Designing Ex Situ Conservation Strategies for Butia capitata [Mart. (Becc.) Arecaceae], a Threatened Palm Tree from Brazilian Savannah Biome, through Zygotic Embryo Cryopreservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Fruit Characterization
2.3. Cryopreservation of Zygotic Embryos
2.4. Acclimatization
3. Results and Discussion
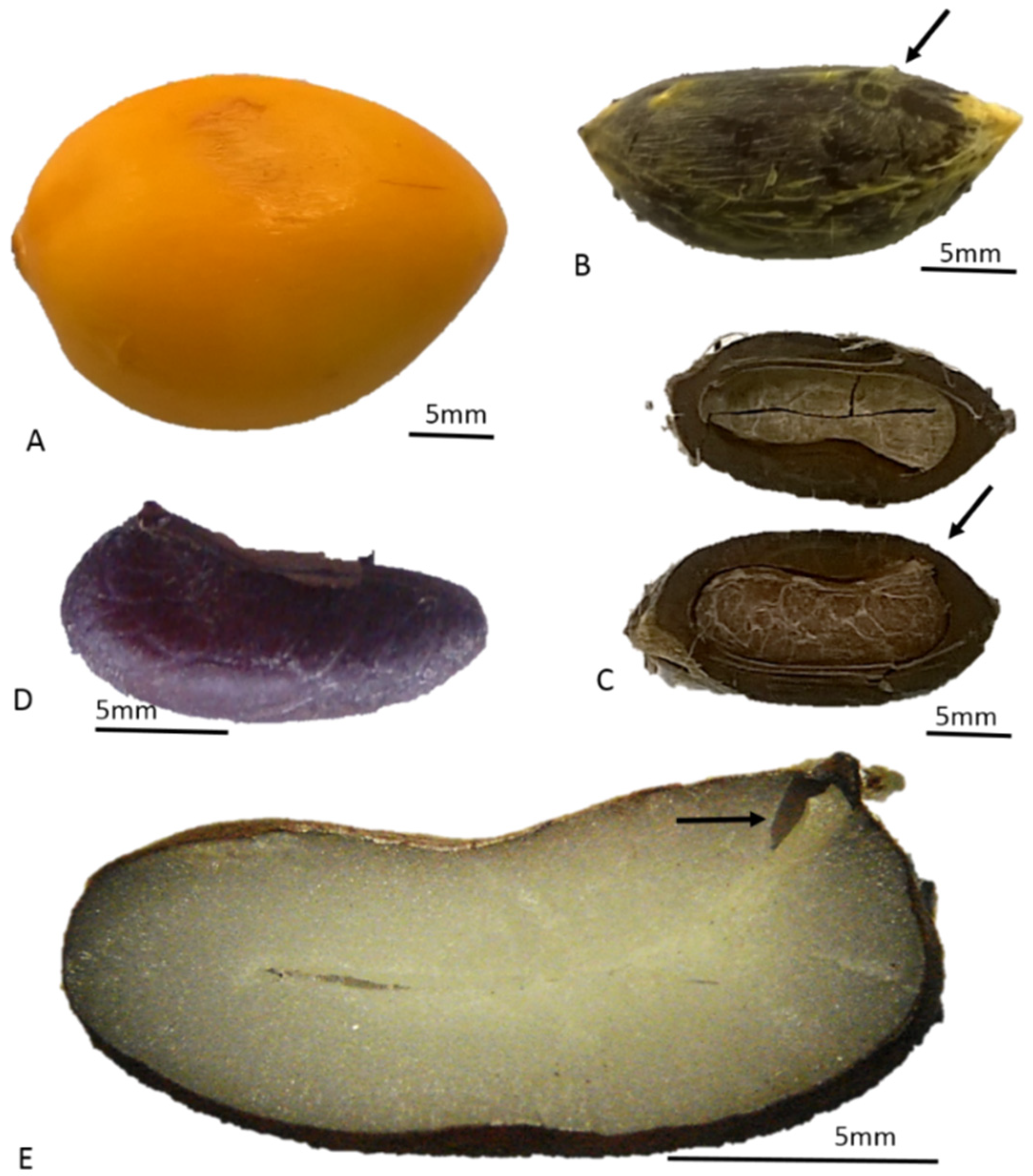
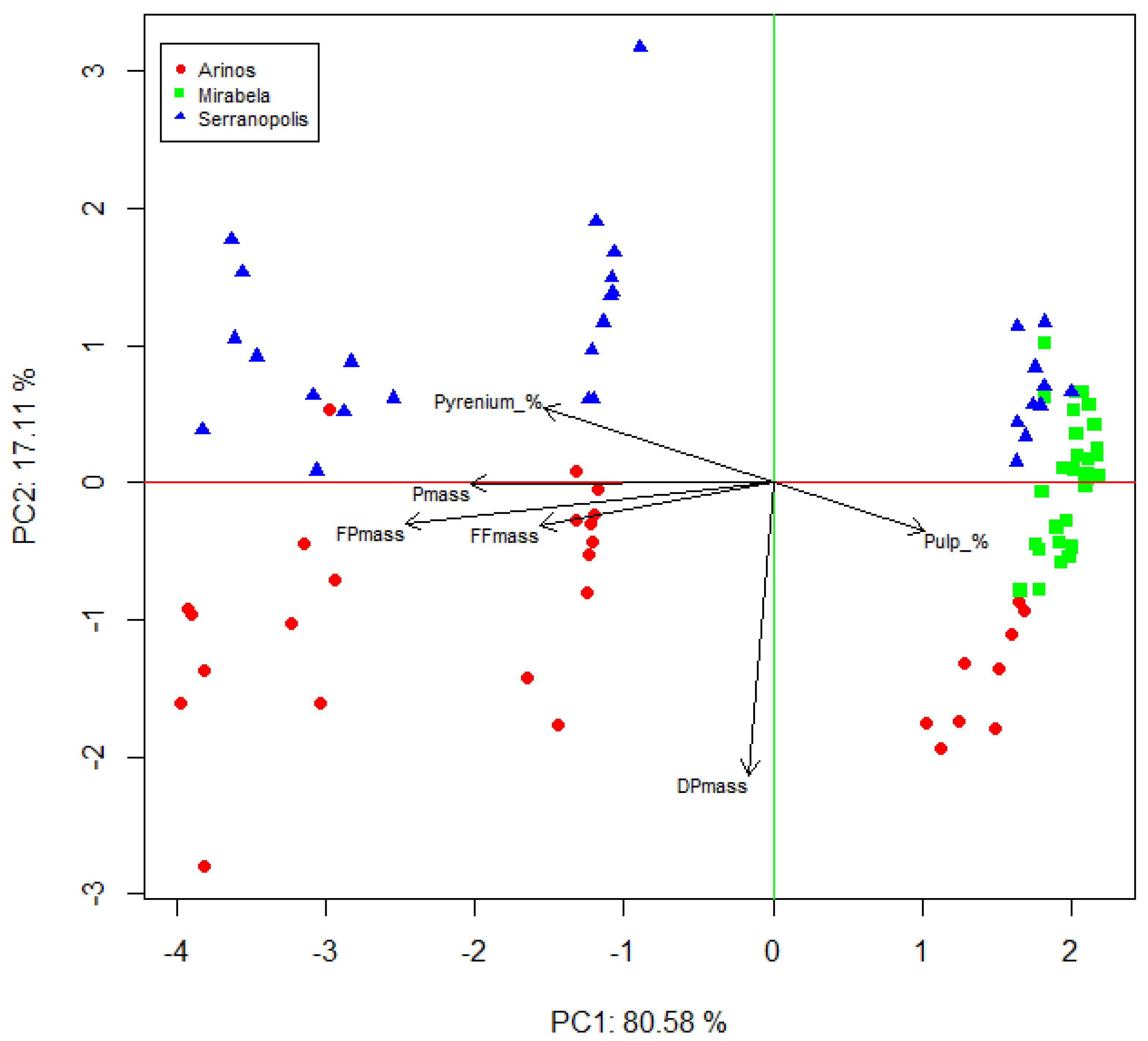
3.1. Fruit Characterization
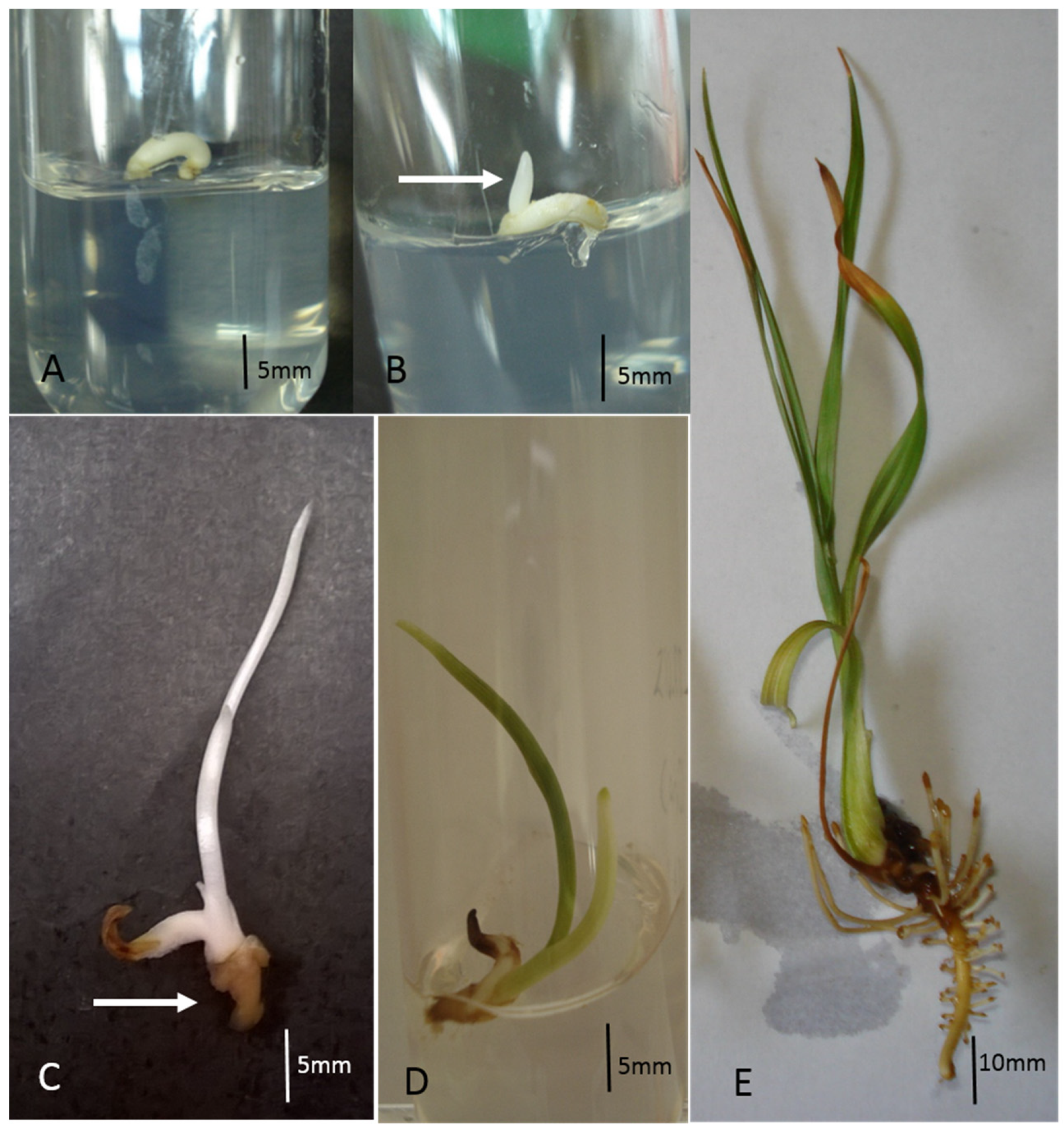
3.2. Cryopreservation of Zygotic Embryos
3.3. Acclimatization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soares, K.P.; Longhi, S.J.A. New Butia (BECC.) BECC. Species (Arecaceae) of Rio Grande do Sul, Brazil. Ciênc. Florest. 2011, 21, 203–208. [Google Scholar] [CrossRef]
- Lorenzi, H.; Sousa, H.; Costa, J.T.M.; Cerqueira, L.S.C.; Ferreira, E. Árvore Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil; Instituto Plantarum: Nova Odessa, Brazil, 2004; p. 432. [Google Scholar]
- Lorenzi, H.; Noblick, L.R.; Kahn, F.; Ferreira, E. Flora Brasileira—Arecaceae (Palmeiras); Instituto Plantarum: Nova Odessa, Brazil, 2010; p. 384. [Google Scholar]
- Lima, V.V.F.; Da Silva, P.A.D.; Scariot, A. Boas Práticas de Manejo Para o Extrativismo Sustentável do Coquinho Azedo; Embrapa Recursos Genéticos e Biotecnologia: Brasília, Brazil, 2010; p. 60. [Google Scholar]
- Mercadante-Simões, M.O.; Fonseca, R.S.; Ribeiro, L.M.; Nunes, Y.R.F. Reproductive biology of Butia Capitata (Mart.) Beccari (Arecaceae) in a savanna area of the north of Minas Gerais. Brazil. Ver. Unimontes Cient 2006, 8, 143–149. [Google Scholar]
- Fior, C.S.; Rodrigues, L.R.; Leonhardt, C.; Schwarz, S.F. Superação de dormência em sementes de Butia capitata. Ciênc. Rural 2011, 41, 1150–1153. [Google Scholar] [CrossRef]
- Lopes, P.S.N.; Aquino, C.F.; Magalhães, H.M.; Júnior, D.S.B. Tratamento físico-químico para superação de dormência em sementes de Butia capitata (Martius) Beccari. Pesq. Agrop. Trop. 2011, 41, 120–125. [Google Scholar] [CrossRef][Green Version]
- Silva, P.A.D.; Scariot, A. Phenology, biometric parameters and productivity of fruits of the palm Butia capitata (Mart.) Beccari in the Brazilian Cerrado in the north of the state of Minas Gerais. Acta Bot Bras. 2013, 27, 580–589. [Google Scholar] [CrossRef]
- Magalhaes, H.M.; Pinheiro, L.R.; Silveira, F.A.; Menezes, M.; Dos Santos, J.B.; Resende, L.V.; Pasqual, M. Genetic diversity of endangered populations of Butia capitata: Implications for conservation. Afr. J. Biotech. 2015, 14, 888–900. [Google Scholar]
- Ribeiro, R.A.; Rodrigues, F.M. Genética da conservação em espécies vegetais do cerrado. Rev. Ciênc. Méd. Biol. 2006, 5, 253–260. [Google Scholar]
- Wang, M.R.; Lambardi, M.; Engelmann, F.; Pathirana, R.; Panis, B.; Volk, G.M.; Wang, Q.C. Advances in cryopreservation of in vitro-derived propagules: Technologies and explant sources. Plant Cell Tissue Organ Cult. 2021, 144, 7–20. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Volk, G.M. Challenges in implementing plant shoot tip cryopreservation technologies. Plant Cell Tissue Organ Cult. 2021, 144, 21–34. [Google Scholar] [CrossRef]
- Benelli, C. Plant Cryopreservation: A Look at the Present and the Future. Plants 2021, 10, 2744. [Google Scholar] [CrossRef]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. An intermediate category of seed storage behaviour? J. Exp. Bot. 1990, 41, 1167–1174. [Google Scholar] [CrossRef]
- Dias, D.S.; Lopes, P.S.N.; Ribeiro, L.M.; Oliveira, L.A.A.; Mendes, E.V.; Carvalho, V.S. Tolerance of desiccation and cryopreservation of Butia capitata palm seeds. Seed Sci. Technol. 2015, 43, 90–100. [Google Scholar] [CrossRef]
- Salomão, N.A.; Santos, I.R.I. Criopreservação de Germoplasma de Espécies Frutíferas Nativas; Documentos 361; Embrapa Recursos Genéticos e Biotecnologia: Brasília, Brazil, 2018; p. 28. [Google Scholar]
- Fior, C.S.; Campos, S.S.; Schwarz, S.F. Tolerância à dessecação e armazenamento em temperatura sub-zero de sementes de Butia odorata (Barb. Rodr.) Noblick. Iheringia 2020, 75, e2020019. [Google Scholar] [CrossRef]
- Engelmann, F. Plant cryopreservation progress and prospects. Vitr. Cell. Dev. Biol. Plant 2004, 40, 427–433. [Google Scholar] [CrossRef]
- Vollmer, R.; Espirilla, J.; Villagaray, R.; Cárdenas, J.; Castro, M.; Sánchez, J.C.; Manrique-Carpintero, N.; Ellis, D.; Anglin, N.L. Cryopreservation of Potato Shoot Tips for Long-Term Storage. Methods Mol. Biol. 2021, 2354, 21–54. [Google Scholar] [CrossRef] [PubMed]
- Chin, H.F.; Krishnapillay, B.; Alang, Z.C. Cryopreservation of Veitchia and Howea palm embryos: Non-development of the haustorium. Cryo Lett. 1988, 9, 372–379. [Google Scholar]
- Gantait, S.; Sinniah, U.R.; Suranthran, P.; Palanyandy, S.R.; Subramaniam, S. Improved cryopreservation of oil palm (Elaeis guineensis Jacq.) polyembryoids using droplet vitrification approach and assessment of genetic fidelity. Protoplasma 2015, 252, 89–101. [Google Scholar] [CrossRef]
- Oliveira, R.A.D.; Neves, S.C.; Ribeiro, L.M.; Lopes, P.S.N.; Silvério, F.O. Storage, oil quality and cryopreservation of babassu palm seeds. Ind. Crops Prod. 2016, 91, 332–339. [Google Scholar] [CrossRef]
- Luis, Z.G.; Scherwinski-Pereira, J.E. A simple and efficient protocol for the cryopreservation of zygotic embryos of macaw palm [Acrocomia aculeata (Jacq.) Lodd. ex Mart.], a tropical species with a capacity for biofuel production. Cryoletters 2017, 38, 7–16. [Google Scholar]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciênc. Agrotecnol. 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F.A. A revised medium for rapid gowth and bioassays with tabaco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Faria, J.P.; Almeida, F.; Silva, L.C.R.; Vieira, R.F.; Agostini-Costa, T.S. Caracterização da Polpa do Coquinho-azedo (Butia capitata var capitata). Rev. Bras. Frut. 2008, 30, 827–829. [Google Scholar] [CrossRef]
- Moura, R.C.; Lopes, P.S.N.; Brandão Junior, D.S.; Gomes, J.G.; Pereira, M.B. Fruit and seed biometry of Butia capitata (Mart.) Beccari (Arecaceae), in the natural vegetation of the North of Minas Gerais, Brazil. Biota. Neotrop. 2010, 10, 415–419. [Google Scholar] [CrossRef]
- Oliveira, N.C.C.; Lopes, P.S.N.; Ribeiro, L.M.; Mercandante-Simões, M.O.; Oliveira, L.A.A.; Silvério, F.O. Seed structure, germination, and reserve mobilization in Butia capitata (Arecaceae). Trees 2013, 27, 1633–1645. [Google Scholar] [CrossRef]
- Pedron, F.A.; Menezes, J.P.; Menezes, N.L. Biometrical parameters of fruit, endocarp and seed of pindo palm. Cienc. Rural 2004, 34, 585–586. [Google Scholar] [CrossRef]
- Rivas, M.; Barilani, A. Diversidad, potencial productivo y reproductivo de los palmares de Butia capitata (Mart.) Becc. de Uruguay. Agrociencia 2004, 8, 11–21. [Google Scholar] [CrossRef]
- Barbosa, R.I.; Lima, A.D.; Mourão Junior, M. Biometria de frutos do Buriti (Mauritia flexuosa L.F.—Arecaceae): Produção de polpa e óleo em uma área de savana em Roraima. Amazônia Ciênc. Desenv. 2010, 5, 71–85. [Google Scholar]
- Reis, R.G.E.; Bezerra, A.M.E.; Gonçalves, N.G.; Magnum, S.P.; Freitas, J.B.S. Biometria e efeito da temperatura e tamanho das sementes na protrusão do pecíolo cotiledonar de carnaúba. Rev. Ciênc. Agron. 2010, 41, 81–86. [Google Scholar]
- Nunes, A.M.; Fachinello, J.C.; Radmann, E.B.; Bianchi, V.J.; Schwartz, E. Caracteres morfológicos e físico-químicos de butiazeiros (Butia capitata) na região de Pelotas, Brasil. Interciência 2010, 35, 500–505. [Google Scholar]
- Hoffmann, J.F.; Barbieri, R.L.; Rombaldi, C.V.; Chavesa, F.C. Butia spp. (Arecaceae): An overview. Sci. Hortic. 2014, 179, 122–131. [Google Scholar] [CrossRef]
- N’nan, O.; Borges, M.; Konan, J.L.K.; Hocher, V.; Verdeil, J.L.; Tregear, J.; Malaurie, B. A simple protocol for cryopreservation of zygotic embryos of ten accessions of coconut (Cocos nucifera L.). In Vitr. Cell. Dev. Biol. Plant 2012, 48, 160–166. [Google Scholar] [CrossRef]
- Sisunandar, S. Cryopreservation for Germplasm Conservation: Progress Report on Indonesian Elite Mutant Coconut “Kopyor”. In Proceeding International Conference on Global Resource Conservation; Universitas Brawijaya: Malang, Indonesia, 2013; pp. 83–87. [Google Scholar]
- Camillo, J.; Luis, Z.G.; Scherwinski-Pereira, J.E. Tolerância de sementes de dendezeiro à criopreservação. Pesq. Agrop. Bras. 2009, 44, 211–215. [Google Scholar] [CrossRef]
- Steinmacher, D.A.; Saldanha, C.W.; Clement, C.R.; Guerra, M.P. Cryopreservation of peach palm zygotic embryos. Cryoletters 2007, 28, 13–22. [Google Scholar] [PubMed]
- Dickie, J.B.; Balick, M.J.; Linington, I.M. Experimental investigations into the feasibility of ex situ preservation of palm seeds: An alternative strategy for biological conservation of this economically important plant family. Biodiv. Cons. 1992, 1, 112–119. [Google Scholar] [CrossRef]
- Martins-Corder, M.P.; Saldanha, C.W. Germinação de sementes e crescimento de plântulas de diferentes progênies de Euterpe edulis Mart. Rev. Árvore 2006, 30, 693–699. [Google Scholar] [CrossRef]
- Lédo, A.S.; Gomes, K.K.P.; Barboza, S.B.S.C.; Vieira, G.S.S.; Tupinambá, E.A.; Aragão, W.M. Cultivo in vitro de embriões zigóticos e aclimatação de plântulas de coqueiro-anão. Pesq. Agrop. Bras. 2007, 42, 147–154. [Google Scholar] [CrossRef][Green Version]
- Angelo, P.C.S.; Moraes, L.A.C.; Lopes, R.; Sousa, N.R.; Cunha, R.N.V.; Quisen, R.C. In vitro rescue of interspecific embryos from Elaeis guineensis x E. oleifera (Arecaceae) Rev. Biol. Trop. 2011, 59, 1081–1088. [Google Scholar]
- Pádua, M.S.S.; Paiva, L.V.; Silva, L.G.T.; Silva, L.C.; Stein, V. In Vitro Development and Acclimatization of Dendezeiro (Elaeis guineensis). Rev. Árvore 2014, 38, 1095–1102. [Google Scholar] [CrossRef]
- Donnelly, D.; Tisdall, L. Acclimatization strategies for micropropagated plants. In Micropropagation of Woody Plants; Ahuja, M.R., Ed.; Kluwer Academic Publishers: Amsterdam, The Netherlands, 1993; pp. 153–166. [Google Scholar] [CrossRef]
- Mohammed, M.; Munir, M.; Ghazzawy, H.S. Design and Evaluation of a Smart Ex Vitro Acclimatization System for Tissue Culture Plantlets. Agronomy 2023, 13, 78. [Google Scholar] [CrossRef]
| Population | Moisture Content * | ||
|---|---|---|---|
| Pulp | Seed | Zygotic Embryo | |
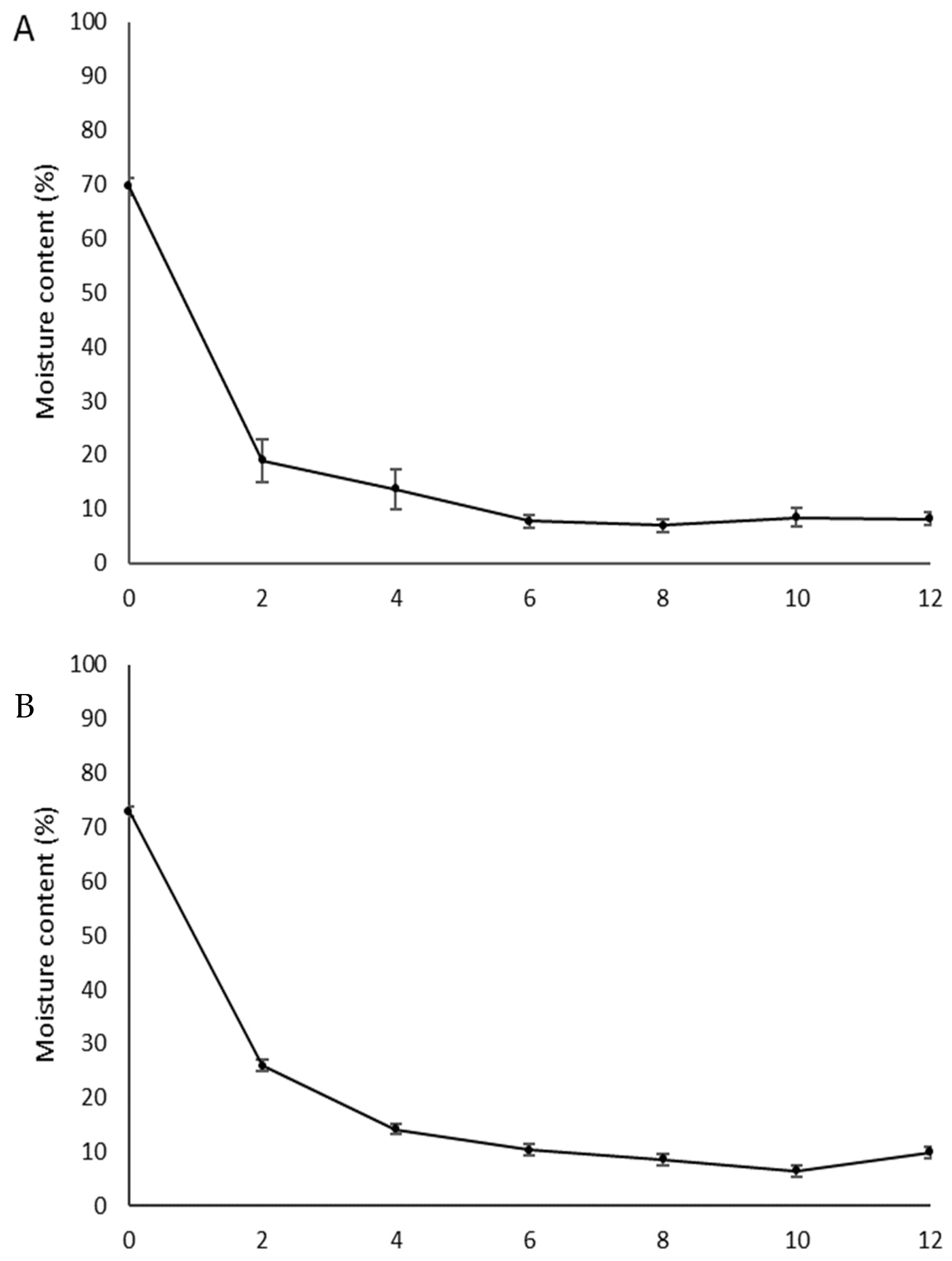
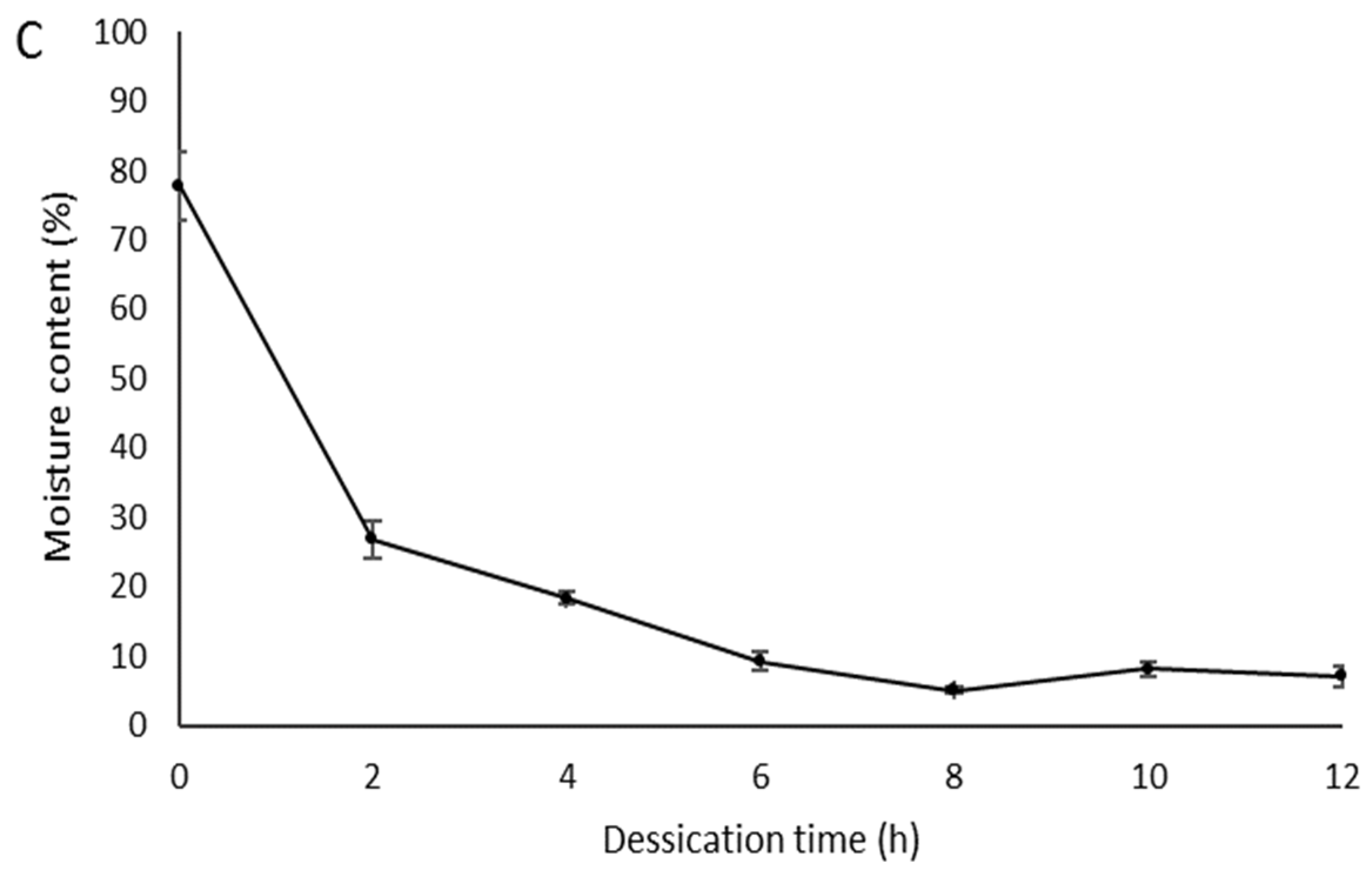
| Arinos | 79.0 ± 2.0 c | 6.2 ± 9.0 a | 18.4 ± 8.0 a |
| Mirabela | 82.4 ± 1.0 b | 5.3 ± 2.0 a | 7.5 ± 8.0 b |
| Serranópolis | 86.2 ± 2.0 a | 6.7 ± 8.0 a | 14.4 ± 8.0 a |
| CV (%) | 2.5 | 18.9 | 30.4 |
| Biometric Parameters | Populations | CV (%) | F | ||
|---|---|---|---|---|---|
| Arinos | Mirabela | Serranópolis | |||
| Fruit length (mm) | 34.3 ± 0.2 A | 26.3 ± 0.1 C | 28.4 ± 0.2 B | 4.7 | 269.1 ** |
| Fruit width (mm) | 22.7 ± 0.2 A | 21.6 ± 0.2 B | 21.6 ± 0.2 B | 6.1 | 6.9 ** |
| Fruit fresh mass (g) | 8.2 ± 1.6 A | 6.5 ± 0.1 B | 6.6 ± 1.6 B | 14.9 | 24.78 ** |
| Pulp thickness (mm) | 4.8 ± 0.07 A | 4.7 ± 0.1 A | 4.8 ± 0.1 A | 12.2 | 0.3 ns |
| Pulp fresh mass (g) | 6.2 ± 0.1 A | 5.1 ± 0.1 B | 4.9 ± 0.1 B | 15.3 | 20.2 ** |
| Pulp dry mass (g) | 1.3 ± 0.03 A | 0.8 ± 0.02 B | 0.6 ± 0.03 C | 18.9 | 91.7 ** |
| Pyrenium length (mm) | 26.4 ± 0.2 A | 21.7 ± 0.2 C | 23.0 ± 0.2 B | 5.5 | 100.5 ** |
| Pyrenium width (mm) | 11.4 ± 0.2 A | 10.8 ± 0.1 B | 11.3 ± 0.1 AB | 7.8 | 4.5 * |
| Pyrenium fresh mass (g) | 2.0 ± 0.5 A | 1.3 ± 0.2 C | 1.6 ± 0.4 B | 21.5 | 24.5 ** |
| Endocarp thickness (mm) | 2.1± 0.07 B | 1.9 ± 0.06 B | 2.4 ± 0.05 A | 15.6 | 11.8 ** |
| Seed length (mm) | 16.0 ± 0.2 A | 12.4 ± 0.1 C | 13.8 ± 0.05 B | 7.7 | 83.2 ** |
| Seed width (mm) | 6.2 ± 0.1 A | 6.3 ± 0.1 A | 5.9 ± 0.1 A | 10.2 | 2.9 ns |
| Zygotic embryo length (mm) | 3.1 ± 0.07 B | 3.3 ± 0.1 AB | 3.5 ± 0.7 A | 13.6 | 4.5 * |
| Zygotic embryo width (mm) | 0.9 ± 0.02 B | 0.8 ± 0.03 B | 0.9 ± 0.03 A | 15.3 | 6.5 ** |
| Population | Desiccation Time (h) | Germination (%) * | Aerial Part Formation (Shoot Only) (%) * | Normal Plant Formation (Complete Plant) (%) * | |||
|---|---|---|---|---|---|---|---|
| (−) LN | (+) LN | (−) LN | (+) LN | (−) LN | (+) LN | ||
| Arinos | 0 | 76.6 ± 8.0 aA | 0.0 bB | 46.6 ± 9.0 aA | 0.0 cB | 46.6 ± 9.0 aA | 0.0 cB |
| 2 | 56.6 ± 9.0 aA | 23 ± 8.0 bB | 30.0 ± 8.0 aA | 16.6 ± 5.0 bcA | 30.0 ± 8.0 aA | 13.3 ± 4.0 bcA | |
| 4 | 70.0 ± 8.0 aA | 86.6 ± 6.0 aA | 50.0 ± 9.0 aA | 50.0 ± 9.0 abA | 40.0 ± 9.0 aA | 50.0 ± 9.0 aA | |
| 6 | 60.0 ± 9.0 aA | 76.6 ± 8.0 aA | 33.3 ± 8.0 aB | 60.0 ± 9.0 aA | 33.0 ± 8.0 aB | 60.0 ± 9.0 aA | |
| 8 | 63.3 ± 9.0 aA | 76.6 ± 8.0 aA | 26.6 ± 7.0 aA | 46.6 ± 9.0 abA | 26.0 ± 7.0 aA | 46.0 ± 9.0 abA | |
| 10 | 70.0 ± 8.0 aA | 80.0 ± 7.0 aA | 46.6 ± 9.0 aA | 60.0 ± 9.0 aA | 46.6 ± 9.0 aA | 60.0 ± 9.0 aA | |
| 12 | 60.0 ± 9.0 aB | 86.6 ± 6.0 aA | 30.0 ± 8.0 aA | 63.3 ± 9.0 aA | 26.0 ± 9.0 aA | 60.0 ± 9.0 aA | |
| Mirabela | 0 | 76.6 ± 7.0 aA | 0.0 cB | 46.6 ± 9.0 aA | 0.0 ± 0 cB | 46.6 ± 9.0 aA | 0.0 ± 0 cB |
| 2 | 56.6 ± 5.0 aA | 40.0 ± 5.0 bA | 30.0 ± 7.0 aA | 10.0 ± 3.0 cA | 30 ± 7.0 aA | 10 ± 3.0 cB | |
| 4 | 70.0 ± 5.0 aA | 43.3 ± 5.0 bB | 50.0 ± 9.0 aA | 26.6 ± 7.0 cbB | 43.3 ± 9.0 aA | 23.3 ± 6.0 bcA | |
| 6 | 60.0 ± 5.0 aB | 93.3 ± 3.0 aA | 33.3 ± 8.0 aB | 66.6 ± 8.0 aA | 30 ± 8.0 aB | 63.3 ± 8.0 aA | |
| 8 | 60.0 ± 5.0 aA | 73.3 ± 4.0 abA | 26.6 ± 7.0 aB | 53.3 ± 9.0 abA | 26.6 ± 7.0 aB | 53.3 ± 9.0 abA | |
| 10 | 70.0 ± 5.0 aA | 66.6 ± 5.0 abA | 46.6 ± 9.0 aA | 46.6 ± 9.0 abA | 43.3 ± 9.0 aA | 46.6 ± 9.0 abA | |
| 12 | 60.0 ± 5.0 aA | 60.0 ± 5.0 abA | 30.0 ± 7.0 aA | 26.6 ± 7.0 cbA | 26.6 ± 7.0 aA | 23.3 ± 7.0 bcA | |
| Serranópolis | 0 | 86.6 ± 6.0 aA | 0.0 cB | 73.3 ± 7.0 aA | 0.0 ± 0 cB | 70 ± 7.0 aA | 0.0 ± 0 cB |
| 2 | 70.0 ± 8.0 aA | 36.5 ± 9.0 bB | 56.6 ± 9.0 aA | 16.6 ± 5.0 cbB | 56.6 ± 9.0 aA | 16.6 ± 5.0 bcB | |
| 4 | 70.0 ± 8.0 aA | 76.6 ± 8.0 aA | 56.6 ± 9.0 aA | 56.6 ± 8.0 aA | 53.3 ± 9.0 aA | 56.6 ± 8.0 aA | |
| 6 | 83.3 ± 7.0 aA | 60.0 a ± 9.0 bB | 60.0 ± 9.0 aA | 50 ± 9.0 abA | 56.6 ± 9.0 aA | 43.3 ± 8.0 abA | |
| 8 | 86.6 ± 6.0 aA | 73.3 ± 8.0 aA | 76.6 ± 7.0 aA | 66.6 ± 8.0 aA | 73.3 ± 7.0 aA | 63.3 ± 8.0 aA | |
| 10 | 63.3 ± 9.0 aA | 70.0 ± 8.0 aA | 50.0 ± 9.0 aA | 63.3 ± 8.0 aA | 46.6 ± 8.0 aA | 63.3 ± 8.0 aA | |
| 12 | 73.3 ± 8.0 aA | 60.0 a ± 9.0 bA | 66.6 ± 8.0 aA | 66.6 ± 9.0 aA | 66.6 ± 9.0 aA | 50 ± 9.0 abA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frugeri, G.C.; Nogueira, G.F.; Souza, A.L.X.d.; Scherwinski-Pereira, J.E. Designing Ex Situ Conservation Strategies for Butia capitata [Mart. (Becc.) Arecaceae], a Threatened Palm Tree from Brazilian Savannah Biome, through Zygotic Embryo Cryopreservation. Int. J. Plant Biol. 2023, 14, 612-624. https://doi.org/10.3390/ijpb14030047
Frugeri GC, Nogueira GF, Souza ALXd, Scherwinski-Pereira JE. Designing Ex Situ Conservation Strategies for Butia capitata [Mart. (Becc.) Arecaceae], a Threatened Palm Tree from Brazilian Savannah Biome, through Zygotic Embryo Cryopreservation. International Journal of Plant Biology. 2023; 14(3):612-624. https://doi.org/10.3390/ijpb14030047
Chicago/Turabian StyleFrugeri, Giuliano Carvalho, Gabriela Ferreira Nogueira, André Luís Xavier de Souza, and Jonny Everson Scherwinski-Pereira. 2023. "Designing Ex Situ Conservation Strategies for Butia capitata [Mart. (Becc.) Arecaceae], a Threatened Palm Tree from Brazilian Savannah Biome, through Zygotic Embryo Cryopreservation" International Journal of Plant Biology 14, no. 3: 612-624. https://doi.org/10.3390/ijpb14030047
APA StyleFrugeri, G. C., Nogueira, G. F., Souza, A. L. X. d., & Scherwinski-Pereira, J. E. (2023). Designing Ex Situ Conservation Strategies for Butia capitata [Mart. (Becc.) Arecaceae], a Threatened Palm Tree from Brazilian Savannah Biome, through Zygotic Embryo Cryopreservation. International Journal of Plant Biology, 14(3), 612-624. https://doi.org/10.3390/ijpb14030047






