Psychopathological Comorbidities in Children and Adolescents with Feeding and Eating Disorders: An Italian Clinical Study
Abstract
1. Introduction
1.1. Exploring the Psychopathology of FED with the K-SADS-PL
1.2. Study Objectives
2. Materials and Methods
2.1. Procedure
- Depressive disorder/bipolar disorder: mild (minimal symptoms beyond diagnostic criteria, manageable distress, minor impairment), moderate (symptoms and impairment between mild and severe), and severe (excessive symptoms, seriously distressing, significant interference).
- Attention deficit/hyperactivity disorder (ADHD): mild, moderate, and severe (see above).
- Conduct disorder: mild, moderate, and severe (see above)
- Oppositional defiant disorder: mild (symptoms in one setting), moderate (symptoms in at least two environments), and severe (symptoms in three or more environments).
- OCD: good or fair insight (recognizes OCD beliefs may not be true), poor insight (believes OCD beliefs are probably true), and absent insight/delusional beliefs (fully convinced OCD beliefs are true).
2.2. Statistical Analysis
3. Results
3.1. Types of Diagnosed Eating Disorders
- -
- 37 (51.3%) restrictive AN (AN-R);
- -
- 9 (12.5%) binging/purging AN (AN-B/P);
- -
- 10 (13.9%) other specified feeding and eating disorders (OSFED), of which 8 (80%) had atypical AN (AN-A) and 2 (20%) had low-frequency or limited duration of BN;
- -
- 5 (6.9%) ARFID;
- -
- 3 (4.2%) BED;
- -
- 3 (4.2%) unspecified feeding and eating disorder (UFED);
- -
- 2 (2.7%) BN.
3.2. Family History
3.3. Descriptive Analysis of Comorbid Psychopathologies
3.4. Comorbid Psychopathologies Distribution
3.5. Relationship Between FED, Registered BMI, and Severity of Comorbid Psychopathology Detected by K-SADS-PL
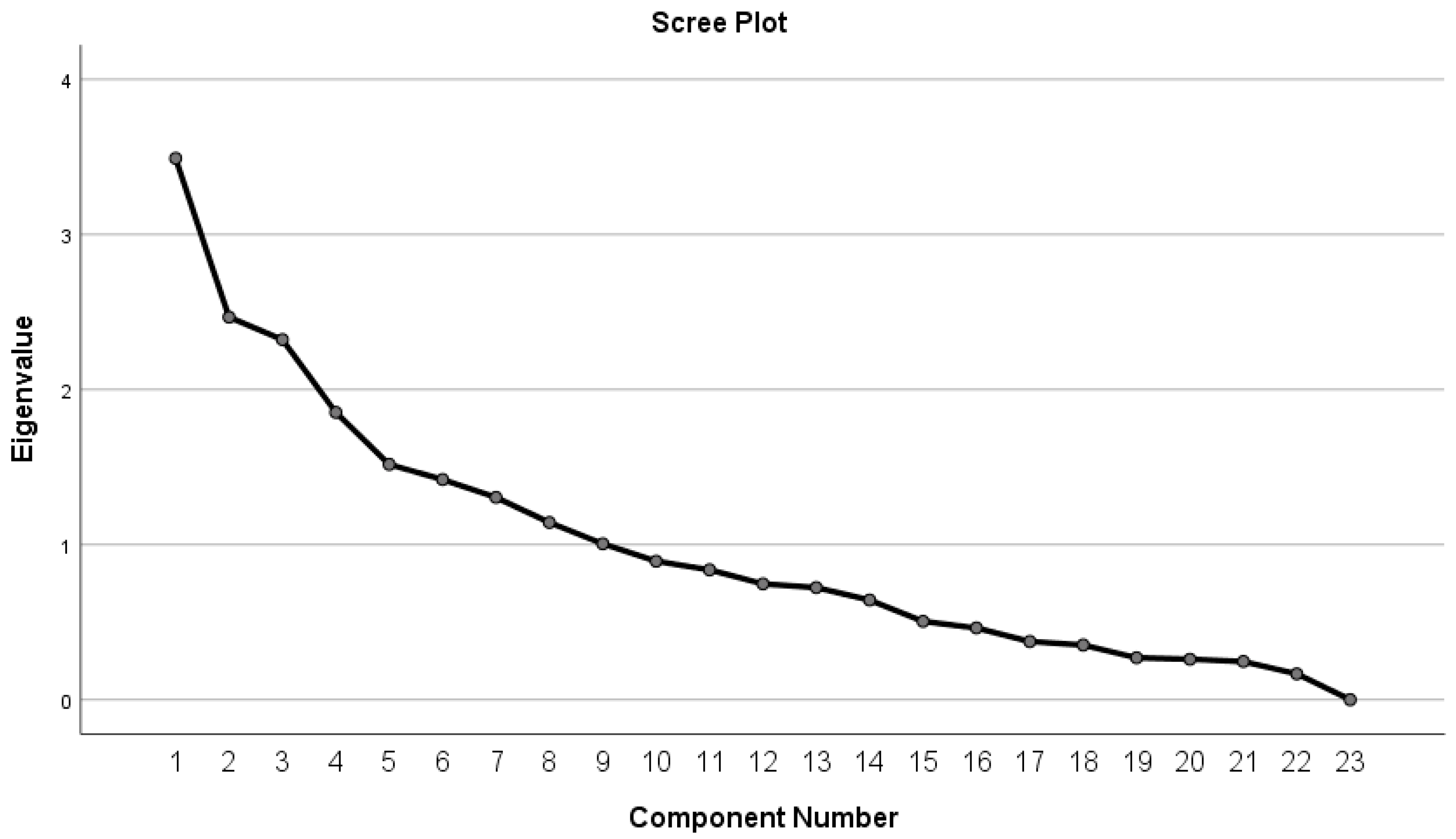
- Principal Component Analysis
- ANOVA
- Main Effects
- Post hoc Comparisons
- BN vs. AN-B/P: The factor scores for individuals with BN were significantly different from those with AN-B/P, with a mean difference of −3.67 (p < 0.001).
- BN vs. AN-R: Similarly, individuals with BN differed significantly from those with the restrictive subtype of AN-R, with a mean difference of −3.98 (p < 0.001).
- BN vs. AN-A: There was a significant difference between BN and the group diagnosed with (AN-A), with a mean difference of −3.07 (p < 0.001).
- BN vs. ARFID: The factor scores for BN individuals also significantly differed from those with avoidant/restrictive food intake disorder (ARFID), with a mean difference of −3.94 (p < 0.001).
- BN vs. BED: Individuals with BN had significantly different factor scores compared to those with BED, with a mean difference of −4.52 (p < 0.001).
4. Discussion
4.1. Characterization of Mood Disorders
4.2. Characterization of Anxiety Disorders
4.3. Characterization of OCD
4.4. Characterization of ADHD
4.5. Characterization of Distruptive, Impulse-Control, and Conduct Disorders
4.6. Characterization of Psychotic Symptoms
4.7. Practical Implication
4.8. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Anxiety Disorders |
| ADHD | Attention Deficit/Hyperactivity Disorder |
| AN | Anorexia Nervosa |
| AN-A | Atypical Anorexia Nervosa |
| AN-B/P | Binge Purging Anorexia Nervosa |
| ANCOVA | Analysis of Covariance |
| ANOVA | Analysis of Variance |
| AN-R | Restrictive Anorexia Nervosa |
| ARFID | Avoidant Restrictive Food Intake Disorder |
| BD | Bipolar Disorder |
| BD-I | Bipolar I Disorder |
| BD-II | Bipolar II Disorder |
| BED | Binge Eating Disorder |
| BMI | Body mass index |
| BN | Bulimia Nervosa |
| BN-A | Atypical Bulimia Nervosa |
| CBCL | Child Behavior Check List |
| CBT | Cognitive–Behavioral Therapy |
| CD | Conduct Disorder |
| CDI | Children’s Depression Inventory |
| DBT | Dialectical Behavior Therapy |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders, fifth edition |
| DSRSC | Depression Self Rating Scale for Children |
| ED | Elimination Disorders |
| ERP | Exposure and Response Prevention |
| FED | Feeding and Eating Disorders |
| GAD | Generalized Anxiety Disorder |
| JASP | Jeffreys’s Amazing Statistics Program |
| K-SADS-PL | Kiddie Schedule for Affective Disorders and Schizophrenia, Present and Lifetime Version |
| MASC | Multidimensional Anxiety Scale for Children |
| MDD | Major Depressive Disorder |
| OCD | Obsessive–Compulsive Disorder |
| ODD | Oppositional Defiant Disorder |
| OSFED | Other Specified Feeding and Eating Disorder |
| PCA | Principal Component Analysis |
| SAD | Separation Anxiety Disorder |
| SCARED | Screen for Child Anxiety Related Emotional Disorders |
| SNAP | Swanson, Noland, and Pelham |
| SUD | Substance Use Disorder |
| TD | Movement–Tic Disorders |
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5-TRTM, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Faccio, E. Le Identità Corporee: Quando L’immagine di sé fa Star Male/Elena Faccio; Presentazione di Alessandro Salvini: Firenze, Italy, 2007; p. xiii+229. Available online: http://digitocs.unibo.it/orti.php?id=BID_2544731 (accessed on 9 March 2024).
- Zanetti, T. Mirror Mirror on the Wall, Whos the Thinnest of Them All? Reflections on Anorexia Nervosa in Adolescence; Eating Disorders in the 21st Century Series; Nova Biomedical: New York, NY, USA, 2014. [Google Scholar]
- ISS. Comunicato Stampa N°20/2022-Giornata del Fiocchetto Lilla sui Disturbi Alimentari, Aggiornata la Mappa dei Servizi Sanitari, ad Oggi Sono Oltre Cento i Centri Accreditati. 2022. Available online: https://www.iss.it/cov19-atterraggio/-/asset_publisher/yX1afjCDBkWH/content/comunicato-stampa-n%C2%B020-2022-giornata-del-fiocchetto-lilla-sui-disturbi-alimentari-aggiornata-la-mappa-dei-servizi-sanitari-ad-oggi-sono-oltre-cento-i-centri-accreditati (accessed on 10 February 2024).
- Zuccotti, G.V. Manuale di Pediatria-la Pratica Clinica, 2nd ed.; Società Editrice Esculapio: Bologna, Italy, 2016; p. 1239. [Google Scholar]
- Silén, Y.; Keski-Rahkonen, A. Worldwide prevalence of DSM-5 eating disorders among young people. Curr. Opin. Psychiatry 2022, 35, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Dalla Ragione, L.; Vanzetta, R. Social Fame: Adolescenza, Social Media e Disturbi Alimentari/Laura Dalla Ragione, Raffaela Vanzetta. Prefazione di Fiorenza Sarzanini; Il Pensiero Scientifico: Roma, Italy, 2023; p. 276. Available online: http://digitocs.unibo.it/orti.php?id=BID_9095432 (accessed on 9 March 2024).
- Spina Giulia. Ospedalebambingesu.it. Disturbi del Comportamento Alimentare: Boom di Casi con la Pandemia da SARS-CoV-2. Available online: https://www.ospedalebambinogesu.it/disturbi-del-comportamento-alimentare-boom-di-casi-con-la-pandemia-da-sars-cov-2-149481/ (accessed on 15 January 2024).
- Nada, A.; Delbon, P.; Masetti, M.; Shayma, A.; Di Maio, S.; Millimaggi, G.; Brunocilla, E.; Franceschelli, A.; Leopoldo Ruggiero, L.; Cassani, C.; et al. L’utilizzo dei Social Network Come Fattore di Rischio per L’insorgenza Precoce dei Disturbi Della Nutrizione e Dell’alimentazione. 9 May 2022. Available online: https://issuu.com/edizioniscriptamanent/docs/rima_1_22 (accessed on 9 March 2024).
- Mushtaq, T.; Ashraf, S.; Hameed, H.; Irfan, A.; Shahid, M.; Kanwal, R.; Aslam, M.A.; Shahid, H.; Noor, K.E.; Shazly, G.A.; et al. Prevalence of Eating Disorders and Their Association with Social Media Addiction among Youths. Nutrients 2023, 15, 4687. [Google Scholar] [CrossRef]
- Bressi, C.; Invernizzi, G. Manuale di Psichiatria e Psicologia Clinica/Cinzia Bressi, Giordano Invernizzi, 5th ed.; McGraw-Hill: Milano, Italy, 2017; p. xxvi+836. Available online: http://digitocs.unibo.it/orti.php?id=BID_4797801 (accessed on 20 February 2024).
- Fairburn, C.G.; Cooper, Z.; Doll, H.A.; Welch, S.L. Risk Factors for Anorexia Nervosa: Three Integrated Case-Control Comparisons. Arch. Gen. Psychiatry 1999, 56, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Kovács-Tóth, B.; Oláh, B.; Szabó, I.K.; Túry, F. Adverse childhood experiences increase the risk for eating disorders among adolescents. Front. Psychol. 2022, 13, 1063693. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9791097/ (accessed on 12 March 2024). [CrossRef] [PubMed]
- Bulik, C.M.; Coleman, J.R.I.; Hardaway, J.A.; Breithaupt, L.; Watson, H.J.; Bryant, C.D.; Breen, G. Genetics and Neurobiology of Eating Disorders. Nat. Neurosci. 2022, 25, 543–554. [Google Scholar] [CrossRef]
- Troop, N.A. Helplessness, mastery and the development of eating disorders: Exploring the links between vulnerability and precipitating factors. Eat. Weight Disord. Stud. Anorexia Bulim. Obes. 2012, 17, e274–e281. [Google Scholar] [CrossRef]
- Ministero Della Salute. DNA-Dati Epidemiologici. 2023. Available online: https://www.salute.gov.it/portale/saluteMentale/dettaglioContenutiSaluteMentale.jsp?lingua=italiano&id=6029&area=salute%20mentale&menu=DNA (accessed on 11 February 2024).
- Siracusano, A.; Troisi, A.; Marino, V.; Tozzi, F. Comorbilità nei disturbi della condotta alimentare: Revisione critica della letteratura. Nòos 2003, 1, 7–23. [Google Scholar]
- Kaufman, J.; Birmaher, B.; Brent, D.; Rao, U.; Flynn, C.; Moreci, P.; Williamson, D.; Ryan, N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial Reliability and Validity Data. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 980–988. [Google Scholar] [CrossRef]
- Makino, T.; Suzuki, F.; Nishiyama, T.; Ishibashi, S.; Nakamichi, H.; Iida, T.; Higashi, T.; Fukumoto, S.; Kurata, S.; Mizuno, Y.; et al. Psychometrics of the kiddie schedule for affective disorders and schizophrenia present and lifetime version for DSM-5 in Japanese outpatients: International Journal of Methods in Psychiatric Research. Int. J. Methods Psychiatry Res. 2023, 32, e1957. [Google Scholar] [CrossRef]
- Dun, Y.; Li, Q.-R.; Yu, H.; Bai, Y.; Song, Z.; Lei, C.; Li, H.-H.; Gong, J.; Mo, Y.; Li, Y.; et al. Reliability and validity of the Chinese version of the kiddie-schedule for affective disorders and schizophrenia-present and lifetime version DSM-5 (K-SADS-PL-C DSM-5). J. Affect. Disord. 2022, 317, 72–78. [Google Scholar] [CrossRef]
- Marques, C.C.; Matos, A.P.; do Céu Salvador, M.; Arnarson, E.Ö.; Craighead, W.E. Reliability and Validity of the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Portuguese Version: Child Psychiatry & Human Development. Child. Psychiatry Hum. Dev. 2022, 53, 1119–1128. [Google Scholar]
- Kolaitis, G.; Zaravinos-Tsakos, F.; Rokas, I.-M.; Syros, I.; Tsakali, A.; Belivanaki, M.; Giannakopoulos, G. Navigating young minds: Reliability and validity of the Greek version of kiddie–schedule for affective disorders and schizophrenia–present and lifetime DSM-5 version (K-SADS-PL-GR-5). BMC Psychiatry 2023, 23, 614. [Google Scholar] [CrossRef] [PubMed]
- Birmaher, B.; Ehmann, M.; Axelson, D.A.; Goldstein, B.I.; Monk, K.; Kalas, C.; Kupfer, D.; Gill, M.K.; Leibenluft, E.; Bridge, J.; et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS-PL) for the Assessment of Preschool Children- A Preliminary Psychometric Study. J. Psychiatr. Res. 2009, 43, 680–686. [Google Scholar] [CrossRef]
- Błachno, M.; Bryńska, A.; Tomaszewicz-Libudzic, C.; Jagielska, G.; Srebnicki, T.; Wolańczyk, T. The influence of obsessive compulsive symptoms on the course of anorexia nervosa. Psychiatry Pol. 2014, 48, 429–439. [Google Scholar]
- Mohammadi, M.R.; Mostafavi, S.; Hooshyari, Z.; Khaleghi, A.; Ahmadi, N.; Molavi, P.; Kian, A.A.; Safavi, P.; Delpisheh, A.; Talepasand, S.; et al. Prevalence, correlates and comorbidities of feeding and eating disorders in a nationally representative sample of Iranian children and adolescents. Int. J. Eat. Disord. 2020, 53, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Kalayci, B.M.; Nalbant, K.; Akdemir, D. Autistic Traits and Social Responsiveness: The Relationship Between Autistic Traits and Comorbid Psychiatric Symptoms in Adolescents with Anorexia Nervosa. Noro Psikiyatr. Arsivi 2021, 58, 283–288. [Google Scholar] [CrossRef]
- Kaufman, J.; Sogos, C. K-SADS-PL DSM-5®: Intervista Diagnostica per la Valutazione dei Disturbi Psicopatologici in Bambini e Adolescenti; Erickson: Portland, OR, USA, 2019; Available online: http://digitocs.unibo.it/orti.php?id=BID_5662922 (accessed on 24 February 2024).
- Smink, F.R.E.; van Hoeken, D.; Hoek, H.W. Epidemiology of eating disorders: Incidence, prevalence and mortality rates. Curr. Psychiatry Rep. 2012, 14, 406–414. [Google Scholar] [CrossRef]
- Brewerton, T.D.; Gavidia, I.; Suro, G.; Perlman, M.M. Associations between major depressive and bipolar disorders and eating disorder, PTSD, and comorbid symptom severity in eating disorder patients. Eur. Eat. Disord. Rev. 2024, 32, 188–200. [Google Scholar] [CrossRef]
- Fornaro, M.; Daray, F.M.; Hunter, F.; Anastasia, A.; Stubbs, B.; De Berardis, D.; Shin, J.I.; Husain, M.I.; Dragioti, E.; Fusar-Poli, P.; et al. The prevalence, odds and predictors of lifespan comorbid eating disorder among people with a primary diagnosis of bipolar disorders, and vice-versa: Systematic review and meta-analysis. J. Affect. Disord. 2021, 280, 409–431. [Google Scholar] [CrossRef]
- Barakat, S.; McLean, S.A.; Bryant, E.; Le, A.; Marks, P.; Touyz, S.; Maguire, S. Risk factors for eating disorders: Findings from a rapid review. J. Eat. Disord. 2023, 11, 8. [Google Scholar] [CrossRef]
- Visser, H.; van Megen, H.; van Oppen, P.; Hoogendoorn, A.; Glas, G.; Neziroglu, F.; van Balkom, A. The impact of poor insight on the course of Obsessive-Compulsive Disorder in patients receiving naturalistic treatment. J. Obs.-Compuls. Relat. Disord. 2017, 13, 42–48. [Google Scholar] [CrossRef]
- DeSocio, J.E. Challenges in diagnosis and treatment of comorbid eating disorders and mood disorders. Perspect. Psychiatry Care 2019, 55, 494–500. [Google Scholar] [CrossRef]
- Steiger, H. Eating disorders and the serotonin connection: State, trait and developmental effects. J. Psychiatry Neurosci. 2004, 29, 20–29. [Google Scholar]
- Hay, P.; Girosi, F.; Mond, J. Prevalence and sociodemographic correlates of DSM-5 eating disorders in the Australian population. J. Eat. Disord. 2015, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Hocaoglu, C. Eating Disorders with Comorbidity Anxiety Disorders. In Eating Disorders-A Paradigm of the Biopsychosocial Model of Illness; IntechOpen: Rijeka, Croatia, 2017; Available online: https://www.intechopen.com/chapters/52740 (accessed on 13 May 2024).
- Garcia, S.C.; Ma, M.E.M.; Keel, P.K.; Burt, S.A.; Neale, M.C.; Boker, S.; Klump, K.L. Increased rates of eating disorders and their symptoms in women with major depressive disorder and anxiety disorders. Int. J. Eat. Disord. 2020, 53, 1844–1854. [Google Scholar] [CrossRef]
- Sander, J.; Moessner, M.; Bauer, S. Depression, Anxiety and Eating Disorder-Related Impairment: Moderators in Female Adolescents and Young Adults. Int. J. Environ. Res. Public Health 2021, 18, 2779. [Google Scholar] [CrossRef] [PubMed]
- Kaye, W.H.; Bulik, C.M.; Thornton, L.; Barbarich, N.; Masters, K.; Group PFC. Comorbidity of Anxiety Disorders with Anorexia and Bulimia Nervosa. Am. J. Psychiatry 2004, 161, 2215–2221. [Google Scholar] [CrossRef]
- Godart, N.T.; Flament, M.F.; Curt, F.; Perdereau, F.; Lang, F.; Venisse, J.L.; Halfon, O.; Bizouard, P.; Loas, G.; Corcos, M.; et al. Anxiety disorders in subjects seeking treatment for eating disorders: A DSM-IV controlled study. Psychiatry Res. 2003, 117, 245–258. [Google Scholar] [CrossRef]
- Swinbourne, J.; Hunt, C.; Abbott, M.; Russell, J.; St Clare, T.; Touyz, S. The comorbidity between eating disorders and anxiety disorders: Prevalence in an eating disorder sample and anxiety disorder sample. Aust. N. Z. J. Psychiatry 2012, 46, 118–131. [Google Scholar] [CrossRef]
- Khalil, R.B.; Bou-Orm, I.R.; Tabet, Y.; Souaiby, L.; Azouri, H. Disgust and fear: Common emotions between eating and phobic disorders. Eat. Weight Disord. Stud. Anorexia Bulim. Obes. 2020, 25, 79–86. [Google Scholar] [CrossRef]
- Wilson, G.T.; Grilo, C.M.; Vitousek, K.M. Psychological treatment of eating disorders. Am. Psychol. 2007, 62, 199–216. [Google Scholar] [CrossRef]
- Hambleton, A.; Pepin, G.; Le, A.; Maloney, D.; Touyz, S.; Maguire, S. Psychiatric and medical comorbidities of eating disorders: Findings from a rapid review of the literature. J. Eat. Disord. 2022, 10, 132. [Google Scholar] [CrossRef]
- Serpell, L.; Livingstone, A.; Neiderman, M.; Lask, B. Anorexia nervosa: Obsessive–compulsive disorder, obsessive–compulsive personality disorder, or neither? Clin. Psychol. Rev. 2002, 22, 647–669. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tong, G.; Wang, Y.; Ruan, H.; Zheng, Z.; Cheng, J.; Wang, Z. Task fMRI studies investigating inhibitory control in patients with obsessive-compulsive disorder and eating disorders: A comparative meta-analysis. World J. Biol. Psychiatry 2024, 25, 26–42. [Google Scholar] [CrossRef]
- Drakes, D.H.; Fawcett, E.J.; Rose, J.P.; Carter-Major, J.C.; Fawcett, J.M. Comorbid obsessive-compulsive disorder in individuals with eating disorders: An epidemiological meta-analysis. J. Psychiatr. Res. 2021, 141, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Steinglass, J.; Walsh, B.T. Habit learning and anorexia nervosa: A cognitive neuroscience hypothesis. Int. J. Eat. Disord. 2006, 39, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Villa, F.; Crippa, A.; Rosi, E.; Nobile, M.; Brambilla, P.; Delvecchio, G. ADHD and eating disorders in childhood and adolescence: An updated minireview. J. Affect. Disord. 2023, 321, 265–271. [Google Scholar] [CrossRef]
- Capusan, A.J.; Bendtsen, P.; Marteinsdottir, I.; Larsson, H. Comorbidity of Adult ADHD and Its Subtypes With Substance Use Disorder in a Large Population-Based Epidemiological Study. J. Atten. Disord. 2019, 23, 1416–1426. [Google Scholar] [CrossRef]
- Porfirio, M.-C.; Giovinazzo, S.; Cortese, S.; Giana, G.; Lo-Castro, A.; Mouren, M.-C.; Curatolo, P.; Purper-Ouakil, D. Role of ADHD symptoms as a contributing factor to obesity in patients with MC4R mutations. Med. Hypotheses 2015, 84, 4–7. [Google Scholar] [CrossRef]
- Bryant, E.; Spielman, K.; Le, A.; Marks, P.; Touyz, S.; Maguire, S. Screening, assessment and diagnosis in the eating disorders: Findings from a rapid review. J. Eat. Disord. 2022, 10, 78. [Google Scholar] [CrossRef]
- Keel, P.K.; Holm-Denoma, J.M.; Crosby, R.D. Clinical Significance and Distinctiveness of Purging Disorder and Binge Eating Disorder. Int. J. Eat. Disord. 2011, 44, 311–316. [Google Scholar] [CrossRef]
- Fernández-Aranda, F.; Jiménez-Murcia, S.; Álvarez-Moya, E.M.; Granero, R.; Vallejo, J.; Bulik, C.M. Impulse control disorders in eating disorders: Clinical and therapeutic implications. Compr. Psychiatry 2006, 47, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Kaye, W. Neurobiology of Anorexia and Bulimia Nervosa Purdue Ingestive Behavior Research Center Symposium Influences on Eating and Body Weight over the Lifespan: Children and Adolescents. Physiol. Behav. 2008, 94, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Motevalli, S. The Relationship between Adverse Childhood Experiences and Conduct Disorder among Primary School Students. Int. J. School Health 2024, 11, 221–231. [Google Scholar]
- Poletti, M.; Preti, A.; Raballo, A. Eating Disorders and Psychosis as Intertwined Dimensions of Disembodiment: A Narrative Review. Clin. Neuropsychiatry 2022, 19, 187–192. [Google Scholar] [PubMed]
- Aya, V.; Ulusoy, K.; Cardi, V. A systematic review of the ‘eating disorder voice’ experience. Int. Rev. Psychiatry Abingdon Engl. 2019, 31, 347–366. [Google Scholar] [CrossRef] [PubMed]
- Noordenbos, G.; Aliakbari, N.; Campbell, R. The relationship among critical inner voices, low self-esteem, and self-criticism in eating disorders. Eat. Disord. 2014, 22, 337–351. [Google Scholar] [CrossRef]
- Barton, R.; Aouad, P.; Hay, P.; Buckett, G.; Russell, J.; Sheridan, M.; Brakoulias, V.; Touyz, S. Distinguishing delusional beliefs from overvalued ideas in Anorexia Nervosa: An exploratory pilot study. J. Eat. Disord. 2022, 10, 85. [Google Scholar] [CrossRef]
| Theoretical Models | Relationship Between FED and Psychiatric and Medical Comorbidities |
|---|---|
| First model | FED as the primary disorder, leading to the development of comorbidities |
| Second model | Pre-existing disorders increase vulnerability to the development of FED |
| Third model | Latent mood or anxiety disorders emerge during or after FED onset |
| Fourth model | Shared neurobiological deficits (neuroendocrine, brain dysfunctions) contribute to FED and comorbidities |
| Fifth model | FED and other psychiatric disorder arise from shared etiological factors (genetics, environment) |
| Family History of Psychiatric Disorders | Number of Patients (n, %) |
|---|---|
| FED | 22 (30.6%) |
| Mood Disorders | 15 (20.8%) |
| Anxiety Disorders | 7 (9.7%) |
| Psychotic Disorder | 2 (2.8%) |
| Substance Use Disorders | 2 (2.8%) |
| Personality Disorder | 1 (1.4%) |
| Diagnostic Category | Family History of Psychiatric Disorder (n, %) |
|---|---|
| BED and UFED | 2 (66.7%) |
| AN-R | 20 (54.1%) |
| BN and OSFED | 1 (50%) |
| ARFID | 1 (20%) |
| AN-B/P | 1 (11.1%) |
| Patients who presented to the first outpatient visit | 0 (p = 0.2; X2 = 10.4) |
| N (%) | Distribution—N (%) | Main Symptoms | |
|---|---|---|---|
| Mood Disorders | 48 (66.5) | Dysthymia—24 (33.3) MDD—16 (22.2) BD-II—8 (11.1) | Persistent low mood, generalized fatigue, cognitive disturbances, psychomotor impairments, reduced self-awareness, excessive feelings of guilt |
| Anxiety Disorders | 63 (87.5) | GAD—35 (48.6) PD—15 (20.8) SAD—15 (20.8) | Panic attacks, catastrophic thoughts about separation from parental figures, nightmares |
| Phobic Disorders | 53 (73.6) | Specific phobias—45 (62.5) Social phobia—22 (30.6) Agoraphobia—22 (30.6) | Anticipatory anxiety, panic attacks in crowded contexts and avoidance behaviors mainly in connection with social situations related to food or exposure of their body |
| OCD | 34 (47.2) | Obsessions—46 (63.7) Compulsive behaviors—42 (58.3) | Symmetry obsessions, aggressive thoughts, fear of contamination and/or disease, hoarding obsessions; compulsions of control, counting, touching, cleaning, repetition, hoarding |
| ADHD | 22 (30.5) | Inattentive phenotype—15 (20.8) Hyperactive/Impulsive phenotype—3 (4.2) Mixed phenotype—4 (5.6) | Difficulty sitting, distractibility, impulsivity, difficulty in sustaining attention |
| Behavioral Disorders | 10 (13.9) | ODD—6 (8.3) CD—2 (2.8) Both—2 (2.8) | Irritable and angry mood, provocative or argumentative behaviors, aspects of vindictiveness, serious rule violation, previous episodes of fraud or theft, histories of aggression |
| Psychotic Symptoms | 29 (40.3) | Delusions—8 (11.1) Hallucinations—5 (6.9) Both—16 (22.2) | Delusions of guilt or sin, reference, persecution, thought insertion, nihilism, grandiosity/omnipotence and influence; auditory hallucinations, described as voices calling the subject’s name and having an imperative and/or commenting role; visual, tactile, and olfactory hallucinations |
| Tic Disorders | 0 (0) | Not found—0 (0) | - |
| Elimination Disorders | 0 (0) | Not found—0 (0) | - |
| No-FED Diagnosis (N%) | AN-B/P (N%) | AN-R (N%) | ARFID (N%) | BED (N%) | BN (N%) | OSFED (N%) | UFED (N%) | Total (N%) | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Depressive Disorders | ||||||||||
| MDD | 0 (0) | 2 (2.8) | 7 (9.7) | 1 (1.4) | 0 (0) | 2 (2.8) | 4 (5.6) | 1 (1.4) | 16 (22.2) | X2 (7.72) 9.447; p = 0.200 |
| Dysthymia | 1 (1.4) | 3 (4.2) | 14 (19.4) | 1 (1.4) | 1 (1.4) | 0 (0) | 3 (4.2) | 1 (1.4) | 24 (33.3) | X2 (7.72) 1.788; p = 0.800 |
| Bipolar Disorders | ||||||||||
| BD-I | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | p = 1.000 |
| BD-II | 0 (0) | 1 (1.4) | 4 (5.6) | 0 (0) | 0 (0) | 1 (1.4) | 2 (2.8) | 0 (0) | 8 (11.1) | X2 (7.72) 4.979; p = 0.700 |
| Anxiety Disorders | ||||||||||
| Panic Disorder | 0 (0) | 3 (4.2) | 3 (4.2) | 1 (1.4) | 1 (1.4) | 1 (1.4) | 5 (6.9) | 1 (1.4) | 12 (20.8) | X2 (7.72) 12.035; p = 0.090 |
| SAD | 1 (1.4) | 1 (1.4) | 5 (6.9) | 1 (1.4) | 1 (1.4) | 1 (1.4) | 3 (4.2) | 2 (2.8) | 15 (20.8) | X2 (7.72) 7.650; p = 0.400 |
| GAD | 2 (2.8) | 4 (5.6) | 16 (22.2) | 1 (1.4) | 2 (2.8) | 1 (1.4) | 7 (9.7) | 2 (2.8) | 35 (48.6) | X2 (7.72) 5.135; p = 0.600 |
| Social Phobia | 1 (1.4) | 3 (4.2) | 11 (15.3) | 1 (1.4) | 1 (1.4) | 0 (0) | 3 (4.2) | 2 (2.8) | 22 (30.6) | X2 (7.72) 3.054; p = 0.900 |
| Agoraphobia | 0 (0) | 2 (2.8) | 9 (12.5) | 1 (1.4) | 1 (1.4) | 2 (2.8) | 6 (8.3) | 1 (1.4) | 22 (30.6) | X2 (7.72) 10.824; p = 0.100 |
| Specific Phobia | 2 (2.8) | 6 (8.3) | 20 (27.8) | 4 (5.6) | 3 (4.2) | 2 (2.8) | 6 (8.3) | 2 (2.8) | 45 (62.5) | X2 (7.72) 11.382; p = 0.700 |
| OCD | 1 (1.4) | 3 (4.2) | 18 (25.0) | 1 (1.4) | 1 (1.4) | 1 (1.4) | 6 (8.3) | 3 (4.2) | 34 (47.2) | X2 (7.72) 6.692; p = 0.500 |
| ADHD | 0 (0) | 4 (5.6) | 8 (11.1) | 0 (0) | 3 (4.2) | 1 (1.4) | 4 (5.6) | 2 (2.8) | 22 (30.6) | X2 (7.72) 15.168; p = 0.100 |
| Distruptive, Impulse- Control, CD | ||||||||||
| ODD | 0 (0) | 0 (0) | 5 (6.9) | 0 (0) | 1 (1.4) | 1 (1.4) | 1 (1.4) | 0 (0) | 8 (11.1) | X2 (7.72) 15.531; p = 0.400 |
| CD | 0 (0) | 0 (0) | 1 (1.4) | 0 (0) | 1 (1.4) | 1 (1.4) | 0 (0) | 1 (1.4) | 4 (5.6) | X2 (7.72) 18.515; p = 0.009 |
| Psychotic Symptoms | ||||||||||
| Delusions | 0 (0) | 4 (5.6) | 13 (18.1) | 0 (0) | 0 (0) | 0 (0) | 5 (6.9) | 2 (2.8) | 24 (33.3) | X2 (7.72) 9.447; p = 0.200 |
| Hallucinations | 0 (0) | 2 (2.8) | 11 (15.3) | 0 (0) | 1 (1.4) | 0 (0) | 4 (5.6) | 2 (2.8) | 20 (27.8) | X2 (7.72) 7.107; p = 0.400 |
| Other Disorders | ||||||||||
| Elimination Disorders | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | p = 1.000 |
| Tic Disorders | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | p = 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Califano, M.; Pruccoli, J.; Cavallino, O.; Lenzi, A.; Parmeggiani, A. Psychopathological Comorbidities in Children and Adolescents with Feeding and Eating Disorders: An Italian Clinical Study. Pediatr. Rep. 2025, 17, 61. https://doi.org/10.3390/pediatric17030061
Califano M, Pruccoli J, Cavallino O, Lenzi A, Parmeggiani A. Psychopathological Comorbidities in Children and Adolescents with Feeding and Eating Disorders: An Italian Clinical Study. Pediatric Reports. 2025; 17(3):61. https://doi.org/10.3390/pediatric17030061
Chicago/Turabian StyleCalifano, Maria, Jacopo Pruccoli, Oliviero Cavallino, Alessandra Lenzi, and Antonia Parmeggiani. 2025. "Psychopathological Comorbidities in Children and Adolescents with Feeding and Eating Disorders: An Italian Clinical Study" Pediatric Reports 17, no. 3: 61. https://doi.org/10.3390/pediatric17030061
APA StyleCalifano, M., Pruccoli, J., Cavallino, O., Lenzi, A., & Parmeggiani, A. (2025). Psychopathological Comorbidities in Children and Adolescents with Feeding and Eating Disorders: An Italian Clinical Study. Pediatric Reports, 17(3), 61. https://doi.org/10.3390/pediatric17030061








