Eosinophilic Esophagitis in a 3-Year-Old Girl with Spinal Muscular Atrophy Type 1: The First Reported Case
Abstract
1. Introduction
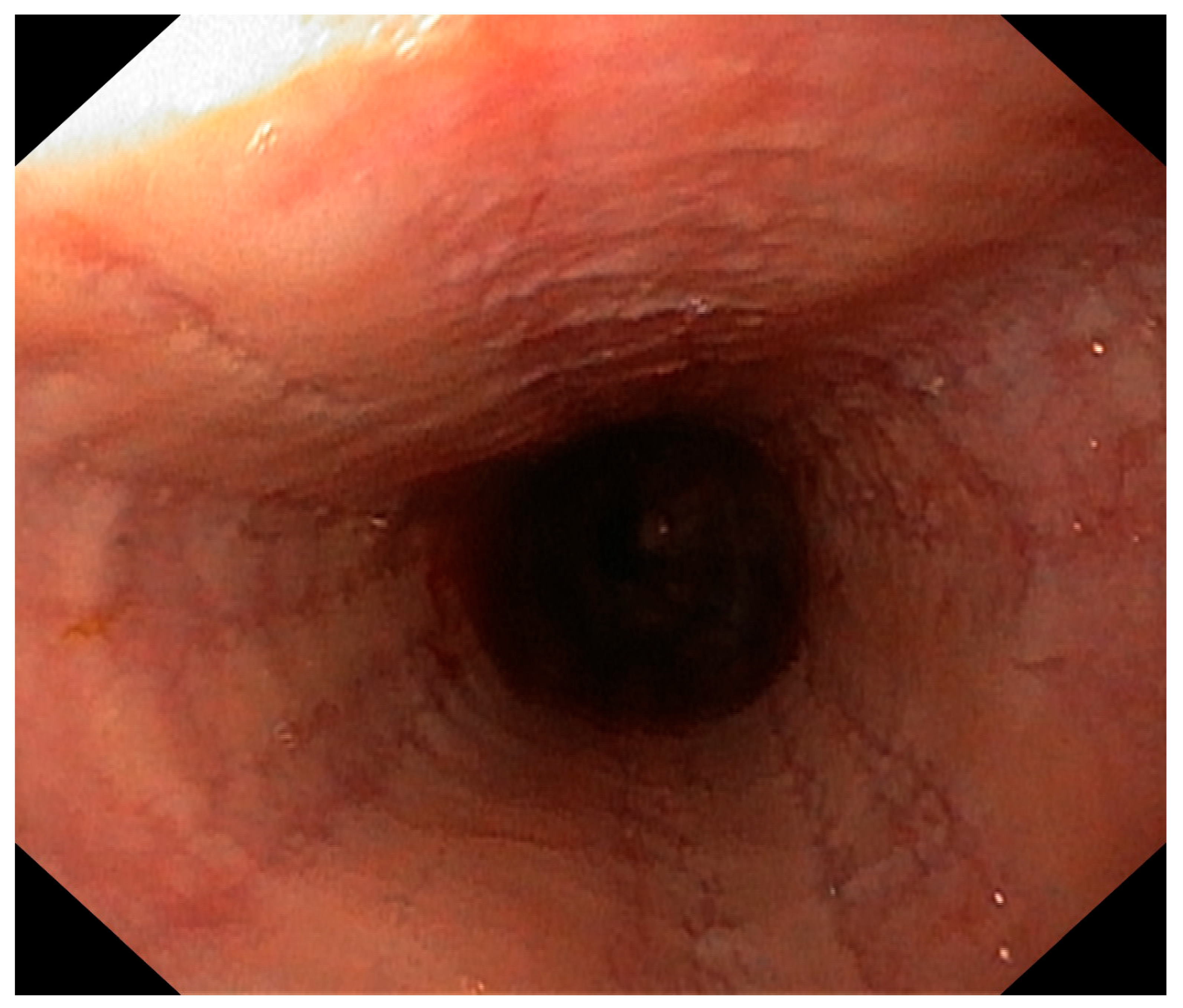
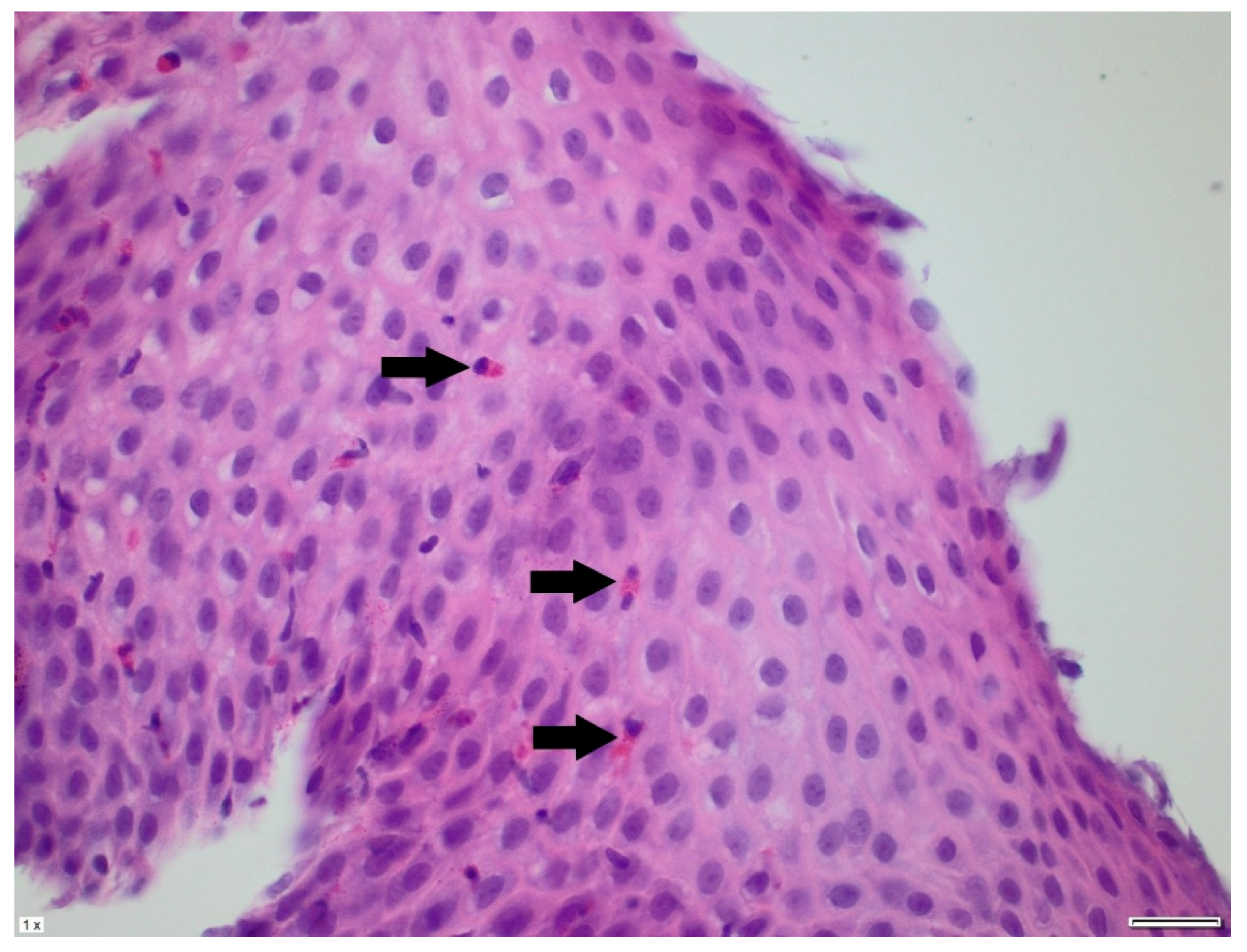
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SMA 1 | spinal muscular atrophy type 1 |
| EoE | eosinophilic esophagitis |
| GI | gastrointestinal |
| GERD | gastroesophageal reflux disease |
| SMN 1 | survival motor neuron 1 gene |
| CHOP INTEND | Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders |
| SMA 2 | spinal muscular atrophy type type 2 |
| EHF | extensively hydrolyzed protein formula |
| AAF | amino acid-based formulas |
References
- Lally, C.; Jones, C.; Farwell, W.; Reyna, S.P.; Cook, S.F.; Flanders, W.D. Indirect estimation of the prevalence of spinal muscular atrophy Type I, II, and III in the United States. Orphanet J. Rare Dis. 2017, 12, 175. [Google Scholar] [CrossRef]
- Mercuri, E.; Sumner, C.J.; Muntoni, F.; Darras, B.T.; Finkel, R.S. Spinal muscular atrophy. Nat. Rev. Dis. Primers 2022, 8, 52. [Google Scholar] [CrossRef]
- Cances, C.; Vlodavets, D.; Comi, G.P.; Masson, R.; Mazurkiewicz-Bełdzińska, M.; Saito, K.; Zanoteli, E.; Dodman, A.; El-Khairi, M.; Gorni, K.; et al. Natural history of Type 1 spinal muscular atrophy: A retrospective, global, multicenter study. Orphanet J. Rare Dis. 2022, 17, 300. [Google Scholar] [CrossRef] [PubMed]
- Glanzman, A.; Mazzone, E.; Main, M.; Pelliccioni, M.; Wood, J.; Swoboda, K.; Scott, C.; Pane, M.; Messina, S.; Bertini, E.; et al. The Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND): Test development and reliability. Neuromuscul. Disord. 2010, 20, 155–161. [Google Scholar] [CrossRef]
- Cooper, K.; Nalbant, G.; Sutton, A.; Harnan, S.; Thokala, P.; Chilcott, J.; McNeill, A.; Bessey, A. Systematic Review of Newborn Screening Programmes for Spinal Muscular Atrophy. Int. J. Neonatal Screen. 2024, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Barni, S.; Arasi, S.; Mastrorilli, C.; Pecoraro, L.; Giovannini, M.; Mori, F.; Liotti, L.; Saretta, F.; Castagnoli, R.; Caminiti, L.; et al. Pediatric eosinophilic esophagitis: A review for the clinician. Ital. J. Pediatr. 2021, 47, 230. [Google Scholar] [CrossRef]
- Navarro, P.; Arias, Á.; Arias-González, L.; Laserna-Mendieta, E.J.; Ruiz-Ponce, M.; Lucendo, A.J. Systematic review with meta-analysis: The growing incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment. Pharmacol. Ther. 2019, 49, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Moawad, F.J. Eosinophilic Esophagitis: Incidence and Prevalence. Gastrointest. Endosc. Clin. N. Am. 2018, 28, 15–25. [Google Scholar] [CrossRef]
- Allin, K.H.; Poulsen, G.; Melgaard, D.; Frandsen, L.T.; Jess, T.; Krarup, A.L. Eosinophilic oesophagitis in Denmark: Population-based incidence and prevalence in a nationwide study from 2008 to 2018. United Eur. Gastroenterol. J. 2022, 10, 640–650. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Molina-Infante, J.; Arias, Á.; Von Arnim, U.; Bredenoord, A.J.; Bussmann, C.; Dias, J.A.; Bove, M.; González-Cervera, J.; Larsson, H.; et al. Guidelines on eosinophilic esophagitis: Evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur. Gastroenterol. J. 2017, 5, 335–358. [Google Scholar] [CrossRef]
- Capucilli, P.; Cianferoni, A.; Grundmeier, R.W.; Spergel, J.M. Comparison of comorbid diagnoses in children with and without eosinophilic esophagitis in a large population. Ann. Allergy Asthma Immunol. 2018, 121, 711–716. [Google Scholar] [CrossRef]
- Abonia, J.P.; Wen, T.; Stucke, E.M.; Grotjan, T.; Griffith, M.S.; Kemme, K.A.; Collins, M.H.; Putnam, P.E.; Franciosi, J.P.; von Tiehl, K.F.; et al. High prevalence of eosinophilic esophagitis in patients with inherited connective tissue disorders. J. Allergy Clin. Immunol. 2013, 132, 378–386. [Google Scholar] [CrossRef]
- Pagliara, C.; Zambaiti, E.; Antoniello, L.M.; Gamba, P. Eosinophilic Esophagitis in Esophageal Atresia: Is It Really a New Disease? Children 2022, 9, 1032. [Google Scholar] [CrossRef]
- Amil-Dias, J.; Oliva, S.; Papadopoulou, A.; Thomson, M.; Gutiérrez-Junquera, C.; Kalach, N.; Orel, R.; Auth, M.K.; Nijenhuis-Hendriks, D.; Strisciuglio, C.; et al. Diagnosis and management of eosinophilic esophagitis in children: An update from the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN). J. Pediatr. Gastroenterol. Nutr. 2024, 79, 394–437. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, S.; Cohen, A.B.; Rahbar, R.D.; Rubinstein, E.; Rosen, R. Characterization of Eosinophilic Esophagitis in Infants and Toddlers. J. Pediatr. Gastroenterol. Nutr. 2023, 77, 86–92. [Google Scholar] [CrossRef]
- Yeo, C.J.J.; Darras, B.T. Overturning the Paradigm of Spinal Muscular Atrophy as Just a Motor Neuron Disease. Pediatr. Neurol. 2020, 109, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Durkin, E.T.; Schroth, M.K.; Helin, M.; Shaaban, A.F. Early laparoscopic fundoplication and gastrostomy in infants with spinal muscular atrophy type I. J. Pediatr. Surg. 2008, 43, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Coratti, G.; Ricci, M.; Capasso, A.; D’amico, A.; Sansone, V.; Bruno, C.; Messina, S.; Ricci, F.; Mongini, T.; Coccia, M. Prevalence of Spinal Muscular Atrophy in the Era of Disease-Modifying Therapies: An Italian Nationwide Survey. Neurology 2023, 100, 522–528. [Google Scholar] [CrossRef]
- Farrar, M.A.; Vucic, S.; Johnston, H.M.; du Sart, D.; Kiernan, M.C. Pathophysiological insights derived by natural history and motor function of spinal muscular atrophy. J. Pediatr. 2013, 162, 155–159. [Google Scholar] [CrossRef]
- Fuller, H.R.; Shorrock, H.K.; Gillingwater, T.H.; Pigott, A.; Smith, V.; Kulshrestha, R.; Sewry, C.S.; Willis, T.A. Two Cases of Spinal Muscular Atrophy Type II with Eosinophilic Oesophagitis. J. Neuromuscul. Dis. 2017, 4, 357–362. [Google Scholar] [CrossRef]
- Day, J.W.; Howell, K.; Place, A.; Long, K.; Rossello, J.; Kertesz, N.; Nomikos, G. Advances and limitations for the treatment of spinal muscular atrophy. BMC Pediatr. 2022, 22, 632. [Google Scholar] [CrossRef] [PubMed]
- Erwin, E.A.; James, H.R.; Gutekunst, H.M.; Russo, J.M.; Kelleher, K.J.; Platts-Mills, T.A. Serum IgE measurement and detection of food allergy in pediatric patients with eosinophilic esophagitis. Ann. Allergy Asthma Immunol. 2010, 104, 496–502. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Result | Reference Range | Unit |
|---|---|---|---|
| Red blood cells | 3.8 | 3.6–5.1 | ×106/mm3 |
| Hemoglobin | 11.3 | 10.2–14.2 | g/dL |
| Hematocrit | 34.8 | 31.0–40.5 | % |
| Platelets | 211 | 140–440 | ×103/mm3 |
| WBC | 15.3 | 5.5–15.5 | ×103/mm3 |
| NEU # | 4.65 | 1.5–8.5 | ×103/mm3 |
| LYM # | 10.02 | 2.0–8.0 | ×103/mm3 |
| EOS # | 0.28 | 0.02–0.65 | ×103/mm3 |
| CRP (C-reactive protein) | 0.24 | <0.50 | mg/dL |
| Albumin | 4.0 | 3.8–5.4 | g/dL |
| AST | 42 | <56 | U/L |
| ALT | 17 | <39 | U/L |
| sIgE Bovine serum albumin | 22.75 | <0.35 | kU/L |
| sIgE Casein | 2.72 | <0.35 | kU/L |
| sIgE Beta-lactoglobulin | 9.45 | <0.35 | kU/L |
| sIgE Alpha-lactalbumin | 6.45 | <0.35 | kU/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzec, A.; Jarocka-Cyrta, E.; Ruskań-Bakun, M. Eosinophilic Esophagitis in a 3-Year-Old Girl with Spinal Muscular Atrophy Type 1: The First Reported Case. Pediatr. Rep. 2025, 17, 80. https://doi.org/10.3390/pediatric17040080
Marzec A, Jarocka-Cyrta E, Ruskań-Bakun M. Eosinophilic Esophagitis in a 3-Year-Old Girl with Spinal Muscular Atrophy Type 1: The First Reported Case. Pediatric Reports. 2025; 17(4):80. https://doi.org/10.3390/pediatric17040080
Chicago/Turabian StyleMarzec, Aleksandra, Elżbieta Jarocka-Cyrta, and Marta Ruskań-Bakun. 2025. "Eosinophilic Esophagitis in a 3-Year-Old Girl with Spinal Muscular Atrophy Type 1: The First Reported Case" Pediatric Reports 17, no. 4: 80. https://doi.org/10.3390/pediatric17040080
APA StyleMarzec, A., Jarocka-Cyrta, E., & Ruskań-Bakun, M. (2025). Eosinophilic Esophagitis in a 3-Year-Old Girl with Spinal Muscular Atrophy Type 1: The First Reported Case. Pediatric Reports, 17(4), 80. https://doi.org/10.3390/pediatric17040080







