Abstract
The mulberry tree (Morus alba) is a perennial and fast-growing tree distributed worldwide under different climatic conditions. Most of the world’s silk production (>90%) is facilitated by the feeding of silkworm larvae on the leaves of mulberry (Morus alba L.) varieties. Therefore, exploration of the protocol for improving the propagation efficiency and increasing the reproductive capacity of M. alba varieties could be of great significance. This study aimed to determine the effect of four concentrations (0, 100, 200 and 400 mg L−1) each of naphthaleneacetic acid (NAA), indole-3-butyric acid (IBA), and zinc sulfate (0, 100 and 200 mg L−1), supplied separately or combined, on the rooting and growth of mulberry cuttings. M. alba cuttings were immersed for 5 s in each solution using the quick-dip method and subsequently, the cuttings were dried and planted in plastic pots and maintained in a greenhouse for 60 days. The number of leaves (NL), longest root size (LRS), longest stem size (LSS), number of rooted cuttings (NRC), number of stems per tree (NSP), rooting percentage (RP), wet root weight (WRW), dry root weight (DRW), wet stem weight (WSW), dry stem weight (DSW), wet leaf weight (WLW) and dry leaf weight (DLW) were evaluated. The results obtained showed an increase in all growth parameters of the mulberry cuttings. Treatments of hormones (IBA and NAA) and Zn sulfate were effective on LSS, LRS and WSW. The highest values of LSS were obtained for the treatments T5, T6, T14, T15, T16 and T18. Moreover, T5, T12 and T10 showed the highest values of LRS. The highest value of WSW was observed for T18, T5, T14, T15 and T16. The highest values of WLW and DLW were observed in T20 and T14. Dry stem weight (DSW) was high in T18 and T14. The application of NAA (at 200 mg L−1), IBA (200 and 400 mg L−1) and Zn sulfate (200 and 400 mg L−1), either alone or in double combination, can be a suitable and reliable method for mulberry propagation.
1. Introduction
The mulberry tree (Morus alba L.) is a perennial and fast-growing tree distributed worldwide under different climatic conditions. M. alba has great economic importance and its production is focused on the improvement of its foliage for the feeding of the silkworm (Bombyx mori Linnaeus, 1758), which converts M. alba into silk protein [1]. M. alba fruits are tasty and are widely used in the production of jellies, pulps, juices, ice creams, and wines [2,3]. M. alba fruits contain several health-promoting compounds, such as carbohydrates, vitamins, minerals, amino acids, and antioxidants [4]. In addition, the roots and stem bark of M. alba are a rich source of phenolic compounds, such as maclurin, rutin, isoquercitrin, and resveratrol, and have purgative and astringent properties [5]. Mulberry leaves are a rich source of (poly)phenolic substances, including phenolic acids and flavonoids such as caffeic acid, caffeoylquinic acids, kaempferol-3-O-(6-malonyl)–glucoside, quercetin-3-O-(6-malonyl)–glucoside, and quercetin-3-O-glucoside [6].
Plants of the Morus genus are valuable and are popular in ancient herbal medicine with extensive pharmacological potential to cure various ailments. While phytochemicals from plants of the Morus genus have already been extensively analysed, there may still be unknown compounds that play a role in the plant’s biological function, which needs to be elucidated [7]. Preparations made from the leaves have been used for treating elevated glucose levels in blood, high blood pressure, inflammation, cancer, and as a cough suppressant. Leaves are dried and used, as infusions of the dried leaves are rich in amino acids, steroids, flavonoids, vitamins and proteins; therefore, the dried leaf infusions areused as a supplement for general wellness [8]. The root bark of Morus plants has been used for the treatment of cough, yellow sputum, bronchitis, xerophthalmia, nephritis, pulmonary diseases, and incised wounds. They have also been used for anti-inflammatory, diuretic, anti-tussive, and anti-pyretic purposes in oriental medicine [9]. Stem bark is used as an anti-rheumatic, anti-spasmodic, diuretic, hypotensive, and pectoral medicine. The stem bark is also used as a purgative and vermifuge agent [10]. Specifically, the methanolic extract of Morus alba has a strong antidopaminergic impact, which suggests it may be used to alleviate psychotic symptoms alongside pharmaceutical treatments and manage psychotic disorders [11]. Around 14 compounds were isolated from the ethanolic extract of the root bark of Morus alba, primarily flavonoids, such asmulberrofuran D, mulberrofuran G, and mulberrofuran K., morusin, kuwanon G, kuwanon H, and their other derivatives. When tested against human immunodeficiency virus (HIV-1), only morusin 4′-glucoside, kuwanon H, and morusin were found to be effective against the virus [12]. Black mulberry consumption by patients with lipid disorders can affect heart health risk factors such as apolipoprotein concentration, blood pressure, and inflammatory markers [13].
The adventitious root formation in hardwood cuttings is a key physiological process for the propagation of many plant species of economic importance. Despite the intensive control of environmental factors, high economic losses still occur because of insufficient rooting [14]. The propagation of M. alba can be performed using different sexual or asexual methods. Sexual reproduction is accomplished through seed preparation and planting, whereas asexual multiplication includes grafting, sprouting, cuttings, and tissue culture. Because of the existence of mixed and randomized pollination in M. alba, the sexual reproduction method is not appropriate for large-scale propagation. In addition, some methods of asexual transplantation and tissue culture demand high production and maintenance costs [15]. Therefore, shoot-cutting with the use of growth regulators appears to be the most suitable option for mulberry propagation. It is urgent and necessary to develop new techniques of M. alba propagation using biotechnological tools, to guarantee the conservation of this species and to increase its productivity [1].
Auxin activity was classically defined as the competence to stimulate elongation in coleoptile and stem sections, and also rooting. Auxin plays an essential role in the formation of adventitious roots by increasing root primordia initiation and growth via cell division, as well as promoting the hydrolysis of starch and the mobilization of sugars and nutrients to the cutting base. In addition, auxin not only acts through linear pathways but is also involved in many cross-talk responses with other phytohormones [16,17]. Natural auxins, such as indole-3-acetic acid (IAA), phenylacetic acid (PAA) and indole-3-butyric acid (IBA) regulate cell division, cell growth, ethylene biosynthesis, root development, leaf formation, apical dominance, differentiation of vascular tissues, and fruit setting [18]. Synthetic auxins such as 1-naphthaleneacetic acid (NAA) induce similar physiological responses to natural auxins in bioassays [19]. In particular, IBA and NAA are the most important auxins for the rooting of cuttings, as most plant species respond positively to their action [20,21]. IBA is more stable than IAA and persists longer in the plant tissues [22]. The use of IBA generally causes root production in a larger number of cuttings, a faster rooting onset and a significant improvement in root quality and seedling production [23,24]. Henrique et al. [25] evaluated the effect of different growth regulators on the rooting of Pinus caribaea and found that an IBA + paclobutrazol treatment produced a higher percentage of rooted cuttings than those treated with NAA in different concentrations. Similarly, Singh et al. [26] observed that IBA (2000 mg L−1) application improved all growth parameters in M. alba cuttings. The study of the exogenous application of natural (IAA, PAA and IBA) and synthetic (NAA) auxins on the growth and metabolism of the green microalga Chlorella vulgaris showed that IAA and IBA had the highest biological activity at 0.1 μM, whereas PAA and NAA had the highest stimulatory effect on the number of cells at 1 µM [27].
Mineral nutrients can be an important source of several cofactors for adventitious root production, and their use in appropriate amounts might be critical for a satisfactory rooting rate of cuttings. Zinc (Zn) is a micronutrient that contributes to tryptophan biosynthesis, the amino acid precursor for IAA production [28]. In addition, Zn takes part in the biosynthesis of essential enzymes, protein synthesis, carbohydrate regulation and starch production [29], as well as in proper root differentiation, all of which are essential cuttingpropagation processes [30,31]. Thus, the use of a combination of Zn with synthetic auxin-derivatives for the rooting of cuttings can not only increase the production of roots but also improve other growth parameters. Although the seedling propagation of different mulberry varieties using plant regulators is well-known, little is known about the action of these hormones combined with mineral compounds, such as Zn.
Given the above, this study was conducted to assess if the rooting and growth parameters in M. alba can be increased by the addition of Zn and different IBA and NAA concentrations at two cutting times.
2. Materials and Methods
The study was conducted at the Iran Silk Research Centre (ISRC), Rasht, Iran. The harvesting of branches for preparing M. alba seedlings of the Kairyo-nezumigaeshi variety was carried out using twenty healthy adult trees of uniform size, maintained with daily irrigation. For this purpose, after a light pruning in the previous winter, no harvest was performed until the collection of branches for cutting preparation. The branches were collected, identified and stored in the dark at a low temperature (<10 °C). The medium regions of the tree lateral branches were evenly cut to prepare the cuttings. Each cutting had at least five buds and was 20–25 cm long × 1.0–1.5 cm in diameter. The experiment was conducted using afactorial design with three randomized replications (three cuttings per replicate). Two cutting times (March and April), and twenty combinations of different concentrations of two plant hormones and Zn sulfate (T1 to T25) were used as factors in this experiment. The Kairyo-nezumigaeshi, as the main mulberry variety of region, was studied. The treatments included four different concentrations (0, 100, 200 and 400 mg L−1) of NAA and IBA and three concentrations (0, 200 and 400 mg L−1) of Zn sulfate. The effect of each compound on plant growth was evaluated separately and combined. The different solutions were prepared by dissolving the amount of each hormone in 10 mL of methanol and adding distilled water to make up a final solution of 1 L. The basal ends of the cuttings obtained from M. alba matrices were immediately immersed in the respective solutions using the quick-dip method, which consists of the dissolution of auxin in alcohol and the immersion of ~3 cm of the cut end of the cutting in the solution for 1min. The cuttings were dried for 15 min and then planted in plastic pots (20 × 10 cm) filled with a mixture of soil + bovine manure and sand (1:3; v/v). The pots were maintained in a greenhouse with periodic irrigation, a relative air humidity of between 75–80% and a controlled temperature of approximately 25 °C for 60 days (Figure 1). The same methods of maintaining climatic conditions were used.

Figure 1.
Mulberry cuttings with NAA, IBA, and Zn sulfate. (a) Mulberry cuttings in pots with soil + bovine manure and sand (1:3; v/v), and (b,c) the growth of mulberry cuttings in the greenhouse at 75–80% relative humidity and 25 °C.
For the measurement of growth parameters, the rooted cuttings in each treatment were collected and removed from the experimental units twice (in March and April). A cutting was considered rooted when it exhibited one or more roots with a length longer than 1 cm [13]. The standard maintenance protocols followed for normal survival and growing. Twelve growth parameters were measured: number of leaves (NL); the longest root size (LRS); the longest stem size (LSS); number of rooted cuttings (NRC); number of stems per tree (NSP); rooting percentage (RP); wet root weight (WRW); dry root weight (DRW); wet stem weight (WSW); dry stem weight (DSW); wet leaf weight (WLW); and dry leaf weight (DLW), using a measuring tape with an accuracy of 0.1 cm and a digital scale with an accuracy of 0.01 g [23]. The data were collected from all of the treatments together. Data were tested for normality and homogeneity of variance using the Kolmogorov–Smirnov test. Because the test of homogeneity of variances and test of normality of data are prerequisites for an analysis of variance (ANOVA), these statistical analyses were performed to inform the ANOVA. The analysis of variance was performed considering the cutting time (March and April) as a factor (independent variable) and the biometric parameters × treatments as response variables (dependent variables). Differences of statistical significance between means were evaluated with the LSD test (p < 0.05), using the statistical package SAS 2000 (SAS Institute Inc., Cary, NC, USA).
3. Results
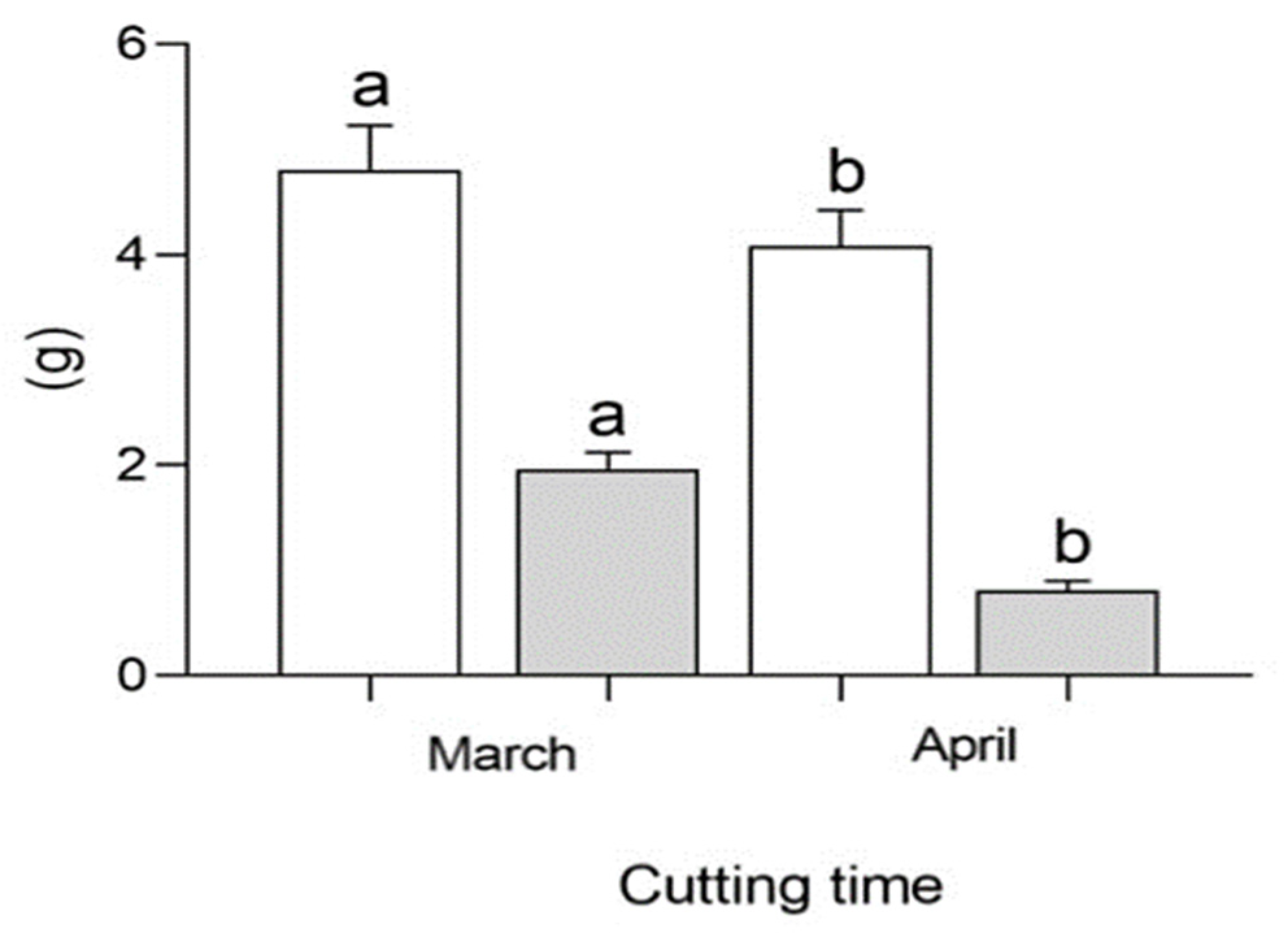
The analysis of variance showed that the effect of cutting time was significant for NL, WRW and DRW (Table 1). The application of hormone + Zn sulfate evidenced the interaction of most parameters, such as NL, LSS, NRC, RP, LRS, WRW, DRW, WLW, DLW and WSW. The interaction between cutting time × hormone + Zn sulfate treatments did not show differences in the two years. In Figure 2, wet root weight (WRW) and dry root weight (DRW) are shown in white and gray, respectively. Both of these parameters increased at the first cutting time (March) compared to the second cutting time (April). The cutting time also influenced the rooting and growth parameters of the cuttings (Table 2). The number of leaves increased in March, whereas the number of rooted cuttings was only higher in April. The rooting percentage increased in April. The wet and dry weight of the leaves was significantly different between cutting times.

Table 1.
Analysis of Variance (ANOVA) for growth parameters of mulberry cuttings considering cutting time, hormone (NAA, IBA) and Zn sulfate treatments. NL, number of leaves; LSS, longest stem size; NRC, number of rooted cuttings; RP, rooting percentage; NSP, number of stems per tree; LRS, longest root size; WRW, wet root weight; DRW, dry root weight; WLW, wet leaf weight; DLW, dry leaf weight; WSW, wet stem weight; DSW, dry stem weight. * and ** indicate statistical significance at p < 0.05 and p < 0.01, respectively, by LSD test.
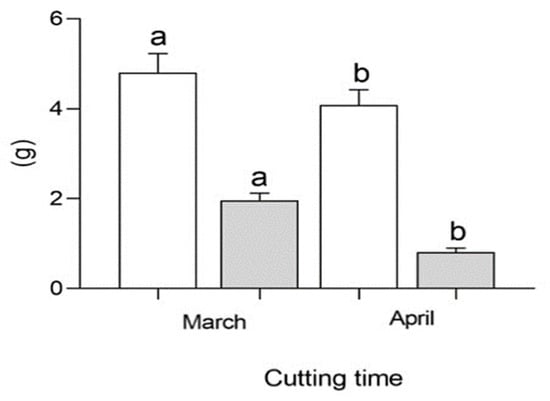
Figure 2.
Comparison of wet root weight (WRW, white) and dry root weight (DRW, gray) of mulberry cuttings with NAA, IBA and Zn sulfate in successive years. Different letters for any of the traits (white and gray) indicate differences of statistical significance (p < 0.05) by LSD test.

Table 2.
Influence of cutting time on the growth parameters of mulberry cuttings. NL, number of leaves; NRC, number of rooted cuttings; RP, rooting percentage. Different letters indicate differences with a statistical significance (p < 0.05) by LSD test.
Treatments of hormones and Zn sulfate were effective for LSS, LRS and WSW (Table 3). The highest values of LSS were obtained for T5, T6, T14, T15, T16 and T18. Moreover, T5, T12 and T10 showed the highest values of LRS. The highest value of WSW was observed for T18, which was significantly similar to T5, T14, T15 and T16 and there were not significant differences between these treatments (Table 3).

Table 3.
Longest stem size (LSS), longest root size (LRS), and wet stem weight (WSW) of mulberry cuttings with different concentrations of NAA, IBA, and Zn sulfate. Different letters indicate differences with a statistical significance (p < 0.05) by LSD test.
The LSD statistic, placed at the bottom of each column, can be used to compare the mean of treatments. The highest mean NL per cutting was observed for T5, T12 and T19. We also observed the lowest mean number of leaves per cutting in T17, T8 and T4. Similarly, the NRC was significantly higher in T1, T11, T14, T18 and T19. The lowest NRC values were found for T7. The RP showed a trend similar to the NRC and the highest values were observed in T1, T11, T14, T18 and T19. However, regarding the WRW, the cuttings subjected to T10 showed the highest values, which were similar to the values observed for T1, T2, T5, T6, T18, T19 and T20. Moreover, the cuttings subjected to the IBA treatment alone (100 mg L−1) showed the highest DRW, however the value decreased with the addition of Zn sulfate. The highest values of WLW and DLW (dry leaf weight) were observed in T14 and T19. Dry stem weight (DSW) was high in T14 and T18 (Table 4).

Table 4.
Effects of hormones and Zn sulfate on growth parameters of mulberry cuttings. NL, number of leaves; NRC, number of rooted cuttings; RP, rooting percentage; WRW, wet root weight; DRW, dry root weight; WLW, wet leaf weight; DLW, dry leaf weight; DSW, dry stem weight. Different letters indicate differences with a statistical significance (p < 0.05) by LSD test.
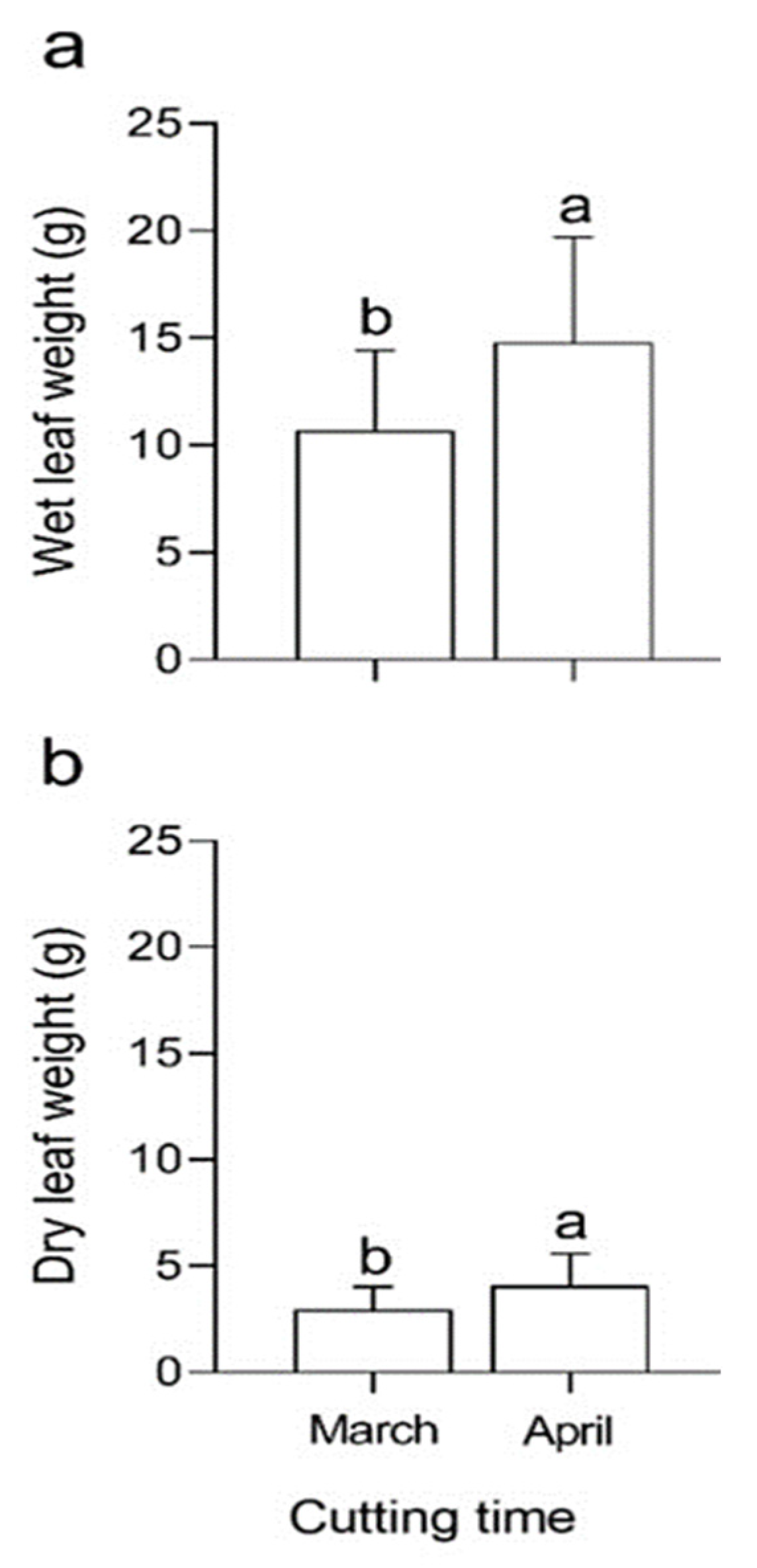
In general, the highest values of WLW were recorded in April (especially in T7 and T14), when compared to March (Figure 3). T14 differed by approximately 40% compared to T9, which showed the lowest DLW values.
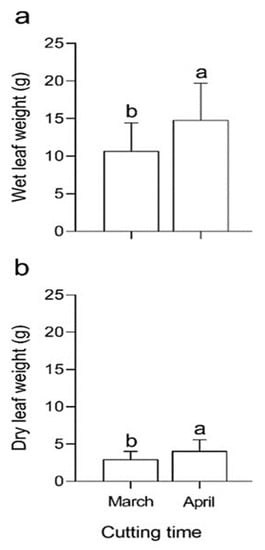
Figure 3.
Comparison of (a) wet leaf weight (WLW) and (b) dry leaf weight (DLW) of mulberry cuttings with NAA, IBA and Zn sulfate in successive years. Different letters for any of the traits indicate differences of statistical significance (p < 0.05) by LSD test.
4. Discussion
The rooting of cuttings and growth parameters of M. alba appear to depend on both the applied growth regulators and the cutting time. High concentrations of IBA activate mitotic genes that are generally regulated by auxins, which might stimulate cell division [32]. In addition, because IBA is an auxin precursor, it might act in regulating IAA levels by β-oxidation [33]. Sometimes, this conjugation process may be irreversibly inactivated by oxidation, regulating adventitious root growth [22,33]. It is well-known that IBA induces the development of adventitious roots in cuttings. However, to obtain the best rooting percentages, IBA concentrations should be adjusted depending on the type of treatment and the genetic material. Chandrashekar et al. [34] found positive effects of growth regulators on S-36 M. alba after a 24 h treatment with IBA (25 and 50 mg L−1) and NAA (50 and 75 mg L−1). Similarly, Brondani et al. [35] observed that Eucalyptus benthamii Maiden &Cambage cuttings also responded positively to rooting at IBA concentrations ≥ 2000 mg L−1. Gehlot et al. [36] found the maximum rooting percentage in Azadirachta indica A Juss at 250 mg L−1 IBA. This hormone concentration increased the lateral growth in the basal region of mini cuttings and resulted in the differentiation of a larger number of primordial roots. During external contact with the cell, IBA induces changes in the metabolism of enzymes, carbohydrates, RNA, DNA and proteins in the rooting zone, which might either inhibit or promote root growth, especially during cell division and differentiation [37]. Indole-3-butyric acid (IBA) is the most widely used root promoting chemical in the nursery trade, along with 1-naphthaleneacetic acid (NAA), because it is nontoxic over a wide range of concentrations [38]. A concentration of 2000 to 4000 ppm IBA will cause good rooting for most shrubs and evergreens, but a lower concentration of NAA was also mentioned for rooting [39]. Adventitious root formation in woody plants is associated with the action of endogenous auxin and can be triggered by the application of exogenous auxin, such as 1-naphthylacetic acid (NAA). NAA is used to influence/induce and to ensure a greater rooting capacity of cuttings and the better establishment of numerous shrubs and trees. NAA could even effectively improve the survival rate of cuttings or shorten the rooting period [40].
We observed the highest stem, as well as the longest root length; these results not only showed the positive effect of growth regulators on the rooting percentage and the production of high-quality roots but also the positive effect of the combination of these compounds with Zn sulfate. Zinc acts as a cofactor of various enzymes in catalytic processes, the production of chlorophyll [41], the synthesis of carbohydrates and proteins, the metabolism of nucleic acids and lipids, auxin formation and cell multiplication [42,43]. Zinc activates tryptophan synthetase, the enzyme responsible for the synthesis of tryptophan in the indoleacetic acid (IAA) biochemical pattern, which is a heteroauxine [44]. Indole-3-butyric acid (IBA) is a similar auxin that differs from IAA only in the length of its chain, which contains additional -CH2 groups [45]. Two major pathways for IAA biosynthesis have been proposed: the tryptophan (Trp)-independent and Trp-dependent pathways. In Trp-dependent IAA biosynthesis, four pathways have been postulated in plants: (i) the indole-3-acetamide (IAM) pathway; (ii) the indole-3-pyruvic acid (IPA) pathway; (iii) the tryptamine (TAM) pathway; and (iv) the indole-3-acetaldoxime (IAOX) pathway [46]. The two-step conversion of Trp to IAA is the main auxin biosynthesis pathway that plays an essential role in many developmental processes. TAA1 (tryptophan aminotransferase of Arabidopsis 1), an aminotransferase, catalyzes the formation of indole-3-pyruvic acid (IPA) from L-tryptophan (L-Trp), the first step in a previously proposed auxin biosynthetic pathway [47]. Zhao [48] reported tryptophan is first converted to indole-3-pyruvate (IPA) by the TAA family of amino transferases, and subsequently IAA is produced from IPA by the YUC family of flavin monooxygenases. Erland and Saxena [49] also indicated tryptophan primarily as a precursor for auxin and indoleamines, among other metabolites, and the mediation of auxin action by the indoleamines as a one-way interaction. They proposed that these processes run in both directions, with auxin modifying indoleamine biosynthesis and the melatonin–serotonin balance, contributing to its effects on plant morphogenesis, and that tryptophan also functions as an inductive signal to mediate diverse phytochemical and morphogenetic pathways. Karakeçili et al. [45] indicated that auxins (IBA and IAA) loading ZnO nanoparticles could be used as efficient nanocarriers in agricultural applications. In addition, Zn plays a key role in maintaining cell membrane integrity, preventing the oxidation of growth hormones and controlling the production of reactive oxygen species [50,51]. Therefore, our results showed that the addition of different concentrations of Zn sulfate and hormones favored rooting and increased the growth and biomass allocation of roots and aerial parts in M. alba trees, without showing phytotoxicity. Similar results were obtained by Nejad et al. [52], who observed that Zn sulfate (50 μmol) combined with IBA synergistically increased the leaf area and fresh and dry weight of shoots and roots in beans.
Regarding the comparison of growth characteristics between cutting time, it was evidenced that the highest number of rooted cuttings coincided with the highest rooting percentage. However, the highest RP correlated significantly with the highest wet leaf weight only in April, which might be due to the higher water retention of the leaves, and consequently of the cuttings, and favored fungal proliferation. Moreover, the higher leaf retention of water did not influence the wet root weight in April, which was lower than in March. The interaction between the applied treatments × cutting time evidenced that climatic characteristics might vary from one cutting time to another, affecting the rooting and other growth parameters of M. alba trees. It is likely that the warm weather in March and April favored the rooting of the cuttings and the growth and biomass allocation of roots and shoots. The results could also be related to the cold storage period and/or the different temperatures in March and April. Singh et al. [26] observed a poor rooting in M. alba cuttings planted during the coldest season, while they found a higher number of sprouted cuttings with a longer root length and a larger number of leaves in August. In addition, Cristofori et al. [53] also found that rooting ability was related to the interaction between year and cutting time.
Even if IBA, NAA, and Zn had positive effects on the rooting and growth of mulberry cuttings, M. alba rooting varied significantly depending on the type and concentration of the hormone used. The application of NAA (at 200 mg L−1), IBA (200 and 400 mg L−1) and Zn sulfate (200 and 400 mg L−1), either alone or in combination, can be a suitable and reliable method for mulberry propagation. Specifically, we demonstrated that the positive effect of IBA + Zn sulfate was more marked, compared to the other treatments tested. Nevertheless, this study has some limitations because, for successful root formation, factors such as cutting origin, type of phytohormone applied, nutritional composition, age of the matrix, and season of cutting can all play a significant role in the propagation of M. alba. For this reason, all the synergic effects and actions of these parameters are complicated to predict, and they should be better investigated in the future. The results of this study could help to mitigate the high economic losses due to low mulberry rooting, that in turn has a severe impact on the sericulture industry.
Author Contributions
R.S.: conceptualization, project administration, experimental design; P.S.: growth analysis, editing; M.P.: methodology, physiological analyses, writing; E.A.V.: determination of rooting potential, editing; A.S. (Alireza Seidavi): supervision, writing, resources; N.A.A.: writing, statistical analysis; Z.S.: general methodology, editing; A.S. (Adriano Sofo): supervision, writing and editing of the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to Iran Silk Research Centre (ISRC), Rasht, Iran which supported this study through funding. Partial financial supports by the Rasht Branch, Islamic Azad University, Rasht, Iran, grant number 17.16.1.462 is gratefully acknowledged. This work was partly supported by an OECD Co-operative Research Programme grant: Biological Resource Management for Sustainable Agricultural Systems. Directorate: T AD/CRP; Contract: JA00091460.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
A.S. (Adriano Sofo) would like to thank A.S. (Alireza Seidavi) for the mutual collaboration between the University of Basilicata and Rasht Branch, Islamic Azad University, Rasht, Iran.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mallick, P.; Ghosh, S.; Chattaraj, S.; Sikdar, S.R. Isolation of mesophyll protoplast from Indian mulberry (Morus alba L.) cv. S1635. J. Environ. Sociobiol. 2016, 13, 217–222. [Google Scholar]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackey, A.S.; Xiao, L.; Tahir, H.E. Effect of Lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-Acid-Fermented mulberry juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Budiman, A.; Praditasari, A.; Rahayu, D.; Aulifa, D.L. Formulation of antioxidant gel from black mulberry fruit extract (Morus nigra L.). J. Pharm. Bioallied Sci. 2019, 11, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Farahani, M.; Salehi-Arjmand, H.; Khadivi, A.; Akramian, M. Chemical characterization and antioxidant activities of Morus alba var. nigra fruits. Sci. Hort. 2019, 253, 120–127. [Google Scholar] [CrossRef]
- Gao, M.Z.; Cui, Q.; Wang, L.T.; Meng, Y.; Yu, L.; Li, Y.Y.; Fu, Y.J. A green and integrated strategy for enhanced phenolic compounds extraction from mulberry (Morus alba L.) leaves by deep eutectic solvent. Microchem. J. 2020, 154, 104598. [Google Scholar] [CrossRef]
- Thabti, I.; Elfalleh, W.; Hannachi, H.; Ferchichi, A.; Campos, M.G. Identification and quantification of phenolic acids and flavonol glycosides in Tunisian Morus species by HPLC-DAD and HPLC-MS. J. Func. Food. 2012, 4, 367–374. [Google Scholar] [CrossRef]
- Yadav, S.; Nair, N.; Biharee, A.; Morris Prathap, V.; Majeed, J. Updated ethnobotanical notes, phytochemistry and phytopharmacology of plants belonging to the genus Morus (Family: Moraceae). Phytomedicine Plus 2022, 2, 100120. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, E.M.; Amorós, A.; Hernández, F.; Martínez, J.J. Physicochemical properties of white (Morus alba) and black (Morus nigra) mulberry leaves, a new food supplement. J. Food Nutr. Res. 2017, 5, 253–261. [Google Scholar]
- Wei, H.; Zhu, J.J.; Liu, X.Q.; Feng, W.H.; Wang, Z.M.; Yan, L.H. Review of bioactive compounds from root barks of Morus plants (Sang-Bai-Pi) and their pharmacological effects. Cogent Chem. 2016, 2, 1212320. [Google Scholar] [CrossRef]
- Miljković, V.M.; Nikolić, G.S.; Nikolić, L.B.; Arsić, B.B. Morus species through centuries in pharmacy and as food. Savrem. Tehnol. 2014, 3, 111–115. [Google Scholar] [CrossRef] [Green Version]
- Hussain, F.; Rana, Z.; Shafique, H.; Malik, A.; Hussain, Z. Phytopharmacological potential of different species of Morus alba and their bioactive phytochemicals: A review. Asian Pac. J. Trop. Biomed. 2017, 7, 950–956. [Google Scholar] [CrossRef]
- Zafar, M.S.; Muhammad, F.; Javed, I.; Akhtar, M.; Khaliq, T.; Aslam, B.; Waheed, A.; Yasmin, R.; Zafar, H. White mulberry (Morus alba): A brief phytochemical and pharmacological evaluations account. Int. J. Agric. Biol. 2013, 15, 612–620. [Google Scholar]
- KeshtkarAghababaee, S.; Vafa, M.R.; Shidfar, F.; Gohari, M.R.; Katebi, D.; Mohammadi, V. Effect of black mulberry (Morus nigra L.) consumption on serum concentrations of lipoproteins, Apo-A1, Apo-B and hs-CRP and blood pressure in dyslipidemic patients. Iran. J. Nutr. Sci. Food Technol. 2014, 8, 67–81. [Google Scholar]
- Ahkami, A.H.; Melzer, M.; Ghaffari, M.R.; Pollmann, S.; Javid, M.G.; Shahinnia, F.; Hajirezaei, M.R.; Druege, U. Distribution of indole-3-Acetic acid in Petunia hybrida shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation. Planta 2013, 238, 499–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyuncu, F.; Senel, E. Rooting of black mulberry (Morus nigra) hardwood cuttings. J. Fruit Ornam. Plant. Res. 2003, 11, 53–57. [Google Scholar]
- Hoermayer, L.; Montesinos, J.C.; Marhava, P.; Benková, E.; Yoshida, S.; Friml, J. Wounding-Induced changes in cellular pressure and localized auxin signalling spatially coordinate restorative divisions in roots. Proc. Natl. Acad. Sci. USA 2020, 117, 15322–15331. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Mo, Y.W.; Wang, S.B.; Zhang, Z. Auxin efflux carriers, MiPINs, are involved in adventitious root formation of mango cotyledon segments. Plant Physiol. Biochem. 2020, 150, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Finet, C.; Jaillais, Y. Auxology: When auxin meets plant evo–devo. Dev. Biol. 2012, 369, 19–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imin, N.; Nizamidin, M.; Daniher, D.; Nolan, K.E.; Rose, R.J.; Rolfe, B.G. Proteomic analysis of somatic embryogenesis in Medicago truncatula explants culture grown under 6-benzylaminopurine and 1-Naphthaleneacetic acid treatments. Plant Physiol. 2005, 137, 1250–1260. [Google Scholar] [CrossRef] [Green Version]
- Kaewchangwat, N.; Thanayupong, E.; Jarussophon, S.; Niamnont, N.; Yata, T.; Prateepchinda, S.; Unger, O.; Han, B.H.; Suttisintong, K. Coumarin-Caged compounds of 1-naphthaleneacetic acid as light-Responsive controlled-Release plant root stimulators. J. Agric. Food Chem. 2020, 68, 6268–6279. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Amin, T. Effects of location, gender and indole butyric acid on rooting of Laurus nobilis L. semi-Hardwood stem cuttings. Agric. Sci. Technol. 2020, 12, 260–263. [Google Scholar] [CrossRef]
- Costa, C.T.; Almeida, M.R.; Ruedell, C.M.; Schwambach, J.; Maraschin, F.S.; Fett-Neto, A.G. When stress and development go hand in hand: Main hormonal controls of adventitious rooting in cuttings. Front. Plant Sci. 2013, 4, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, K.K.; Choudhary, T.; Kumar, A. Effect of various concentrations of IBA and NAA on the rooting of stem cuttings of mulberry (Morus Alba L.) under mist house condition in Garhwal Hill region. Indian J. Hill Farm. 2014, 27, 74–77. [Google Scholar]
- Abdel-Rahman, S.; Abdul-Hafeez, E.; Saleh, A.M. Improving rooting and growth of Conocarpus erectus stem cuttings using indole-3-Butyric acid (IBA) and some biostimulants. Sci. J. Flowers Ornam. Plants 2020, 7, 109–129. [Google Scholar] [CrossRef]
- Henrique, A.; Campinhos, E.N.; Ono, E.O.; Pinho, S.Z. Effect of plant growth regulators in the rooting of Pinus cuttings. Braz. Arch. Biol. Technol. 2006, 49, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.K.; Dev Kishan, J.; Mehta, S.K. Rootability of hardwood cuttings of mulberry (Morus alba L.) influenced by planting time and growing conditions under valley condition of Garhwal Himalayas. Plant Arch. 2015, 15, 1031–1036. [Google Scholar]
- Niczyporuk, A.P.; Bajguz, A. The effect of natural and synthetic auxins on the growth, metabolite content and antioxidant response of green alga Chlorella vulgaris (Trebouxiophyceae). Plant Growth Regul. 2014, 73, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Latoh, L.P.; Gomes, E.N.; Zuffellato-Ribas, K.C. Can indolebutyric and fulvic acids induce adventitious rhizogenesis on mini-cuttings from Brazilian native tibouchinas? Ornam. Hortic. 2019, 25, 42–48. [Google Scholar] [CrossRef]
- Abdallah, A.; Mekdad, A.; Shaaban, A. Integrative applications of nitrogen, zinc, and boron to nutrients-Deficient soil improves sugar beet productivity and technological sugar contents under semi-arid conditions. J. Plant Nutr. 2020, 43, 1935–1950. [Google Scholar]
- Kumar-Tewari, R.; Kumar, P.; Sharma, P.N. Morphology and physiology of zinc-Stressed mulberry plants. J. Plant Nutr. Soil Sci. 2008, 171, 286–294. [Google Scholar] [CrossRef]
- dos Santos, L.R.; da Silva, B.R.S.; Pedron, T.; Batista, B.L.; da Silva Lobato, A.K. 24-Epibrassinolide improves root anatomy and antioxidant enzymes in soybean plants subjected to zinc stress. J. Soil Sci. Plant Nutr. 2020, 20, 105–124. [Google Scholar] [CrossRef]
- Qu, Y.; Liu, X.; Zhang, X.; Tang, Y.; Hu, Y.; Chen, S.; Xiang, L.; Zhang, Q. Transcriptional regulation of Arabidopsis copper amine oxidase (CuAO) in indole-3-butyric acid-induced lateral root development. Plant Growth Regul. 2019, 9, 287–297. [Google Scholar] [CrossRef]
- Aryal, B.; Huynh, J.; Schneuwly, J.; Siffert, A.; Liu, J.; Alejandro, S.; Ludwid-Müller, J.; Martinoia, E.; Geisler, M. ABCG36/PEN3/PDR8 is an exporter of the auxin precursor, indole-3-butyric acid, and involved in auxin-controlled development. Front. Plant Sci. 2019, 10, 899. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.S.; Radakrishnan, S.; Sikdar, A.K.; Datta, R.K.; Shetty, H.S. Effect of growth regulators on the propagation of S-36 mulberry stem cuttings. Indian For. 1996, 122, 525–527. [Google Scholar]
- Brondani, G.E.; Baccarin, F.J.B.; Ondas, H.W.W.; Stape, J.L.; Gonçalves, A.N.; Almeida, M. Low temperature, IBA concentrations and optimal time for adventitious rooting of Eucalyptus benthamii mini-cuttings. J. For. Res. 2012, 23, 583–592. [Google Scholar] [CrossRef]
- Gehlot, A.; Gupta, R.K.; Tripathi, A.; Arya, I.D.; Arya, S. Vegetative propagation of Azadirachtaindica: Effect of auxin and rooting media on adventitious root induction in mini-cuttings. Adv. For. Sci. 2014, 1, 1–9. [Google Scholar]
- Ruppert, D.C. Hormone concentrations. Comb. Proc. Int. Plant Prop. Soc. 1974, 24, 349–350. [Google Scholar]
- Kentelky, E.; Jucan, D.; Cantor, M.; Szekely-Varga, Z. Efficacy of different concentrations of NAA on selected ornamental woody shrubs cuttings. Horticulturae 2021, 7, 464. [Google Scholar] [CrossRef]
- Poston, A.L. Cutting Propagation and Container Production of Rudy Haag Burning Bush [Euonymus Alatus Rudy Haag]. Master’s Thesis, University of Kentucky, Lexington, KY, USA, 2007. [Google Scholar]
- Meng, Y.; Xing, L.; Li, K.; Wei, Y.; Wang, H.; Mao, J.; Dong, F.; Ma, D.; Zhang, Z.; Han, M.; et al. Genome-Wide identification, characterization and expression analysis of novel long non-Coding RNAs that mediate IBA-Induced adventitious root formation in apple rootstocks. Plant Growth Regul. 2019, 87, 287–302. [Google Scholar] [CrossRef]
- Ebrahim, A.M.; Alnajjar, A.O.; Mohammed, M.E.; Idris, A.M.; Mohammed, M.E.; Michalke, B. Investigation of total zinc contents and zinc-protein profile in medicinal plants traditionally used for diabetes treatment. BioMetals 2020, 33, 65–74. [Google Scholar] [CrossRef]
- Gai, A.P.C.; Santos, D.S.; Vieira, E.A. Effects of zinc excess on antioxidant metabolism, mineral content and initial growth of Handroanthusimpetiginosus (Mart. ex DC.) Mattos and Tabebuia roseoalba (Ridl.) Sandwith. Environ. Exp. Bot. 2017, 144, 88–99. [Google Scholar] [CrossRef]
- Zayed, Z.E.; El-Dawayati, M.M.; Hussien, F.A.; Saber, T.Y. Enhanced in vitro multiplication and rooting of date palm cv. yellow maktoum by zinc and copper ions. Plant Arch. 2020, 20, 517–528. [Google Scholar]
- Castillo-González, J.; Ojeda-Barrios, D.; Hernández-Rodríguez, A.; Cecilia González-Franco, A.; Robles-Hernández, L.; López-Ochoa, G.R. Zinc metalloenzymes in plants. Interciencia 2018, 43, 242–248. [Google Scholar]
- Karakeçili, A.; Korpayev, S.; Dumanoğlu, H.; Alizadeh, S. Synthesis of indole-3-Acetic acid and indole-3-Butyric acid loaded zinc oxide nanoparticles: Effects on rhizogenesis. J. Biotech. 2019, 303, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Mano, Y.; Nemoto, K. The pathway of auxin biosynthesis in plants. J. Experim. Bot. 2012, 63, 2853–2872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, Y.; Ferrer, J.L.; Ljung, K.; Pojer, F.; Hong, F.; Long, J.A.; Li, L.; Moreno, J.E.; Bowman, M.E.; Ivans, L.J.; et al. Rapid synthesis of auxin via a new tryptophan-Dependent pathway is required for shade avoidance in Plants. Cell 2008, 133, 164–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y. Auxin biosynthesis: A simple two-Step pathway converts tryptophan to Indole-3-Acetic Acid in plants. Mol. Plant 2012, 5, 334–338. [Google Scholar] [CrossRef] [Green Version]
- Erland, L.A.E.; Saxena, P. Auxin driven indoleamine biosynthesis and the role of tryptophan as an inductive signal in Hypericum perforatum (L.). PLoS ONE 2019, 14, e0223878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, E.A.; Galvão, F.C.A.; Barros, A.L. Influence of water limitation on the competitive interaction between two Cerrado species and the invasive grass Brachiariabrizantha cv. Piatã. Plant Physiol. Biochem. 2019, 135, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, D.; Merkulova, E.A.; Naya, L.; Horie, T.; Kanno, Y.; Seo, M.; Ohsumi, Y.; Masclaux-Daubresse, C.; Yoshimoto, K. Autophagy increases zinc bioavailability to avoid light-mediated reactive oxygen species production under zinc deficiency. Plant Physiol. 2020, 182, 284–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nejad, R.H.; Najafi, F.; Arvin, P.; Firuzeh, R. Study different levels of zinc sulfate (ZnSO4) on fresh and dry weight, leaf area, relative water content and total protein in bean (Phaseolus vulgaris L.) plant. Bull. Environ. Pharm. Life Sci. 2014, 13, 144–151. [Google Scholar]
- Cristofori, V.; Rouphael, Y.; Rugini, E. Collection time, cutting age, IBA and putrescine effects on root formation in Corylus avellana L. cuttings. Sci. Hort. 2010, 124, 189–194. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).