Abstract
Salinity stress is among the key challenges for sustainable food production. It is continuously increasing against the backdrop of constant climate change and anthropogenic practices leading to a huge drop in soil, water, and cultivated crop quality and productivity. Halotolerant plants represent hot spots for endophytic bacteria which may have mechanisms to overcome salt stress. This research initiative aims to highlight the possible exploitation of bacterial endophytes as a microbial biotechnology tool in the productive success of plants exposed to saline stress. We started by solely studying the mechanisms of stress tolerance by plants and halotolerant bacteria. After that, we focused on the beneficial mechanisms of endophytic bacteria in salt stress mitigation. On one side, potent bacterium works by promoting plant performances by facilitating the plant’s nutrient uptake (P, K, Zn, N, and Fe) and by promoting the production of growth hormones (IAA and CKs). On the other side, they balance stress phytohormones (ABA, JA, GA, and ACC) produced by plants in case of soil salt augmentation. The selected potent endophytic bacteria could be exploited and applied to ameliorate the production and salt tolerance of food crops. Lastly, we elucidated deeper advanced technologies including (i) genomics unveiling the plant’s culture-dependent and culture-independent microbiomes, (ii) metabolomics focusing on genes’ metabolic pathways to discover novel secondary metabolites, (iii) transcriptomics studying gene expression, and (iv) proteomics delimiting proteins expressed in stress alleviation. These technologies have been used to understand the plant–bacterial mechanisms of interaction to combat salt stress.
1. Introduction
The human population is projected to reach around 10 billion people within the next 30 years [1,2,3]. This inflation of population threatens global food security and human nutrition, especially since agricultural production is subjected to multiple environmental factors, such as salinity [4], temperature [5], drought [6], presence of toxic metals, and/or organic contaminants among various other stresses [3]. Excess salt concentrations affect 7% of the world’s land involving 20% of cultivable fields and approximately 70% of dry land [7,8]. Salt accumulation is increasing constantly owing to anthropogenic practices and global warming coupled with natural disasters [9,10,11], leading to a huge drop in soil, water, and cultivated crop quality and productivity [12]. Excess in Na+, Ca2+, Mg2+, and SO42− ions and alkaline pH constitutes a prevalent indicator of a hypersaline environment [13,14,15]. Soil salinization is often measured by calculating electrical conductivity (EC), and when it exceeds 4 deci-Siemens per meter (dS × m−1) the soil is considered saline [16]. Manishankar et al. [17] cited in their review that, depending on the soil type, the increase in sodium concentrations is responsible for modifying the soil texture by decreasing its dispersion, porosity, and permeability to air, water, and fertilizers [17]. Likewise, depending on the plant species and the growth stage, it may be affected by ionic and osmotic stresses (~200 mM NaCl) sometimes leading to its subsequent mortality [18,19,20,21]. The osmotic stress alters the cell’s water content; it is triggered by plants immediately after excess NaCl detection. Ionic stress takes place days after NaCl occurrence; it depends on the frequency of Na+ and Cl− ions accumulated inside the plant cells [22]. Spontaneously growing plants in saline biotopes are known as halophytes and they developed various mechanisms of adaptation to high-salt concentrations (>400 mM) [23,24]. The common processes of soil desalinization by halophytic plants are uptake, accumulation, and/or exclusion of excess salts, maintaining ionic balance using Na+/K+ transporters, lowering the transpiration rate, hydraulic conductance, and stomatal openings, as well as the expression of genes responsible for salt stress alleviation [25,26]. Thus, to conduct healthy, cost-effective, and biological salt-tolerant agriculture, it is important to use naturally salt-tolerant plants and/or to exploit the plant’s associated rhizospheric and endophytic microorganisms in order to enhance endogenous and exogenous plant salt stress tolerance [24,27,28]. These beneficial microbes promote host plant tolerance to soil salinity, increase soil fertility, promote plant growth, and eliminate excess salt in their host plants [29,30]. The mechanisms used by these microorganisms to induce tolerance are osmotic balance, compatible solutes synthesis, exopolysaccharides production, a lipidic layer of Gram-negative bacteria, bacterial consortium interactions, and genetic improvement/modifications of secondary metabolites by regulating the expression of plant stress genes [31,32,33,34,35,36,37,38,39,40] as will be further explored throughout this review.
2. Effects of Salinity on Plants
Plants subjected to high concentrations of salt show several symptoms at all stages of their growth [41]. The morphological reduction of plant growth rate may be caused by a tremendous decrease in the root and leaf surfaces leading to a deficiency in water and nutrient assimilation and a disturbance in photosynthesis, respectively [42,43]. Halophytic plants have an astonishing ability to cope with extreme hypersaline environments contrary to glycophytes being salt sensitive. Vaishnav et al. [22] stated that most vegetable plants are glycophytes and most cereals and legumes are halophytes (Figure 1).
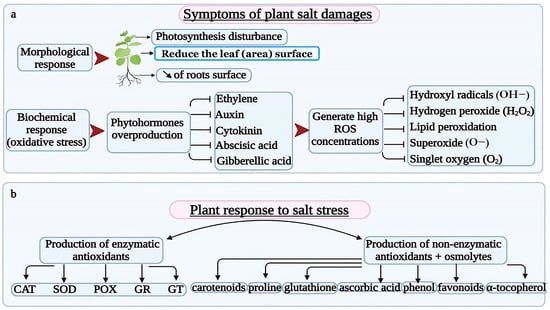
Figure 1.
Plant salt stress repercussions and mechanisms of salt alleviation. (a) symptoms of plant salt damage. (b) plant responses to salt stress.
The plant’s response to salt stress starts with discerning stress signals via membrane receptors. These signals act harmonically to alleviate harmful salt concentrations. The study of biochemical responses gives an in-depth knowledge of the plant’s immune response to salt stress. For instance, oxidative stress is activated in case of anionic and osmotic damage [44,45]. It induces changes in the plant’s physiological response by producing phytohormones, namely ethylene, auxin, cytokinin, gibberellic, and abscisic acids [46,47]. These plant disorders generate high concentrations of ROS (Reactive Oxygen Species) including hydroxyl radicals (OH−), hydrogen peroxide (H2O2), lipid peroxidation, superoxide (O−), and singlet oxygen (O2) which are responsible for plant cells, proteins, and DNA damages [48,49,50]. ROS overproduction occurs in mitochondria, chloroplasts, peroxisomes, and apoplast organs [51]. When they exceed the threshold level, ROS become harmful to plant organs, tissues, and principal cell constituents (proteins, lipids, chlorophylls, nucleic acids, etc.) [52,53]. Stressed plants produce antioxidant compounds to lower ROS concentrations by breaking down and eliminating free radicals [54,55,56]. Enzymatic antioxidants include catalase (CAT), superoxide dismutase (SOD), peroxidases (POX), glutathione reductase (GR), and glutathione transferase (GT) [57,58,59]. They work in an organized system to alleviate oxidative damage caused by ROS [60,61,62]. Other non-enzymatic antioxidants and osmolytes, namely carotenoids, proline, glutathione, ascorbic acid, phenols, flavonoids, and α-tocopherol are involved in ROS scavenging by promoting osmotic balance and preserving the plant’s protein structures [63,64,65]. These resistance mechanisms work together to improve plant adaptation to salt damage [66,67] (Figure 1).
3. Effects of Salinity on Plant Microbiomes
Salt stress shapes rhizospheric and resident endophytic microbiota in plants [68]. Therefore, numerous studies have targeted the microbiomes of halophytes and glycophytes revealing a unique microbiome population that is selected by plants grown under salt stress [69,70]. Salinity has also been documented to be among the main factors regulating the bacterial community associated with the roots of halophytes [71]. The microbiomes of plants under salt stress are supposed to provide candidates that can help efforts to counteract salt stress’s harmful effects on cultivated crops [70].
4. Genes Involved in Plant Protection against Salt
Plant tolerance to salinity has been linked to the presence in their genomes of genes that limit salt uptake by the roots from the soil, limit salt transport throughout the plant, adjust ionic and osmotic balance in plant cells, and regulate the onset of senescence [72]. Therefore, genes that control sodium uptake and transport regulation have been documented in rice [73]. In lentil salt stress, tolerance has been linked to genes that enhance proline accumulation and modulate photosynthetic traits [74]. Numerous genes for salt stress tolerance are now being isolated from halophytes, glycophytes, and plants’ wild relatives [70,75,76].
5. Bacterial Adaptation to High Salt Levels
Bacteria are endowed with various acclimatization mechanisms to hypersaline conditions [5]. For example, osmotic pressure is a phenomenon occurring between bacterial intracellular and extracellular mediums to maintain an equivalent ion efflux across the cellular membrane [42,67]. When bacteria absorb extra amounts of salt, Na+ ions remain blocked in the extracellular medium until achieving an osmotic balance [31]. Otherwise, several bacteria could accumulate and/or synthesize compatible solutes including amino acids with their derivatives (proline, betaine, choline, glutamate, etc.), polyols (mannitol, sorbitol, glycerol, etc.), and sugars in huge amounts [32,33]. These compatible solutes do not have a specific charge, and they do not interfere with osmosis. Instead, they act by increasing the volume of cytoplasm and water in bacteria allowing them to grow and tolerate extreme conditions [77,78,79]. Certain groups of bacteria produce exopolysaccharides (EPS) to form biofilms responsible for maintaining bacterial moisture in case of high NaCl concentrations [36]. Furthermore, the lipid layer existing in the membrane of Gram-negative bacteria helps them improve salinity tolerance [37]. Numerous scientists have also demonstrated that the synergetic interactions in a bacterial consortium are a powerful tool for salt stress mitigation [38]. The genetic improvement/modification of secondary metabolites responsible for salt stress toleration in halotolerant bacteria helps improve their adaptability [2]. Other mechanistic lines of bacterial salt resistance are still not fully understood because endophytic metabolites are constantly changing depending on internal and external conditions [36] (Figure 2).
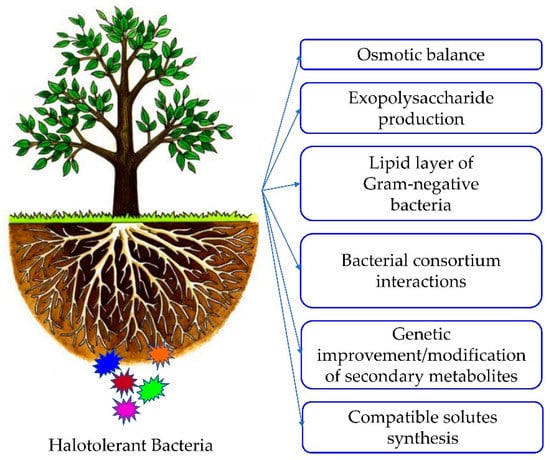
Figure 2.
Mechanisms of bacterial adaptation to high salt levels.
6. Plants–Endophytes–Salt Interactions
Apart from the plant and bacteria’s separate mechanisms of salt adaptation, plants could improve their salt stress tolerance through symbiotic associations with rhizospheric and endophytic bacterial communities [23,27,37]. Soil salinity affects rhizosphere communities and creates a natural selection pressure that is positive for halotolerant bacteria [39]. Soil salinity affects rhizospheric communities and creates a positive selection of halotolerant bacteria. This specific group has a significant role in improving both soil and plant health [80].
The rhizosphere is the primary source of endophytes [27], where competitive bacteria are attracted by plant exudates engaging only powerful and beneficial bacteria [39,81]. Bacterial entrance into the host plant occurs either by the secretion of specific enzymes such as cellulase and pectinase which allows them to break down the root cortex [28], or by wounds existing naturally during the propagation of secondary roots and/or caused by phytopathogens [82]. The most common definition of endophytic bacteria includes all those that, during part or all of their life cycle, invade the internal tissues of plants (leaves, flowers, seeds, stems, and roots) without causing disease and that can confer benefits to their host [83]. For a more detailed definition and history of endophytes, I invite you to read the book chapter in Slama et al. [30]. Shastry et al. [84] reported that the endophytic Enterobacter cloacae gene Ghats I encodes pectinesterases and cellulases enzymes having a crucial role in bacterial entry inside the host plant’s tissues [84].
Rhizospheric and endophytic bacteria positively implicate their associated plants using similar mechanisms [82,85]. However, endophytes have attracted the greatest attention due to their direct interaction with the host plant, less competition with other microbes, and better performance to remove biotic and abiotic stresses [86,87]. In this mutual interaction, plants benefit from bacterial abilities by enhancing nutrient availability and uptake apart from the amelioration of their adaptive and immune systems [48]. Bacteria benefit from available nutrients and protection from external aggressions [29].
Considerable attention has been recently dedicated to applicable projects focusing on the screening of bacterial endophytes as natural biofertilizers and plant protectors [24,28]. In the case of salt stress, it is preferable to use halophytic plants growing in saline environments [88,89]. They constitute the best model for the isolation of bacterial endophytes helping in the elimination of excess salt concentrations [24,90,91,92,93]. The isolated halotolerant bacteria could be inoculated to non-halophytic and/or domestic crops to help them cope with excess salt problems [39,94,95]. It is an effective, eco-friendly, economical, and safe approach [96,97,98]. For instance, the endophytic Enterobacter sp. isolated from the halophytic plant Psoralea corylifolia L. enhanced the seed vigor index and salt tolerance of the non-host plant Triticum aestivum [97]. Sun et al. [37] used the endophytic bacterium Pantoea alhagi NX-11 to mitigate salt concentrations of rice seedlings. Similarly, Pantoea agglomerans conferred salt stress resistance of durum wheat and improved its growth in such a harsh environment [45] (Figure 3). An in silico study conducted by Dąbrowska et al. [98] of the RSH (RelA/SpoT Homologs) Gene family and expression analysis in response to PGPR bacteria and salinity in Brassica napus provide a strong basis for future studies targeting plant salt tolerance. The strain Staphylococcus epidermis (P-30) proved effective under 20% salt concentration and was endowed with multiple PGP potential [99,100]. In another study, the strain Brevibacterium linens RS16 was able to enhance rice salt resistance [101].
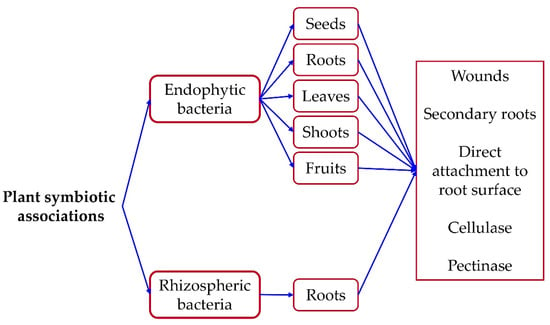
Figure 3.
Mechanisms of bacterial penetration inside plant tissues.
7. Diversification of Endophytic Bacteria Colonizing Halotolerant Plants
7.1. Endophytic Lifestyle and Taxonomic Diversification
Endophytic bacterial communities are strongly diversified depending on their lifestyle into obligate and facultative categories [102]. Obligate bacteria are those connected to plants during their entire lifespan. They are related to the host plant in nutrition and they are transmitted by seeds or vectors only [103]. Facultative bacteria are more autonomous, they could grow inside and outside the host plant without being affected [104]. In most cases, obligate endophytes are culture-independent unlike facultative bacteria being culture-dependent [39].
Saline soil comprises a microbiome belonging to the domains of bacteria, archaea, and eukarya (fungi). Particularly, the domain of bacteria dominates due to its high halotolerant bacterial diversification [105]. The most prevalent bacterial phyla in host plants are Proteobacteria (~50%) comprising the bacterial genera Pseudomonas 42P4 and Enterobacter 64S1 alleviating tomato saline stress [106], Firmicutes (~10%) such as Bacillus sp. isolated from mangrove trees, which improved the percentage of germination and seedling growth when inoculated to rice plants [107] and halotolerant Bacillus subtilis Y16 which enhanced Helianthus annuus L. to manage salt stress [108], Bacteroidetes (~10%) including the Flavobacterium crocinum HYN0056T species having an upregulation potential of salt and drought stresses of Arabidopsis [109], and Actinobacteria (~10%) namely Streptomyces jiujiangensus isolated from mangrove trees promote the growth of Oryza Sativa seedlings subjected to 200 mM NaCl salinity [110].
7.2. Factors Influencing the Bacterial Colonization
The endophytic bacterial diversification is mainly influenced by the host plant’s species, physiological status growth stage, and surrounding environment [111]. For instance, Frank et al. [112] illustrated that the endophytic composition varies based on the type of colonized plant tissue, where a remarkable difference could be noticed between species colonizing underground or aerial plant tissues. Plants growing in soil containing high-salt concentrations recruit endophytic bacteria with the capacity to tolerate and alleviate such stress [24]. Borruso et al. [113] conveyed that in harsh environments, the identity of the plant species plays a minor role when compared to the soil contribution in shaping the endophytic communities. However, Szymańska et al. [114] demonstrated that the bacterial composition of a halophytic plant (Salicornia europaea) was not affected by high salt levels in the soil.
8. Endophytic Bacterial Mechanisms of Salt Mitigation
On the one hand, endophytic bacteria improve crop growth and yield, and, on the other, stimulate plant defense mechanisms [115,116]. The following section will give a better understanding of endophytic bacterial strategies to assist their host plant in enhancing salt mitigation.
8.1. Nutrient Uptake Amelioration under Salt Stress
Bacterial mechanisms to ameliorate plant performances at high-salt concentrations involve the contribution of essential nutrient acquisition and the stimulation of plant biomass production. For instance, zinc (Zn), phosphate (P), and potassium (K) exist mostly in insoluble forms in the soil [24,28]. Therefore, bacterial nutrient solubilization is crucial for plant growth [117]. Various bacterial genera ensured P, Zn, and K solubilization. They include Bacillus, Azotobacter, Pantoea, Pseudomonas, Proteus, Providencia, Serratia, Klebsiella, Enterobacter, Acidothiobacillus, Paenibacillus, and many other genera [15,27,118,119]. Nitrogen (N) is another fundamental element for plants. Biological N-fixation occurs by transforming the atmospheric nitrogen into a plant-assimilable form [7]. Rhizobium is the most well-known bacterial genera responsible for N-fixation, and, even in the presence of high NaCl concentrations, it forms nodules in the roots of its host plant where nitrogen is transformed into ammonia using a nitrogenase enzyme [120]. Hanin et al. [121] proved the intervention of halotolerant N-fixing bacteria in preserving membrane ionic balance. Iron predominantly exists in an insoluble form (Fe3+) in soil and is implicated in several enzymatic reactions in plants and bacteria [39]. Endophytic bacteria produce siderophores to chelate iron and facilitate its plant uptake [122]. For instance, numerous scientists demonstrated the effectiveness of Bacillus genera in iron chelation under a saline environment [123]. Moreover, Slama et al. [28] conducted a large siderophore screening study on 117 endophytic bacteria isolated from the halophytic plant Limoniastrum monopetalum. The results showed that 95% of these bacteria produced siderophores with most of them being of the Bacillus sp. species [28] (Figure 4).
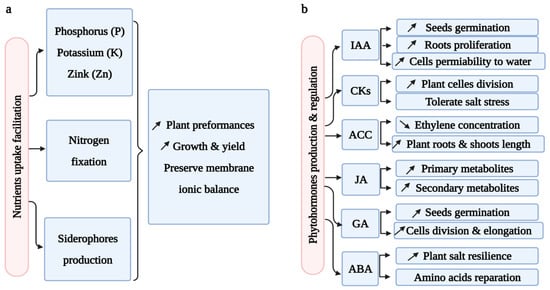
Figure 4.
Bacterial mechanisms of salt stress tolerance. (a) nutrient uptake facilitation. (b) phytohormone production and regulation. Upward and downward arrows mean increase and decrease of options.
8.2. Phytohormone Production and Regulation under Salt Stress
Phytohormones are organic compounds produced at low concentrations by plants and beneficial microbes to boost plant growth and yield [124]. The modulation of phytohormone levels helps in plant proliferation [125].
Auxin indole-3-acetic acid (IAA) is a significant phytohormone produced by a vast array of endophytic bacterial genera colonizing halophytic plants such as Marinobacterium, Bacillus, Sinorhizobium, Arthrobacter, and Pseudomonas [126]. In the case of salinity stress, IAA acts by increasing plant seed germination, root proliferation, and cell permeability to water, and by decreasing cell wall pressure [22]. In another study, Soleimani et al. [127] studied the effects of IAA-producing bacteria in improving NaCl toleration. The study revealed that the Arthrobacter siccitolerans strain adjusted the IAA expression levels to cope with the salt stress imposed on the host plant.
Cytokinins (CKs) are produced by endophytic bacteria to enhance plant cell divisions and to tolerate environmental stresses including high-salt concentrations [128,129]. Indeed, the plant’s inoculation with microbes producing phytohormones including cytokinins helped to inhibit the adverse effects of salt and contributed to the amelioration of all plant growth stages [130].
In the 1-aminocyclopropane-1-carboxylate (ACC) deaminase reaction, S-adenosyl methionine (SAM) is converted by the 1-aminocyclopropane-1-carboxylate synthase (ACS) enzyme to ACC, the immediate precursor of ethylene [131]. Ethylene acts as a plant growth hormone at low concentrations. However, plant stress exposure generates high concentrations of ethylene, transforming it into a plant growth inhibitor which could lead to plant death [132]. Endophytic bacteria could accumulate ACC produced by plants and transform it into ammonia and α-ketobutyrate, leading to a drop in ethylene concentrations, a regulation of plant growth, and protection from saline and any other imposed stresses [133]. Otherwise, ACC is used for nitrogen assimilation in the form of ammonia [39]. A plethora of plant growth-promoting bacteria (PGPB) were able to produce the ACC deaminase enzyme, and they include Pseudomonas, Bacillus, Acinetobacter, Enterobacter, Arthrobacter, Serratia, Brevibacterium, Corynebacterium, Planococcus, Micrococcus, Exiguobacterium, Burkholderia, Halomonas, Zhihengliuella, Alcaligenes, Ochrobactrum, Klebsiella, and Oceanimona genera [134,135,136]. Singh and Jha [137] demonstrated the great effects of the halotolerant Serratia sp. bacterium in lowering the ethylene levels of wheat cultivated in saline soil. This strain enhanced the plant growth rate by facilitating nutrient uptake and increasing wheat roots and shoot length. Yadav et al. [15] reported that P. simiae AU5 mutant overproducing ACC deaminase was effective in decreasing salt stress and ethylene concentrations in mung bean plants as compared to the P. simiae AU5 wild strain.
Halotolerant bacteria are also able to produce and alter high abscisic acid (ABA) levels in plants in case of abiotic stress occurrence [39,138]. Interestingly, Shahzad et al. [139] stated that the endophytic Bacillus amyloliquefaciens strain RWL-1 produced ABA to ameliorate plant salt resilience. It allowed the reparation of essential amino acids and induced salicylic acid production by the host rice plant.
Jasmonic acid (JA) is a signal molecule promoting the production of a plant’s primary and secondary metabolites to ameliorate their tolerance to both biotic and abiotic stresses [140]. In previous work, Liu et al. [141] conveyed that Bacillus amyloliquefaciens FZB42 conferred salt tolerance to the host Arabidopsis plant by upregulating the plant’s JA pathways. Moreover, an earlier study found that the bacterial inoculation of plants increases the JA gene expression of host plants [142]. Gibberellic acid (GA) is another plant hormone produced by several endophytes as well. It is responsible for plant cell division and elongation [38], seed germination, and fruit maintenance [143]. PGPB-inoculated plants under salinity stress promote plant growth throughout the production of several phytohormones including GA. For example, the Pseudomonas putida H-2-3 strain improved the performances of the host soybean plant cultivated in hypersaline soil [144] (Figure 4).
9. Molecular Analysis of Plant–Bacterial Mechanisms of Salt Mitigation
Valuable omics tools (genomics, metagenomics, transcriptomics, and proteomics) have been recently integrated in order to identify and delimit the diversification, roles, and ways of communication within the endophytic bacterial consortium and with their host plants [145]. For instance, genomic identification deeply elucidates the culture-dependent and culture-independent microbiomes in a specific plant, which helps in understanding their mechanisms of action [91,118]. Reportedly, the whole genome of the halotolerant strain Bacillus fexus KLBMP 4941 was sequenced to determine the biosynthetic gene clusters (BGCs) involved in salt alleviation [146]. In the same context, metagenomic studies are charged with delimiting the metabolic pathways of genes encoding for known and novel secondary metabolites allowing bacterial adaptation to harsh salinity [147,148]. Similarly, the transcriptomic study acts by revealing genes expressed by bacteria exposed to salt stress [149,150]. This phenomenon was well described by Dong et al. [151] who carried out a transcriptomic analysis on bacteria colonizing A. thaliana grown under salt stress. The endophytic bacteria expressed various genes responsible for the salt stress resistance of their host plant. Lastly, the proteomic study gives an insight into the proteins of interest expressed by bacteria for stress mitigation. Genomic sequence and comparative proteomic analysis were realized to determine the response of Micrococcus luteus strain SA211 to Lithium (Li) [152,153,154]. The M. luteus strain SA211 was able to adapt by over synthesizing proteins responsible for coping with LiCl stress. Generally, the use of advanced and novel protocols permits the discovery of novel natural products, which could be useful in bacterial adaptation to extreme environments as well as in plant and human health [153]. The above-mentioned high throughput molecular sequencing techniques are extremely valuable to form mutants endowed with high salt resistance capacities and their further biological inoculation in glycophyte plants in order to confer salt tolerance [138] (Figure 5).
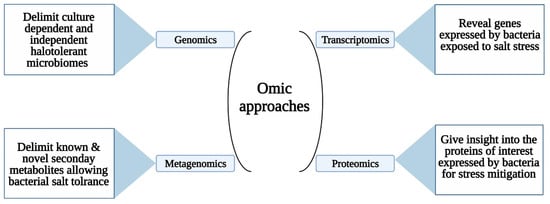
Figure 5.
Omic approaches enhance knowledge about bacterial mechanisms of salt stress adaptation and mitigation.
10. Conclusions
Plant–endophyte interaction is a very complex mechanism controlled by a network of signals, hormones, enzymes, volatile compounds, genes, and metabolites working in tandem to ensure a mutual relationship. Our work described the mechanisms of salt mitigation by plants and bacteria. However, it focused mostly on the advantages provided by endophytic bacteria to improve plant tolerance to saline conditions. We discussed the mechanisms of nutrient uptake amelioration and phytohormone production and the regulation of potent bacteria helping in salt mitigation. Moreover, we highlighted the advanced molecular and omics tools’ contribution in revealing the mechanisms of salinity stress. Potent salt-tolerant bacteria isolated from halotolerant plants could be further exploited in agronomy in order to improve agricultural plants’ resistance to increasing salt concentrations in soil and water. This biological remediation is easy, cost-effective, and environmentally friendly.
Author Contributions
Conceptualization, H.B.S., A.C.B. and L.B.; methodology, L.B.; software, R.A., A.C.B. and H.B.S.; validation, H.B.S., L.L., O.B. and L.B.; formal analysis, O.B., L.L., A.C.B. and L.B.; investigation, H.B.S. and L.B.; resources, F.N.A., O.B. and L.L.; data curation, R.A. and A.C.B.; writing—original draft preparation, H.B.S. and L.B.; writing—review and editing, H.B.S., A.C.B. and L.B.; visualization, F.N.A., H.B.S. and L.L.; supervision, L.B.; project administration, L.B.; funding acquisition, F.N.A., O.B. and L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Magallon, K.J.; Dinneny, J.R. Environmental Stress: Salinity Ruins a Plant’s Day in the Sun. Curr. Biol. 2019, 29, R360–R362. [Google Scholar] [CrossRef] [PubMed]
- Sunita, K.; Mishra, I.; Mishra, J.; Prakash, J.; Arora, N.K. Secondary Metabolites from Halotolerant Plant Growth Promoting Rhizobacteria for Ameliorating Salinity Stress in Plants. Front. Microbiol. 2020, 11, 567768. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, P.H.R.; da Silva, F.B.; de Abreu, C.M.; da Silva, G.J. Plant growth promoting rhizobacteria in amelioration of abiotic stresses: A functional interplay and prospective. In Plant Growth-Promoting Microbes for Sustainable Biotic and Abiotic Stress Management; Springer: Cham, Switzerland, 2021; pp. 25–49. [Google Scholar]
- Frikha Dammak, D.; Zarai, Z.; Najah, S.; Abdennabi, R.; Belbahri, L.; Rateb, M.E.; Mejdoub, H.; Maalej, S. Antagonistic Properties of Some Halophilic Thermoactinomycetes Isolated from Superficial Sediment of a Solar Saltern and Production of Cyclic Antimicrobial Peptides by the Novel Isolate Paludifilum halophilum. BioMed Res. Int. 2017, 2017, 1205258. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumar, R.; Jain, V.; Jain, S. Differential Behavior of the Antioxidant System in Response to Salinity Induced Oxidative Stress in Salt-Tolerant and Salt-Sensitive Cultivars of Brassica juncea L. Biocatal. Agric. Biotechnol. 2018, 13, 12–19. [Google Scholar] [CrossRef]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of Plant Response to Salt and Drought Stress and Their Alteration by Rhizobacteria. Plant Soil. 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Kerbab, S.; Silini, A.; Chenari Bouket, A.; Cherif-Silini, H.; Eshelli, M.; El Houda Rabhi, N.; Belbahri, L. Mitigation of NaCl Stress in Wheat by Rhizosphere Engineering Using Salt Habitat Adapted PGPR Halotolerant Bacteria. Appl. Sci. 2021, 11, 1034. [Google Scholar] [CrossRef]
- Goswami, M.; Deka, S. Plant growth-promoting rhizobacteria—Alleviators of abiotic stresses in soil: A review. Pedosphere 2020, 30, 40–61. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Sharma, A.K.; Agarwal, P.; Varma, A.; Tuteja, N. Volatiles and Food Security; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Gondek, M.; Weindorf, D.C.; Thiel, C.; Kleinheinz, G. Soluble Salts in Compost and Their Effects on Soil and Plants: A Review. Compost Sci. Util. 2020, 28, 59–75. [Google Scholar] [CrossRef]
- Reints, J.; Dinar, A.; Crowley, D. Dealing with Water Scarcity and Salinity: Adoption of Water Efficient Technologies and Management Practices by California Avocado Growers. Sustainability 2020, 12, 3555. [Google Scholar] [CrossRef]
- Ilangumaran, G.; Smith, D.L. Plant Growth Promoting Rhizobacteria in Amelioration of Salinity Stress: A Systems Biology Perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef]
- Vaishnav, A.; Hansen, A.P.; Agrawal, P.K.; Varma, A.; Choudhary, D.K. Biotechnological perspectives of Legume–Rhizobium symbiosis. In Rhizobium Biology and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 247–256. [Google Scholar]
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; Ahmed, A.-H. Plant Growth Promoting Bacteria as an Alternative Strategy for Salt Tolerance in Plants: A Review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.N.; Kaur, T.; Kour, D.; Rana, K.L.; Yadav, N.; Rastegari, A.A.; Kumar, M.; Paul, D.; Sachan, S.G.; Saxena, A.K. Chapter 16—Saline microbiome: Biodiversity, ecological significance, and potential role in amelioration of salt stress. In New and Future Developments in Microbial Biotechnology and Bioengineering; Rastegari, A.A., Yadav, A.N., Yadav, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 283–309. [Google Scholar]
- Zhai, Y.; Huang, M.; Zhu, C.; Xu, H.; Zhang, Z. Evaluation and Application of the AquaCrop Model in Simulating Soil Salinity and Winter Wheat Yield under Saline Water Irrigation. Agronomy 2022, 12, 2313. [Google Scholar] [CrossRef]
- Manishankar, P.; Wang, N.; Köster, P.; Alatar, A.A.; Kudla, J. Calcium Signaling during Salt Stress and in the Regulation of Ion Homeostasis. J. Exp. Bot. 2018, 69, 4215–4226. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, K.E.; MacNeill, G.J.; Gerwing, P.D.; Greenberg, B.M. Phytoremediation of salt-impacted soils and use of plant growth-promoting rhizobacteria (PGPR) to enhance phytoremediation. In Phytoremediation; Springer: Berlin/Heidelberg, Germany, 2017; pp. 19–51. [Google Scholar]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant Salt-Tolerance Mechanism: A Review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Bhardwaj, A.K.; Mishra, V.K.; Singh, A.K.; Arora, S.; Srivastava, S.; Singh, Y.P.; Sharma, D.K. Soil Salinity and Land Use-Land Cover Interactions with Soil Carbon in a Salt-Affected Irrigation Canal Command of Indo-Gangetic Plain. Catena 2019, 180, 392–400. [Google Scholar] [CrossRef]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Wirth, S.; Egamberdieva, D. Comparing Symbiotic Performance and Physiological Responses of Two Soybean Cultivars to Arbuscular Mycorrhizal Fungi under Salt Stress. Saudi J. Biol. Sci. 2019, 26, 38–48. [Google Scholar] [CrossRef]
- Vaishnav, A.; Shukla, A.K.; Sharma, A.; Kumar, R.; Choudhary, D.K. Endophytic Bacteria in Plant Salt Stress Tolerance: Current and Future Prospects. J. Plant Growth Regul. 2019, 38, 650–668. [Google Scholar] [CrossRef]
- Li, Y.; Kong, Y.; Teng, D.; Zhang, X.; He, X.; Zhang, Y.; Lv, G. Rhizobacterial Communities of Five Co-Occurring Desert Halophytes. PeerJ 2018, 6, e5508. [Google Scholar] [CrossRef]
- Slama, H.; Cherif-Silini, H.; Chenari Bouket, A.; Qader, M.; Silini, A.; Yahiaoui, B.; Alenezi, F.; Luptakova, L.; Triki, M.; Vallat, A.; et al. Screening for Fusarium Antagonistic Bacteria from Contrasting Niches Designated the Endophyte Bacillus Halotolerans as Plant Warden against Fusarium. Front. Microbiol. 2019, 9, 3236. [Google Scholar] [CrossRef]
- Bharti, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant Growth Promoting Rhizobacteria Dietzia Natronolimnaea Modulates the Expression of Stress Responsive Genes Providing Protection of Wheat from Salinity Stress. Sci. Rep. 2016, 6, 34768. [Google Scholar] [CrossRef]
- Saghafi, D.; Delangiz, N.; Lajayer, B.A.; Ghorbanpour, M. An Overview on Improvement of Crop Productivity in Saline Soils by Halotolerant and Halophilic PGPRs. 3 Biotech 2019, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Cheffi, M.; Chenari Bouket, A.; Alenezi, F.N.; Luptakova, L.; Belka, M.; Vallat, A.; Rateb, M.E.; Tounsi, S.; Triki, M.A.; Belbahri, L. Olea europaea L. Root Endophyte Bacillus Velezensis OEE1 Counteracts Oomycete and Fungal Harmful Pathogens and Harbours a Large Repertoire of Secreted and Volatile Metabolites and Beneficial Functional Genes. Microorganisms 2019, 7, 314. [Google Scholar] [CrossRef]
- Slama, H.; Triki, M.; Chenari Bouket, A.; Mefteh, F.; Alenezi, F.; Luptakova, L.; Cherif-Silini, H.; Vallat, A.; Oszako, T.; Gharsallah, N.; et al. Microorganisms Screening of the High-Rhizosphere Competent Limoniastrum monopetalum’ Culturable Endophyte Microbiota Allows the Recovery of Multifaceted and Versatile Biocontrol Agents. Microorganisms 2019, 7, 249. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Slama, H.B.; Cherif-Silini, H.; Bouket, A.C.; Silini, A.; Alenezi, F.N.; Luptakova, L.; Vallat, A.; Belbahri, L. Biotechnology and Bioinformatics of Endophytes in Biocontrol, Bioremediation, and Plant Growth Promotion. In Endophytes: Mineral Nutrient Management; Springer: Cham, Switzerland, 2021; Volume 3, pp. 181–205. [Google Scholar]
- Aung, K.; Jiang, Y.; He, S.Y. The Role of Water in Plant–Microbe Interactions. Plant J. 2018, 93, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Elsakhawy, T.A.; Nashwa, A.H.; Ghazi, A.A. The Potential Use of Ectoine Produced by a Moderately Halophilic Bacteria Chromohalobacter salexigens KT989776 for Enhancing Germination and Primary Seedling of Flax “Linum usitatissimum L.” under Salinity Conditions. Biotechnol. J. Int. 2019, 23, 1–12. [Google Scholar] [CrossRef]
- Pan, J.; Peng, F.; Xue, X.; You, Q.; Zhang, W.; Wang, T.; Huang, C. The Growth Promotion of Two Salt-Tolerant Plant Groups with PGPR Inoculation: A Meta-Analysis. Sustainability 2019, 11, 378. [Google Scholar] [CrossRef]
- Liu, X.; Luo, Y.; Li, Z.; Wang, J.; Wei, G. Role of Exopolysaccharide in Salt Stress Resistance and Cell Motility of Mesorhizobium Alhagi CCNWXJ12–2 T. Appl. Microbiol. Biotechnol. 2017, 101, 2967–2978. [Google Scholar] [CrossRef]
- Pichler, H.; Emmerstorfer-Augustin, A. Modification of Membrane Lipid Compositions in Single-Celled Organisms—From Basics to Applications. Methods 2018, 147, 50–65. [Google Scholar] [CrossRef]
- Pal, K.K.; Dey, R.; Sherathia, D.N.; Mangalassery, S.; Kumar, A.; Rupapara, R.B.; Mandaliya, M.; Rawal, P.; Bhadania, R.A.; Thomas, M. Alleviation of Salinity Stress in Peanut by Application of Endophytic Bacteria. Front. Microbiol. 2021, 12, 791. [Google Scholar] [CrossRef]
- Sun, L.; Lei, P.; Wang, Q.; Ma, J.; Zhan, Y.; Jiang, K.; Xu, Z.; Xu, H. The Endophyte Pantoea Alhagi NX-11 Alleviates Salt Stress Damage to Rice Seedlings by Secreting Exopolysaccharides. Front. Microbiol. 2020, 10, 3112. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Druzhinina, I.S.; Pan, X.; Yuan, Z. Microbially Mediated Plant Salt Tolerance and Microbiome-Based Solutions for Saline Agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef] [PubMed]
- Stringlis, I.A.; Proietti, S.; Hickman, R.; Van Verk, M.C.; Zamioudis, C.; Pieterse, C.M. Root Transcriptional Dynamics Induced by Beneficial Rhizobacteria and Microbial Immune Elicitors Reveal Signatures of Adaptation to Mutualists. Plant J. 2018, 93, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Enespa; Prakash, J.; Chandra, P. Halophilic Microbes from Plant Growing Under the Hypersaline Habitats and Their Application for Plant Growth and Mitigation of Salt Stress. In Plant Microbiomes for Sustainable Agriculture; Yadav, A.N., Singh, J., Rastegari, A.A., Yadav, N., Eds.; Sustainable Development and Biodiversity; Springer: Cham, Switzerland, 2020; pp. 317–349. [Google Scholar]
- Trușcă, M.; Gâdea, Ș.; Vidican, R.; Stoian, V.; Vâtcă, A.; Balint, C.; Stoian, V.A.; Horvat, M.; Vâtcă, S. Exploring the Research Challenges and Perspectives in Ecophysiology of Plants Affected by Salinity Stress. Agriculture 2023, 13, 734. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil Salinity: A Serious Environmental Issue and Plant Growth Promoting Bacteria as One of the Tools for Its Alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Chakraborty, K.; Basak, N.; Bhaduri, D.; Ray, S.; Vijayan, J.; Chattopadhyay, K.; Sarkar, R.K. Ionic Basis of Salt Tolerance. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 325–362. [Google Scholar]
- Kashyap, P.L.; Solanki, M.K.; Kushwaha, P.; Kumar, S.; Srivastava, A.K. Biocontrol Potential of Salt-Tolerant Trichoderma and Hypocrea Isolates for the Management of Tomato Root Rot under Saline Environment. J. Soil Sci. Plant Nutr. 2020, 20, 160–176. [Google Scholar] [CrossRef]
- Cherif-Silini, H.; Thissera, B.; Bouket, A.C.; Saadaoui, N.; Silini, A.; Eshelli, M.; Alenezi, F.N.; Vallat, A.; Luptakova, L.; Yahiaoui, B. Durum Wheat Stress Tolerance Induced by Endophyte Pantoea agglomerans with Genes Contributing to Plant Functions and Secondary Metabolite Arsenal. Int. J. Mol. Sci. 2019, 20, 3989. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Gładysz, O.; Goliński, P. Participation of Phytohormones in Adaptation to Salt Stress. In Plant Hormones under Challenging Environmental Factors; Ahammed, G.J., Yu, J.-Q., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 75–115. [Google Scholar]
- Farvardin, A.; González-Hernández, A.I.; Llorens, E.; García-Agustín, P.; Scalschi, L.; Vicedo, B. The Apoplast: A Key Player in Plant Survival. Antioxidants 2020, 9, 604. [Google Scholar] [CrossRef]
- Liu, J.; Fu, C.; Li, G.; Khan, M.N.; Wu, H. ROS Homeostasis and Plant Salt Tolerance: Plant Nanobiotechnology Updates. Sustainability 2021, 13, 3552. [Google Scholar] [CrossRef]
- Singh, R.; Upadhyay, A.K.; Chandra, P.; Singh, D.P. Sodium Chloride Incites Reactive Oxygen Species in Green Algae Chlorococcum humicola and Chlorella vulgaris: Implication on Lipid Synthesis, Mineral Nutrients and Antioxidant System. Bioresour. Technol. 2018, 270, 489–497. [Google Scholar] [CrossRef]
- Kang, S.-M.; Shahzad, R.; Bilal, S.; Khan, A.L.; Park, Y.-G.; Lee, K.-E.; Asaf, S.; Khan, M.A.; Lee, I.-J. Indole-3-Acetic-Acid and ACC Deaminase Producing Leclercia Adecarboxylata MO1 Improves Solanum lycopersicum L. Growth and Salinity Stress Tolerance by Endogenous Secondary Metabolites Regulation. BMC Microbiol. 2019, 19, 80. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, H.; Zhou, J.-M.; Smith, S.M.; Li, J. Malate Circulation: Linking Chloroplast Metabolism to Mitochondrial ROS. Trends Plant Sci. 2020, 25, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, T.K.; Bashir, T.; Hashem, A.; Abd_Allah, E.F.; Khan, A.L.; Al-Harrasi, A.S. Early Events in Plant Abiotic Stress Signaling: Interplay between Calcium, Reactive Oxygen Species and Phytohormones. J. Plant Growth Regul. 2018, 37, 1033–1049. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, Y. Apoplastic Proteases-Powerful Weapons against Pathogen Infection in Plants. Plant Commun. 2020, 1, 100085. [Google Scholar] [CrossRef] [PubMed]
- Del Río, L.A.; López-Huertas, E. ROS Generation in Peroxisomes and Its Role in Cell Signaling. Plant Cell Physiol. 2016, 57, 1364–1376. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Unraveling Salt Stress Signaling in Plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef]
- Cai, J.; Chen, T.; Wang, Y.; Qin, G.; Tian, S. SlREM1 Triggers Cell Death by Activating an Oxidative Burst and Other Regulators. Plant Physiol. 2020, 183, 717–732. [Google Scholar] [CrossRef]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Antioxidant Responses of Wheat Plants under Stress. Genet. Mol. Biol. 2016, 39, 1–6. [Google Scholar] [CrossRef]
- Podgórska, A.; Burian, M.; Szal, B. Extra-Cellular but Extra-Ordinarily Important for Cells: Apoplastic Reactive Oxygen Species Metabolism. Front. Plant Sci. 2017, 8, 1353. [Google Scholar] [CrossRef]
- Che-Othman, M.H.; Jacoby, R.P.; Millar, A.H.; Taylor, N.L. Wheat Mitochondrial Respiration Shifts from the Tricarboxylic Acid Cycle to the GABA Shunt under Salt Stress. New Phytol. 2020, 225, 1166–1180. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R.M. Plant Growth under Water/Salt Stress: ROS Production; Antioxidants and Significance of Added Potassium under Such Conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef]
- Szymańska, S.; Tyburski, J.; Piernik, A.; Sikora, M.; Mazur, J.; Katarzyna, H. Raising Beet Tolerance to Salinity through Bioaugmentation with Halotolerant Endophytes. Agronomy 2020, 10, 1571. [Google Scholar] [CrossRef]
- Dar, M.I.; Naikoo, M.I.; Rehman, F.; Naushin, F.; Khan, F.A. Proline accumulation in plants: Roles in stress tolerance and plant development. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Springer: Berlin/Heidelberg, Germany, 2016; pp. 155–166. [Google Scholar]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A. Azospirillum Lipoferum FK1 Confers Improved Salt Tolerance in Chickpea (Cicer arietinum L.) by Modulating Osmolytes, Antioxidant Machinery and Stress-Related Genes Expression. Environ. Exp. Bot. 2019, 159, 55–65. [Google Scholar] [CrossRef]
- Lee, H.-G.; Kim, H.-S.; Oh, J.-Y.; Lee, D.-S.; Yang, H.-W.; Kang, M.-C.; Kim, E.-A.; Kang, N.; Kim, J.; Heo, S.-J. Potential Antioxidant Properties of Enzymatic Hydrolysates from Stichopus japonicus against Hydrogen Peroxide-Induced Oxidative Stress. Antioxidants 2021, 10, 110. [Google Scholar] [CrossRef]
- Fromm, S.; Senkler, J.; Eubel, H.; Peterhänsel, C.; Braun, H.-P. Life without Complex I: Proteome Analyses of an Arabidopsis Mutant Lacking the Mitochondrial NADH Dehydrogenase Complex. J. Exp. Bot. 2016, 67, 3079–3093. [Google Scholar] [CrossRef]
- Schmidt, R.R.; Weits, D.A.; Feulner, C.F.; van Dongen, J.T. Oxygen Sensing and Integrative Stress Signaling in Plants. Plant Physiol. 2018, 176, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Vita, F.; Sabbatini, L.; Sillo, F.; Ghignone, S.; Vergine, M.; Guidi Nissim, W.; Fortunato, S.; Salzano, A.M.; Scaloni, A.; Luvisi, A.; et al. Salt stress in olive tree shapes resident endophytic microbiota. Front. Plant Sci. 2022, 13, 992395. [Google Scholar] [CrossRef]
- Monteiro, D.A.; Fazolato, C.S.B.; Martinz, L.F.; Rachid, C.T.C.C. The bacteriome of the halophyte Atriplex nummularia (old man saltbush) in salt-affected soils—An ecological model. FEMS Microbiol. Ecol. 2022, 98, fiac135. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Q.; Liu, J.; Wang, L.; Wu, X.; Zhao, Z.; Wang, N.; Gao, Z. Suaeda salsa Root-Associated Microorganisms Could Effectively Improve Maize Growth and Resistance under Salt Stress. Microbiol. Spectr. 2022, 10, e0134922. [Google Scholar] [CrossRef]
- Vu, M.T.; Geraldi, A.; Do, H.D.K.; Luqman, A.; Nguyen, H.D.; Fauzia, F.N.; Amalludin, F.I.; Sadila, A.Y.; Wijaya, N.H.; Santoso, H.; et al. Soil Mineral Composition and Salinity Are the Main Factors Regulating the Bacterial Community Associated with the Roots of Coastal Sand Dune Halophytes. Biology 2022, 11, 695. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Gadelha, C.G.; Coutinho, I.A.C.; Pinheiro, S.K.P.; Miguel, E.C.; Carvalho, H.H.; Lopes, L.S.; Gomes-Filho, E. Sodium uptake and transport regulation, and photosynthetic efficiency maintenance as the basis of differential salt tolerance in rice cultivars. Environ. Exp. Bot. 2021, 192, 104654. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Romeo, F.; Marra, F.; Mallamaci, C.; Hussain, M.I.; Muscolo, A. Salinity tolerance of lentil is achieved by enhanced proline accumulation, lower level of sodium uptake and modulation of photosynthetic traits. J. Agron. Plant Sci. 2022, 208, 40–52. [Google Scholar] [CrossRef]
- Wang, D.; Yang, N.; Zhang, C.; He, W.; Ye, G.; Chen, J.; Wei, X. Transcriptome analysis reveals molecular mechanisms underlying salt tolerance in halophyte Sesuvium portulacastrum. Front. Plant Sci. 2022, 13, 973419. [Google Scholar] [CrossRef]
- Kapazoglou, A.; Gerakari, M.; Lazaridi, E.; Kleftogianni, K.; Sarri, E.; Tani, E.; Bebeli, P.J. Crop Wild Relatives: A Valuable Source of Tolerance to Various Abiotic Stresses. Plants 2023, 12, 328. [Google Scholar] [CrossRef]
- Weinisch, L.; Kühner, S.; Roth, R.; Grimm, M.; Roth, T.; Netz, D.J.; Pierik, A.J.; Filker, S. Identification of Osmoadaptive Strategies in the Halophile, Heterotrophic Ciliate Schmidingerothrix Salinarum. PLoS Biol. 2018, 16, e2003892. [Google Scholar] [CrossRef]
- Kohler, C.; Lourenço, R.F.; Bernhardt, J.; Albrecht, D.; Schüler, J.; Hecker, M.; Gomes, S.L. A Comprehensive Genomic, Transcriptomic and Proteomic Analysis of a Hyperosmotic Stress Sensitive α-Proteobacterium. BMC Microbiol. 2015, 15, 71. [Google Scholar] [CrossRef]
- Chandra, P.; Singh, E. Applications and mechanisms of plant growth-stimulating rhizobacteria. In Plant-Microbe Interaction: An Approach to Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; pp. 37–62. [Google Scholar]
- Etesami, H.; Glick, B.R. Halotolerant Plant Growth–Promoting Bacteria: Prospects for Alleviating Salinity Stress in Plants. Environ. Exp. Bot. 2020, 178, 104124. [Google Scholar] [CrossRef]
- Tian, X.-Y.; Zhang, C.-S. Illumina-Based Analysis of Endophytic and Rhizosphere Bacterial Diversity of the Coastal Halophyte Messerschmidia Sibirica. Front. Microbiol. 2017, 8, 2288. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A Review on the Plant Microbiome: Ecology, Functions, and Emerging Trends in Microbial Application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Batra, P.; Barkodia, M.; Ahlawat, U.; Sansanwal, R.; Sharma, T.; Wati, L. Endophytes: An Environmental Friendly Bacteria for Plant Growth Promotion. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1899–1911. [Google Scholar] [CrossRef]
- Shastry, R.P.; Welch, M.; Rai, V.R.; Ghate, S.D.; Sandeep, K.; Rekha, P.D. The Whole-Genome Sequence Analysis of Enterobacter Cloacae Strain Ghats1: Insights into Endophytic Lifestyle-Associated Genomic Adaptations. Arch. Microbiol. 2020, 202, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Kandel, S.L.; Joubert, P.M.; Doty, S.L. Bacterial Endophyte Colonization and Distribution within Plants. Microorganisms 2017, 5, 77. [Google Scholar] [CrossRef]
- Lata, R.; Chowdhury, S.; Gond, S.K.; White, J.F., Jr. Induction of Abiotic Stress Tolerance in Plants by Endophytic Microbes. Lett. Appl. Microbiol. 2018, 66, 268–276. [Google Scholar] [CrossRef]
- Etesami, H.; Beattie, G.A. Mining Halophytes for Plant Growth-Promoting Halotolerant Bacteria to Enhance the Salinity Tolerance of Non-Halophytic Crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef]
- Yamamoto, K.; Shiwa, Y.; Ishige, T.; Sakamoto, H.; Tanaka, K.; Uchino, M.; Tanaka, N.; Oguri, S.; Saitoh, H.; Tsushima, S. Bacterial Diversity Associated with the Rhizosphere and Endosphere of Two Halophytes: Glaux maritima and Salicornia europaea. Front. Microbiol. 2018, 9, 2878. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Q.; Hou, J.; Tu, C.; Luo, Y.; Christie, P. Whole Genome Analysis of Halotolerant and Alkalotolerant Plant Growth-Promoting Rhizobacterium Klebsiella sp. D5A. Sci. Rep. 2016, 6, 26710. [Google Scholar] [CrossRef]
- Andrés-Barrao, C.; Lafi, F.F.; Alam, I.; De Zélicourt, A.; Eida, A.A.; Bokhari, A.; Alzubaidy, H.; Bajic, V.B.; Hirt, H.; Saad, M.M. Complete Genome Sequence Analysis of Enterobacter sp. SA187, a Plant Multi-Stress Tolerance Promoting Endophytic Bacterium. Front. Microbiol. 2017, 8, 2023. [Google Scholar] [CrossRef]
- Mehnaz, D.; Mukhtar, S.; Ishaq, A.; Hassan, S.; Abdulla, K.; Mirza, M.S. Comparison of Microbial Communities Associated with Halophyte (Salsola stocksii) and Non-Halophyte (Triticum aestivum) Using Culture-Independent Approaches. Pol. J. Microbiol. 2017, 66, 353–364. [Google Scholar]
- Szymańska, S.; Sikora, M.; Hrynkiewicz, K.; Tyburski, J.; Tretyn, A.; Gołębiewski, M. Choosing Source of Microorganisms and Processing Technology for next Generation Beet Bioinoculant. Sci. Rep. 2021, 11, 2829. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.; Patel, R.M.; Parekh, V.B. Culturable Endophytic Bacteria from Halotolerant Salicornia brachata L.: Isolation and Plant Growth Promoting Traits. Indian J. Appl. Microbiol. 2018, 21, 10–21. [Google Scholar]
- Furtado, B.U.; Golębiewski, M.; Skorupa, M.; Hulisz, P.; Hrynkiewicz, K. Bacterial and Fungal Endophytic Microbiomes of Salicornia europaea. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef] [PubMed]
- Sessitsch, A.; Pfaffenbichler, N.; Mitter, B. Microbiome Applications from Lab to Field: Facing Complexity. Trends Plant Sci. 2019, 24, 194–198. [Google Scholar] [CrossRef]
- Arora, N.K.; Fatima, T.; Mishra, J.; Mishra, I.; Verma, S.; Verma, R.; Verma, M.; Bhattacharya, A.; Verma, P.; Mishra, P. Halo-Tolerant Plant Growth Promoting Rhizobacteria for Improving Productivity and Remediation of Saline Soils. J. Adv. Res. 2020, 26, 69–82. [Google Scholar] [CrossRef]
- Sorty, A.M.; Meena, K.K.; Choudhary, K.; Bitla, U.M.; Minhas, P.S.; Krishnani, K.K. Effect of Plant Growth Promoting Bacteria Associated with Halophytic Weed (Psoralea corylifolia L.) on Germination and Seedling Growth of Wheat under Saline Conditions. Appl. Biochem. Biotechnol. 2016, 180, 872–882. [Google Scholar] [CrossRef]
- Dąbrowska, G.B.; Turkan, S.; Tylman-Mojżeszek, W.; Mierek-Adamska, A. In Silico Study of the RSH (RelA/SpoT Homologs) Gene Family and Expression Analysis in Response to PGPR Bacteria and Salinity in Brassica napus. Int. J. Mol. Sci. 2021, 22, 10666. [Google Scholar] [CrossRef]
- Glynou, K.; Nam, B.; Thines, M.; Maciá-Vicente, J.G. Facultative Root-Colonizing Fungi Dominate Endophytic Assemblages in Roots of Nonmycorrhizal Microthlaspi Species. New Phytol. 2018, 217, 1190–1202. [Google Scholar] [CrossRef]
- Das, P.; Behera, B.K.; Meena, D.K.; Azmi, S.A.; Chatterjee, S.; Meena, K.; Sharma, A.P. Salt Stress Tolerant Genes in Halophilic and Halotolerant Bacteria: Paradigm for Salt Stress Adaptation and Osmoprotection. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 642–658. [Google Scholar]
- Chatterjee, P.; Kanagendran, A.; Samaddar, S.; Pazouki, L.; Sa, T.-M.; Niinemets, Ü. Inoculation of Brevibacterium linens RS16 in Oryza sativa Genotypes Enhanced Salinity Resistance: Impacts on Photosynthetic Traits and Foliar Volatile Emissions. Sci. Total Environ. 2018, 645, 721–732. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Pu, H.; Liu, S.; Kan, J.; Jin, C. Recent Advances in Endophytic Exopolysaccharides: Production, Structural Characterization, Physiological Role and Biological Activity. Carbohydr. Polym. 2017, 157, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, P.; Kashyap, P.L.; Bhardwaj, A.K.; Kuppusamy, P.; Srivastava, A.K.; Tiwari, R.K. Bacterial Endophyte Mediated Plant Tolerance to Salinity: Growth Responses and Mechanisms of Action. World J. Microbiol. Biotechnol. 2020, 36, 26. [Google Scholar] [CrossRef] [PubMed]
- Suarez, C.; Cardinale, M.; Ratering, S.; Steffens, D.; Jung, S.; Montoya, A.M.Z.; Geissler-Plaum, R.; Schnell, S. Plant Growth-Promoting Effects of Hartmannibacter Diazotrophicus on Summer Barley (Hordeum vulgare L.) under Salt Stress. Appl. Soil Ecol. 2015, 95, 23–30. [Google Scholar] [CrossRef]
- Yaish, M.W.; Al-Lawati, A.; Jana, G.A.; Vishwas Patankar, H.; Glick, B.R. Impact of Soil Salinity on the Structure of the Bacterial Endophytic Community Identified from the Roots of Caliph Medic (Medicago truncatula). PLoS ONE 2016, 11, e0159007. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodriguez, M.M.; Pontin, M.; Piccoli, P.; Lobato Ureche, M.A.; Gordillo, M.G.; Funes-Pinter, I.; Cohen, A.C. Halotolerant Native Bacteria Enterobacter 64S1 and Pseudomonas 42P4 Alleviate Saline Stress in Tomato Plants. Physiol. Plant. 2022, 174, e13742. [Google Scholar] [CrossRef] [PubMed]
- Dutta, B.; Datta, A.; Dey, A.; Ghosh, A.K.; Bandopadhyay, R. Establishment of Seed Biopriming in Salt Stress Mitigation of Rice Plants by Mangrove Derived Bacillus sp. Biocatal. Agric. Biotechnol. 2023, 48, 102626. [Google Scholar] [CrossRef]
- Hamid, S.; Ahmad, I.; Akhtar, M.J.; Iqbal, M.N.; Shakir, M.; Tahir, M.; Rasool, A.; Sattar, A.; Khalid, M.; Ditta, A.; et al. Bacillus Subtilis Y16 and Biogas Slurry Enhanced Potassium to Sodium Ratio and Physiology of Sunflower (Helianthus annuus L.) to Mitigate Salt Stress. Environ. Sci. Pollut. Res. 2021, 28, 38637–38647. [Google Scholar] [CrossRef]
- Kim, J.; Woo, O.-G.; Bae, Y.; Keum, H.L.; Chung, S.; Sul, W.J.; Lee, J.-H. Enhanced Drought and Salt Stress Tolerance in Arabidopsis by Flavobacterium Crocinum HYN0056T. J. Plant Biol. 2020, 63, 63–71. [Google Scholar] [CrossRef]
- Suksaard, P.; Pathom-aree, W.; Duangmal, K. Diversity and Plant Growth Promoting Activities of Actinomycetes from Mangroves. Chiang Mai J. Sci. 2017, 44, 1210–1223. [Google Scholar]
- Song, L.; Yang, S.; Liu, H.; Xu, J. Geographic and Environmental Sources of Variation in Bacterial Community Composition in a Large-Scale Municipal Landfill Site in China. Appl. Microbiol. Biotechnol. 2017, 101, 761–769. [Google Scholar] [CrossRef]
- Frank, A.C.; Saldierna Guzmán, J.P.; Shay, J.E. Transmission of Bacterial Endophytes. Microorganisms 2017, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Borruso, L.; Bacci, G.; Mengoni, A.; De Philippis, R.; Brusetti, L. Rhizosphere Effect and Salinity Competing to Shape Microbial Communities in Phragmites australis (Cav.) Trin. Ex-Steud. FEMS Microbiol. Lett. 2014, 359, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, S.; Dąbrowska, G.B.; Tyburski, J.; Niedojadlo, K.; Piernik, A.; Hrynkiewicz, K. Boosting the Brassica napus L. Tolerance to Salinity by the Halotolerant Strain Pseudomonas stutzeri ISE12. Environ. Exp. Bot. 2019, 163, 55–68. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant Growth-Promoting Bacterial Endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Saidi, S.; Cherif-Silini, H.; Bouket, A.C.; Silini, A.; Eshelli, M.; Luptakova, L.; Alenezi, F.N.; Belbahri, L. Improvement of Medicago Sativa Crops Productivity by the Co-Inoculation of Sinorhizobium Meliloti–Actinobacteria under Salt Stress. Curr. Microbiol. 2021, 78, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Otieno, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant Growth Promotion Induced by Phosphate Solubilizing Endophytic Pseudomonas Isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef]
- Belbahri, L.; Chenari Bouket, A.; Rekik, I.; Alenezi, F.N.; Vallat, A.; Luptakova, L.; Petrovova, E.; Oszako, T.; Cherrad, S.; Vacher, S. Comparative Genomics of Bacillus amyloliquefaciens Strains Reveals a Core Genome with Traits for Habitat Adaptation and a Secondary Metabolites Rich Accessory Genome. Front. Microbiol. 2017, 8, 1438. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Qi, P.; Wang, T.; Chi, X.; Wang, M.; Chen, M.; Chen, N.; Pan, L. Role of Halotolerant Phosphate-Solubilising Bacteria on Growth Promotion of Peanut (Arachis hypogaea) under Saline Soil. Ann. Appl. Biol. 2019, 174, 20–30. [Google Scholar] [CrossRef]
- Bertrand, A.; Gatzke, C.; Bipfubusa, M.; Lévesque, V.; Chalifour, F.P.; Claessens, A.; Rocher, S.; Tremblay, G.F.; Beauchamp, C.J. Physiological and Biochemical Responses to Salt Stress of Alfalfa Populations Selected for Salinity Tolerance and Grown in Symbiosis with Salt-Tolerant Rhizobium. Agronomy 2020, 10, 569. [Google Scholar] [CrossRef]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New Insights on Plant Salt Tolerance Mechanisms and Their Potential Use for Breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef]
- Navarro-Torre, S.; Barcia-Piedras, J.M.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Camacho, M.; Caviedes, M.A.; Pajuelo, E.; Rodríguez-Llorente, I.D. Assessing the Role of Endophytic Bacteria in the Halophyte Arthrocnemum macrostachyum Salt Tolerance. Plant Biol. 2017, 19, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kesaulya, H.; Hasinu, J.V.; Tuhumury, G.N. Potential of Bacillus Spp Produces Siderophores Insuppressing Thewilt Disease of Banana Plants. Proc. IOP Conf. Ser. Earth Environ. Sci. 2018, 102, 012016. [Google Scholar] [CrossRef]
- Verma, P.; Yadav, A.N.; Khannam, K.S.; Kumar, S.; Saxena, A.K.; Suman, A. Molecular Diversity and Multifarious Plant Growth Promoting Attributes of Bacilli Associated with Wheat (Triticum aestivum L.) Rhizosphere from Six Diverse Agro-Ecological Zones of India. J. Basic Microbiol. 2016, 56, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Charles, T.C.; Glick, B.R. Endophytic phytohormones and their role in plant growth promotion. In Functional Importance of the Plant Microbiome; Springer: Berlin/Heidelberg, Germany, 2017; pp. 89–105. [Google Scholar]
- Li, H.Q.; Jiang, X.W. Inoculation with Plant Growth-Promoting Bacteria (PGPB) Improves Salt Tolerance of Maize Seedling. Russ. J. Plant Physiol. 2017, 64, 235–241. [Google Scholar] [CrossRef]
- Soleimani, R.; Alikhani, H.A.; Towfighi, H.; Khavazi, K.; Pourbabaee, A.A. Isolated Bacteria from Saline–Sodic Soils Alter the Response of Wheat under High Adsorbed Sodium and Salt Stress. Int. J. Environ. Sci. Technol. 2017, 14, 143–150. [Google Scholar] [CrossRef]
- Großkinsky, D.K.; Tafner, R.; Moreno, M.V.; Stenglein, S.A.; De Salamone, I.E.G.; Nelson, L.M.; Novák, O.; Strnad, M.; Van Der Graaff, E.; Roitsch, T. Cytokinin Production by Pseudomonas fluorescens G20-18 Determines Biocontrol Activity against Pseudomonas syringae in Arabidopsis. Sci. Rep. 2016, 6, 23310. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.S.; Mekureyaw, M.F.; Pandey, C.; Roitsch, T. Role of Cytokinins for Interactions of Plants with Microbial Pathogens and Pest Insects. Front. Plant Sci. 2020, 10, 1777. [Google Scholar] [CrossRef]
- Egamberdieva, D. Alleviation of Salt Stress by Plant Growth Regulators and IAA Producing Bacteria in Wheat. Acta Physiol. Plant. 2009, 31, 861–864. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S. ACC Deaminase Producing Bacteria with Multifarious Plant Growth Promoting Traits Alleviates Salinity Stress in French Bean (Phaseolus vulgaris) Plants. Front. Microbiol. 2019, 10, 1506. [Google Scholar] [CrossRef]
- Fuertes-Mendizábal, T.; Bastías, E.I.; González-Murua, C.; González-Moro, M. Nitrogen Assimilation in the Highly Salt-and Boron-Tolerant Ecotype Zea mays L. Amylacea. Plants 2020, 9, 322. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Srivastava, A.K.; Tiwari, S.P.; Kumar, S. Microbes for Climate Resilient Agriculture; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Chen, L.; Dodd, I.C.; Theobald, J.C.; Belimov, A.A.; Davies, W.J. The Rhizobacterium Variovorax Paradoxus 5C-2, Containing ACC Deaminase, Promotes Growth and Development of Arabidopsis thaliana via an Ethylene-Dependent Pathway. J. Exp. Bot. 2013, 64, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Siddikee, M.A.; Sundaram, S.; Chandrasekaran, M.; Kim, K.; Selvakumar, G.; Sa, T. Halotolerant Bacteria with ACC Deaminase Activity Alleviate Salt Stress Effect in Canola Seed Germination. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 237–241. [Google Scholar] [CrossRef]
- Sarkar, A.; Pramanik, K.; Mitra, S.; Soren, T.; Maiti, T.K. Enhancement of Growth and Salt Tolerance of Rice Seedlings by ACC Deaminase-Producing Burkholderia sp. MTCC 12259. J. Plant Physiol. 2018, 231, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Jha, P.N. Alleviation of Salinity-Induced Damage on Wheat Plant by an ACC Deaminase-Producing Halophilic Bacterium Serratia sp. SL-12 Isolated from a Salt Lake. Symbiosis 2016, 69, 101–111. [Google Scholar] [CrossRef]
- Rodríguez-Llorente, I.D.; Pajuelo, E.; Navarro-Torre, S.; Mesa-Marín, J.; Caviedes, M.A. Bacterial Endophytes from Halophytes: How Do They Help Plants to Alleviate Salt Stress? In Saline Soil-Based Agriculture by Halotolerant Microorganisms; Springer: Berlin/Heidelberg, Germany, 2019; pp. 147–160. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Waqas, M.; Kang, S.-M.; Lee, I.-J. Inoculation of Abscisic Acid-Producing Endophytic Bacteria Enhances Salinity Stress Tolerance in Oryza Sativa. Environ. Exp. Bot. 2017, 136, 68–77. [Google Scholar] [CrossRef]
- Liu, S.; Tian, Y.; Jia, M.; Lu, X.; Yue, L.; Zhao, X.; Jin, W.; Wang, Y.; Zhang, Y.; Xie, Z.; et al. Induction of Salt Tolerance in Arabidopsis thaliana by Volatiles from Bacillus amyloliquefaciens FZB42 via the Jasmonic Acid Signaling Pathway. Front. Microbiol. 2020, 11, 562934. [Google Scholar] [CrossRef]
- Liu, S.; Hao, H.; Lu, X.; Zhao, X.; Wang, Y.; Zhang, Y.; Xie, Z.; Wang, R. Transcriptome Profiling of Genes Involved in Induced Systemic Salt Tolerance Conferred by Bacillus amyloliquefaciens FZB42 in Arabidopsis thaliana. Sci. Rep. 2017, 7, 10795. [Google Scholar] [CrossRef]
- Cassells, A.C.; Rafferty-McArdle, S.M. Priming of plant defences by PGPR against fungal and bacterial plant foliar pathogens. In Bacteria in Agrobiology: Stress Management; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–26. [Google Scholar]
- Bhise, K.K.; Dandge, P.B. Mitigation of Salinity Stress in Plants Using Plant Growth Promoting Bacteria. Symbiosis 2019, 79, 191–204. [Google Scholar] [CrossRef]
- Kang, S.-M.; Radhakrishnan, R.; Khan, A.L.; Kim, M.-J.; Park, J.-M.; Kim, B.-R.; Shin, D.-H.; Lee, I.-J. Gibberellin Secreting Rhizobacterium, Pseudomonas putida H-2-3 Modulates the Hormonal and Stress Physiology of Soybean to Improve the Plant Growth under Saline and Drought Conditions. Plant Physiol. Biochem. 2014, 84, 115–124. [Google Scholar] [CrossRef]
- Ha-Tran, D.M.; Nguyen, T.T.M.; Hung, S.-H.; Huang, E.; Huang, C.-C. Roles of Plant Growth-Promoting Rhizobacteria (PGPR) in Stimulating Salinity Stress Defense in Plants: A Review. Int. J. Mol. Sci. 2021, 22, 3154. [Google Scholar] [CrossRef]
- Wang, T.-T.; Ding, P.; Chen, P.; Xing, K.; Bai, J.-L.; Wan, W.; Jiang, J.-H.; Qin, S. Complete Genome Sequence of Endophyte Bacillus flexus KLBMP 4941 Reveals Its Plant Growth Promotion Mechanism and Genetic Basis for Salt Tolerance. J. Biotechnol. 2017, 260, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Mora-Ruiz, M.D.R.; Font-Verdera, F.; Orfila, A.; Rita, J.; Rosselló-Móra, R. Endophytic Microbial Diversity of the Halophyte Arthrocnemum macrostachyum across Plant Compartments. FEMS Microbiol. Ecol. 2016, 92, fiw145. [Google Scholar] [CrossRef] [PubMed]
- Tamosiune, I.; Baniulis, D.; Stanys, V. Role of Endophytic Bacteria in Stress Tolerance of Agricultural Plants: Diversity of Microorganisms and Molecular Mechanisms. In Probiotics Agroecosystem; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–29. [Google Scholar]
- Das, P.; Behera, B.K.; Chatterjee, S.; Das, B.K.; Mohapatra, T. De Novo Transcriptome Analysis of Halotolerant Bacterium Staphylococcus sp. Strain P-TSB-70 Isolated from East Coast of India: In Search of Salt Stress Tolerant Genes. PLoS ONE 2020, 15, e0228199. [Google Scholar] [CrossRef]
- Kushwaha, P.; Kashyap, P.L.; Kuppusamy, P.; Srivastava, A.K.; Tiwari, R.K. Functional Characterization of Endophytic Bacilli from Pearl Millet (Pennisetum glaucum) and Their Possible Role in Multiple Stress Tolerance. Plant Biosyst. Int. J. Deal. Aspects Plant Biol. 2020, 154, 503–514. [Google Scholar] [CrossRef]
- Dong, Z.-Y.; Rao, M.P.N.; Wang, H.-F.; Fang, B.-Z.; Liu, Y.-H.; Li, L.; Xiao, M.; Li, W.-J. Transcriptomic Analysis of Two Endophytes Involved in Enhancing Salt Stress Ability of Arabidopsis thaliana. Sci. Total Environ. 2019, 686, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Martínez, F.L.; Rajal, V.B.; Irazusta, V.P. Genomic Characterization and Proteomic Analysis of the Halotolerant Micrococcus luteus SA211 in Response to the Presence of Lithium. Sci. Total Environ. 2021, 785, 147290. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Shin, W.; Siddikee, M.A.; Joe, M.M.; Benson, A.; Kim, K.; Selvakumar, G.; Kang, Y.; Jeon, S.; Samaddar, S.; Chatterjee, P. Halotolerant Plant Growth Promoting Bacteria Mediated Salinity Stress Amelioration in Plants. Korean J. Soil Sci. Fertil. 2016, 49, 355–367. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).