Affective Neuroscience Personality Scale (ANPS) in Children with Internalizing Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instruments
2.3. Procedure
2.4. Statistical Analysis
3. Results
3.1. Distribution of Neuroemotional Systems for the Age and Sex Variables
3.2. Intercorrelations Between the ANPS Scales in the Control and Clinical Groups
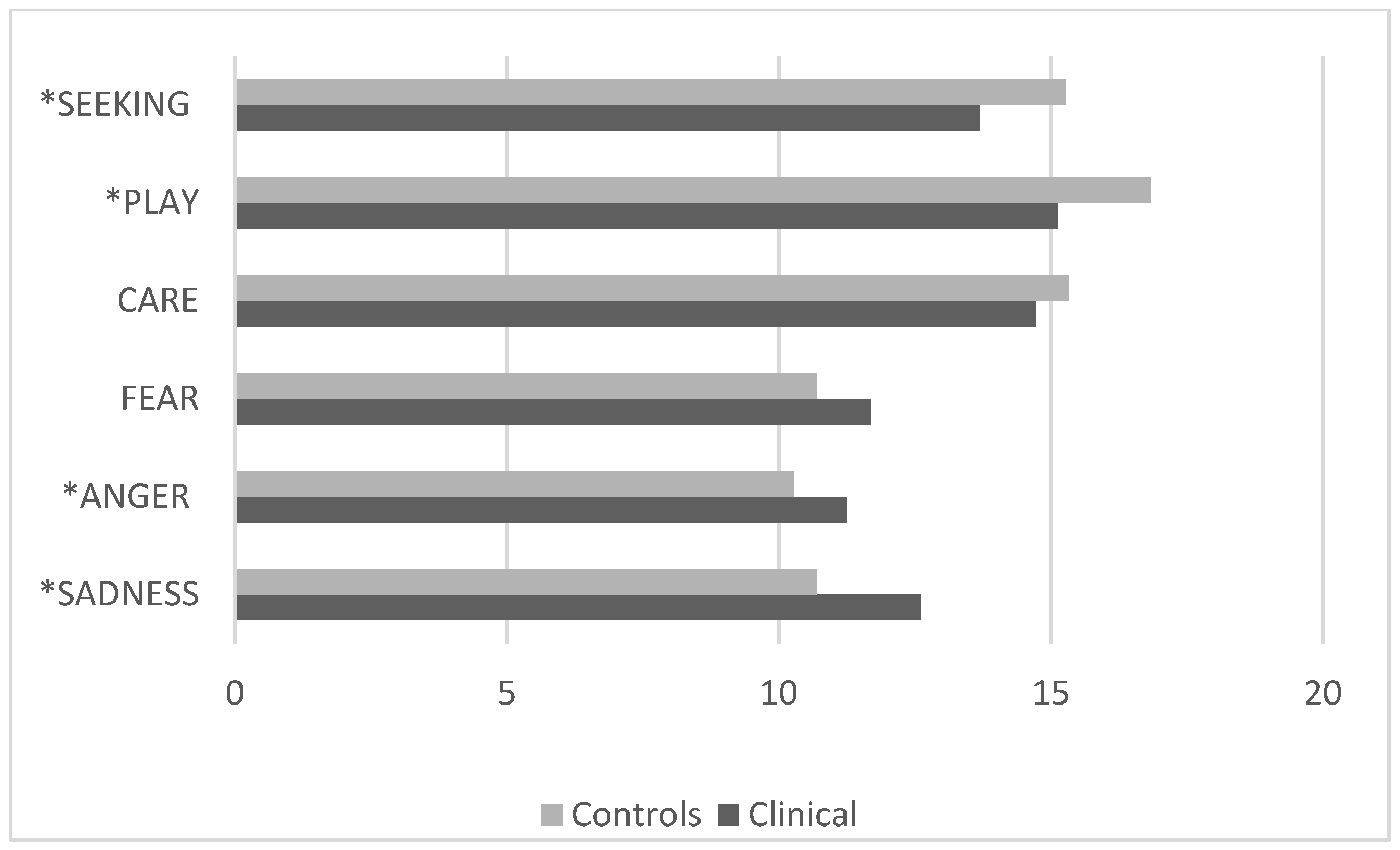
3.3. Comparison Between the Control Group and the Clinical Group with Internalizing Disorders
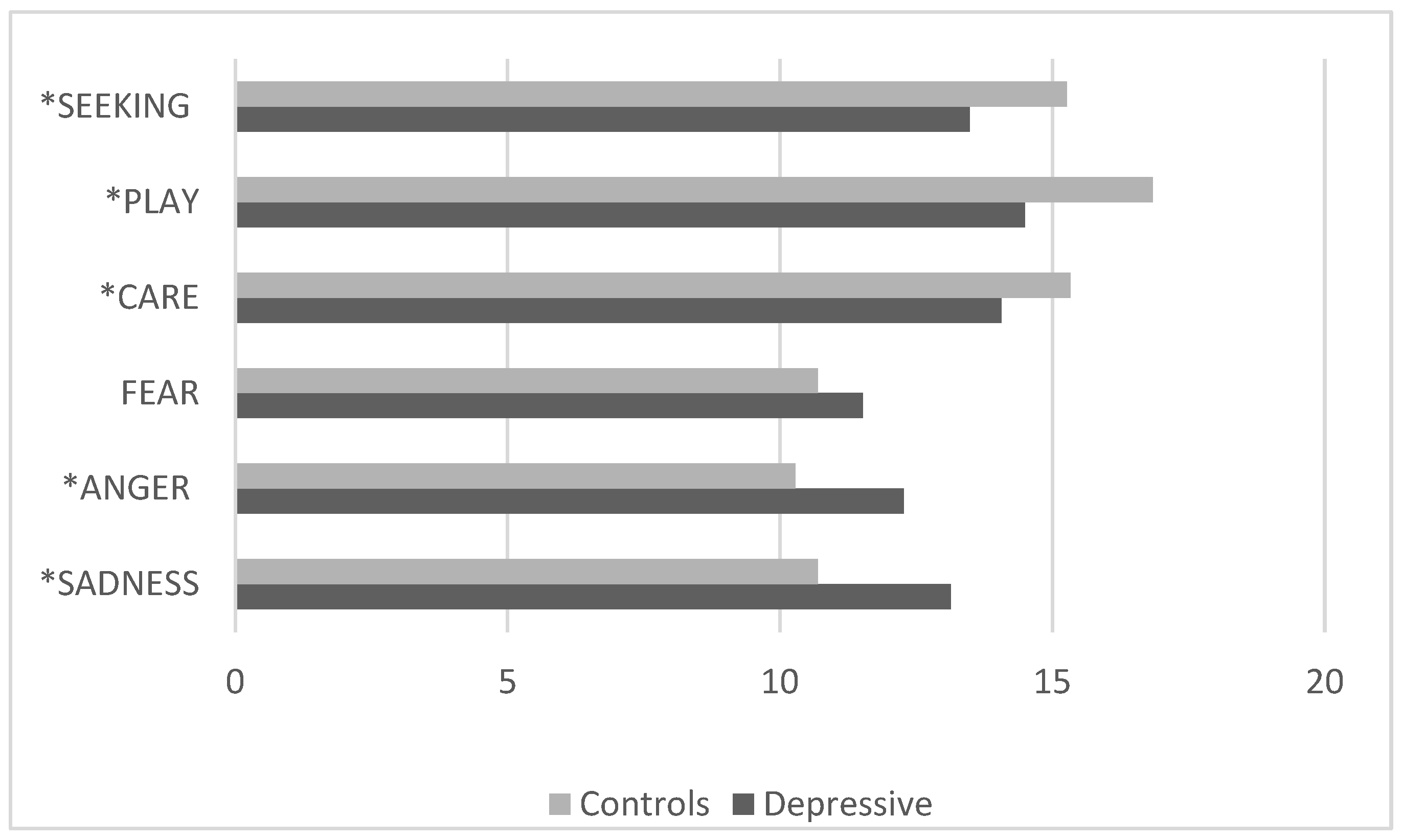
3.4. Comparison Between the Control Group and the Clinical Group with Depressive Disorders
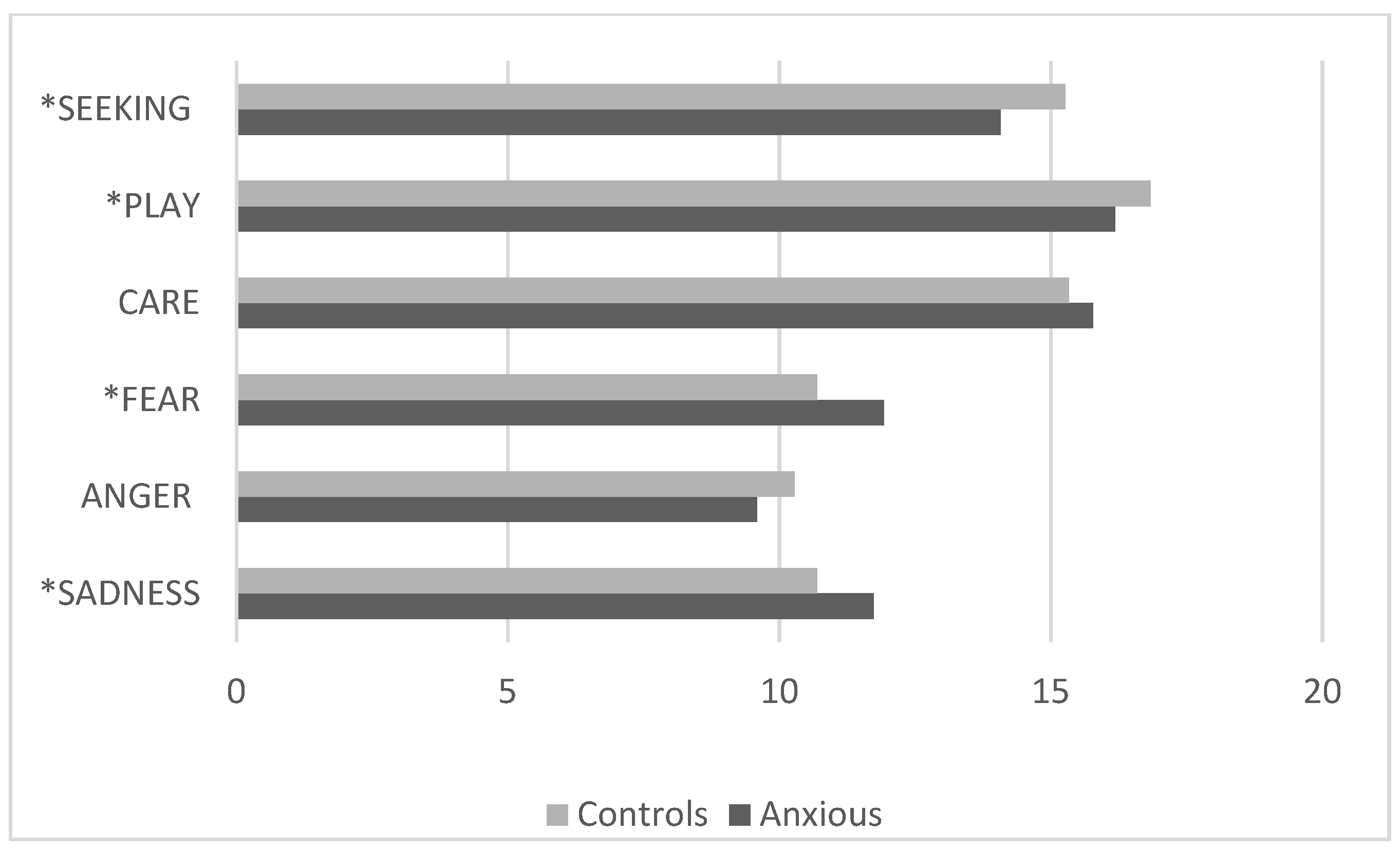
3.5. Comparison Between the Control Group and the Clinical Group with Anxiety Disorders
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANPS | Affective Neuroscience Personality Scales |
References
- Panksepp, J. Affective Neuroscience: The Foundations of Human and Animal Emotions; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Coenen, V.A.; Schlaepfer, T.E. Panksepp’s SEEKING System Concepts and Their Implications for the Treatment of Depression with Deep-Brain Stimulation. Neuropsychoanalysis 2012, 14, 43–45. [Google Scholar] [CrossRef]
- Panksepp, J.; Biven, L. The Archaeology of Mind: Neuroevolutionary Origins of Human Emotion; Norton: New York, NY, USA, 2012. [Google Scholar]
- Panksepp, J. On the embodied neural nature of core emotional affects. J. Conscious. Stud. 2005, 12, 158–184. [Google Scholar]
- Heidbreder, C.; Gewiss, M.; Demot, B.; Mertens, I.; De Witte, P. Balance of glutamate and dopamine in the nucleus accumbens modulates self-stimulation behavior after injection of cholecystokinin and neurotensin in the rat brain. Peptides 1992, 13, 441–449. [Google Scholar] [CrossRef]
- Yeomans, J.S.; Mathur, A.; Tampakeras, M. Rewarding brain stimulation: Role of tegmental cholinergic neurons that activate dopamine neurons. Behav. Neurosci. 1993, 107, 1077–1087. [Google Scholar] [CrossRef]
- Siegel, A. The Neurobiology of Aggression and Rage; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Damasio, A.R.; Grabowski, T.J.; Bechara, A.; Damasio, H.; Ponto, L.L.; Parvizi, J.; Hichwa, R.D. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat. Neurosci. 2000, 3, 1049–1056. [Google Scholar] [CrossRef]
- Brandão, M.L.; Anseloni, V.Z.; Pandossio, J.E.; De Araujo, J.E.; Castilho, V.M. Neurochemical mechanisms of the defensive behavior in the dorsal midbrain. Neurosci. Biobehav. Rev. 1999, 23, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Brandão, M.L.; Borelli, K.G.; Nobre, M.J.; Santos, J.M.; Albrechet-Souza, L.; Oliveira, A.R.; Martinez, R.Z. Gabaergic regulation of the neural organization of fear in the midbrain tectum. Neurosci. Biobehav. Rev. 2005, 29, 1299–1311. [Google Scholar] [CrossRef]
- Brandão, M.L.; Zanoveli, J.M.; Ruiz-Martinez, R.C.; Oliveira, L.C.; Landeira-Fernandez, J. Different patterns of freezing behavior organized in the periaqueductal gray of rats: Association with different types of anxiety. Behav. Brain. Res. 2008, 188, 1–13. [Google Scholar] [CrossRef]
- Panksepp, J. The psychoneurology of fear: Evolutionary perspectives and the role of animal models in understanding human anxiety. In Handbook of Anxiety: The Neurobiology of Anxiety; Burrows, G.D., Roth, M., Noyes, R., Jr., Eds.; Elsevier: Amsterdam, The Netherlands, 1990; Volume 3, pp. 3–58. [Google Scholar]
- Amano, K.; Notani, M.; Iseki, H.; Kawabatake, H.; Tanikawa, T.; Kawamura, H.; Kitamura, K. Homovanillic acid concentration of the third ventricular CSF before and after electrical stimulation of the midbrain central gray and the periventricular gray in humans. In Modern Concepts in Psychiatric Surgery, Proceedings of the 5th World Congress of Psychiatric Surgery, Boston, MA, USA, 21–25 August 1978; Elsevier: Amsterdam, The Netherlands, 1979; Volume 1, p. 65. [Google Scholar]
- Kinsley, C.H.; Lambert, K.G. The maternal brain. Sci. Am. 2006, 294, 72–79. [Google Scholar] [CrossRef]
- Kinsley, C.H.; Lambert, K.G. Reproduction-induced neuroplasticity: Natural behavioural and neuronal alterations associated with the production and care of offspring. Neuroendocrinology 2008, 20, 515–525. [Google Scholar] [CrossRef]
- Jirkowski, G.F.; Caldwell, J.D.; Pilgrim, C.; Stumpf, W.E.; Pedersen, C.A. Changes for immunostain for oxytocin in forebrain of the female rat during late pregnancy, parturition and early lactation. Cell Tissue Res. 1989, 256, 411–417. [Google Scholar]
- Numan, M. Neural control of maternal behavior. In Mammalian Parenting; Hrasnergor, N.A., Bridges, R.S., Eds.; Oxford University Press: New York, NY, USA, 1990; pp. 321–359. [Google Scholar]
- Panksepp, J. Brain emotional circuits and psychopathologies. In Emotions and Psychopathology; Clynes, M., Panksepp, J., Eds.; Plenum: New York, NY, USA, 1988; pp. 37–76. [Google Scholar]
- Bejjani, B.-P.; Damier, P.; Arnulf, I.; Thivard, L.; Bonnet, A.-M.; Dormont, D.; Cornu, P.; Pidoux, B.; Samson, Y.; Agid, Y. Transient acute depression induced by high-frequency deep-brain stimulation. N. Engl. J. Med. 1999, 340, 1476–1480. [Google Scholar] [CrossRef] [PubMed]
- Vanderschuren, L.J. How the brain makes play fun. Am. J. Play 2010, 2, 315–337. [Google Scholar]
- Panksepp, J.; Crepeau, L.; Clynes, M. Effects of CRF on separation distress in juvenile play. Soc. Neurosci. Abstr. 1987, 13, 1320. [Google Scholar]
- Guerra, D.J.; Colonnello, V.; Panksepp, J. The neurobiology of RAGE and anger & psychiatric implications with a focus on depression. In Multiple Facets of Anger: Getting Mad or Restoring Justice? Pahlavan, F., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2011. [Google Scholar]
- Panksepp, J. Emotional endophenotypes in evolutionary psychiatry. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 774–784. [Google Scholar] [CrossRef]
- Gottesman, I.I.; Gould, T.D. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am. J. Psychiatry 2003, 160, 636–645. [Google Scholar] [CrossRef]
- Brown, S.M.; Hariri, A.R. Neuroimaging studies of serotonin gene polymorphisms: Exploring the interplay of genes, brain, and behavior. Cogn. Affect. Behav. Neurosci. 2006, 6, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Roozendaal, B.; McEwen, B.S.; Chattarji, S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 2009, 10, 423–433. [Google Scholar] [CrossRef]
- Goodman, M.; New, A.S.; Triebwasser, J.; Collins, K.A.; Siever, L. Phenotype, endophenotype, and genotype comparisons between borderline personality disorder and major depressive disorder. J. Pers. Disord. 2010, 24, 38–59. [Google Scholar] [CrossRef]
- Pearlson, G.D.; Calhoun, V. Structural and functional magnetic resonance imaging in psychiatric disorders. Can. J. Psychiatry 2007, 52, 158–166. [Google Scholar] [CrossRef]
- Savitz, J.B.; Drevets, W.C. Imaging phenotypes of major depressive disorder: Genetic correlates. Neuroscience 2009, 164, 300–330. [Google Scholar] [CrossRef] [PubMed]
- Panksepp, J. Affective consciousness: Core emotional feelings in animals and humans. Conscious. Cogn. 2005, 14, 30–80. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.L.; Panksepp, J.; Normansell, L. The affective neuroscience personality scales: Normative data and implications. Neuropsychoanalysis. 2003, 5, 57–69. [Google Scholar] [CrossRef]
- Polanczyk, G.V.; Salum, G.A.; Sugaya, L.S.; Caye, A.; Rohde, L.A. Annual Research Review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J. Child. Psychol. Psychiatry 2015, 56, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.; Camilleri, N.; Eberhardt, J.; Umla-Runge, K.; Newbury-Birch, D. A systematic review and meta-analysis on the prevalence of mental disorders among children and adolescents in Europe. Eur. Child Adolesc. Psychiatry 2022, 30, 1–18. [Google Scholar] [CrossRef]
- Coenen, V.A.; Schlaepfer, T.E.; Maedler, B.; Panksepp, J. Cross-species affective functions of the medial forebrain bundle—Implications for the treatment of affective pain and depression in humans. Neurosci. Biobehav. Rev. 2011, 35, 1971–1981. [Google Scholar] [CrossRef]
- Panksepp, J.; Watt, J. Why does depression hurt? Ancestral primary-process separation-distress (PANIC) and diminished brain reward (SEEKING) processes in the genesis of depressive affect. Psychiatry 2011, 74, 5–14. [Google Scholar] [CrossRef]
- Bowlby, J. A Secure Base: Parent-Child Attachment and Healthy Human Development; Routledge: London, UK, 1988. [Google Scholar]
- Nelson, E.E.; Panksepp, J. Brain substrates of infant–mother attachment: Contributions of opioids, oxytocin, and norepinephrine. Neurosci. Biobehav. Rev. 1998, 22, 437–452. [Google Scholar] [CrossRef]
- Horesh, N.; Klomek, A.B.; Apter, A. Stressful life events and major depressive disorders. Psychiatry Res. 2008, 160, 192–199. [Google Scholar] [CrossRef]
- Mullins, L.L.; Siegel, L.J.; Hodges, K. Cognitive problem-solving and life event correlates of depressive symptoms in children. J. Abnorm. Child Psychol. 1985, 13, 305–314. [Google Scholar] [CrossRef]
- Zellner, M.R.; Watt, D.F.; Solms, M.; Panksepp, J. Affective neuroscientific and neuropsychoanalytic approaches to two intractable psychiatric problems: Why depression feels so bad and what addicts really want. Neurosci. Biobehav. Rev. 2011, 35, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Panksepp, J.; Sacks, D.S.; Crepeau, L.J.; Abbott, B.B. The psycho and neurobiology of fear systems in the brain. In Fear, Avoidance, and Phobias; Denny, M.R., Ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1991; pp. 7–59. [Google Scholar]
- Zanoveli, J.M.; Brandão, M.L. The dorsal periaqueductal and basolateral amygdala are necessary for the expression of conditioned place avoidance induced by semicarbazide stimulation of the dorsal periaqueductal region. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1715–1721. [Google Scholar] [CrossRef]
- Gloor, P. The Temporal Lobe and Limbic System; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Nashold, B.S.; Wilson, W.P.; Slaughter, G. Sensations evoked by stimulation of the midbrain of man. J. Neurosurg. 1969, 30, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Panksepp, J. Mood changes. In Handbook of Clinical Neurology; Vinken, P., Bruyn, G., Klawans, H., Eds.; Elsevier: Amsterdam, The Netherlands, 1985. [Google Scholar]
- Burrows, G.D.; Roth, M.; Noyes, R., Jr. Handbook of Anxiety. In The Neurobiology of Anxiety; Elsevier: Amsterdam, The Netherlands, 1990; Volume 3. [Google Scholar]
- Panksepp, J. Gray zones at the emotion–cognition interface: A commentary. Cogn. Emot. 1990, 4, 289–302. [Google Scholar] [CrossRef]
- Adamec, R.E.; Young, B. Neuroplasticity in specific limbic system circuits may mediate specific kindling induced changes in animal affect-implications for understanding anxiety associated with epilepsy. Neurosci. Biobehav. Rev. 2000, 24, 705–723. [Google Scholar] [CrossRef] [PubMed]
- Maren, S. Long-term potentiation in the amygdala: A mechanism for emotional learning and memory. Trends Neurosci. 1999, 22, 561–567. [Google Scholar] [CrossRef]
- Montag, C.; Elhai, J.D.; Davis, K.L. A comprehensive review of studies using the Affective Neuroscience Personality Scales in the psychological and psychiatric sciences. Neurosci. Biobehav. Rev. 2021, 125, 160–167. [Google Scholar] [CrossRef]
- Davis, K.L.; Panksepp, J. The brain’s emotional foundations of human personality and the Affective Neuroscience Personality Scales. Neurosci. Biobehav. Rev. 2011, 35, 1946–1958. [Google Scholar] [CrossRef]
- Marengo, D.; Davis, K.L.; Gradwohl, G.Ö.; Montag, C. A meta-analysis on individual differences in primary emotional systems and Big Five personality traits. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- He, Z.; Lu, F.; Sheng, W.; Han, S.; Pang, Y.; Chen, Y.; Chen, H. Abnormal functional connectivity as neural biological substrate of trait and state characteristics in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 102, 109949. [Google Scholar] [CrossRef]
- Montag, C.; Widenhorn-Müller, K.; Panksepp, J.; Kiefer, M. Individual differences in Affective Neuroscience Personality Scale (ANPS) primary emotional traits and depressive tendencies. Compr. Psychiatry 2017, 73, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J.; van der Merwe, L.; Ramesar, R. Dysthymic and anxiety-related personality traits in bipolar spectrum illness. J. Affect. Disord. 2008, 109, 305–311. [Google Scholar] [CrossRef]
- Savitz, J.; van der Merwe, L.; Ramesar, R. Hypomanic, cyclothymic and hostile personality traits in bipolar spectrum illness: A family-based study. J. Psychiatr. Res. 2008, 42, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Talarowska, M.; Wysiadecki, G.; Chodkiewicz, J. Affective neuroscience personality scales and early maladaptive schemas in depressive disorders. Int. J. Environ. Res. Public Health 2022, 19, 8062. [Google Scholar] [CrossRef]
- Brienza, L.; Zennaro, A.; Vitolo, E.; Andò, A. Affective Neuroscience Personality Scale (ANPS) and clinical implications: A systematic review. J. Affect. Disord. 2023, 320, 178–195. [Google Scholar] [CrossRef]
- Panksepp, J. The long-term psychobiological consequences of infant emotions: Prescriptions for the twenty-first century. Infant. Ment. Health 2001, 22, 132–173. [Google Scholar] [CrossRef]
- Levi, G.; Sogos, C.; Mazzei, E.; Paolesse, C. Depressive disorder in preschool children: Patterns of affective organization. Child. Psychiatry Hum. Dev. 2001, 32, 55–69. [Google Scholar] [CrossRef]
- Cupellaro, S.; Colonnello, V.; Sogos, C.; Ubertini, C.; Pankseep, J.; Levi, G. La valutazione dei sistemi neuroemotivi nei bambini. Studio esplorativo dell’Affective Neuroscience Personality Scale (ANPS) in età scolare. Psicol. Clin. Dello Sviluppo. 2021, 1, 51–67. [Google Scholar] [CrossRef]
- Barbaranelli, C.; Caprara, G.V.; Rabasca, A.; Pastorelli, C.A. questionnaire for measuring the Big Five in late childhood. Pers. Individ. Dif. 2003, 34, 645–664. [Google Scholar] [CrossRef]
- Pahlavan, F.; Mouchiroud, C.; Zenasni, F.; Panksepp, J. Validation de l’adaptation française de l’échelle neuro-affective de personnalité [French validation of the Affective Neuroscience Personality Scales (ANPS)]. Eur. Rev. Appl. Psychol. 2008, 58, 155–163. [Google Scholar] [CrossRef]
- Abella, V.; Panksepp, J.; Manga, D.; Bárcena, C.; Iglesias, J.A. Spanish validation of the Affective Neuroscience Personality Scales. Span. J. Psychol. 2011, 14, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Pingault, J.B.; Falissard, B.; Côté, S.; Berthoz, S. A new approach of personality and psychiatric disorders: A short version of the Affective Neuroscience Personality Scales. PLoS ONE 2012, 7, e41489. [Google Scholar] [CrossRef] [PubMed]
- Barrett, F.S.; Robins, R.W.; Janata, P.A. brief form of the Affective Neuroscience Personality Scales. Psychol. Assess. 2013, 25, 826. [Google Scholar] [CrossRef] [PubMed]
- Giacolini, T.; Ardizzone, I.; Davis, K.L.; Ferrara, M.; Picconi, L.; Terrinoni, A.; Sabatello, U. Brain emotional systems: The Italian version of the ANPS—Affective Neuroscience Personality Scales 2.4 (reliability and validity). Clin. Neuropsychiatry 2017, 14, 263–274. [Google Scholar]
- Pascazio, L.; Bembich, S.; Nardone, I.B.; Vecchiet, C.; Guarino, G.; Clarici, A. Validation of the Italian translation of the Affective Neuroscience Personality Scales. Psychol. Rep. 2015, 116, 97–115. [Google Scholar] [CrossRef]
- Davis, K.L.; Panksepp, J. The Emotional Foundations of Personality: A Neurobiological and Evolutionary Approach; WW Norton and Company: New York, NY, USA, 2018. [Google Scholar]
- Kaufman, J.; Birmaher, B.; Rao, U.; Ryan, N.; Kaufman, J.; Birmaher, B.; Rao, U.; Ryan, N.; A Cura Di Sogos, C.; Di Noia, S.P.; et al. 2019 K-SADS-PL DSM-5 Intervista Diagnostica per La Valutazione Dei Disturbi Psicopatologici in Bambini e Adolescenti. In Schedule for Affective Disorders and Schizophrenia for School-Aged Children: Present and Lifetime Version (K-SADS-PL) DSM-5; Erickson, Ed.; Erickson: Trento, Italy, 2019. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Derntl, B.; Finkelmeyer, A.; Eickhoff, S.; Kellermann, T.; Falkenberg, D.I.; Schneider, F.; Habel, U. Multidimensional assessment of empathic abilities: Neural correlates and gender differences. Psychoneuroendocrinology 2010, 35, 67–82. [Google Scholar] [CrossRef]
- Schulte-Rüther, M.; Markowitsch, H.J.; Shah, N.J.; Fink, G.R.; Piefke, M. Gender differences in brain networks supporting empathy. Neuroimage 2008, 42, 393–403. [Google Scholar] [CrossRef]
- Brensilver, M.; Negriff, S.; Mennen, F.E.; Trickett, P.K. Longitudinal relations between depressive symptoms and externalizing behavior in adolescence: Moderating effects of maltreatment experience and gender. J. Clin. Child Adolesc. Psychol. 2011, 40, 607–617. [Google Scholar] [CrossRef]
- Gjerde, P.F.; Block, J.; Block, J.H. Depressive symptoms and personality during late adolescence: Gender differences in the externalization-internalization of symptom expression. J. Abnorm. Psychol. 1988, 97, 475–486. [Google Scholar] [CrossRef]
- Masten, A.S.; Roisman, G.I.; Long, J.D.; Burt, K.B.; Obradović, J.; Riley, J.R.; Boelcke-Stennes, K.; Tellegen, A. Developmental cascades: Linking academic achievement and externalizing and internalizing symptoms over 20 years. Dev. Psychol. 2005, 41, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, T.E.; Caspi, A.; Rutter, M.; Silva, P.A. Sex Differences in Antisocial Behavior; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Silverthorn, P.; Frick, P.J. Developmental pathways to antisocial behavior: The delayed-onset pathway in girls. Dev. Psychopathol. 1999, 11, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Erskine, H.E.; Ferrari, A.J.; Nelson, P.; Polanczyk, G.V.; Flaxman, A.D.; Vos, T.; Whiteford, H.A.; Scott, J.G. Epidemiological modelling of attention-deficit/hyperactivity disorder and conduct disorder for the Global Burden of Disease Study 2010. J. Child. Psychol. Psychiatry 2013, 54, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Ginott, H. Group Psychotherapy with Children: The Theory and Practice of Play Therapy; McGraw-Hill Books: London, UK, 1994. [Google Scholar]
- Schaefer, C.E. Play therapy for psychic trauma in children. In Handbook of pPlay Therapy, Volume Two: Advances and Innovations; O’Connor, K.J., Schaefer, C., Eds.; John Wiley & Sons: New York, NY, USA, 1994; pp. 297–319. [Google Scholar]
- Landreth, G.L. Self-expressive communication. In The Therapeutic Powers of Play; Schaefer, C.E., Ed.; Jason Aronson: Lanham, MD, USA, 1993. [Google Scholar]
- Mol Lous, A.; de Wit, C.A.; De Bruyn, E.E.; Riksen-Walraven, J.M. Depression markers in young children’s play: A comparison between depressed and non-depressed 3- to 6-year-olds in various play situations. J. Child. Psychol. Psychiatry 2002, 43, 1029–1038. [Google Scholar] [CrossRef]
- Nelson, L.J.; Hart, C.H.; Yang, C.; Wu, P.; Jin, S. An examination of the behavioral correlates of subtypes of nonsocial play among Chinese preschoolers. Merrill Palmer Q. 2012, 58, 77–109. [Google Scholar] [CrossRef]
- Bratton, S.C.; Ray, D.; Rhine, T.; Jones, L. The efficacy of play therapy with children: A meta-analytic review of treatment outcomes. Prof. Psychol. Res. Pract. 2005, 36, 376–390. [Google Scholar] [CrossRef]
- Leblanc, M.; Ritchie, M. A meta-analysis of play therapy outcomes. Couns. Psychol. Q. 2001, 14, 149–163. [Google Scholar] [CrossRef]
- O’Connor, K.J.; Shaefer, C.E.; Braverman, L.D. Handbook of Play Therapy, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Cautin, R.L.; Overholser, J.C.; Goetz, P. Assessment of mode of anger expression in adolescent psychiatric inpatients. Adolescence 2001, 36, 163–170. [Google Scholar]
- Kosterman, R.; Hawkins, J.D.; Mason, W.A.; Herrenkohl, T.I.; Lengua, L.J.; McCauley, E. Assessment of behavior problems in childhood and adolescence as predictors of early adult depression. J. Psychopathol. Behav. Assess. 2010, 32, 118–127. [Google Scholar] [CrossRef]
- Mason, W.A.; Kosterman, R.; Hawkins, J.D.; Herrenkohl, T.I.; Lengua, L.J.; McCauley, E. Predicting depression, social phobia, and violence in early adulthood from childhood behavior problems. J. Am. Acad. Child. Adolesc. Psychiatry 2004, 43, 307–315. [Google Scholar] [CrossRef]
- Roland, E. Aggression, depression and bullying others. Aggress. Behav. 2002, 28, 198–206. [Google Scholar] [CrossRef]
- Sijtsema, J.J.; Oldehinkel, A.J.; Veenstra, R.; Verhulst, F.C.; Ormel, J. Effects of structural and dynamic family characteristics on the development of depressive and aggressive problems during adolescence. The TRAILS study. Eur. Child. Adolesc. Psychiatry 2014, 23, 499–513. [Google Scholar] [CrossRef]
- Weiss, B.; Catron, T. Specificity of the comorbidity of aggression and depression in children. J. Abnorm. Child. Psychol. 1994, 22, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Angold, A.L.; Costello, E.J. Depressive comorbidity in children and adolescents: Empirical, theoretical, and methodological issues. J. Psychiatry 1993, 150, 1779–1791. [Google Scholar] [CrossRef]
- Brady, E.U.; Kendall, P.C. Comorbidity of anxiety and depression in children and adolescents. Psychol. Bull. 1992, 111, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Seligman, L.D.; Ollendick, T.H. Comorbidity of anxiety and depression in children and adolescents: An integrative review. Clin. Child. Fam. Psychol. Rev. 1998, 1, 125–144. [Google Scholar] [CrossRef]
- Bittner, A.; Goodwin, R.D.; Wittchen, H.U.; Beesdo, K.; Höfler, M.; Lieb, R. What Characteristics of Primary Anxiety Disorders Predict Subsequent Major Depressive Disorder? J. Clin. Psychiatry 2004, 65, 618–626. [Google Scholar] [CrossRef]
- Chorpita, B.F.; Daleiden, E.L. Tripartite dimensions of emotion in a child clinical sample: Measurement strategies and implications for clinical utility. J. Consult. Clin. Psychol. 2002, 70, 1150–1160. [Google Scholar] [CrossRef]
- Cole, D.A.; Peeke, L.G.; Martin, J.M.; Truglio, R.; Seroczynski, A.D. A longitudinal look at the relation between depression and anxiety in children and adolescents. J. Consult. Clin. Psychol. 1998, 66, 451. [Google Scholar] [CrossRef]
- Merikangas, K.R.; Avenevoli, S. Epidemiology of mood and anxiety disorders in children and adolescents. In Textbook in Psychiatric Epidemiology; Tsuang, M.T., Tohen, M., Eds.; Wiley-Liss: Hoboken, NJ, USA, 2002. [Google Scholar]
- Biederman, J.; Petty, C.R.; Hirshfeld-Becker, D.R.; Henin, A.; Faraone, S.V.; Fraire, M.; Henry, B.; McQuade, J.; Rosenbaum, J.F. Developmental trajectories of anxiety disorders in offspring at high risk for panic disorder and major depression. Psychiatry Res. 2007, 153, 245–252. [Google Scholar] [CrossRef]
- Foley, D.L.; Pickles, A.; Maes, H.M.; Silberg, J.L.; Eaves, L.J. Course and short-term outcomes of separation anxiety disorder in a community sample of twins. J. Am. Acad. Child. Adolesc. Psychiatry 2004, 43, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Keenan, K.; Feng, X.; Hipwell, A.; Klostermann, S. Depression begets depression: Comparing the predictive utility of depression and anxiety symptoms to later depression. J. Child. Psychol. Psychiatry 2009, 50, 1167–1175. [Google Scholar] [CrossRef]
- Warner, V.; Wickramaratne, P.; Weissman, M.M. The role of fear and anxiety in the familial risk for major depression: A three-generation study. Psychol. Med. 2008, 38, 1543. [Google Scholar] [CrossRef] [PubMed]
- Panksepp, J.; Fuchs, T.; Iacobucci, P. The basic neuroscience of emotional experiences in mammals: The case of subcortical FEAR circuitry and implications for clinical anxiety. Appl. Anim. Behav. Sci. 2011, 129, 1–17. [Google Scholar] [CrossRef]
- Davis, M. Neural systems involved in fear and anxiety measured with fear potentiated startle. Am. Psychol. 2006, 61, 741–756. [Google Scholar] [CrossRef]
- Maren, S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: Cautions and caveats. Eur. J. Neurosci. 2008, 28, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Quirk, G.J.; Mueller, D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 2008, 33, 56–72. [Google Scholar] [CrossRef]
- Carlsson, K.; Petersson, K.M.; Lundqvist, D.; Karlsson, A.; Ingvar, M.; Öhman, A. Fear and the amygdala: Manipulation of awareness generates differential cerebral responses to phobic and fear-relevant (but non-feared) stimuli. Emotion 2004, 4, 340. [Google Scholar] [CrossRef]
- Etkin, A.; Wager, T.D. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry 2007, 164, 1476–1488. [Google Scholar] [CrossRef]
- Öhman, A. The role of the amygdala in human fear: Automatic detection of threat. Psychoneuroendocrinology 2005, 30, 953–958. [Google Scholar] [CrossRef]
- Shin, L.M.; Liberzon, I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010, 35, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Scaini, S.; Centorame, G.; Lissandrello, F.; Sardella, S.; Stazi, M.A.; Fagnani, C.; Brombin, C.; Battaglia, M. The role of genetic and environmental factors in covariation between anxiety and anger in childhood. Eur. Child. Adolesc. Psychiatry 2021, 30, 607–617. [Google Scholar] [CrossRef]
- Essau, C.A.; Ishikawa, S.-I.; Sasagawa, S.; Sato, H.; Okajima, I.; Otsui, K.; Georgiou, G.A.; O’Callaghan, J.; Michie, F. Anxiety symptoms among adolescents in Japan and England: Their relationship with self-construals and social support. Depress. Anxiety 2011, 28, 509–518. [Google Scholar] [CrossRef]
- Nantel-Vivier, A.; Pihl, R.O.; Côté, S.; Tremblay, R.E. Developmental association of prosocial behaviour with aggression, anxiety and depression from infancy to preadolescence. J. Child. Psychol. Psychiatry 2014, 55, 1135–1144. [Google Scholar] [CrossRef]
- Okuyucu, M.; Kaya, H.; Konyalıoğlu, F.S.; Göka, E. Prosocial behavior, anxiety levels, and psychosocial risk factors in adolescents with generalized anxiety disorder: A comparison with healthy controls. Psychiatry Behav. Sci. 2024, 14, 123. [Google Scholar] [CrossRef]
- Itoi, K.; Sugimoto, N. The brainstem noradrenergic systems in stress, anxiety and depression. J. Neuroendocrinol. 2010, 22, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.J. The serotonergic system in mood disorders and suicidal behaviour. Philos. Trans. R Soc. Lond. B Biol. Sci. 2013, 368, 20120537. [Google Scholar] [CrossRef]
- Caspi, A.; Sugden, K.; Moffitt, T.E.; Taylor, A.; Craig, I.W.; Harrington, H.; McClay, J.; Mill, J.; Martin, J.; Braithwaite, A.; et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science 2003, 301, 386–389. [Google Scholar] [CrossRef]
- Risch, N.; Herrell, R.; Lehner, T.; Liang, K.Y.; Eaves, L.; Hoh, J.; Merikangas, K.R. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. JAMA 2009, 301, 2462–2471. [Google Scholar] [CrossRef]
- Chen, F.S.; Kumsta, R.; von Dawans, B.; Monakhov, M.; Ebstein, R.P.; Heinrichs, M. Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proc. Natl. Acad. Sci. USA 2011, 108, 19937–19942. [Google Scholar] [CrossRef]
- Troisi, A.; Frazzetto, G.; Carola, V.; Di Lorenzo, G.; Coviello, M.; D’Amato, F.R.; Moles, A.; Siracusano, A.; Gross, C. Social hedonic capacity is associated with the A118G polymorphism of the mu-opioid receptor gene (OPRM1) in adult healthy volunteers and psychiatric patients. Soc. Neurosci. 2011, 6, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Zai, C.C.; Muir, K.E.; Nowrouzi, B.; Shaikh, S.A.; Choi, E.; Berall, L.; Trépanier, M.-O.; Beitchman, J.H.; Kennedy, J.L. Possible genetic association between vasopressin receptor 1B and child aggression. Psychiatry Res. 2012, 200, 784–788. [Google Scholar] [CrossRef] [PubMed]
| SEEKING | FEAR | CARE | ANGER | PLAY | SADNESS | |
|---|---|---|---|---|---|---|
| SEEKING | 1.00 | −0.09 | 0.13 | −0.17 | 0.27 | −0.18 |
| FEAR | −0.09 | 1.00 | 0.16 | 0.20 | −0.18 | 0.65 |
| CARE | 0.13 | 0.16 | 1.00 | −0.09 | 0.32 | 0.20 |
| ANGER | −0.17 | 0.20 | −0.09 | 1.00 | −0.18 | 0.26 |
| PLAY | 0.27 | −0.18 | 0.32 | −0.18 | 1.00 | −0.23 |
| SADNESS | −0.18 | 0.65 | 0.20 | 0.26 | −0.23 | 1.00 |
| SEEKING | FEAR | CARE | ANGER | PLAY | SADNESS | |
|---|---|---|---|---|---|---|
| SEEKING | 1.00 | 0.14 | 0.28 | −0.07 | 0.48 | 0.08 |
| FEAR | 0.14 | 1.00 | 0.31 | 0.22 | 0.05 | 0.42 |
| CARE | 0.28 | 0.31 | 1.00 | −0.09 | 0.45 | 0.20 |
| ANGER | −0.07 | 0.22 | −0.09 | 1.00 | −0.20 | 0.44 |
| PLAY | 0.48 | 0.05 | 0.45 | −0.20 | 1.00 | −0.04 |
| SADNESS | 0.08 | 0.42 | 0.20 | 0.44 | −0.04 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cupellaro, S.; Colonnello, V.; Sabatello, U.; Ubertini, C.; Sogos, C. Affective Neuroscience Personality Scale (ANPS) in Children with Internalizing Disorders. Pediatr. Rep. 2025, 17, 55. https://doi.org/10.3390/pediatric17030055
Cupellaro S, Colonnello V, Sabatello U, Ubertini C, Sogos C. Affective Neuroscience Personality Scale (ANPS) in Children with Internalizing Disorders. Pediatric Reports. 2025; 17(3):55. https://doi.org/10.3390/pediatric17030055
Chicago/Turabian StyleCupellaro, Simone, Valentina Colonnello, Ugo Sabatello, Chiara Ubertini, and Carla Sogos. 2025. "Affective Neuroscience Personality Scale (ANPS) in Children with Internalizing Disorders" Pediatric Reports 17, no. 3: 55. https://doi.org/10.3390/pediatric17030055
APA StyleCupellaro, S., Colonnello, V., Sabatello, U., Ubertini, C., & Sogos, C. (2025). Affective Neuroscience Personality Scale (ANPS) in Children with Internalizing Disorders. Pediatric Reports, 17(3), 55. https://doi.org/10.3390/pediatric17030055







