects of Drought on Forests and Rangelands in the United States: A Comprehensive Science Synthesis, GTR WO-93b; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 2016.
- Ontl, T.A.; Janowiak, M.K.; Swanston, C.W.; Daley, J.; Handler, S.; Cornett, M.; Hagenbuch, S.; Handrick, C.; McCarthy, L.; Patch, N. Forest management for carbon sequestration and climate adaptation. J. For. 2020, 118, 86–101. [Google Scholar] [CrossRef]
- Niinemets, Ü. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. For. Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Bingham, M.A.; Simard, S.W. Do mycorrhizal network benefits to survival and growth of interior Douglas-fir seedlings increase with soil moisture stress? Ecol. Evol. 2011, 1, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Kaarakka, L.; Cornett, M.; Domke, G.; Ontl, T.; Dee, L.E. Improved forest management as a natural climate solution: A review. Ecol. Solut. Evid. 2021, 2, e12090. [Google Scholar] [CrossRef]
- Basso, A.S.; Miguez, F.E.; Laird, D.A.; Horton, R.; Westgate, M. Assessing potential of biochar for increasing water-holding capacity of sandy soils. Glob. Chang. Biol. Bioenergy 2013, 5, 132–143. [Google Scholar] [CrossRef]
- Richard, R.P.; Potvin, L.R.; Kane, E.S.; Handler, S.D.; Smith, P.J.; Peterson, D. Biochar and wood ash amendments for forestry in the Lake States: Initial results. J. For. 2018, 116, 222–227. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Lehmann, J.; Engelhard, M.H. Natural oxidation of back carbon in soils: Changes in molecular form and surface charge along a climosequence. Geochim. Cosmochim. 2008, 72, 1598–1610. [Google Scholar] [CrossRef]
- Joseph, S.D.; Camps-Arbestain, M.; Lin, Y.; Munroe, P.; Chia, C.H.; Hook, J.; van Zwieten, L.; Kimber, S.; Cowie, A.; Singh, B.P.; et al. An investigation into the reactions of biochar in soil. Aust. J. Soil Res. 2010, 48, 501–515. [Google Scholar] [CrossRef]
- Page-Dumroese, D.S.; Robichaud, P.R.; Brown, R.E.; Tirocke, J.M. Water repellency of two forest soils after biochar addition. Trans. ASABE 2015, 58, 335–342. [Google Scholar]
- Laird, D.A.; Fleming, P.; Davis, D.D.; Horton, R.; Wang, B.; Karlen, D.L. Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 2010, 158, 443–449. [Google Scholar] [CrossRef]
- Juno, E.; Ibánez, I. Biochar application and soil transfer in tree restoration: A meta-analysis and field experiment. Ecol. Restor. 2021, 39, 158–167. [Google Scholar] [CrossRef]
- Grau-Andrés, R.; Pingree, M.R.A.; Öquist, M.G.; Wardle, D.A.; Nilsson, M.C.; Gundale, M.J. Biochar increases tree biomass in a managed boreal forest, but does not alter N2O, CH4, and CO2 emissions. GCB Bioenergy 2021, 13, 1329–1342. [Google Scholar] [CrossRef]
- Palviainen, M.; Aaltonen, H.; Laurén, A.; Köster, K.; Berninger, F.; Ojala, A.; Pumpanen, J. Biochar amendment increases tree growth in nutrient-poor, young Scots pine stands in Finland. For. Ecol. Manag. 2020, 474, 118362. [Google Scholar] [CrossRef]
- Thomas, S.C. Biochar effects on germination and radicle extension in temperate tree seedlings under field conditions. Can. J. For. Res. 2021, 51, 10–17. [Google Scholar] [CrossRef]
- Slesak, R.A.; Kelso, S.G.; Windmuller-Campione, M.A. Effect of biochar and manual vegetation control on early growth and survival of planted jack pine (Pinus banksiana Lamb.) seedlings in northern Minnesota. For. Sci. 2022, 68, 104–112. [Google Scholar] [CrossRef]
- Slesak, R.A.; Windmuller-Campione, M.A. Limited effects of biochar application and periodic irrigation on jack pine (Pinus banksiana) seedling growth in northern Minnesota, USA. Can. J. For. Res. 2023. [Google Scholar] [CrossRef]
- Bieser, J.M.H.; Thomas, S.C. Biochar and high-carbon wood ash effects on soil and vegetation in a boreal clearcut. Can. J. For. Res. 2019, 49, 1124–1134. [Google Scholar] [CrossRef]
- Sarauer, J.L.; Page-Dumroese, D.S.; Coleman, M.D. Soil greenhouse gas, carbon content, and tree growth response to biochar amendment in western United States forests. GCB Bioenergy 2019, 11, 660–671. [Google Scholar] [CrossRef]
- Robertson, S.J.; Michael Rutherford, P.; López-Gutiérrez, J.C.; Massicotte, H.B. Biochar enhances seedling growth and alters root symbioses and properties of sub-boreal forest soils. Can. J. Soil Sci. 2012, 92, 329–340. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Pinto, J.R.; Heiskanen, J.; Tervahauta, A.; McBurney, K.G.; Page-Dumroese, D.S.; Englund, K. Biochar can be a suitable replacement for Sphagnum peat in nursery production of Pinus ponderosa seedlings. Forests 2018, 9, 232. [Google Scholar] [CrossRef]
- Silva, M.I.; Mackowiak, C.; Minogue, P.; Reis, A.F.; da Veiga Moline, E.F. Potential impacts of using sewage sludge biochar on the growth of plant forest seedlings. Cienc. Rural 2017, 47, e20160064. [Google Scholar] [CrossRef]
- Simiele, M.; De Zio, E.; Montagnoli, A.; Terzaghi, M.; Chiatante, D.; Scippa, G.S.; Trupiano, D. Biochar and/or compost to enhance nursery-produced seedling performance: A potential tool for forest restoration programs. Forests 2022, 13, 550. [Google Scholar] [CrossRef]
- Heiskanen, J.; Tammeorg, P.; Dumroese, R.K. Growth of Norway spruce seedlings after transplanting into silty soil amended with biochar: A bioassay in a growth chamber—Short Communication. J. For. Sci. 2013, 59, 125–129. [Google Scholar] [CrossRef]
- Sarauer, J.L.; Coleman, M.D. Biochar as a growing media component for containerized production of douglas-fir. Can. J. For. Res. 2018, 48, 581–588. [Google Scholar] [CrossRef]
- Mulcahy, D.N.; Mulcahy, D.L.; Dietz, D. Biochar soil amendment increases tomato seedling resistance to drought in sandy soils. J. Arid Environ. 2013, 88, 222–225. [Google Scholar] [CrossRef]
- Gullap, M.K.; Severoglu, S.; Karabacak, T.; Yazici, A.; Ekinci, M.; Turan, M.; Yildirim, E. Biochar derived from hazelnut shells mitigates the impact of drought stress on soybean seedlings. N. Z. J. Crop Hortic. Sci. 2022, 1–19. [Google Scholar] [CrossRef]
- Hafeez, Y.; Iqbal, S.; Jabeen, K.; Shahzad, S.; Jahan, S.; Rasul, F. Effect of biochar application on seed germination and seedling growth of Glycine max (L.) merr. under drought stress. Pak. J. Bot. 2017, 49, 7–13. [Google Scholar]
- Yildirim, E.; Ekinci, M.; Turan, M. Impact of biochar in mitigating the negative fffect of drought stress on cabbage seedlings. J. Soil Sci. Plant Nutr. 2021, 21, 2297–2309. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Ibrahim, M.; Riaz, M.; Arif, M.S.; Hafeez, F.; Al-Wabel, M.I.; Shahzad, A.N. Biochar soil amendment on alleviation of drought and salt stress in plants: A critical review. Environ. Sci. Pollut. Res. 2017, 24, 12700–12712. [Google Scholar] [CrossRef]
- Hansen, V.; Hauggaard-Nielsen, H.; Petersen, C.T.; Mikkelsen, T.N.; Müller-Stöver, D. Effects of gasification biochar on plant-available water capacity and plant growth in two contrasting soil types. Soil Tillage Res. 2016, 161, 1–9. [Google Scholar] [CrossRef]
- Mannan, M.A.; Mia, S.; Halder, E.; Dijkstra, F.A. Biochar application rate does not improve plant water availability in soybean under drought stress. Agric. Water Manag. 2021, 253, 106940. [Google Scholar] [CrossRef]
- Fujita, S.; Watanabe, H.; Marozas, V.; Tamai, Y.; Satoh, F.; Koike, T. Effects of biochar and litter on water relations of Japanese black pine (Pinus thunbergii) seedlings. J. For. Res. 2020, 25, 76–82. [Google Scholar] [CrossRef]
- Matt, C.P.; Keyes, C.R.; Dumroese, R.K. Biochar effects on the nursery propagation of 4 northern Rocky Mountain native plant species. Nativ. Plants J. 2018, 19, 14–26. [Google Scholar] [CrossRef]
- Lyu, S.; Du, G.; Liu, Z.; Zhao, L.; Lyu, D. Effects of biochar on photosystem function and activities of protective enzymes in Pyrus ussuriensis Maxim. under drought stress. Acta Physiol. Plant. 2016, 38, 220. [Google Scholar] [CrossRef]
- Lo Piccolo, E.; Becagli, M.; Lauria, G.; Cantini, V.; Ceccanti, C.; Cardelli, R.; Massai, R.; Remorini, D.; Guidi, L.; Landi, M. Biochar as a soil amendment in the tree establishment phase: What are the consequences for tree physiology, soil quality and carbon sequestration? Sci. Total Environ. 2022, 844, 157175. [Google Scholar] [CrossRef]
- Zoghi, Z.; Hosseini, S.M.; Kouchaksaraei, M.T.; Kooch, Y.; Guidi, L. The effect of biochar amendment on the growth, morphology and physiology of Quercus castaneifolia seedlings under water-deficit stress. Eur. J. For. Res. 2019, 138, 967–979. [Google Scholar] [CrossRef]
- Heydari, M.; Hajinia, S.; Jafarian, N.; Karamian, M.; Mosa, Z.; Asgharzadeh, S.; Rezaei, N.; Guidi, L.; Valkó, O.; Prévosto, B. Synergistic use of biochar and the plant growth-promoting rhizobacteria in mitigating drought stress on oak (Quercus brantii Lindl.) seedlings. For. Ecol. Manag. 2023, 531, 120793. [Google Scholar] [CrossRef]
- Licht, J.; Smith, N. The influence of lignocellulose and hemicellulose biochar on photosynthesis and water use efficiency in seedlings from a Northeastern U.S. pine-oak ecosystem. J. Sustain. For. 2018, 37, 25–37. [Google Scholar] [CrossRef]
- Ashton, M.S.; Kelty, M.J. The Practice of Silviculture: Applied Forest Ecology; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Handler, S.; Marcinkowski, K.; Janowiak, M.; Swanston, C. Climate Change Field Guide for Northern Minnesota Forests: Site-Level Considerations and Adaptation, USDA Northern Forests Climate Hub Technical Report #2; University of Minnesota, College of Food, Agricultural, and Natural Resource Sciences: St. Paul, MN, USA, 2017. [Google Scholar]
- Li, L.; Zhang, Y.J.; Novak, A.; Yang, Y.; Wang, J. Role of biochar in improving sandy soil water retention and resilience to drought. Water 2021, 13, 407. [Google Scholar] [CrossRef]
- Handler, S.; Duveneck, M.J.; Iverson, L.; Peters, E.; Scheller, R.M.; Wythers, K.R.; Brandt, L.; Butler, P.; Janowiak, M.; Shannon, P.D.; et al. Minnesota Forest Ecosystem Vulnerability Assessment and Synthesis: A Report from the Northwoods Climate Change Response Framework Project; United States Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2014. [Google Scholar]
- Rudolph, T.D.; Laidly, P.R. Pinus banksiana Lamb. Jack Pine. In Silvics of North America, Volume 1; Agriculture Handbook, 654; Burns, R.M., Honkala, B.H., Eds.; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1990; pp. 280–293. [Google Scholar]
- NOAA National Centers for Environmental Information. Daily Summary Data for Cloquet, Minnesota USA. Available online: https://www.ncdc.noaa.gov/cdo-web/ (accessed on 26 July 2023).
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–41. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Pinheiro, J.; Bates, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Toczydlowski, A.J.Z.; Slesak, R.A.; Venterea, R.T.; Spokas, K.A. Pyrolysis temperature has greater effects on carbon and nitrogen biogeochemistry than biochar feedstock when applied to a sandy forest soil. For. Ecol. Manag. 2023, 534, 120881. [Google Scholar] [CrossRef]
- Zhao, P.; Palviainen, M.; Köster, K.; Berninger, F.; Bruckman, V.J.; Pumpanen, J. Effects of biochar on fluxes and turnover of carbon in boreal forest soils. Soil Sci. Soc. Am. J. 2019, 83, 126–136. [Google Scholar] [CrossRef]
- Thiel, D.; Nagy, L.; Beierkuhnlein, C.; Huber, G.; Jentsch, A.; Konnert, M.; Kreyling, J. Uniform drought and warming responses in Pinus nigra provenances despite specific overall performances. For. Ecol. Manag. 2012, 270, 200–208. [Google Scholar] [CrossRef]
- Taeger, S.; Sparks, T.H.; Menzel, A. Effects of temperature and drought manipulations on seedlings of Scots pine provenances. Plant Biol. 2015, 17, 361–372. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, F.; Gou, X.; Fonti, P.; Xia, J.; Cao, Z.; Liu, J.; Wang, Y.; Zhang, J. Seasonal variations in leaf-level photosynthesis and water use efficiency of three isohydric to anisohydric conifers on the Tibetan Plateau. Agric. For. Meteorol. 2021, 308–309, 108581. [Google Scholar] [CrossRef]
- Brix, H. Effects of plant water stress on photosynthesis and survival of four conifers. Can. J. For. Res. 1979, 9, 160–165. [Google Scholar] [CrossRef]
- Sujeeun, L.; Thomas, S.C. Biochar mitigates allelopathic effects in temperate trees. Ecol. Appl. 2023, 33, e2832. [Google Scholar] [CrossRef]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.; Lima, I.M.; Lamb, M.C.; Mcaloon, A.J.; et al. Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef]
- Weng, Z.H.; Van Zwieten, L.; Tavakkoli, E.; Rose, M.T.; Singh, B.P.; Joseph, S.; Macdonald, L.M.; Kimber, S.; Morris, S.; Rose, T.J.; et al. Microspectroscopic visualization of how biochar lifts the soil organic carbon ceiling. Nat. Commun. 2022, 13, 5177. [Google Scholar] [CrossRef]
- Brtnicky, M.; Datta, R.; Holatko, J.; Bielska, L.; Gusiatin, Z.M.; Kucerik, J.; Kintl, A.; Sudoma, M.; Ahmed, N.; Pecina, V. A critical review of the possible adverse effects of biochar in the soil environment. Sci. Total Environ. 2021, 796, 148756. [Google Scholar] [CrossRef]


| Property | Value |
|---|---|
| C (w%) | 84.04 |
| H (w%) | 2.47 |
| N (w%) | 0.48 |
| O (w%) | 7.18 |
| Ash (w%) | 5.83 |
| VM (w%) | 15.06 |
| Fixed C (w%) | 79.12 |
| H:C molar ratio | 0.35 |
| O:C molar ratio | 0.064 |
| pH | 7.36 |
| Conductivity (S/m) | 432 |
| Liming (%CCE) | 3.5 |
| CEC (cmol cations per kg char) | 1.6 |
| Change in Diameter (mm) | Change in Height (cm) | ||
|---|---|---|---|
| Term | Df | p-Value | p-Value |
| Covariate (initial measurement) | 1 | 0.9 | 0.002 |
| Biochar | 2 | 0.515 | 0.076 |
| Water | 1 | <0.001 | <0.001 |
| Biochar * Water | 2 | 0.641 | 0.503 |
| Photo | Trans | Cond | ||
|---|---|---|---|---|
| Model Term | Num df | p-Value | p-Value | p-Value |
| (Intercept) | 1 | <0.0001 | <0.0001 | <0.0001 |
| Week | 6 | <0.0001 | <0.0001 | <0.0001 |
| Biochar | 2 | 0.1292 | 0.7237 | 0.6806 |
| Water | 1 | <0.0001 | <0.0001 | <0.0001 |
| Week * Biochar | 12 | 0.7719 | 0.9668 | 0.83 |
| Week * Water | 6 | 0.1484 | 0.394 | 0.0075 |
| Biochar * Water | 2 | 0.6529 | 0.9517 | 0.8995 |
| Week * Biochar * Water | 12 | 0.9897 | 0.9924 | 0.9414 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reuling, L.F.; Toczydlowski, A.J.Z.; Slesak, R.A.; Windmuller-Campione, M.A. Effects of Biochar on Drought Tolerance of Pinus banksiana Seedlings. Int. J. Plant Biol. 2023, 14, 811-824. https://doi.org/10.3390/ijpb14030060
Reuling LF, Toczydlowski AJZ, Slesak RA, Windmuller-Campione MA. Effects of Biochar on Drought Tolerance of Pinus banksiana Seedlings. International Journal of Plant Biology. 2023; 14(3):811-824. https://doi.org/10.3390/ijpb14030060
Chicago/Turabian StyleReuling, Laura F., Alan J. Z. Toczydlowski, Robert A. Slesak, and Marcella A. Windmuller-Campione. 2023. "Effects of Biochar on Drought Tolerance of Pinus banksiana Seedlings" International Journal of Plant Biology 14, no. 3: 811-824. https://doi.org/10.3390/ijpb14030060
APA StyleReuling, L. F., Toczydlowski, A. J. Z., Slesak, R. A., & Windmuller-Campione, M. A. (2023). Effects of Biochar on Drought Tolerance of Pinus banksiana Seedlings. International Journal of Plant Biology, 14(3), 811-824. https://doi.org/10.3390/ijpb14030060






