Evaluation of the Allelopathic Activity of Albizia procera (Roxb.) Benth. as a Potential Source of Bioherbicide to Control Weeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Extract Preparation and Growth Bioassay
2.3. Isolation of A. procera Plant Extracts
2.4. Statistical Analysis
3. Results
3.1. Allelopathic Effect of A. procera Plant Extracts
3.2. Purification of Allelopathic Compounds from A. procera Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Anthony, S. Agroforestree Database: A Tree Reference and Selection Guide Version 4.0. 2009. Available online: http://www.worldagroforestry.org/sites/treedbs/treedatabases.asp (accessed on 15 September 2022).
- Zich, F.A.; Hyland, B.P.M.; Whiffen, T.; Kerrigan, R.A. “Albizia procera”. Australian Tropical Rainforest Plants Edition 8 (RFK8); Centre for Australian National Biodiversity Research (CANBR): Acton, ACT, Australia, 2020; Retrieved 1 March 2021.
- Mahfuza, K.; Hajera, K.; Ekramul, I.; Shahnaj, P. Analgesic, antibacterial and central nervous system depressant activities of A. procera leaves. Asian Pac. J. Top. Biomed. 2014, 4, 279–284. [Google Scholar]
- Parrotta, J.A.; Roshekto, J.M. A Publication of the Forest, Farm, and Community Tree Network (FACT Net); Winrock International: Washington, DC, USA, 1997. [Google Scholar]
- Das, D.K.; Alam, M.K. Trees of Bangladesh; Bangladesh Forest Research Institute (BFRI): Chittagong, Bangladesh, 2001.
- Luna, R.K. Plantation Trees; International Book Distributors: Dehra Dun, India, 1996. [Google Scholar]
- Pradhan, A.; Bhuyan, S.; Chhetri, K.; Mandal, S.; Bhattacharyya, A. Saponins from A. procera extract: Surfactant activity and preliminary analysis. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128778. [Google Scholar] [CrossRef]
- Troup, R.S. The Silviculture of Indian Trees; Clarendon Press: Oxford, UK, 1921; Volume 2. [Google Scholar]
- Alam, M.K.; Mohiuddin, M.; Basak, S.R. Village trees of Bangladesh: Diversity and economic aspects. Bangladesh J. For. Sci. 1996, 25, 21–36. [Google Scholar]
- Khan, M.S.; Alam, M.K. Homestead Flora of Bangladesh; Forestry Division, Bangladesh Agricultural Research Council (BARC): Dhaka, Bangladesh, 1996.
- Gupta, G.; Yadav, R.S.; Maurya, D. Decomposition and nitrogen dynamics of tree pruned biomass under A. procera based agroforestry system in semi-arid region of bundelkhand, India. Curr. World Environ. 2017, 12, 3. [Google Scholar] [CrossRef]
- Parotta, J.A.; Roshetko, J.M. Albizia procera–White Siris for Reforestation and Agroforestry; FACT Net 97–01; Winrock International: Washington, DC, USA, 1997. [Google Scholar]
- Asolker, L.V.; Kakkar, K.K.; Chakre, O.J. Supplement to Glossary of Indian Medicinal Plants. Part-I (A-K); National Institute of Science Communication: New Delhi, India, 2000; pp. 116–117. [Google Scholar]
- Sarkar, A.K. Studies on In Vitro Regeneration and Related Biochemical Investigations in A. procera Benth. Ph.D. Thesis, Rani Durgavati University, Jabalpur, India, 2007. [Google Scholar]
- Shaik, A.; Yalavarthi, P.; Bannoth, C. Role of anti-fertility medicinal plants on male & female reproduction. J. Complement. Altern. Med. Res. 2017, 3, 1–22. [Google Scholar]
- Anand, D.; Sathish, M.; Dhivya, L.S. In vitro α-amylase and α-glucosidase inhibitor activities of A. procera stem bark. Asian J. Pharm. Clin. Res. 2018, 11, 344–347. [Google Scholar]
- Sangeetha, M.; Chamundeeswari, D.; Babu, C.S.; Rose, C.; Gopal, V. Attenuation of oxidative stress in arthritic rats by ethanolic extract of A. procera benth bark through modulation of the expression of inflammatory cytokines. J. Ethnopharmacol. 2020, 250, 112435. [Google Scholar] [CrossRef] [PubMed]
- Pasala, L.P.K.; Reddy, S.S.N.; Silvia, Y.; Reddy, A.D.; Sampath, N.; Dorababu, N.V.L.; Mulukuri, K.T.S.; Kumar, M.S.; Chandana, C.S.; Chetty, M.; et al. Molecular docking and in vivo immunomodulatory activity of A. procera bark on doxorubicin induced immunosuppressive rats. J. King Saud. Univ. Sci. 2022, 34, 101828. [Google Scholar] [CrossRef]
- Kokila, K.; Priyadharshini, S.D.; Sujatha, V. Phytopharmacological properties of albizia species: A review. Int. J. Pharm. Pharm. Sci. 2013, 5, 70–73. [Google Scholar]
- Srivastava, V.; Kumar, V.S.; Panwar, S.; Deep, P.; Verma, S. A brief review on phytopharmacological reports on Albizia procera. Asian J. Pharm. Sci. 2020, 6, 144–149. [Google Scholar] [CrossRef]
- Perveen, S.; Yousaf, M.; Mushtaq, M.N.; Sarwar, N.; Khan, M.; Nadeem, S.M. Bioherbicidal potential of some allelopathic agroforestry and fruit plant species against Lepidium sativum. Soil Environ. 2019, 38, 119–126. [Google Scholar] [CrossRef]
- Chauhan, B.S. Recommendations for improved weed management. Front. Agron. 2020, 1, 1–4. [Google Scholar]
- Gharde, Y.; Singh, P.K.; Dubey, R.P.; Gupta, P.K. Assessment of yield and economic losses in agriculture due to weeds in India. Crop Prot. 2018, 107, 12–18. [Google Scholar] [CrossRef]
- Kraehmer, H.; Laber, B.; Rosinger, C.; Schulz, A. Herbicides as weed control agents: State of the art: I. Weed control research and safener technology: The path to modern agriculture. Plant Physiol. 2014, 166, 1119–1131. [Google Scholar] [CrossRef]
- Kraehmer, H.; Van Almsick, A.; Beffa, R.; Dietrich, H.; Eckes, P.; Hacker, E.; Hain, R.; Strek, H.J.; Stuebler, H.; Willms, L. Herbicides as weed control agents: State of the art: II. Recent achievements. Plant Physiol. 2014, 166, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Bocker, T.; Mohring, N.; Finger, R. Herbicide free agriculture? A bio-economic modelling application to Swiss wheat production. Agric. Syst. 2019, 173, 378–392. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef]
- Gasnier, C.; Dumont, C.; Benachour, N.; Clair, E.; Chagnon, M.C.; Sferalini, G.E. Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology 2009, 262, 184–191. [Google Scholar] [CrossRef]
- Moss, S.; Ulber, L.; den Hoed, I. A herbicide resistance risk matrix. Crop Prot. 2019, 115, 13–19. [Google Scholar] [CrossRef]
- Heap, I. The International Survey of Herbicide Resistant Weeds. Available online: www.weedscience.org (accessed on 30 August 2022).
- Heap, I.; Duke, S.O. Overview of glyphosate-resistant weeds worldwide. Pest. Manag. Sci. 2018, 74, 1040–1049. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, W.; Ain, Q.; Siddiqui, M.B.; Hakeem, K.R. Cytotoxic allelochemicals induce ultrastructural modifications in Cassia tora L. and mitotic changes in Allium cepa L.: A weed versus weed allelopathy approach. Protoplasma 2019, 256, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Ustuner, T.; Sakran, L.; Almhemed, K. Effect of herbicides on living organisms in the ecosystem and available alternative control methods. Int. J. Sci. Res. Publ. 2020, 10, 622–632. [Google Scholar]
- Thawabteh, A.; Juma, S.; Bader, M.; Karaman, D.; Scrano, L.; Bufo, S.A.; Karaman, R. The biological activity of natural alkaloids against herbivores, cancerous cells and pathogens. Toxins 2019, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- Soltys, D.; Krasuska, U.; Bogatek, R.; Gniazdow, A. Allelochemicals as bio-herbicides present and perspectives. In Herbicides Current Research and Case Studies in Use; Price, A.J., Kelton, J.A., Eds.; In Tech: Rijeka, Croatia, 2013; pp. 517–542. [Google Scholar]
- Mendes, I.D.S.; Rezende, M.O.O. Assessment of the allelopathic effect of leaf and seed extracts of Canavalia ensiformis as post emergent bioherbicides: A green alternative for sustainable agriculture. J. Environ. Sci. Health Part B 2014, 49, 374–380. [Google Scholar] [CrossRef]
- Bailey, K.L. The bioherbicide approach to weed control using plant pathogens. In Integrated Pest Management; Academic Press: Cambridge, MA, USA, 2014; pp. 245–266. [Google Scholar]
- Cordeau, S.; Triolet, M.; Wayman, S.; Steinberg, C.; Guillemin, J.P. Bioherbicides: Dead in the water? A review of the existing products for integrated weed management. Crop Prot. 2016, 87, 44–49. [Google Scholar] [CrossRef]
- El-Darier, S.M.; Abdelaziz, H.A.; El-Dien, M.H.Z. Effect of soil type on the allelotoxic activity of Medicago sativa L. residues in Vicia faba L. agroecosystems. J. Taibah Univ. Sci. 2014, 8, 84–89. [Google Scholar] [CrossRef][Green Version]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef]
- Hossen, K.; Ozaki, K.; Teruya, T.; Kato-Noguchi, H. Three active phytotoxic compounds from the leaves of Albizia richardiana (Voigt.) King and Prain for the development of bioherbicides to control weeds. Cells 2021, 10, 2385. [Google Scholar] [CrossRef]
- Hossen, K.; Das, K.R.; Asato, Y.; Teruya, T.; Kato-Noguchi, H. Allelopathic activity and characterization of allelopathic substances from Elaeocarpus floribundus Blume leaves for the development of bioherbicides. Agronomy 2022, 12, 57. [Google Scholar] [CrossRef]
- Islam, M.S.; Zaman, F.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Isolation and identification of three potential phytotoxic compounds from Chrysopogon aciculatus (Retz.) Trin. Acta Physiol. Plant 2021, 43, 56. [Google Scholar] [CrossRef]
- Rob, M.; Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic activity and identification of phytotoxic substances from Schumannianthus dichotomus. Plants 2020, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Hossen, K.; Das, K.R.; Okada, S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Allelopathic potential and active substances from Wedelia chinensis (Osbeck). Foods 2020, 9, 1591. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, N.A. Allelopathic effect of leaf extract of two wild plants on seed germination, shoot and root length of two weed species; Portulaca oleracea and Chenopodium murale. Biosci. Biotechnol. Res. Asia 2018, 15, 929–935. [Google Scholar] [CrossRef]
- Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic activity and growth inhibitory substances from Albizia richardiana (Voigt.) King & Prain. Appl. Sci. 2021, 11, 1455. [Google Scholar]
- Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxicity of the novel compound 3-hydroxy-4-oxo-β-dehydroionol and compound 3-oxo-α-ionone from Albizia richardiana (Voigt.) King & Prain. Environ. Technol. Innov. 2021, 23, 101779. [Google Scholar]
- Hossen, K.; Kato-Noguchi, H. Determination of allelopathic properties of Acacia catechu (L.f.) Willd. Not. Bot. Horti. Agrobot. Cluj. Napoca. 2020, 48, 2050–2059. [Google Scholar] [CrossRef]
- Mousavi, S.H.; Alami-Saeid, K.H.; Moshatati, A. Effect of leaf, stem and root extract of alfalfa (Melilotus indicus) on seed germination and seedling growth of wheat (Triticum aestivum). Int J. Agric. Crop Sci. 2013, 5, 44–49. [Google Scholar]
- Liu, J.; Xie, M.; Li, X.; Jin, H.; Yang, X.; Yan, Z.; Su, A.; Qin, B. Main allelochemicals from the rhizosphere soil of Saussurea lappa (Decne.) Sch. Bip. and their effects on plants’ antioxidase systems. Molecules 2018, 23, 2506. [Google Scholar] [CrossRef]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef]
- Irshad, A.; Cheema, Z.A. Influence of some plant water extracts on the germination and seedling growth of barnyard grass (E. crus-galli (L) Beauv). Pak. J. Sci. Ind. Res. 2004, 43, 222–226. [Google Scholar]
- El-Mergawi, R.A.; Al-Humaid, A.I. Searching for natural herbicides in methanol extracts of eight plant species. Bull. Natl. Res. Cent. 2019, 43, 22. [Google Scholar] [CrossRef]
- Chen, F.; Meng, Y.; Shuai, H.; Luo, X.; Zhou, W.; Liu, J.; Shu, K. Effect of plant allelochemicals on seed germination and its ecological significance. Chin. J. Eco. Agric. 2017, 25, 36–46. [Google Scholar]
- Motmainna, M.; Juraimi, A.S.; Uddin, M.K.; Asib, N.B.; Islam, A.K.M.; Hasan, M. Allelopathic potential of Malaysian invasive weed species on weedy rice (Oryza sativa f. spontanea Roshev). Allelopath. J. 2021, 53, 53–68. [Google Scholar] [CrossRef]
- Najafpour, M.M. Oxygen evolving complex in photosystem II: Better than excellent. Dalton Trans. 2011, 40, 9076–9084. [Google Scholar] [CrossRef]
- Lee, S.M.; Radhakrishnan, R.; Kang, S.M.; Kim, J.H.; Lee, I.Y.; Moon, B.Y.; Yoon, B.W.; Lee, I.J. Phytotoxic mechanisms of bur cucumber seed extracts on lettuce with special reference to analysis of chloroplast proteins, phytohormones, and nutritional elements. Ecotoxicol. Environ. Saf. 2015, 122, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Shaul, O. Magnesium transport and function in plants: The tip of the iceberg. Biometals 2002, 15, 309–323. [Google Scholar] [CrossRef] [PubMed]

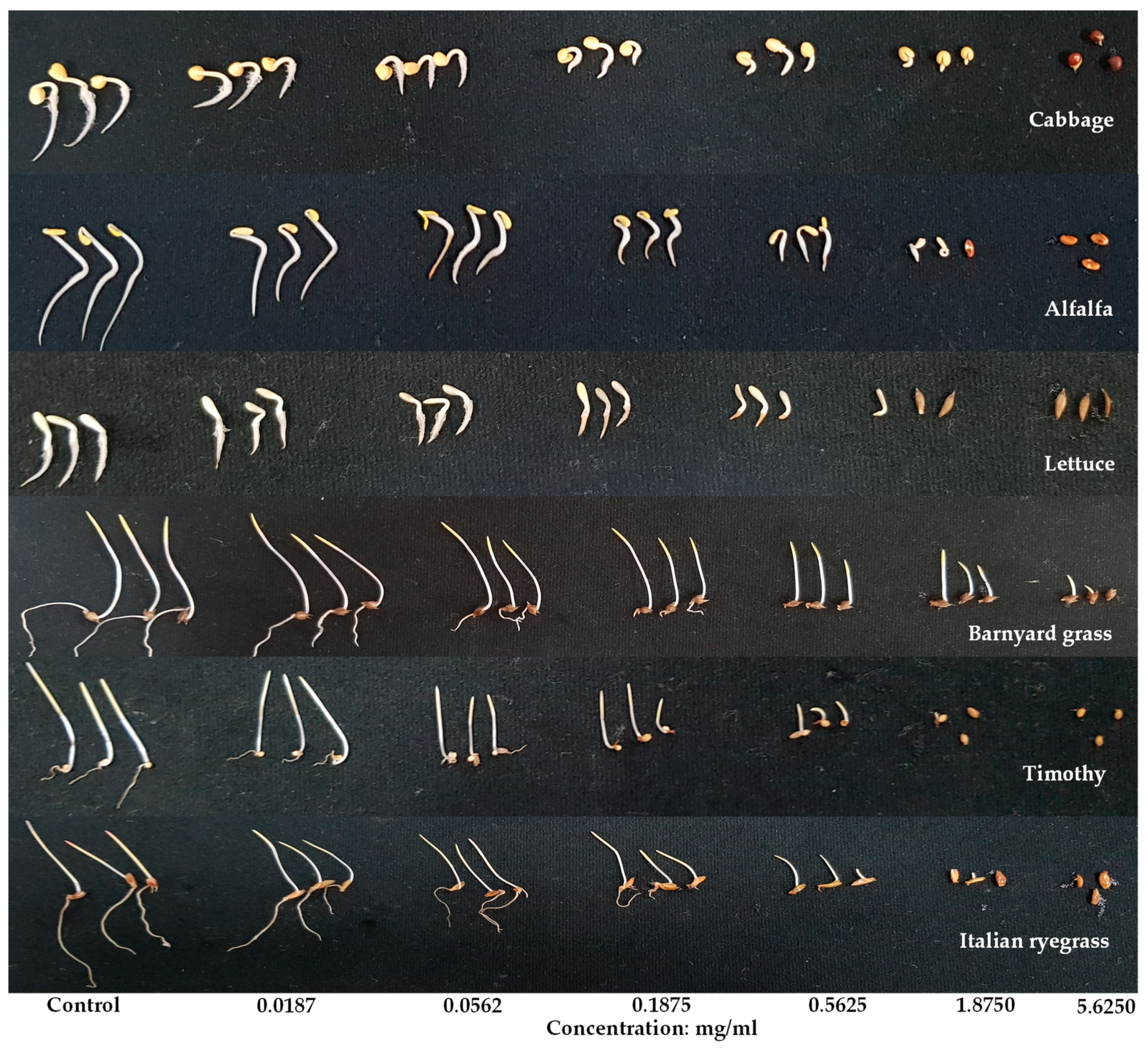
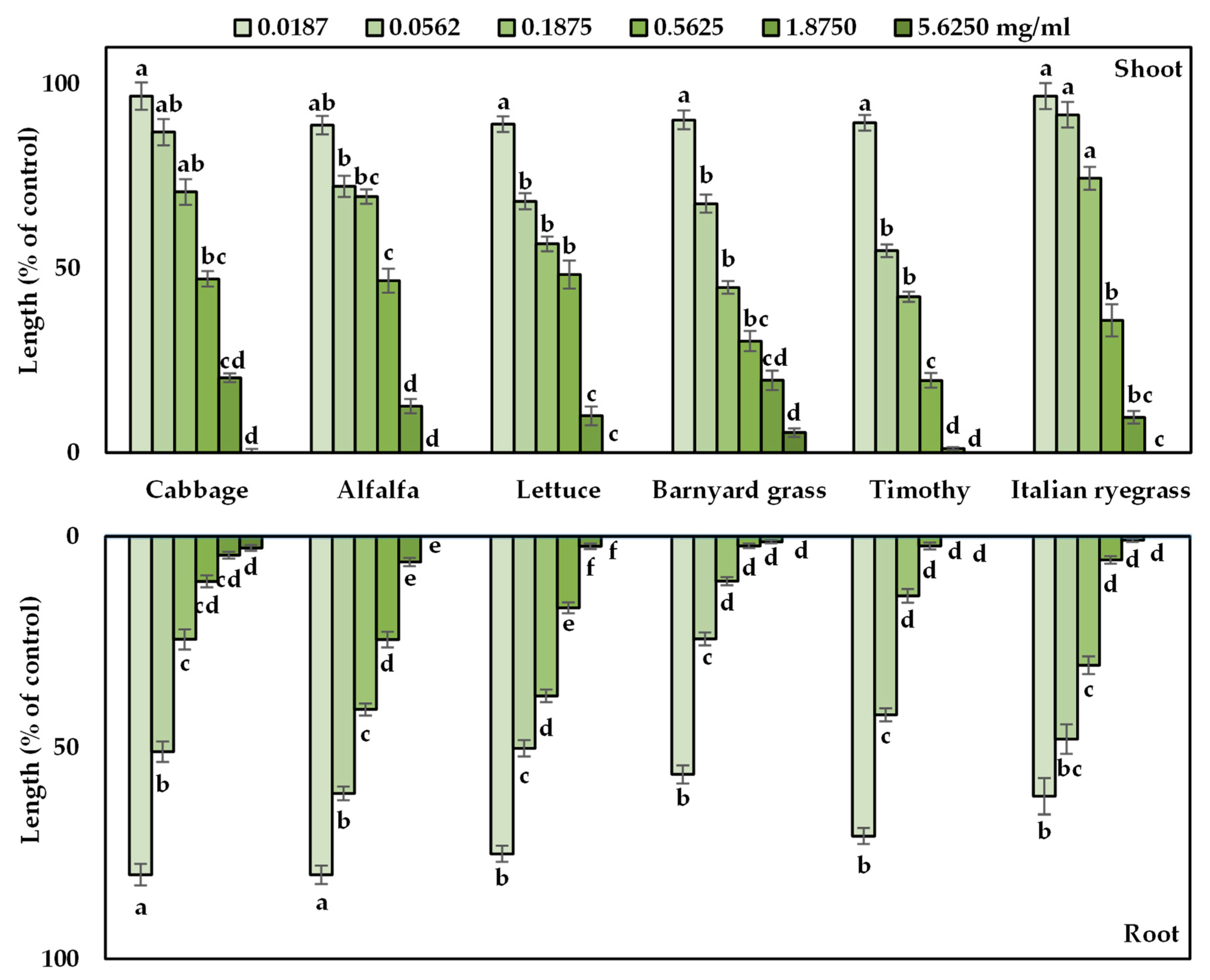
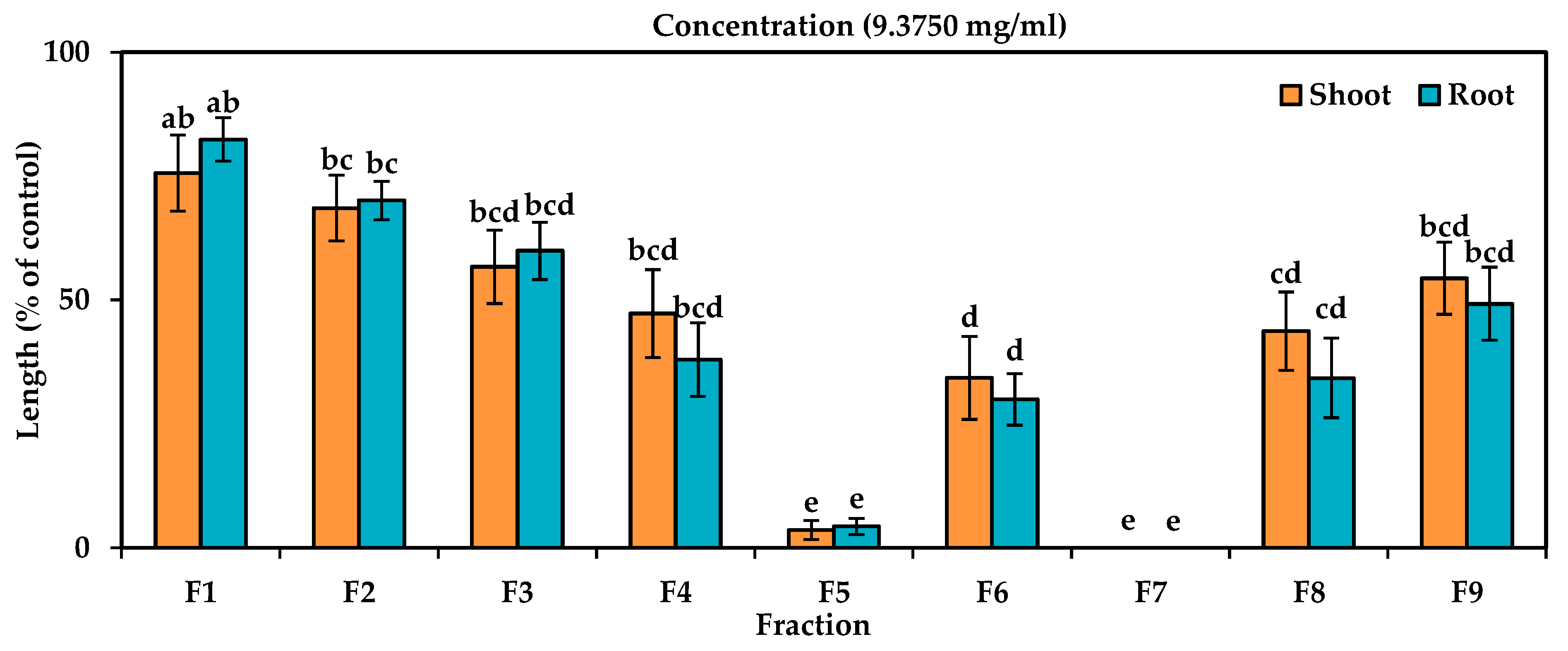

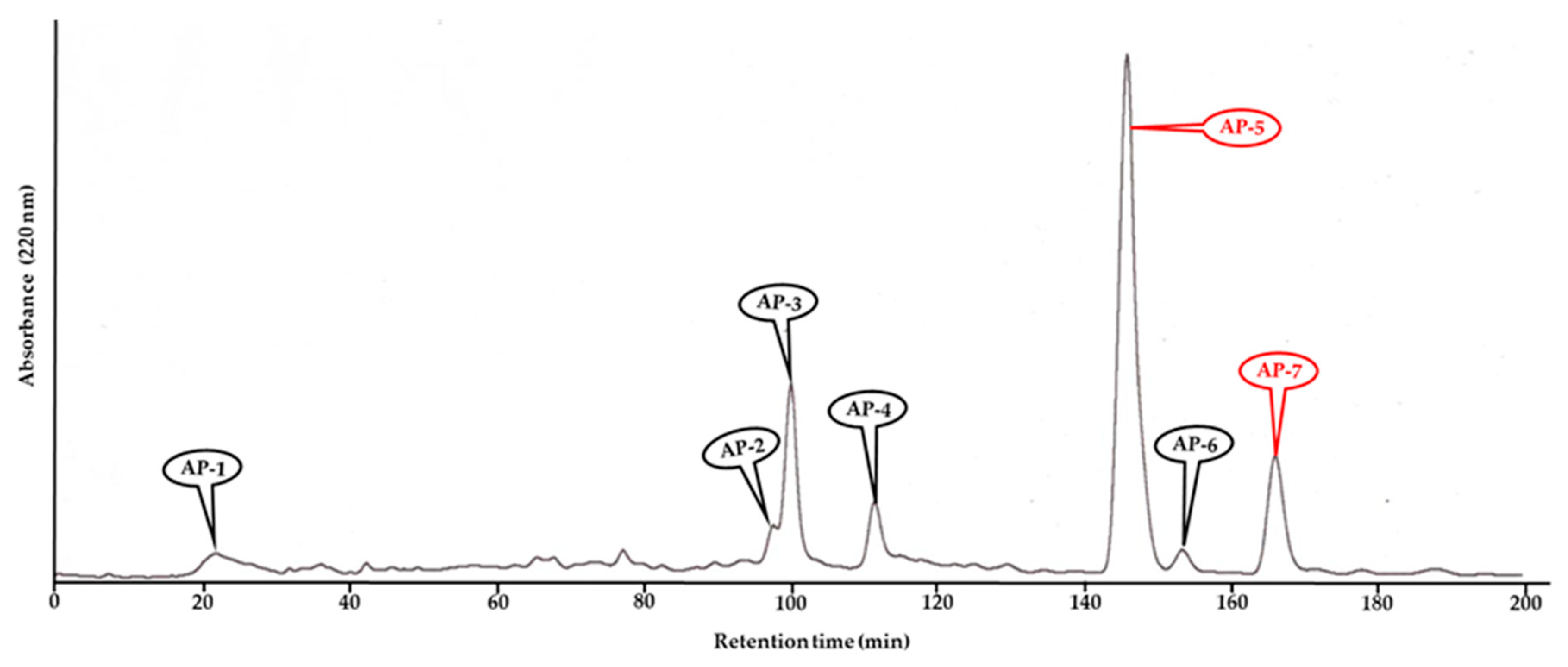
| Test Plant Species | I50 Value (mg/mL) | ||
|---|---|---|---|
| Shoot ** | Root ** | ||
| Dicot | Cabbage | 0.4935 | 0.0823 |
| Alfalfa | 0.4800 | 0.1087 | |
| Lettuce | 0.4500 | 0.0562 | |
| Monocot | Barnyard grass | 0.1425 | 0.0225 |
| Timothy | 0.0862 | 0.0412 | |
| Italian ryegrass | 0.3765 | 0.0476 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossen, K.; Kato-Noguchi, H. Evaluation of the Allelopathic Activity of Albizia procera (Roxb.) Benth. as a Potential Source of Bioherbicide to Control Weeds. Int. J. Plant Biol. 2022, 13, 523-534. https://doi.org/10.3390/ijpb13040042
Hossen K, Kato-Noguchi H. Evaluation of the Allelopathic Activity of Albizia procera (Roxb.) Benth. as a Potential Source of Bioherbicide to Control Weeds. International Journal of Plant Biology. 2022; 13(4):523-534. https://doi.org/10.3390/ijpb13040042
Chicago/Turabian StyleHossen, Kawsar, and Hisashi Kato-Noguchi. 2022. "Evaluation of the Allelopathic Activity of Albizia procera (Roxb.) Benth. as a Potential Source of Bioherbicide to Control Weeds" International Journal of Plant Biology 13, no. 4: 523-534. https://doi.org/10.3390/ijpb13040042
APA StyleHossen, K., & Kato-Noguchi, H. (2022). Evaluation of the Allelopathic Activity of Albizia procera (Roxb.) Benth. as a Potential Source of Bioherbicide to Control Weeds. International Journal of Plant Biology, 13(4), 523-534. https://doi.org/10.3390/ijpb13040042







