Rapid Clinical Response to Omalizumab Treatment in Pediatric Acute Urticaria Associated with Mycoplasma Infection: A Two-Case Report
Abstract
1. Introduction
2. Case Presentation
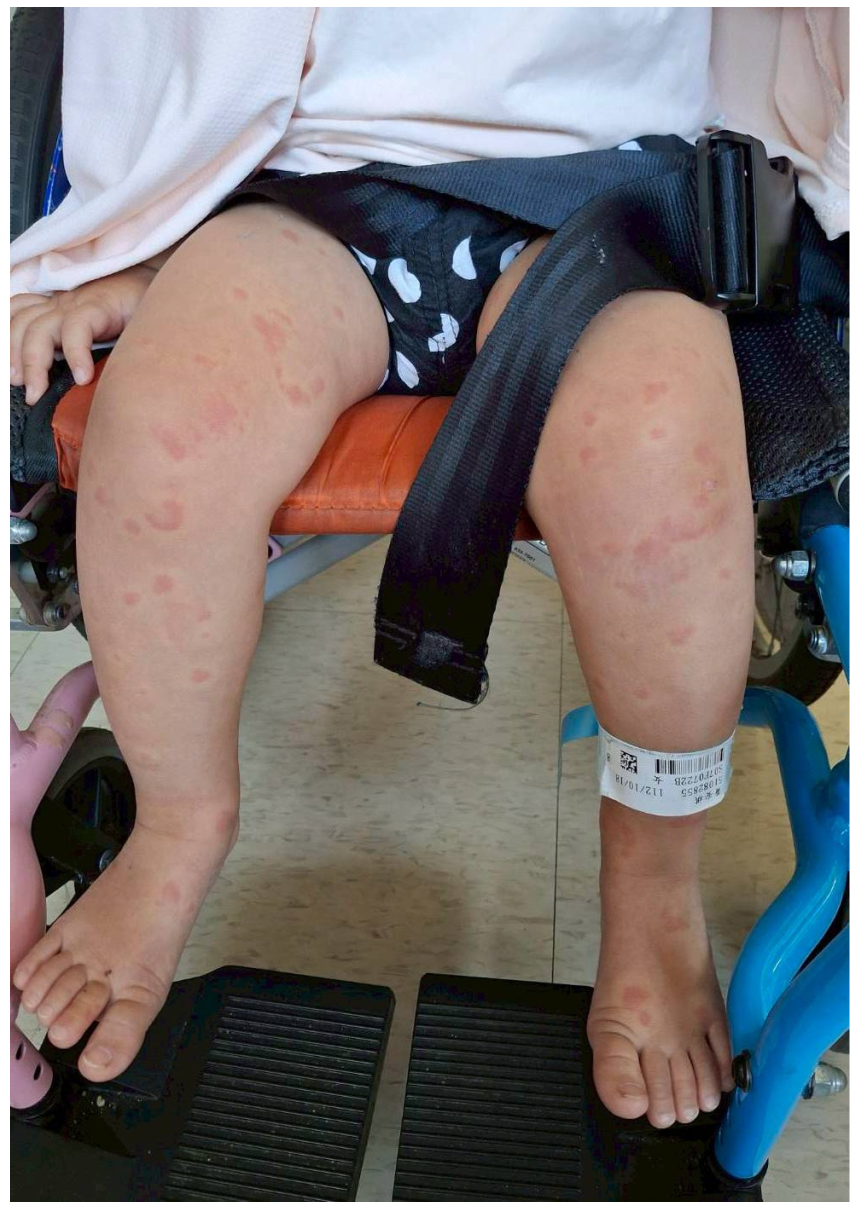
2.1. Case One
2.2. Case Two
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AU | Acute urticaria |
| CU | Chronic urticaria |
| CSU | Chronic spontaneous urticaria |
References
- Zuberbier, T.; Abdul Latiff, A.H.; Abuzakouk, M.; Aquilina, S.; Asero, R.; Baker, D.; Ballmer-Weber, B.; Bangert, C.; Ben-Shoshan, M.; Bernstein, J.A.; et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy 2022, 77, 734–766. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Fok, J.S.; Kocaturk, E.; Li, P.H.; Okas, T.L.; Marcelino, J.; Metz, M. Update on the Treatment of Chronic Spontaneous Urticaria. Drugs 2025, 85, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Kanani, A.; Betschel, S.D.; Warrington, R. Urticaria and angioedema. Allergy Asthma Clin. Immunol. 2018, 14, 59. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.A.; Tsai, C.K.; Kuo, K.C.; Yu, H.R. Rational stepwise approach for Mycoplasma pneumoniae pneumonia in children. J. Microbiol. Immunol. Infect. 2021, 54, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Church, M.K.; Kolkhir, P.; Metz, M.; Maurer, M. The role and relevance of mast cells in urticaria. Immunol. Rev. 2018, 282, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, X.; Bai, J.; Sun, Q.; Qiao, J.; Fang, H. Omalizumab for the Treatment of Refractory Acute Urticaria. Am. J. Ther. 2024, 31, e487–e489. [Google Scholar] [CrossRef] [PubMed]

| Case | Sex/Age, Wt (yr/Kg) | Presentations | Total-IgE (0–100 KU/L) | White Blood Cell Count (4–10 × 109/L) | Neutrophil Count (2–7 × 109/L) | C-Reactive Protein (0–8 mg/L) | Treatment Before Omalizumab |
|---|---|---|---|---|---|---|---|
| 1 | F/4Y5M, 17.8 | Wheals, swelling over trunk | 1357 | 9.01 | 6.06 | 80.54 | Hydrocortisone 300 mg every day for 5 days |
| Case | Sex/Age, Wt (yr/Kg) | Presentations | Total-IgE (0–100 KU/L) | White Blood Cell Count (4–10 × 109/L) | Neutrophil Count (2–7 × 109/L) | C-reactive Protein (0–8 mg/L) | Treatment Before Omalizumab |
|---|---|---|---|---|---|---|---|
| 2 | F/1Y8M, 10 | Wheals, swelling over trunk and extremities | 32.6 | 12.9 | 7.998 | 12.7 | Hydrocortisone 200 mg per day for 4 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Wu, Z.-L.; Huang, Y.-S.; Chiang, C.-T.; Yu, H.-R. Rapid Clinical Response to Omalizumab Treatment in Pediatric Acute Urticaria Associated with Mycoplasma Infection: A Two-Case Report. Pediatr. Rep. 2026, 18, 2. https://doi.org/10.3390/pediatric18010002
Wu Z-L, Huang Y-S, Chiang C-T, Yu H-R. Rapid Clinical Response to Omalizumab Treatment in Pediatric Acute Urticaria Associated with Mycoplasma Infection: A Two-Case Report. Pediatric Reports. 2026; 18(1):2. https://doi.org/10.3390/pediatric18010002
Chicago/Turabian StyleWu, Zhen-Li, Yi-Siang Huang, Chien-Ting Chiang, and Hong-Ren Yu. 2026. "Rapid Clinical Response to Omalizumab Treatment in Pediatric Acute Urticaria Associated with Mycoplasma Infection: A Two-Case Report" Pediatric Reports 18, no. 1: 2. https://doi.org/10.3390/pediatric18010002
APA StyleWu, Z.-L., Huang, Y.-S., Chiang, C.-T., & Yu, H.-R. (2026). Rapid Clinical Response to Omalizumab Treatment in Pediatric Acute Urticaria Associated with Mycoplasma Infection: A Two-Case Report. Pediatric Reports, 18(1), 2. https://doi.org/10.3390/pediatric18010002





