When Genes Reveal the Truth: Alport Syndrome Mimicking Steroid-Resistant Nephrotic Syndrome
Abstract
1. Introduction
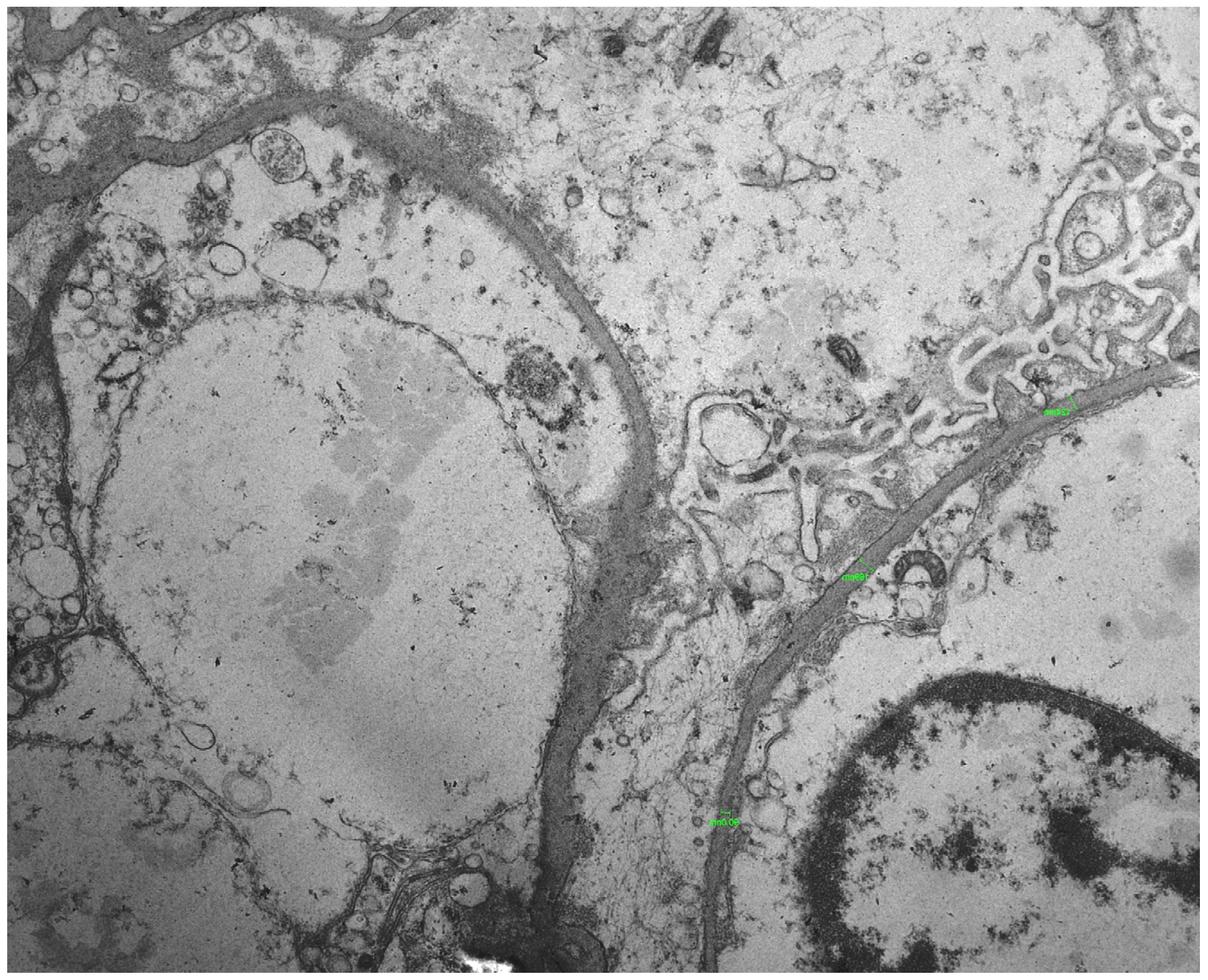
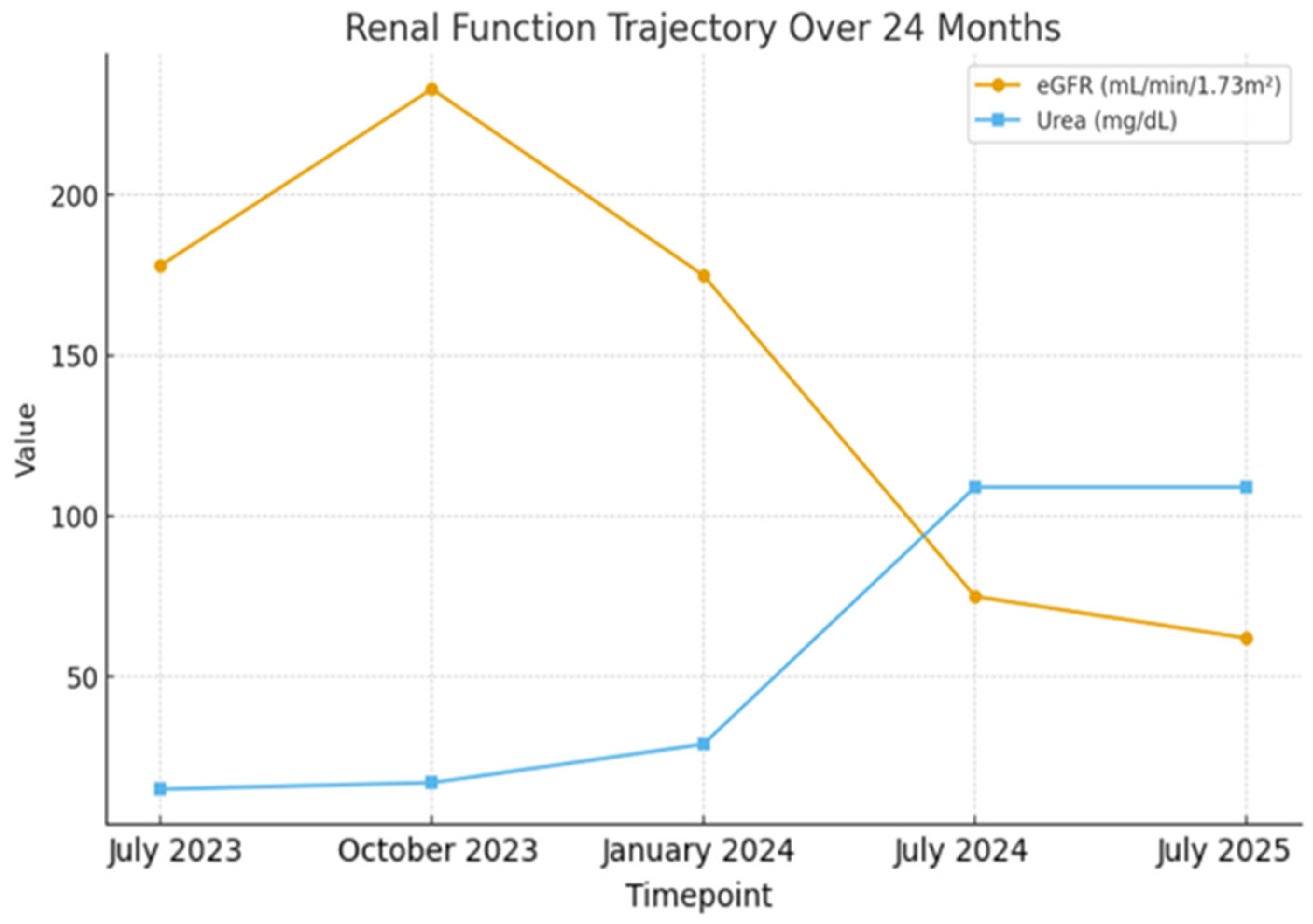
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FSGS | Focal segmental glomerulosclerosis |
| SRNS | Steroid-resistant nephrotic syndrome |
| COL4A3 | Collagen type IV alpha-3 |
| COL4A4 | Collagen type IV alpha-4 |
| COL4A5 | Collagen type IV alpha-5 |
| GBM | Glomerular basement membrane |
References
- Cosgrove, D.; Liu, S. Collagen IV diseases: A focus on the glomerular basement membrane in Alport syndrome. Matrix Biol. 2017, 57–58, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Ćomić, J.; Riedhammer, K.M.; Gunthner, R.; Schaaf, C.W.; Richthammer, P.; Simmendinger, H.; Kieffer, D.; Berutti, R.; Tasic, V.; Abazi-Emini, N.; et al. The multifaceted phenotypic and genotypic spectrum of type-IV-collagen-related nephropathy—A human genetics department experience. Front. Med. 2022, 9, 957733. [Google Scholar] [CrossRef] [PubMed]
- Adone, A.; Anjankar, A. Alport Syndrome: A Comprehensive Review. Cureus 2023, 15, e47129. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Seshan, S.V.; Barisoni, L.; Amann, K.; Bajema, I.M.; Becker, J.U.; Joh, K.; Ljubanovic, D.; Roberts, I.S.; Roelofs, J.J.; et al. Consensus definitions for glomerular lesions by light and electron microscopy: Recommendations from a working group of the Renal Pathology Society. Kidney Int. 2020, 98, 1120–1134. [Google Scholar] [CrossRef] [PubMed]
- Dotis, J.; Ververi, A.; Printza, N. Type IV collagen-related nephropathy as a diagnosis in nephrotic syndrome. Pediatr. Nephrol. 2025, 40, 873. [Google Scholar] [CrossRef] [PubMed]
- Puapatanakul, P.; Isaranuwatchai, S.; Chanakul, A.; Surintrspanont, J.; Iampenkhae, K.; Kanjanabuch, T.; Suphapeetiporn, K.; Charu, V.; Suleiman, H.Y.; Praditpornsilpa, K.; et al. Quantitative assessment of glomerular basement membrane collagen IV α chains in paraffin sections from patients with focal segmental glomerulosclerosis and Alport gene variants. Kidney Int. 2024, 105, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Solanki, K.V.; Hu, Y.; Moore, B.S.; Abedi, V.; Avula, V.; Mirshahi, T.; Strande, N.T.; Bucaloiu, I.D.; Chang, A.R. The Phenotypic Spectrum of COL4A3 Heterozygotes. Kidney Int. Rep. 2023, 8, 2088–2099. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, A.L.; Bitencourt, L.; Paranhos, R.M.; Leitáo, C.A.; Ferreira, G.C.; Simões E Silva, A.C. Alport Syndrome: A Comprehensive Review on Genetics, Pathophysiology, Histology, Clinical and Therapeutic Perspectives. Curr. Med. Chem. 2021, 28, 5602–5624. [Google Scholar] [CrossRef] [PubMed]
- Wildes, D.M.; Fitzsimons, A.; Doyle, B.; Green, A.; Sweeney, C.; Awan, A. An unusual case of nephrotic syndrome. Pediatr. Nephrol. 2024, 39, 3205–3208. [Google Scholar] [CrossRef] [PubMed]
- Lennon, R.; Randles, M.J.; Humphries, M.J. The importance of podocyte adhesion for a healthy glomerulus. Front. Endocrinol. 2014, 5, 160. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, A.; Lipska-Ziętkiewicz, B.S.; Schaefer, F. Exploring the Clinical and Genetic Spectrum of Steroid Resistant Nephrotic Syndrome: The PodoNet Registry. Front. Pediatr. 2018, 6, 200. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Di, H.; Liang, J.; Liu, Z. Effectiveness of renin-angiotensin-aldosterone system blockers in patients with Alport syndrome: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2023, 38, 2485–2493. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, C.; Rheault, M.N. Genetic Basis of Type IV Collagen Disorders of the Kidney. Clin. J. Am. Soc. Nephrol. 2021, 16, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.W.; Dahl, N.K. Clinical Genetic Testing in Nephrology: Core Curriculum 2024. Am. J. Kidney Dis. 2024, 84, 632–645. [Google Scholar] [CrossRef] [PubMed]

| Category | Element/Description | Clinical Significance and Association |
|---|---|---|
| Genetic finding | ||
| Gene (OMIM) | COL4A3 (NM_000091.5, OMIM 120070), Chromosome 2 | Associated with glomerular basement membrane diseases |
| DNA change (rsID) | c.1022G>A (rs200738124) | Substitution of guanine (G) with adenine (A) at position 1022 |
| Protein change | p.Arg341His | Missense change: replacement of arginine (Arg) with histidine (His) at position 341 |
| Carrier status | Heterozygous | The individual carries one mutated copy of the gene |
| Clinical correlation | ||
| Associated diseases | Alport syndrome 2 (autosomal recessive, OΜΙΜ 203780), Alport syndrome 3 (autosomal dominant, OMIM 104200), Benign familial hematuria 2 (autosomal dominant, OMIM 620320) | Variable spectrum of inheritance and severity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Dotis, J.; Kondou, A.; Liapis, G.; Ververi, A.; Kollios, K.; Printza, N. When Genes Reveal the Truth: Alport Syndrome Mimicking Steroid-Resistant Nephrotic Syndrome. Pediatr. Rep. 2026, 18, 3. https://doi.org/10.3390/pediatric18010003
Dotis J, Kondou A, Liapis G, Ververi A, Kollios K, Printza N. When Genes Reveal the Truth: Alport Syndrome Mimicking Steroid-Resistant Nephrotic Syndrome. Pediatric Reports. 2026; 18(1):3. https://doi.org/10.3390/pediatric18010003
Chicago/Turabian StyleDotis, John, Antonia Kondou, George Liapis, Athina Ververi, Konstantinos Kollios, and Nikoleta Printza. 2026. "When Genes Reveal the Truth: Alport Syndrome Mimicking Steroid-Resistant Nephrotic Syndrome" Pediatric Reports 18, no. 1: 3. https://doi.org/10.3390/pediatric18010003
APA StyleDotis, J., Kondou, A., Liapis, G., Ververi, A., Kollios, K., & Printza, N. (2026). When Genes Reveal the Truth: Alport Syndrome Mimicking Steroid-Resistant Nephrotic Syndrome. Pediatric Reports, 18(1), 3. https://doi.org/10.3390/pediatric18010003






